Abstract
Nonspecific and COX-2 selective nonsteroidal anti-inflammatory drugs (NSAIDs) function by inhibiting the cyclooxygenase isoenzymes and effectively reduce pain and inflammation attributed to acute or chronic musculoskeletal pathologies. However, use of NSAIDs as an analgesic is thought to negatively contribute to bone healing. This review strived to provide a thorough unbiased analysis of the current research conducted on animals and humans regarding NSAIDs and their effect on bone healing. Specifically, this review discusses the role of animal models, dosing regiments, and outcome parameters when examining discrepancies about NSAIDS and their effects on bone regeneration. The role of COX-2 in bone regeneration needs to be better defined in order to further elucidate the impact of NSAIDs on bone healing.
Keywords: NSAIDs, bone healing, COX-2, COX-1, fracture
1. Prostaglandins, Cyclooxygenases, and Bone Metabolism
Vertebrate bone is in a constant state of flux between destruction of old tissue and synthesis of new in a process often referred to as bone remodeling. An imbalance in bone remodeling can lead to osteoporosis or osteopetrosis [1]. Prostaglandins synthesized from arachidonic acid via cyclooxygenase activity mediate bone destruction and bone formation. Cyclooxygenase (COX) is the initial enzymatic activity in the conversion of arachidonic acid into prostaglandins. Two distinct forms of cyclooxygenase have been isolated, COX-1 and COX-2. COX-1 is constitutively expressed and is involved in physiological functions such as gastric protection and hemostasis, while COX-2 is inductively expressed and is involved in pathophysiological processes such as pain, inflammation, and fever. While the molecular and physiological connections that might explain this paradox of prostaglandin induced bone destruction and formation are not well understood, the basis of the paradox lies in the fact that prostaglandins can independently stimulate osteoclast and osteoblast activity to, respectively, destroy and synthesize bone [2]. Given the clear-cut effects of prostaglandin metabolism on normal bone physiology, the question arises as to what role cyclooxygenase metabolism and non-steroidal anti-inflammatory drugs (NSAIDs) which inhibit cyclooxygenase activity have during bone healing when new bone is rapidly made and remodeled into mature lamellar bone.
2. Types of Bone Healing
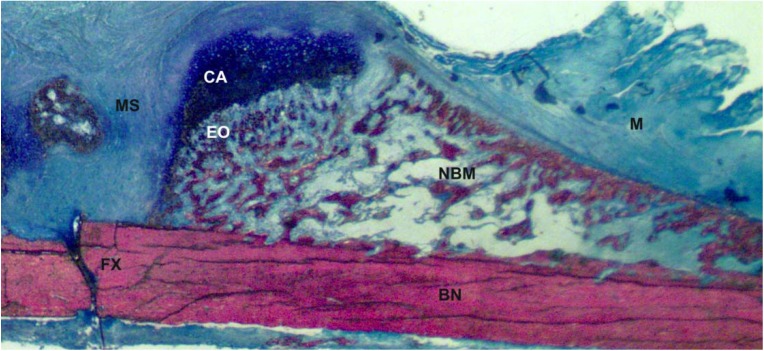
Unlike most mammalian tissues, bone heals by a regenerative process to restore the metabolic and mechanical functions of the injured bone. Bone is a highly perfused tissue so that following a typical bone fracture localized tissue hypoxia and hematoma formation occurs [3]. Inflammation at the fracture site soon follows and is characterized by cellular infiltration with large numbers of T-cells, granulocytes, and CD68-positive mononuclear cells and a paucity of B-lymphocytes [4,5]. It is thought that cell signaling events associated with hypoxia, degranulation of platelets during hematoma formation, or inflammatory cells initiate the bone healing pathway. The fracture site is subsequently invaded with a large number of fibroblast-like cells as the fracture callus forms (Figure 1). The exact origin of these fracture callus cells is unknown but it is likely that the fracture callus cells have multiple origins including proliferating periosteal and endosteal cells, circulating mesenchymal stem cells, pericytes, and muscle-derived mesenchymal stem cells. At each periphery of the fracture callus, a small amount of new bone is formed via intramembraneous ossification by osteoblasts in the periosteum [6]. These sites of new bone act as buttresses for the fracture callus and aid in the initial mechanical stabilization of the fracture. Fracture callus cells adjacent to the newly formed bone and the periosteum differentiate into chondrocytes such that the early fracture callus has new bone at its peripheries, fibroblast-like cells in the middle, and chondrocytes sandwiched in between. The chondrocytes closest to the periosteum and new bone are the first chondrocytes to become hypertrophic and form calcified cartilage. Osteoblast proliferating from the pre-existing bone beneath the calcified cartilage, and in conjunction with angiogenic activity, form bone on the calcified cartilage stratum. This bone formation process is endochondral ossification. As healing progresses, differentiation of the fracture callus cells into chondrocytes proceeds from the periphery of the callus and the original periosteum towards the center and circumferential edges of the callus. Behind the newly differentiating chondrocytes, calcified cartilage formation and endochondral ossification occur until the fracture gap is bridged with newly formed bone. Subsequent bone remodeling enhances the mechanical properties of the newly formed bone tissue and returns the bone into its former shape. This is the normal healing response to a broken bone and is often referred to as secondary healing.
Figure 1.
Upper right quadrant of a longitudinal rat fracture femur section, 2-weeks post-fracture. MS = mesenchymal cells, CA = cartilage, EO = site of endochondral ossification, FX = fracture site, BN = bone, M = muscle, NBM = site of new bone and marrow.
Primary bone fracture healing relies upon the constant and natural remodeling processes of the skeleton to heal a fracture. It is generally not a natural healing event but is the basis of many orthopaedic surgical procedures. In this scenario, the two bone ends of the fracture are juxtaposed into their original configuration and then locked in place by surgically applied plates, rods, pins, screws, or some combination [7]. This causes rigid fixation of the fracture and the natural process of bone remodeling knits the fractured bone ends together. Primary healing is probably spurred by the initial hypoxia, hematoma, and inflammation reactions that occur prior to surgery and the stimulation of remodeling that occurs due to osteonecrosis of the bone ends caused by the hypoxia.
Spinal fusion is an artificial type of bone healing that is used to fuse two or more vertebrae often for arthritic pain relief. For instance, a posterolateral spinal fusion involves dissecting the soft tissues from the dorsal side of the vertebrae and abrading the bone surface of the vertebrae to stimulate periosteal osteogenesis [8]. Bone autograft, generally harvested from the hip, is morselized and packed between the two vertebrae to be fused. The vertebrae are stabilized with various fixation devices that are applied to the dorsal-lateral or ventral-lateral aspects of the vertebrae. The abraded bone surfaces and the autograft bone chips begin forming new bone primarily through an intramembraneous ossification process. However, small islands of cartilage are often observed in the fusion mass indicating that unorganized endochondral ossification also occurs during spinal fusion to form new bone. The ultimate goal is that the new bone formed from the vertebral surfaces and the autograft unite and remodel into a solid bone mass thereby stopping or dramatically reducing movement between the two vertebrae.
Small bone defects, such as burr holes, heal by direct synthesis of new bone to fill the defect. This probably occurs in conjunction with the normal processes of bone remodeling. Similarly, other types of arthrodesis procedures to fuse two or more bones or bone growth into or around implanted devices, such as intramedullary stems for artificial hips, likely relies upon direct bone synthesis and not endochondral ossification that occurs during fracture healing. Thus, NSAID effects on fracture healing may not directly relate to other, specific bone formation processes.
3. Cyclooxygenase Inhibitors
Nonsteroidal anti-inflammatory drugs are commonly prescribed to alleviate acute and chronic musculoskeletal pain. Acute musculoskeletal injuries are characterized by localized tissue swelling, inflammation, and pain and commonly include abnormally stretched or partially torn ligaments and tendons or bone fractures. Chronic musculoskeletal pathologies such as osteoarthritis or Paget's disease are also associated with localized inflammation and tissue swelling. Treatment with NSAIDs reduces local inflammation and pain by inhibiting cyclooxygenase, which is the rate-limiting enzymatic activity in the conversion of arachidonic acid to pro-inflammatory prostaglandins. As a result, NSAID inhibition of cyclooxygenase reduces prostaglandin levels, which impedes inflammation and the attending swelling and pain.
Traditional NSAIDs inhibit the cyclooxygenase activity of both cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) with near equal potency and can induce many side effects. NSAID use can affect blood pressure, ovulation, kidney function, and most frequently the gastrointestinal system [9]. In fact, NSAID use can cause gastrointestinal bleeding and perforations that can be lethal. NSAID side effects were attributed to inhibition of COX-1 because it is constitutively expressed and functions in the protection of gastric mucosa and regulation of platelet aggregation [10,11].
In 1989, a second inducible form of the cyclooxygenase enzyme, cyclooxygenase-2 (COX-2) was discovered [12,13,14]. This discovery spurred interest in defining the connection between cyclooxygenase activity and inflammation in order to understand NSAID use and its side effects. COX-2 expression was found to be induced by tissue injury, certain cell-signaling events, or after noxious stimuli associated with inflammation [15,16,17]. Soon after, NSAID therapy was hypothesized to reduce pain and inflammation by primarily inhibiting COX-2 and not COX-1. As a result, new drugs that selectively inhibit COX-2 were developed. These COX-2 selective inhibitors were hypothesized to reduce gastrointestinal and other side effects because the homeostatic functions of COX-1 would be unaffected. In 1999, celecoxib and rofecoxib became the first COX-2 selective inhibitor drugs available for clinical use. Early clinical trials showed an increased risk for cardiovascular effects caused by rofecoxib, and in 2004 this product was withdrawn from the market, while celecoxib is still available. Celecoxib is still one of the most commonly prescribed drugs in the United States because it has reduced effects on gastric mucosa [18] although it still poses a cardiovascular risk as do other NSAIDs [19].
Because NSAIDs are commonly used to treat skeletal injuries and pain, their effects on fracture healing have been examined by many investigators over the past 35 years [20,21,22,23,24,25,26,27,28]. These studies have shown that the administration of NSAIDs and COX-2 selective NSAIDs can impair or delay bone healing and decrease the mechanical integrity of the healing bone.
4. NSAID Effects on Experimental Models of Bone Healing
4.1. Effects of Traditional NSAIDs on Bone Healing
Many studies have shown that traditional NSAIDS (nonspecific) such as aspirin, indomethacin, and ibuprofen (our definition of NSAID does not include acetaminophen) impede bone healing in various animal models (see Table 1) [20,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48].
Table 1.
NSAID effects on bone healing: animal studies.
| NSAID | Animal; Sex, Age, Weight | Bone Healing Model | Dose(s) mg/kg/day | Drug Administration (Post-Procedure) | Assay(s) | Longest Time Point | Outcome | Comments | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Aspirin | Rat, Male, 45 days | Closed, Unstable, Radius and ulna fractures | 100, 200, 300 | PO for 21 days | Histology | 22 days | Inhibition at highest dose | [23] | |
| Aspirin | Rabbit, N/A | Bone ingrowth, femur | 17, 34 | SQ injection | Histology | 8 weeks | Inhibition at high dose | Porous-coated chrome-cobalt implants; 4.5 mm diameter, 7 mm length | [38] |
| Celecoxib | Mice, 8–10 wks, ≈25 g | Closed, Stable, Tibia fracture | 10, 50 | In mice chow as a peanut butter pellet, PO daily till endpoint | Histology, mechanics | 12 weeks | No effect | [52] | |
| Celecoxib | Rat, Male, 6–9months, 584±62 g | Closed, Stable, Femur fracture | 4 | PO daily till endpoint | Histology, Radiography, Mechanics | 8 weeks | Inhibition | [25] | |
| Celecoxib | Rat, Male, 300 g | Closed, Stable, Femur fracture | 3 | Diet daily till endpoint | Radiography, histology mechanics | 12 weeks | No effect | Drug was given in chocolate | [51] |
| Celecoxib | Rat, Female, 281±20 g | Closed, Stable, Femur fracture | 3, 6 | PO daily till endpoint | Radiography, histology, mechanics | 8 weeks | Inhibition | [73] | |
| Celecoxib | Rat, Female, 272±7 g | Closed, Stable, Femur fracture | 2 , 4, 8 | 5-day before PO, PO daily till endpoint, 7 to 28 days daily, 14 to 28 days daily | Radiography, mechanics | 8 weeks | Inhibition, all doses, over time course, pre 5 day dose had no effect | [64] | |
| Celecoxib | Rat, Female, 250–300 g | Closed, Stable, Femur fracture | 4 | PO BID daily till endpoint | Radiography, histology, mechanics | 5 weeks | Inhibition | [98] | |
| Celecoxib | Rabbit, Male, 4.3–5.4 kg | Spinal fusion | 10 | PO daily till endpoint | Radiography, histology | 8 weeks | No effect | [41] | |
| Diclofenac | Rat, Male, 4–8 months 220–300 g | Closed, Stable, Tibia fracture | 1, 2 | PO daily for 10 days | Radiography, histology | 6 weeks | No effect | [60] | |
| Diclofenac | Rat, Male, 300–350 g | Open, Stable, Tibia fracture | 5 | PO daily for 7 or 21 days | Radiography, mechanics, CT scan | 3 weeks | Inhibition | [42] | |
| Diclofenac | Rat, Male, 30–350 g | Open, Stable, Tibia Osteotomy | 5 | PO daily for 7 days or 21 days | Histology | 3 weeks | 3 week dose inhibited callus maturation | [48] | |
| Etodolac | Rat, Female, 250–300 g | Closed, Stable, Femur fracture | 20 | PO daily till endpoint | Radiography, Mechanics | 3 weeks | Inhibition | [24] | |
| Etodolac | Rat, 12 wks, 250–300 g | Closed, Stable, Femur fracture | 20 | PO daily for 1 week, 3 weeks or during week 3 only | Radiography, mechanics | 3 weeks | Inhibition, when administered for 1 week or 3 weeks | [26] | |
| Flunixin | Rabbit, Female, 2.6–3.0 kg | Closed, Unstable, Tibia fracture | 1.1 | PO daily till endpoint | Mechanics, | 3 weeks | No effect | [58] | |
| Ibuprofen | Mice, Male, 8–10 wks, ≈25 g | Closed, Stable, Tibia fracture | 30 | In mice chow as a peanut butter pellet, PO daily till endpoint | Histology, mechanics | 12 weeks | No effect | [52] | |
| Ibuprofen | Rat, Male, 430–530 g | Closed, Unstable, Tibia fracture | 30–35 | Beginning 1 week prior to surgery and PO for 5 days a week | Callus size, calcium activity | 9 weeks | Ibuprofen activates calcium metabolism & decreases bone mass and composition | [31] | |
| Ibuprofen | Rat, Male, 430–530 g | Closed, Unstable, Tibia fracture | 30–35 | Beginning 1 week prior to surgery and PO for 5 days a week | Histology, mechanics | 9 weeks | Inhibition | [32] | |
| Ibuprofen | Rat, Male, 440–500 g | Closed, Stable, Tibia fracture | 30 | PO for 5 days/week, beginning 3 days post-fracture till endpoint | Histology, mechanics | 12 weeks | No effect | [59] | |
| Ibuprofen | Rat, Female, 375–450 g | Closed, Stable, Femur fracture | 30 | Diet for 4 or 12 weeks | Histology, mechanics | 12 weeks | Inhibition | [49] | |
| Ibuprofen | Rat, Male, 300 g | Closed, Stable, Femur fracture | 30 | PO daily till endpoint | Radiography, | 4 weeks | Inhibition | [45] | |
| Ibuprofen | Rabbit, Male & Female, 4 months, 2.1–3.5 kg | Open, Unstable, Femur osteotomy | 7.5 | PO daily till endpoint | Mechanics | 8 weeks | Inhibition | [34] | |
| Ibuprofen | Rabbit, N/A | Bone ingrowth, femur | 17, 34 | SQ injections | Histology | 8 weeks | Inhibition, both doses, with dose response | Porous-coated chrome-cobalt implants; 4.5 mm diameter, 7 mm length | [38] |
| Ibuprofen | Rabbit, Male, 3.5 kg | Open, Fibula, Osteoto my | 50 | PO three times daily for 28 days | Histology, mechanics | 12 weeks | Inhibition | [28] | |
| Indomethacin | Mice, Male, 8–10 wks, ≈25 g | Closed, Stable, Tibia fracture | 2 | In mice chow as a peanut butter pellet, PO daily till endpoint | Mechanics | 12 weeks | No effect | [52] | |
| Indomethacin | Rat, Male, 50 days, ≈160 g | Tooth extraction | 4 | PO, BID 2mg/kg/day for 5 days | Histology | 3 weeks | Inhibition | NSAID treated rats had delayed healing (1 week delay) | [29] |
| Indomethacin | Rat, Male, Adolesce nt | Closed, Unstable, Femur fracture | 2 | PO daily till endpoint | Radiography, histology, mechanics | 24 days | Inhibition | [20] | |
| Indomethacin | Rat, Male, 195±5 g | Closed, Unstable, Femur fracture | 2 | PO daily till endpoint | Callus weight, histology | 12 days | Indomethacin does not affect collagen metabolism | [55] | |
| Indomethacin | Rat, Male, 210–295 g | Closed, Unstable, Femur fracture | 2 | PO daily till endpoint | Radiography, manual assessment | 94 days | Inhibition | [22] | |
| Indomethacin | Rat, Male, 45 days | Closed, Unstable, | 1, 2, 4 | PO daily for 21 days | Histology | 22 days | Inhibition at all doses | [23] | |
| Indomethacin | Rat, Male, 2, 6–7, or 8–9 months | Fracture by drill hole in caudal vertebra | 4 | Beginning 1 week prior to surgery & daily till endpoint | Histology | 56 days | Inhibited | No effect if Rx was stopped day after lesion was induced | [33] |
| Indomethacin | Rat, Male, 4 wks | Drill hole in calvaria, 0.8mm | 2 | PO daily till 1, 2 or 4 weeks | Radiography, histology | 4 weeks | Inhibition | Dexamethasone was also tested and was found to inhibit bone wound healing more | [35] |
| Indomethacin | Rat, Male, 315–355 g or 329–479 g | Open, Stable, Femur fracture | 2 | 1 hr prior to surgery, PO daily for 3 days | Mechanics | 6 weeks | Inhibition | [107] | |
| Indomethacin | Rat, Male, 67–82 g or 52–64 g | Closed, Unstable, Femur fracture | 0.5, 2 | 2 mg/kg dose was given PO daily for 10 days; A single dose of 0.5 mg/kg was injected at fracture site | Radiography, | 20 days | Inhibition was found with oral and local treatment | Injection at fracture site was given in a poly-orthoester gel | [39] |
| Indomethacin | Rat, Female, 375–450 g | Closed, Stable, Femur fracture | 1 | Diet for 4 or 12 weeks | Histology, mechanics | 12 weeks | Inhibition | [49] | |
| Indomethacin | Rat, Male, 10–12 wks, 370–421 g | Spinal fusion | 3 | SQ injection, 6 days/week till endpoint | Manual assessment | 12 weeks | Inhibition | [79] | |
| Indomethacin | Rat, Female, 294±4 g | Mechanical loading | 0.02, 0.2, 2 | A single dose given 3 hours prior to loading | Histology | 12 days | Partially inhibited | [53] | |
| Indomethacin | Rat, Male, 335–345 g | Open, Stable, Femur osteotom y | 2 | IM for 3 days | Mechanics | 6 weeks | Inhibition | [54] | |
| Indomethacin | Rat, Male, 6–9 months, 584±62 g | Closed, Stable, Femur fracture | 1 | PO daily till endpoint | Radiography, histology, mechanics | 8 weeks | Inhibition | [25] | |
| Indomethacin | Rat, Male, ≈300 g | Closed, Stable, Femur fracture | 1 | PO daily till endpoint | Radiography, histology, mechanics | 12 weeks | No effect by 12 weeks | Indo delayed at 4 wks mechanically | [51] |
| Indomethacin | Rat, Female, ≈226 g | Closed, Stable, Tibia fracture | 0.625 | IP, prior to surgery, BID for 7 days | Radiography, histology, mechanics | 3 weeks | Inhibition | [50] | |
| Indomethacin | Rabbit, 4.5 months, 2.0–2.7 kg | Open, Unstable, Radius & ulna osteotomy | 10, 5 | Fed daily, 6 days a week; high dose for the first 2 weeks and then low dose for next 4 weeks | Radiography, histology | 43 days | Inhibition | [30] | |
| Indomethacin | Rabbit, Male & Female, 4 months, 2.1–3.5 kg | Open, Unstable, Femur osteotomy | 5 | PO daily till endpoint | Mechanics | 8 weeks | Inhibition | [34] | |
| Indomethacin | Rabbit, 4.3–6.0 kg | Open, Unstable, Tibia osteotomy | 50 | In drinking water, 4 days prior to surgery, PO daily till endpoint | Mechanics, bone mineral content | 6 weeks | Inhibition | [36] | |
| Indomethacin | Rabbit, Female, Juvenile | Open, Unstable, Femur osteotomy | 10 | SQ injections, | Radiography, | 6 weeks | Uncertain effect | [56] | |
| Indomethacin | Rabbit, N/A | Bone ingrowth, femur | 1, 2, 3 | SQ injections, daily | Histology | 8 weeks | Inhibition, all doses, with dose response | Porous-coated chrome-cobalt implants; 4.5 mm diameter, 7 mm length | [38] |
| Indomethacin | Rabbit, Male | Bone ingrowth, femur | 10 | SQ injections, daily | Histology | 8 weeks | Inhibition | Radially drilled, cylindrical implants | [37] |
| Indomethacin | Rabbit, 3.5 kg | Open, Unstable, Tibia osteotomy | 12.5 | In drinking water, 4 days prior to surgery, PO daily till endpoint | Histology | 6 weeks | No effect | [61] | |
| Indomethacin | Rabbit, Male, 4.3–5.4 kg | Spinal fusion | 10 | PO daily till endpoint | Radiography, histology | 8 weeks | Inhibition | [41] | |
| Indomethacin | Rabbit, 5 kg | Spinal fusion | 10 | Started 2 or 4 weeks after PO, daily till endpoint | Manual assessment | 6 weeks | Inhibition when treatment was started at 2 weeks PO | [44] | |
| Indomethacin | Rabbit, Male, 3 months, 3.5 kg | Open, Unstable, Ulna fracture | 2 | PO daily till endpoint | Histology, mechanics | 6 weeks | Inhibition | [46] | |
| Indomethacin | Dog, 12–21 kg | Open, Stable, Transsecti on of the 3rd metacarpu s | 5 | PO BID daily for 8 days | Radiography, | 8 weeks | No effect | [57] | |
| Ketorolac | Mice, Male, 8–10 wks. ≈25 g | Closed, Stable, Tibia fracture | 2 | In mice chow as a peanut butter pellet, length not mentioned | Histology, mechanics | 12 weeks | Inhibition at 4 weeks | [52] | |
| Ketorolac | Rat, Male, 335–345 g | Open, Stable, Femur osteotomy | 1 | IM for 3 days | Mechanics | 6 weeks | Inhibition | [54] | |
| Ketorolac | Rat, Male, 425–600 g | Closed, Stable, Femur fracture | 4 | PO daily till endpoint | Histology, mechanics, gene expression | 35 days | Inhibition | [72] | |
| Ketorolac | Rabbit, Male, 3.0 kg | 2 cm defect, Ulna | 2, 4 | PO daily till endpoint | Radiography, histology | 6 weeks | No effect with low dose, high dose inhibition detected between 2nd & 4th week | DBM was added in conjunction to ketorolac | [76] |
| Ketorolac | Rabbit, 4.0–4.5 kg | Spinal fusion | 4 | Continuous infusion (sq pump) for 7 days | Palpation | 6 weeks | Inhibition | 75%, 35%, and 100% fusion in the saline, ketorolac, and ketorolac plus BMP-2 groups, respectively | [71] |
| Meloxicam | Rabbit, Male, 3 month 3.5 kg | Open, Unstable, Ulna osteotomy | 0.3 | PO daily till endpoint | Histology, mechanics | 6 weeks | Inhibition | [46] | |
| Naproxen | Rabbit, Male, 6–12 months, 3.5–4.2 kg | Bone-ingrowth chamber | 110 | Water for 4 weeks | Histology | 4 weeks | Inhibition | Proximal tibia site | [40] |
| NS-398 | Rat, Female, 294±4 g | Mechanical loading | 0.02, 0.2, 2 | A single dose given 3 hours prior to loading | Histology | 12 days | Inhibition | [53] | |
| Parecoxib | Rat, Male, 425–600 g | Closed, Stable, Femur fracture | 0.3, 1.5 | PO daily till endpoint | Histology, mechanics, gene expression | 35 days | Inhibition | [72] | |
| Parecoxib | Rat, Female, ≈226 g | Closed, Stable, Tibia fracture | 0.5 | IP, prior to surgery, BID for 7 days | Radiography, histology, mechanics | 3 weeks | Inhibition | [50] | |
| Piroxicam | Rabbit, Female, 2.6–3.0 kg | Closed, Unstable, Tibia fracture | 0.1, 0.2 | PO daily till endpoint | Mechanics, | 3 weeks | No effect | [58] | |
| Rofecoxib | Mice , Male, 8–10wks. ≈25 g | Closed, Stable, Tibia fracture | 1, 5 | In mice chow as a peanut butter pellet, PO daily till endpoint | Histology, mechanics | 12 weeks | Inhibition at 8 weeks | [52] | |
| Rofecoxib | Mice, Male, 4 months | Open, Stable, Femur osteotomy | 5 | PO daily till endpoint | Radiography, histology, mechanics, laser doppler flow | 32 days | Inhibition, affects blow flow across fracture gap | [75] | |
| Rofecoxib | Rat, Male, 6–9 months, 584±62 g | Closed, Stable, Femur fracture | 3 | PO daily till endpoint | Radiography, histology, mechanics | 8 weeks | Inhibition | [25] | |
| Rofecoxib | Rat, Male, 300 g | Closed, Stable, Femur fracture | 8 | PO BID daily until endpoint | Radiography, | 4 weeks | Inhibition | [45] | |
| Rofecoxib | Rabbit, Male | Closed, Stable, Femur fracturer | 3 | PO daily for 4 weeks | Histology | 4 weeks | Inhibition | Proximal tibia | [40] |
| Rofecoxib | Rabbit, Male, 6–12 months, 3.5–4.2 kg | Bone-ingrowth chamber | 3 | PO daily for 2 weeks, 6 weeks or last 2 weeks | Histology | 6 weeks | Inhibition when administered for 6 weeks, no effect found when treatment was given for 2 weeks | [74] | |
| Rofecoxib | Rabbit, Male, 3 months, 3.5 kg | Open, Unstable, Ulna fracture | 0.5 | PO daily till endpoint | Histology, mechanics | 6 weeks | Inhibition | [46] | |
| Rofecoxib | Rabbit, Male, 3.5 kg | Open, Fibula Osteotomy | 50 | PO 3x daily for 28 days | Histology, | 12 weeks | Inhibition | [28] | |
| Tenoxicam | Rat, Male, ≈100 g | Open, Unstable, Tibia Fracture | 10 | 1 week prior to PO, PO or 48hrs after PO, than daily till endpoint, IM injections | Histology | 4 weeks | Inhibition | [43] |
* PO = post-op; SQ = subcutaneous; BID = twice a day, QID = three times a day; IP = intraperitoneal, IM = Intramuscular
In 1976, Rø et al. were the first to describe the effect of traditional NSAID use on fracture healing outcomes [20]. This study used a closed, non-stabilized femur fracture model in rats to demonstrate that indomethacin treatment delayed fracture healing. More specifically, indomethacin treatment slowed the resolution of the fracture hematoma, increased angulation between bone ends and reduced the biomechanical properties of the bone. Shortly after, Allen et al. supported these conclusions by demonstrating that indomethacin and aspirin caused drug- and dose-dependent delays in bone healing of rat radius and ulna fractures [23].
Research in the following decades continued to support the results of these earlier studies and strongly emphasized the negative effects of traditional NSAIDs on bone healing. For example, Altman et al. demonstrated that ibuprofen (30 mg/kg per day) and indomethacin (1 mg/kg per day) led to decreased mechanical properties and delayed maturation of the callus [49]. In a rat tibia fracture model, indomethacin treatment reduced bone mineral density (BMD) at the fracture site two weeks after fracture [50]. The reduced BMD correlated with decreased ultimate bending moment and bending stiffness at three weeks [27,50]. In 2002, Simon et al. showed that indomethacin treatment in a rat femur fracture model decreased callus mechanical properties at four and six weeks post-fracture. However, the biomechanical properties between control and indomethacin-treatment group fracture calluses were similar by eight weeks [25]. Other investigators have shown that the reduced bone strength associated with indomethacin treatment at earlier time points had dissipated by 12 weeks post-fracture [51,52]. These results demonstrate that the non-selective NSAIDs delay fracture healing but do not have any detrimental effects on the ultimate fracture healing outcome in these animal models. Most studies using osteotomy and bone ingrowth models have demonstrated that indomethacin and ibuprofen treatment retards bone healing [29,33,34,35,36,37,38,48,53,54]. In contrast, only a few studies indicate that NSAIDs have little or no effect on fracture healing outcomes [55,56,57,58,59,60]. One study concluded that indomethacin treatment had no effect on cortical bone healing following a small drill hole defect (2 mm in diameter) [61]. However, the scope of this study was limited to testing a single time point (six weeks) and one outcome parameter. Other studies have shown that non-selective NSAIDs delay fracture healing in larger animal models such as rabbits and dogs [21,28,30].
Bone resorption and formation can be regulated by prostaglandin E2 [62]. Prostaglandin E and F have also been shown to be released after fracture [63]. Data from Simon et al. demonstrates that treatment with nonselective or COX-2 specific NSAIDS at doses comparable to those given to humans (diclofenac, 5 mg/kg or celecoxib, 4 mg/kg) reduced fracture callus levels of prostaglandin E2 and F2α while negatively affecting fracture healing [64]. The reduction of prostaglandins in the environment of the healing bone is thought to contribute to the poor healing of the bone.
Other common side effects of NSAID use include nephrotoxicity, delayed blood clotting, and gastrointestinal bleeding [49,65,66,67]. Chronic NSAID therapy or acute high doses of NSAIDs in animals and humans can cause perforations and gastrointestinal bleeding, which is sometimes lethal [68]. According to Lanas et al. mortalities related to NSAID use and gastrointestinal complication are estimated to be approximately 59 people per 1 million [69]. Though COX-2 selective NSAIDs were developed to avoid this complication and can reduce it, it appears that both COX-1 and COX-2 are necessary to prevent and heal gastrointestinal lesions, respectively [70].
4.2. Effects of COX-2 Selective Inhibitors on Bone Healing.
With the advent of COX-2 selective NSAIDs and their nominal advantages over traditional NSAIDs, physicians began to use them for acute and chronic pain management. As a result, researchers began to study the effects on COX-2 selective NSAIDs on bone healing. The effects of COX-2 inhibitors on bone healing are still highly debated. Many of the existing animal studies have found an inhibitory effect [25,40,46,52,53,64,71,72,73,74,75] but a few studies have found no lasting negative effects [41,51,52,76]. Major factors that may underlie the discrepancies between these studies are the variability in drug dosing, dosing duration, the number of animals used within each study, the age of the animal, the type of fracture model, experimental endpoints, and outcome measurements.
In 1996, Forwood et al. showed that NS-398, a COX-2 selective inhibitor, impaired mechanical loading induced bone formation [53]. More importantly, these results demonstrated that COX-2 was expressed in bone and had an important function. Simon et al. demonstrated that femur fracture healing was severely impaired in COX-2 null mice and in rats treated with celecoxib or rofecoxib; cementing the importance of COX-2 for bone healing [25]. X-ray and histological examination of femur fracture healing in COX-1 null and COX-2 null mice showed an abundant callus undergoing endochondral ossification in COX-1 null mice but an X-ray lucent, cartilaginous callus in the COX-2 null mice with little or no apparent endochondral ossification. Femur fracture healing in rofecoxib treated rats (3 mg/kg, QD) was also severely impaired based upon radiographic and histological observations and torsional mechanical testing. In contrast, celecoxib treatment (4 mg/kg, QD) appeared to be less deleterious to femur fracture healing in the rats based upon the torsional mechanical testing analysis [25]. Data from other researchers supported these results demonstrating that COX-2 inhibitors like celecoxib, rofecoxib, parecoxib, and meloxicam impair fracture healing in mice and rats [46,72,73,74,75]. For instance, Gerstenfeld et al. demonstrated that an oral dose of parecoxib (1.5 mg/kg) delayed femur fracture healing in male rats by decreasing material properties at three weeks after fracture and structural properties at five weeks after fracture [72].
In contrast, a few studies have shown that COX-2 inhibitors have no lasting effect on femur fracture healing [41,51,52]. Brown et al. examined the effects of celecoxib (3 mg/kg/day) on male rat femur fractures [51]. Drugs were administered daily in their food starting one day after fracture and continued until sacrifice. Healing was determined by qualitative histology, radiographic scoring, and mechanical testing at four, eight, and 12 weeks after fracture. The results demonstrated that celecoxib led only to early, negative histological changes, which showed no significant differences by 12 weeks. In a study, by Mullis et al. tibia fracture healing was measured in mice treated daily with peanut butter pellets of celecoxib (10 mg/kg or 50 mg/kg) and rofecoxib (1 mg/kg and 5 mg/kg) [52]. Healing was measured by histological analysis at two weeks and biomechanical testing at four, eight, and 12 weeks. Only the low dose rofecoxib (1 mg/kg) treatment led to decreased mechanical properties at eight weeks after fracture but by 12 weeks no difference was found.
To address these discrepancies, a comprehensive study was performed to determine the dose and time-dependent effects of COX-2 selective NSAID therapies on fracture healing in rats [64]. The previous studies that tested the effects of celecoxib on fracture healing used male rats in which celecoxib has an elimination time of approximately four hours [25,41,51,52,72,75]. In contrast, this study used female rats because the elimination time for celecoxib in female rats is approximately 11 hours which is very similar to that of humans [64]. The data from this study demonstrated that celecoxib given in the human therapeutic range (4 mg/kg, BID) during the early stages of fracture repair significantly reduced femur fracture callus mechanical properties at later stages of healing and increased the number of non-unions [64]. This study also demonstrated that delaying celecoxib treatment until seven days or more after fracture improved healing outcomes. Other studies support these results suggesting that avoiding NSAID treatment early during fracture healing may lessen its negative effects [72,78]. Overall, the study showed that the negative effects of celecoxib treatment on fracture healing were dependent upon drug dose, length of treatment, and when during fracture healing celecoxib treatment occurred. The study results indicate that the earlier, conflicting COX-2 selective NSAID animal studies likely reflect differences in the level and duration of COX-2 inhibition. Ultimately, the data support the conclusion that COX-2 selective NSAID use is detrimental to fracture healing.
4.3. Effects of NSAIDs on Spinal Fusion
Few studies exist which examine the role of NSAID treatment on spinal fusion outcomes in animal models. Most of these studies demonstrate that traditional NSAIDs inhibit spinal fusion success. For instance, Dimar et al. demonstrated that indomethacin treatment delayed healing in rats that underwent a three-level posterior spinal fusion [79]. In this study, 45% of control rat spines were completely fused while only 10% of the indomethacin treated rat spines were completely fused. In a rabbit spinal fusion study, only 18% of the rabbits given indomethacin orally after surgery had successfully fused vertebrae as compared to 64% in the control treatment group [41]. This same study also found that celecoxib treatment (10 mg/kg, daily for eight weeks) had no effect on spinal fusion success. A later rabbit study by the same group found similar negative effects caused by indomethacin treatment even when the treatment was delayed until two weeks after the spinal fusion surgery [44]. Research conducted using other traditional NSAIDs like ketorolac in the rabbit posterolateral spinal fusion model showed that its use decreased spinal fusion success from 75% in controls to 35% in the drug treatment groups [71]. A series of published reports from Scott S. Reuben concerning the use of COX-2 selective NSAIDs for spinal fusion surgery were found to be fraudulent and most have been retracted. As such, the published results of these reports are not discussed. Given the discrepancy between the celecoxib results and those from the indomethacin and ketorolac studies, additional studies are needed to define the effects of COX-2 selective inhibitors on spinal fusion.
5. Human Studies of NSAID Effects on Bone Healing and Formation
NSAID therapy can have a clinically significant effect on bone formation in humans. The negative effect NSAIDs have on bone formation in animals is well documented as described above and in Table 1. However, far less is known about NSAID effects on bone formation in humans. In one area, extensive research has shown that NSAID therapy can inhibit bone formation to reduce the severity or incidence of heterotopic bone formation following hip and femoral neck fractures, after hip arthroplasty, or after certain central nervous system injuries that are often associated with heterotopic ossification in humans [80,81,82,83]. Indeed, NSAIDs are now routinely prescribed for patients following hip athroplasty, and acetabular fractures. The mechanism underlying the NSAID-induced reduction in heterotopic bone formation remains unknown.
Currently, we are unaware of any large, prospective, randomized trials to assess the effects of NSAID therapy on bone fracture healing in humans. A few small studies have examined NSAID use and bone healing success in humans (see Table 2).
Table 2.
NSAID effects on bone healing: human studies.
| Procedure or Injury | Number of Patients | Mean or Median age (yrs) | Follow-up (months) | Drug | Dose(s) | Results | Comments | Ref. |
|---|---|---|---|---|---|---|---|---|
| Fracture, Ankle-joint fracture dislocation |
Exp:1 | Exp:64 | 10 weeks | Indomethacin | 25 mg, QID for 9 weeks | Inhibitory unhealed at 9 weeks |
[84] | |
| Fracture, colles | Exp: 48 Con:50 |
Exp: 62.9 Con: 58.7 |
12 | Flurbiprofen | 50 mg, 3–6 times a day for 14 days | Exp: 50% excellent functional result Con: 94% excellent functional result |
[86] | |
| Fracture, colles | Exp:21 Con:21 |
63 | 12 weeks | Piroxicam | 20 mg/day for 8 weeks | Exp: 28% needed surgery Con: none needed surgery |
[85] | |
| Acetabular fracture | Exp: 41 Con:34 |
Exp: 43 Con:47 |
(Range) Exp:12 Con:11.7 |
Indomethacin | 25 mg, 3x/day for 6 weeks | No difference between grade distribution | Prospective study | [82] |
| Acetabular fracture | Exp: 72 Con: 16 |
Exp: 41 Con: |
Exp: 13 Con: |
Indomethacin | 25 mg, QID for 6 weeks | Exp: 11.1% grade iii or iv ho Con: 37.5% grade iii or iv ho |
Control patient group is small, study was designed to compare radiation vs. NSAID therapy for reduction of ho | [83] |
| Acetabular fracture | Exp: 74 Con:36 |
Exp: 39.5 Con: 38.6 |
3–11 | Indomethacin | 25 mg, QID for 6 weeks | Inhibitory Exp: 29% non-union Con: 7% non-union |
Also compared radiation | [88] |
| Fracture | Exp: 893 Con: 5781 |
Exp: 74 Con:74 |
24 | NSAIDs | 5–7 times a week | No protective effect on subsequent risk of fractures | BMD & fracture risk assessment | [90] |
| Fracture | Exp: 32 Con:67 |
Exp: 35 Con: 38 |
7+ | Ibuprofen or diclofenac | Varied, 1–21 weeks | Inhibitory Exp: 10.74 odds ratio |
Retrospective study | [87] |
| Fracture | Exp1: 214,577 Exp2: 286,850 Con: 214,577 |
Exp1: 55 Exp2:44 Con:55 |
Exp1: 3.4 yrs Exp2: .7 years Con: 2.3 yrs |
NSAIDs | Exp1: 3+ times per week Exp1: 1–2 times per week |
Exp1: 1.47 Exp1 vs. Exp2: 1.04 |
Retrospective study, fracture risk is similar between regular and incidental NSAID users | [91] |
| Fracture, humeral shaft | Exp: 1,032 Con:8,963 |
Exp: 78 Con:77 |
3 | Traditional NSAID | Varied, at least 10 days of treatment in a 30 day period | Exposure to NSAID was associated with nonunion | Retrospective study | [89] |
| Acetabular fracture | Exp: 18 Con: 26 |
Exp: 41 Con: 37 |
12+ | Indomethacin | 25 mg QID for 6 weeks | Exp: 1/18 grade ii ho, 0/18 grade iii or iv Con: 3/26 grade ii ho, 10/26 grade iii or iv ho |
Heterotrophic bone ossification study | [80] |
| Spinal fusion | Exp: 167 Con: 121 |
Exp: 43 Con: 45 |
24+ | Ketorolac | 60 mg IM, then 30 mg/IM very 6–8 h as needed | Inhibitory |
Retrospective study; 4.25-fold increase in non-unions | [92] |
| Exp: 17% non-union Con: 4% non-union |
* exp = experimental; con = control.
One study conducted by Adolphson et al. found that 28% of Colle’s fracture patients who were treated by closed reduction and casting and were treated with piroxicam lost fracture reduction which necessitated subsequent surgical correction. In contrast, all of the Colle’s fracture patients that were treated by closed reduction and casting but with no NSAID therapy healed without surgical intervention [85]. In a double-blind prospective study, flurbiprofen was used in the management of 100 Colles’ fractures [86]. After one year, patients with minimally displaced fractures treated with placebo had significantly better (94%) functional recovery when compared with those treated with flurbiprofen (50%). In contrast, no difference was found between the placebo and flurbiprofen treatment subgroups in the displaced Colles’ fracture group that were treated by surgical fracture reduction after one year.
Retrospective studies indicate that NSAID therapy can significantly impair fracture healing in humans. In a retrospective study designed to assess the effects of reaming on femur fracture healing outcomes, Giannoudis found that the most significant variable affecting femur fracture healing success was NSAID use [87]. The records of 377 femur fracture patients were examined and 32 were found that had developed non-unions. Of the remaining patients, 67 had comparable injuries, co-morbidities, and treatment histories but in these patients, the femur fractures had healed. Of the 32 non-union patients 62.5% had used NSAIDs while only 13.4% of patients with healed femur fractures had used NSAIDs. A compelling retrospective study was performed by Burd et al. [83]. Previously, this group had performed a prospective, randomized trial to compare the efficacy of localized radiation therapy versus systemic indomethacin therapy to reduce the incidence or severity of heterotopic ossification following acetabular fractures [83]. Using this same of set of patients, Burd et al. queried whether the indomethacin therapy affected the healing of any additional long bone fractures in the study patients [88]. They found a non-union rate of 7% in the localized radiation therapy group and a 26% non-union rate in the indomethacin therapy group. One retrospective study, however, failed to identify any negative effect of NSAID use on humerus fracture healing [88]. Approximately 10% of 9,995 humeral shaft fracture patients in the study used NSAIDs sometime in the 90 days after fracture. Only 105 of 9995 patients had a subsequent surgery to treat a non-union and 33 of the 105 non-union patients had used NSAIDs. Other studies also indicate that NSAID use can increase fracture risk and decrease bone mineral density [90,91]. We are unaware of any human studies that have specifically assessed the effects of COX-2 selective inhibitors on fracture healing.
The negative effects of NSAID therapy on spinal fusion success in humans has been documented in a retrospective study. Post-operative ketorolac use significantly impaired spinal fusion success [92]. No study examining COX-2 selective NSAID use and spinal fusion success in humans has been reported besides the aforementioned ones published and subsequent retracted by Reuben. Since ketorolac is a non-specific NSAID, no conclusion can be made as to whether the COX-2 selective NSAIDs will have similar negative effects on spinal fusion success. Recent experiments in rabbits suggest that celecoxib may not have a negative effect on spinal fusion success [41].
6. Role of COX-2 during Bone Healing
Clearly, COX-2 is a positive regulator of fracture healing. However, the mechanisms by which COX-2 promotes healing or by which COX-2 inhibition impairs bone healing are unknown. It is likely that the loss of COX-2 function with NSAID use alters many cellular pathways required for bone healing and therefore multiple molecular pathways are involved.
One theory is that COX-2 function is essential for mesenchymal cell differentiation into osteoblasts, which is necessary for normal fracture healing. Therefore, inhibition of COX-2 activity is expected to negatively impact osteogenesis. Zhang et al. found that bone marrow cell cultures from COX-2 knockout mice produced less osteoblasts than wild-type mice but that treatment with BMP-2 and prostaglandin E2 could reverse this effect [93]. Chikazu et al. also demonstrated that COX-2 contributes to BMP-2 induced osteoblastic differentiation in vitro and in vivo during ectopic bone formation [94]. However, COX-2 null mice have normally formed skeletons and satisfactory post-natal growth indicating that loss of COX-2 activity does not inherently prevent stem cells from differentiating into osteoblasts.
Another theory is that the pain relief afforded by NSAID treatment enables experimental animals to weight bear on the injured limb too early or often, which leads to re-injury and delayed healing. This theory was tested in the rat femur fracture healing model using celecoxib (3 mg/kg/day or 6 mg/kg/day) and acetaminophen (60 mg/kg/day or 300 mg/kg/day) to provide pain relief for 10 days post-fracture [73]. Results from radiographs and mechanical testing demonstrated that celecoxib inhibited healing while acetaminophen did not. In a subsequent study, pain relief from femur fractures in rats treated with acetaminophen, celecoxib (10 mg/kg, BID), and SCIO-469, a p38 kinase-α inhibitor treatment was measured [95]. All the drugs provided significant pain relief. However, only celecoxib treatment was associated with impaired healing in previous studies. These data suggest that analgesia only is not sufficient to account for the negative effects of celecoxib or NSAID treatment on fracture healing. Thus the theory that analgesia induced re-injury causes the delay in healing is not supported by the current experimental evidence.
A third theory is that COX-2 dependent prostaglandins promote angiogenesis that is required for fracture healing [96,97]. Studies have shown that prostaglandins and COX-2 regulate pro-angiogenic microenvironments. These microenvironments are vital to cellular signaling responses and may be important for bone regenerative cells and tissues [98]. Murnaghan et al. supported this theory by showing that rofecoxib treatment reduced blood flow across the fracture gap while impairing fracture healing in a mouse fracture model [75]. Conversely, Xie et al. demonstrated that treatment of COX-2 null mice with a prostaglandin E2 receptor 4 (EP4) agonist increased callus vascularity and restored fracture healing [99]. These data indicate that COX-2 has a role in promoting angiogenesis during fracture healing.
A fourth theory is that loss or inhibition of COX-2 shunts arachidonic acid into the 5-lipoxygenase (5-LO) pathway which alters the inflammation response and negatively affects bone healing [99]. 5-LO is the key enzyme in the production of leukotrienes from arachidonic acid and inhibition of 5-LO accelerates fracture healing in rats [100]. Leukotrienes can decrease osteoblast proliferation and activity in vitro while stimulating osteoclast formation and activity [101,102,103,104,105,106]. Fracture callus leukotriene B4 levels are 4.4-fold higher in COX-2 null mice, consistent with an arachidonic acid shunting mechanism (Manigrasso and O’Connor, submitted). Together, these data indicate that 5-LO activity normally acts to inhibit fracture healing and because loss of COX-2 increases 5-LO activity, healing is impaired.
In the rat closed femur fracture model, celecoxib treatment significantly affects healing in multiple ways [100]. Celecoxib treatment reduced callus cell proliferation rates without altering callus cell numbers, suggesting that cell migration to the fracture site is not affected. Celecoxib treatments also lead to increases in aggrecan and Type II collagen mRNA levels which are indicative of chondrocyte differentiation and cartilage formation. This is consistent with histological and histomorphometric measurements showing formation of a cartilaginous callus in COX-2 deficient animal models. However, Type X collagen mRNA levels were significantly reduced relative to the high the Type II collagen and aggrecan mRNA levels, indicating that the callus chondrocytes in the celecoxib treated rats failed to progress into hypertrophy. This would in turn reduce formation of calcified cartilage, impair endochondral ossification, and prevent healing.
Though there are many theories regarding the role of COX-2 in fracture healing, many basic questions are still unanswered. For instance, we do not know when or in which cells COX-2 is expressed during fracture healing. Nor do we know the repertoire of prostaglandins and other eicosanoids produced during fracture healing or how loss of function in one of the arachidonic acid metabolizing enzymes affects the activity of the remaining enzymes. Understanding these and other basic parameters of arachidonic acid metabolism during fracture healing will provide critical information needed to refine testable hypotheses for elucidating the function of COX-2 during bone regeneration.
7. Summary
This review strived to provide an unbiased analysis of the current literature in regard to non-steroidal anti-inflammatory drugs and their effect on bone healing. Overwhelmingly, this review demonstrates that NSAIDs inhibit or delay fracture healing to a greater or lesser degree, depending on the specific drug, its preparation, mode of administration, and ability to inhibit COX-2. The majority of animal studies and the few human studies that exist support this conclusion. However, further research to determine if the analgesic effects of NSAIDs are beneficial in treating post-traumatic and postoperative wound edema. Since it is increasingly clear that the function of COX-2 is critical for bone regeneration, defining the risk-benefit ratio for NSAID use is critical. Patient co-morbid conditions, such as, fracture severity, advanced age, diabetes, and cardiovascular health, will need to be considered when assessing the risk-benefit ratio. Future research is needed to define the role of COX-2 in bone regeneration and whether NSAID therapy will further impair fracture healing or other regenerative processes in the presence of these co-morbid conditions.
References
- 1.O'Connor J.P., Lysz T. Celecoxib, NSAIDs and the skeleton. Drugs. 2008;44:693–709. doi: 10.1358/dot.2008.44.9.1251573. [DOI] [PubMed] [Google Scholar]
- 2.Radi Z.A., Khan N.K. Effects of cyclooxygenase inhibition on bone, tendon, and ligament healing. Inflamm. Res. 2005;54:358–366. doi: 10.1007/s00011-005-1367-4. [DOI] [PubMed] [Google Scholar]
- 3.Kolar P., Schmidt-Bleek K., Schell H., Gaber T., Toben D., Schmidmaier G., Perka C., Buttgereit F., Duda G.N. The early fracture hematoma and its potential role in fracture healing. Tissue Eng. 2010 doi: 10.1089/ten.TEB.2009.0687. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Andrew J.G., Andrew S.M., Freemont A.J., Marsh D.R. Inflammatory cells in normal human fracture healing. Acta Orthop. 1994;65:462–466. doi: 10.3109/17453679408995493. [DOI] [PubMed] [Google Scholar]
- 5.Oni O.O. The early stages of the repair of adult human diaphyseal fractures. Injury. 1997;28:521–525. doi: 10.1016/s0020-1383(97)00062-4. [DOI] [PubMed] [Google Scholar]
- 6.Einhorn T.A. The cell and molecular biology of fracture healing. Clin. Orthop. Relat. Res. 1998;355:S7–S21. doi: 10.1097/00003086-199810001-00003. [DOI] [PubMed] [Google Scholar]
- 7.Greenbaum M.A., Kanat I.O. Current concepts in bone healing. Review of the literature. J. Am. Podiatr. Med. Assoc. 1993;83:123–129. doi: 10.7547/87507315-83-3-123. [DOI] [PubMed] [Google Scholar]
- 8.Glassman S.D., Carreon L., Djurasovic M., Campbell M.J., Puno R.M., Johnson J.R., Dimar J.R. Posterolateral lumbar spine fusion with INFUSE bone graft. Spine J. 2007;7:44–49. doi: 10.1016/j.spinee.2006.06.381. [DOI] [PubMed] [Google Scholar]
- 9.Langford R.M., Mehta V. Selective cyclooxygenase inhibition: Its role in pain and anaesthesia. Biomed. Pharmacother. 2006;60:323–328. doi: 10.1016/j.biopha.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez E.L., Patrignani P., Tacconelli S., Rodriguez L.A. Variability of risk of upper gastrointestinal bleeding among nonsteroidal anti-inflammatory drugs. Arthritis Rheum. 2010 doi: 10.1002/art.27412. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Lanas A. A review of the gastrointestinal safety data—A gastroenterologist's perspective. Rheumatology. 2010;49(Suppl. 2):ii3–ii10. doi: 10.1093/rheumatology/keq058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simmons D.L., Levy D.B., Yannoni Y., Erikson R.L. Identification of a phorbol ester-repressible v-src-inducible gene. Proc. Natl. Acad. Sci. USA. 1989;86:1178–1182. doi: 10.1073/pnas.86.4.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Banion M.K., Sadowski H.B., Winn V., Young D.A. A serum- and glucocorticoid-regulated 4-kilobase mRNA encodes a cyclooxygenase-related protein. J. Biol. Chem. 1991;266:23261–23267. [PubMed] [Google Scholar]
- 14.Fletcher B.S., Kujubu D.A., Perrin D.M., Herschman H.R. Structure of the mitogen-inducible TIS10 gene and demonstration that the TIS10-encoded protein is a functional prostaglandin G/H synthase. J. Biol. Chem. 1992;267:4338–4344. [PubMed] [Google Scholar]
- 15.Salvemini D., Misko T.P., Masferrer J.L., Seibert K., Currie M.G., Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc. Natl. Acad. Sci. USA. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y., Shaffer A., Portanova J., Seibert K., Isakson P.C. Inhibition of cyclooxygenase-2 rapidly reverses inflammatory hyperalgesia and prostaglandin E2 production. J. Pharmacol. Exp. Ther. 1997;283:1069–1075. [PubMed] [Google Scholar]
- 17.Simmons D.L., Botting R.M., Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- 18.FitzGerald G.A., Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2. N. Engl. J. Med. 2001;345:433–442. doi: 10.1056/NEJM200108093450607. [DOI] [PubMed] [Google Scholar]
- 19.Fitzgerald G.A. Coxibs and cardiovascular disease. N. Engl. J. Med. 2004;351:1709–1711. doi: 10.1056/NEJMp048288. [DOI] [PubMed] [Google Scholar]
- 20.Ro J., Sudmann E., Marton P.F. Effect of indomethacin on fracture healing in rats. Acta Orthop. 1976;47:588–599. doi: 10.3109/17453677608988744. [DOI] [PubMed] [Google Scholar]
- 21.Baratieri A., Deli R. The effect on bone repair of aspirin cones placed in extraction sockets in dogs: A histopathologic study. J. Oral Pathol. 1979;8:198–206. doi: 10.1111/j.1600-0714.1979.tb01886.x. [DOI] [PubMed] [Google Scholar]
- 22.Sudmann E., Dregelid E., Bessesen A., Morland J. Inhibition of fracture healing by indomethacin in rats. Eur. J. Clin. Invest. 1979;9:333–339. doi: 10.1111/j.1365-2362.1979.tb00893.x. [DOI] [PubMed] [Google Scholar]
- 23.Allen H.L., Wase A., Bear W.T. Indomethacin and aspirin: Effect of nonsteroidal anti-inflammatory agents on the rate of fracture repair in the rat. Acta Orthop. 1980;51:595–600. doi: 10.3109/17453678008990848. [DOI] [PubMed] [Google Scholar]
- 24.Endo K., Sairyo K., Kornatsurbara S., Sasa T., Egawa H., Yonekura D., Adachi K., Ogawa T. Cyclooxygenase-2 Inhibitor Inhibits the Fracture Healing. J. Physiol. Anthropol. Appl. Hum. Sci. 2002;21:235–238. doi: 10.2114/jpa.21.235. [DOI] [PubMed] [Google Scholar]
- 25.Simon A.M., Manigrasso M.B., O'Connor J.P. Cyclo-oxygenase 2 function is essential for bone fracture healing. J. Bone Miner. Res. 2002;17:963–976. doi: 10.1359/jbmr.2002.17.6.963. [DOI] [PubMed] [Google Scholar]
- 26.Endo K., Sairyo K., Komatsubara S., Sasa T., Egawa H., Ogawa T., Yonekura D., Murakami R., Yasui N. Cyclooxygenase-2 inhibitor delays fracture healing in rats. Acta Orthop. 2005;76:470–474. doi: 10.1080/17453670510041439. [DOI] [PubMed] [Google Scholar]
- 27.Dimmen S., Engebretsen L., Nordsletten L., Madsen J.E. Negative effects of parecoxib and indomethacin on tendon healing: An experimental study in rats. Knee Surg. Sports Traumatol. Arthrosc. 2009;17:835–839. doi: 10.1007/s00167-009-0763-7. [DOI] [PubMed] [Google Scholar]
- 28.O'Connor J.P., Capo J.T., Tan V., Cottrell J.A., Manigrasso M.B., Bontempo N., Parsons J.R. A comparison of the effects of ibuprofen and rofecoxib on rabbit fibula osteotomy healing. Acta Orthop. 2009;80:597–605. doi: 10.3109/17453670903316769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huusko P.J., Nieminen L.H., Nieminen L.S. The effect of indomethacin on tooth extraction wound healing in rats. Experientia. 1975;31:1056–1058. doi: 10.1007/BF02326957. [DOI] [PubMed] [Google Scholar]
- 30.Sudmann E., Bang G. Indomethacin-induced inhibition of haversian remodelling in rabbits. Acta Orthop. 1979;50:621–627. doi: 10.3109/17453677908991283. [DOI] [PubMed] [Google Scholar]
- 31.Tornkvist H., Lindholm T.S. Effect of ibuprofen on mass and composition of fracture callus and bone—An experimental study on adult rat. Scand. J. Rheumatol. 1980;9:167–171. doi: 10.3109/03009748009098151. [DOI] [PubMed] [Google Scholar]
- 32.Lindholm T.S., Tornkvist H. Inhibitory effect on bone formation and calcification exerted by the anti-inflammatory drug ibuprofen—An experimental study on adult rat with fracture. Scand. J. Rheumatol. 1981;10:38–42. [PubMed] [Google Scholar]
- 33.Elves M.W., Bayley I., Roylance P.J. The effect of indomethacin upon experimental fractures in the rat. Acta Orthop. Scand. 1982;53:35–41. doi: 10.3109/17453678208992176. [DOI] [PubMed] [Google Scholar]
- 34.Tornkvist H., Lindholm T.S., Netz P., Stromberg L., Lindholm T.C. Effect of ibuprofen and indomethacin on bone metabolism reflected in bone strength. Clin. Orthop. Relat. Res. 1984;187:255–259. [PubMed] [Google Scholar]
- 35.Sato S., Kim T., Arai T., Maruyama S., Tajima M., Utsumi N. Comparison between the effects of dexamethasone and indomethacin on bone wound healing. Jpn. J. Pharmacol. 1986;42:71–78. doi: 10.1254/jjp.42.71. [DOI] [PubMed] [Google Scholar]
- 36.Keller J., Bunger C., Andreassen T.T., Bak B., Lucht U. Bone repair inhibited by indomethacin. Effects on bone metabolism and strength of rabbit osteotomies. Acta Orthop. 1987;58:379–383. doi: 10.3109/17453678709146360. [DOI] [PubMed] [Google Scholar]
- 37.Keller J.C., Trancik T.M., Young F.A., St Mary E. Effects of indomethacin on bone ingrowth. J. Orthop. Res. 1989;7:28–34. doi: 10.1002/jor.1100070105. [DOI] [PubMed] [Google Scholar]
- 38.Trancik T., Mills W., Vinson N. The effect of indomethacin, aspirin, and ibuprofen on bone ingrowth into a porous-coated implant. Clin. Orthop. Relat. Res. 1989;249:113–121. [PubMed] [Google Scholar]
- 39.Engesaeter L.B., Sudmann B., Sudmann E. Fracture healing in rats inhibited by locally administered indomethacin. Acta Orthop. Scand. 1992;63:330–333. doi: 10.3109/17453679209154794. [DOI] [PubMed] [Google Scholar]
- 40.Goodman S., Ma T., Trindade M., Ikenoue T., Matsuura I., Wong N., Fox N., Genovese M., Regula D., Smith R.L. COX-2 selective NSAID decreases bone ingrowth in vivo. J. Orthop. Res. 2002;20:1164–1169. doi: 10.1016/S0736-0266(02)00079-7. [DOI] [PubMed] [Google Scholar]
- 41.Long J., Lewis S., Kuklo T., Zhu Y., Riew K.D. The effect of cyclooxygenase-2 inhibitors on spinal fusion. J. Bone Joint Surg. Am. 2002;84A:1763–1768. doi: 10.2106/00004623-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Beck A., Krischak G., Sorg T., Augat P., Farker K., Merkel U., Kinzl L., Claes L. Influence of diclofenac (group of nonsteroidal anti-inflammatory drugs) on fracture healing. Arch. Orthop. Trauma Surg. 2003;123:327–332. doi: 10.1007/s00402-003-0537-5. [DOI] [PubMed] [Google Scholar]
- 43.Giordano V., Giordano M., Knackfuss I.G., Apfel M.I., Gomes R.D. Effect of tenoxicam on fracture healing in rat tibiae. Injury. 2003;34:85–94. doi: 10.1016/s0020-1383(02)00199-7. [DOI] [PubMed] [Google Scholar]
- 44.Riew K.D., Long J., Rhee J., Lewis S., Kuklo T., Kim Y.J., Yukawa Y., Zhu Y. Time-dependent inhibitory effects of indomethacin on spinal fusion. J. Bone Joint Surg. Am. 2003;85A:632–634. doi: 10.2106/00004623-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Leonelli S.M., Goldberg B.A., Safanda J., Bagwe M.R., Sethuratnam S., King S.J. Effects of a cyclooxygenase-2 inhibitor (rofecoxib) on bone healing. Am. J. Orthop. 2006;35:79–84. [PubMed] [Google Scholar]
- 46.Karachalios T., Boursinos L., Poultsides L., Khaldi L., Malizos K.N. The effects of the short-term administration of low therapeutic doses of anti-COX-2 agents on the healing of fractures. An experimental study in rabbits. J. Bone Joint Surg. Br. 2007;89:1253–1260. doi: 10.1302/0301-620X.89B9.19050. [DOI] [PubMed] [Google Scholar]
- 47.Krischak G.D., Augat P., Blakytny R., Claes L., Kinzl L., Beck A. The non-steroidal anti-inflammatory drug diclofenac reduces appearance of osteoblasts in bone defect healing in rats. Arch. Orthop. Trauma Surg. 2007;127:453–458. doi: 10.1007/s00402-007-0288-9. [DOI] [PubMed] [Google Scholar]
- 48.Krischak G.D., Augat P., Sorg T., Blakytny R., Kinzl L., Claes L., Beck A. Effects of diclofenac on periosteal callus maturation in osteotomy healing in an animal model. Arch. Orthop. Trauma Surg. 2007;127:3–9. doi: 10.1007/s00402-006-0202-x. [DOI] [PubMed] [Google Scholar]
- 49.Altman R.D., Latta L.L., Keer R., Renfree K., Hornicek F.J., Banovac K. Effect of nonsteroidal antiinflammatory drugs on fracture healing: A laboratory study in rats. J. Orthop. Trauma. 1995;9:392–400. doi: 10.1097/00005131-199505000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Dimmen S., Nordsletten L., Madsen J.E. Parecoxib and indomethacin delay early fracture healing: A study in rats. Clin. Orthop. Relat. Res. 2009;467:1992–1999. doi: 10.1007/s11999-009-0783-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown K.M., Saunders M.M., Kirsch T., Donahue H.J., Reid J.S. Effect of COX-2-specific inhibition on fracture-healing in the rat femur. J. Bone Joint Surg. Am. 2004;86A:116–123. doi: 10.2106/00004623-200401000-00017. [DOI] [PubMed] [Google Scholar]
- 52.Mullis B.H., Copland S.T., Weinhold P.S., Miclau T., Lester G.E., Bos G.D. Effect of COX-2 inhibitors and non-steroidal anti-inflammatory drugs on a mouse fracture model. Injury. 2006;37:827–837. doi: 10.1016/j.injury.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 53.Forwood M.R. Inducible cyclo-oxygenase (COX-2) mediates the induction of bone formation by mechanical loading in vivo. J. Bone Miner. Res. 1996;11:1688–1693. doi: 10.1002/jbmr.5650111112. [DOI] [PubMed] [Google Scholar]
- 54.Reikeraas O., Engebretsen L. Effects of ketoralac tromethamine and indomethacin on primary and secondary bone healing—An experimental study in rats. Arch. Orthop. Trauma Surg. 1998;118:50–52. doi: 10.1007/s004020050310. [DOI] [PubMed] [Google Scholar]
- 55.Ro J., Langeland N., Sander J. Effect of indomethacin on collagen metabolism of rat fracture callus in vitro. Acta Orthop. 1978;49:323–328. doi: 10.3109/17453677809050082. [DOI] [PubMed] [Google Scholar]
- 56.Shindell R., Lippiello L., Connolly J.F. Uncertain effect of indomethacin on physeal growth injury—Experiments in rabbits. Acta Orthop. 1988;59:46–49. doi: 10.3109/17453678809149343. [DOI] [PubMed] [Google Scholar]
- 57.Mbugua S.W., Skoglund L.A., Lokken P. Effects of phenylbutazone and indomethacin on the post-operative course following experimental orthopaedic surgery in dogs. Acta Vet Scand. 1989;30:27–35. doi: 10.1186/BF03548065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.More R.C., Kody M.H., Kabo J.M., Dorey F.J., Meals R.A. The effects of two nonsteroidal antiinflammatory drugs on limb swelling, joint stiffness, and bone torsional strength following fracture in a rabbit model. Clin. Orthop. Relat. Res. 1989;247:306–312. [PubMed] [Google Scholar]
- 59.Huo M.H., Troiano N.W., Pelker R.R., Gundberg C.M., Friedlaender G.E. The influence of ibuprofen on fracture repair: Biomechanical, biochemical, histologic, and histomorphometric parameters in rats. J. Orthop. Res. 1991;9:383–390. doi: 10.1002/jor.1100090310. [DOI] [PubMed] [Google Scholar]
- 60.Akman S., Gogus A., Sener N., Bilgic B., Aksoy B., Seckin F. Effect of diclofenac sodium on union of tibial fractures in rats. Adv. Ther. 2002;19:119–125. doi: 10.1007/BF02850267. [DOI] [PubMed] [Google Scholar]
- 61.Keller J., Kjaersgaard-Andersen P., Bayer-Kristensen I., Melsen F. Indomethacin and bone trauma. Effects on remodeling of rabbit bone. Acta Orthop. 1990;61:66–69. doi: 10.3109/17453679008993070. [DOI] [PubMed] [Google Scholar]
- 62.Blackwell K.A., Raisz L.G., Pilbeam C.C. Prostaglandins in bone: Bad cop, good cop? Trends Endocrinol. Metab. 2010;21:294–301. doi: 10.1016/j.tem.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dekel S., Lenthall G., Francis M.J. Release of prostaglandins from bone and muscle after tibial fracture—An experimental study in rabbits. J. Bone Joint Surg. Br. 1981;63B:185–189. doi: 10.1302/0301-620X.63B2.7217139. [DOI] [PubMed] [Google Scholar]
- 64.Simon A.M., O'Connor J.P. Dose and time-dependent effects of cyclooxygenase-2 inhibition on fracture-healing. J. Bone Joint Surg. Am. 2007;89:500–511. doi: 10.2106/JBJS.F.00127. [DOI] [PubMed] [Google Scholar]
- 65.Perazella M.A., Buller G.K. NSAID nephrotoxicity revisited: Acute renal failure due to parenteral ketorolac. South Med. J. 1993;86:1421–1424. doi: 10.1097/00007611-199312000-00025. [DOI] [PubMed] [Google Scholar]
- 66.Roth S.H., Tindall E.A., Jain A.K., McMahon F.G., April P.A., Bockow B.I., Cohen S.B., Fleischmann R.M. A controlled study comparing the effects of nabumetone, ibuprofen, and ibuprofen plus misoprostol on the upper gastrointestinal tract mucosa. Arch. Intern. Med. 1993;153:2565–2571. [PubMed] [Google Scholar]
- 67.Splinter W.M., Rhine E.J., Roberts D.W., Reid C.W., MacNeill H.B. Preoperative ketorolac increases bleeding after tonsillectomy in children. Can. J. Anaesth. 1996;43:560–563. doi: 10.1007/BF03011766. [DOI] [PubMed] [Google Scholar]
- 68.Langman M.J., Weil J., Wainwright P., Lawson D.H., Rawlins M.D., Logan R.F., Murphy M., Vessey M.P., Colin-Jones D.G. Risks of bleeding peptic ulcer associated with individual non-steroidal anti-inflammatory drugs. Lancet. 1994;343:1075–1078. doi: 10.1016/s0140-6736(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 69.Lanas A., Perez-Aisa M.A., Feu F., Ponce J., Saperas E., Santolaria S., Rodrigo L., Balanzo J., Bajador E., Almela P., Navarro J.M., Carballo F., Castro M., Quintero E. A nationwide study of mortality associated with hospital admission due to severe gastrointestinal events and those associated with nonsteroidal antiinflammatory drug use. Am. J. Gastroenterol. 2005;100:1685–1693. doi: 10.1111/j.1572-0241.2005.41833.x. [DOI] [PubMed] [Google Scholar]
- 70.Dubois R.W., Melmed G.Y., Henning J.M., Bernal M. Risk of Upper Gastrointestinal Injury and Events in Patients Treated With Cyclooxygenase (COX)-1/COX-2 Nonsteroidal Antiinflammatory Drugs (NSAIDs), COX-2 Selective NSAIDs, and Gastroprotective Cotherapy: An Appraisal of the Literature. J. Clin. Rheumatol. 2004;10:178–189. doi: 10.1097/01.rhu.0000128851.12010.46. [DOI] [PubMed] [Google Scholar]
- 71.Martin G.J., Jr., Boden S.D., Titus L. Recombinant human bone morphogenetic protein-2 overcomes the inhibitory effect of ketorolac, a nonsteroidal anti-inflammatory drug (NSAID), on posterolateral lumbar intertransverse process spine fusion. Spine. 1999;24:2188–2194. doi: 10.1097/00007632-199911010-00003. [DOI] [PubMed] [Google Scholar]
- 72.Gerstenfeld L.C., Thiede M., Seibert K., Mielke C., Phippard D., Svagr B., Cullinane D., Einhorn T.A. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J. Orthop. Res. 2003;21:670–675. doi: 10.1016/S0736-0266(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 73.Bergenstock M., Min W., Simon A.M., Sabatino C., O'Connor J.P. A comparison between the effects of acetaminophen and celecoxib on bone fracture healing in rats. J. Orthop. Trauma. 2005;19:717–723. doi: 10.1097/01.bot.0000184144.98071.5d. [DOI] [PubMed] [Google Scholar]
- 74.Goodman S.B., Ma T., Mitsunaga L., Miyanishi K., Genovese M.C., Smith R.L. Temporal effects of a COX-2-selective NSAID on bone ingrowth. J. Biomed. Mater. Res. 2005;72:279–287. doi: 10.1002/jbm.a.30231. [DOI] [PubMed] [Google Scholar]
- 75.Murnaghan M., Li G., Marsh D.R. Nonsteroidal anti-inflammatory drug-induced fracture nonunion: An inhibition of angiogenesis? J. Bone Joint Surg. Am. 2006;88(Suppl. 3):140–147. doi: 10.2106/JBJS.F.00454. [DOI] [PubMed] [Google Scholar]
- 76.Ho M.L., Chang J.K., Wang G.J. Effects of ketorolac on bone repair: A radiographic study in modeled demineralized bone matrix grafted rabbits. Pharmacology. 1998;57:148–159. doi: 10.1159/000028236. [DOI] [PubMed] [Google Scholar]
- 77.Paulson S.K., Zhang J.Y., Breau A.P., Hribar J.D., Liu N.W., Jessen S.M., Lawal Y.M., Cogburn J.N., Gresk C.J., Markos C.S., Maziasz T.J., Schoenhard G.L., Burton E.G. Pharmacokinetics, tissue distribution, metabolism, and excretion of celecoxib in rats. Drug Metab. Dispos. 2000;28:514–521. [PubMed] [Google Scholar]
- 78.Virchenko O., Skoglund B., Aspenberg P. Parecoxib impairs early tendon repair but improves later remodeling. Am. J. Sports Med. 2004;32:1743–1747. doi: 10.1177/0363546504263403. [DOI] [PubMed] [Google Scholar]
- 79.Dimar J.R., 2nd., Ante W.A., Zhang Y.P., Glassman S.D. The effects of nonsteroidal anti-inflammatory drugs on posterior spinal fusions in the rat. Spine. 1996;21:1870–1876. doi: 10.1097/00007632-199608150-00006. [DOI] [PubMed] [Google Scholar]
- 80.McLaren A.C. Prophylaxis with indomethacin for heterotopic bone—After open reduction of fractures of the acetabulum. J. Bone Joint Surg. Am. 1990;72:245–247. [PubMed] [Google Scholar]
- 81.Gebuhr P., Wilbek H., Soelberg M. Naproxen for 8 days can prevent heterotopic ossification after hip arthroplasty. Clin. Orthop. Relat. Res. 1995:166–169. [PubMed] [Google Scholar]
- 82.Moore K.D., Goss K., Anglen J.O. Indomethacin versus radiation therapy for prophylaxis against heterotopic ossification in acetabular fractures: A randomised, prospective study. J. Bone Joint Surg. Br. 1998;80:259–263. doi: 10.1302/0301-620X.80B2.8157. [DOI] [PubMed] [Google Scholar]
- 83.Burd T.A., Lowry K.J., Anglen J.O. Indomethacin compared with localized irradiation for the prevention of heterotopic ossification following surgical treatment of acetabular fractures. J. Bone Joint Surg. Am. 2001;83A:1783–1788. doi: 10.2106/00004623-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 84.Sudmann E., Hagen T. Indomethacin-induced delayed fracture healing. Arch. Orthop. Traum Surg. 1976;85:151–154. doi: 10.1007/BF00415452. [DOI] [PubMed] [Google Scholar]
- 85.Adolphson P., Abbaszadegan H., Jonsson U., Dalen N., Sjoberg H.E., Kalen S. No effects of piroxicam on osteopenia and recovery after Colles' fracture—A randomized, double-blind, placebo-controlled, prospective trial. Arch. Orthop. Trauma Surg. 1993;112:127–130. doi: 10.1007/BF00449987. [DOI] [PubMed] [Google Scholar]
- 86.Davis T.R., Ackroyd C.E. Non-steroidal anti-inflammatory agents in the management of Colles' fractures. Br. J.Clin. Pract. 1988;42:184–189. [PubMed] [Google Scholar]
- 87.Giannoudis P.V., MacDonald D.A., Matthews S.J., Smith R.M., Furlong A.J., De Boer P. Nonunion of the femoral diaphysis—The influence of reaming and non-steroidal anti-inflammatory drugs. J. Bone Joint Surg. Br. 2000;82:655–658. doi: 10.1302/0301-620x.82b5.9899. [DOI] [PubMed] [Google Scholar]
- 88.Burd T.A., Hughes M.S., Anglen J.O. Heterotopic ossification prophylaxis with indomethacin increases the risk of long-bone nonunion. J. Bone Joint Surg. Br. 2003;85B:700–705. [PubMed] [Google Scholar]
- 89.Bhattacharyya T., Levin R., Vrahas M.S., Solomon D.H. Nonsteroidal antiinflammatory drugs and nonunion of humeral shaft fractures. Arthritis Rheum. 2005;53:364–367. doi: 10.1002/art.21170. [DOI] [PubMed] [Google Scholar]
- 90.Bauer D.C., Orwoll E.S., Fox K.M., Vogt T.M., Lane N.E., Hochberg M.C., Stone K., Nevitt M.C. Aspirin and NSAID use in older women: effect on bone mineral density and fracture risk—Study of Osteoporotic Fractures Research Group. J. Bone Miner. Res. 1996;11:29–35. doi: 10.1002/jbmr.5650110106. [DOI] [PubMed] [Google Scholar]
- 91.van Staa T.P., Leufkens H.G., Cooper C. Use of nonsteroidal anti-inflammatory drugs and risk of fractures. Bone. 2000;27:563–568. doi: 10.1016/s8756-3282(00)00361-6. [DOI] [PubMed] [Google Scholar]
- 92.Glassman S.D., Rose S.M., Dimar J.R., Puno R.M., Campbell M.J., Johnson J.R. The effect of postoperative nonsteroidal anti-inflammatory drug administration on spinal fusion. Spine. 1998;23:834–838. doi: 10.1097/00007632-199804010-00020. [DOI] [PubMed] [Google Scholar]
- 93.Zhang X., Schwarz E.M., Young D.A., Puzas J.E., Rosier R.N., O'Keefe R.J. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J. Clin. Invest. 2002;109:1405–1415. doi: 10.1172/JCI15681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chikazu D., Li X., Kawaguchi H., Sakuma Y., Voznesensky O.S., Adams D.J., Xu M., Hoshio K., Katavic V., Herschman H.R., Raisz L.G., Pilbeam C.C. Bone morphogenetic protein 2 induces cyclo-oxygenase 2 in osteoblasts via a Cbfal binding site: Role in effects of bone morphogenetic protein 2 in vitro and in vivo. J. Bone Miner. Res. 2002;17:1430–1440. doi: 10.1359/jbmr.2002.17.8.1430. [DOI] [PubMed] [Google Scholar]
- 95.Cottrell J.A., Meyenhofer M., Medicherla S., Higgins L., O'Connor J.P. Analgesic effects of p38 kinase inhibitor treatment on bone fracture healing. Pain. 2009;142:116–126. doi: 10.1016/j.pain.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 96.Einhorn T.A., Majeska R.J., Rush E.B., Levine P.M., Horowitz M.C. The expression of cytokine activity by fracture callus. J. Bone Miner. Res. 1995;10:1272–1281. doi: 10.1002/jbmr.5650100818. [DOI] [PubMed] [Google Scholar]
- 97.Hausman M.R., Schaffler M.B., Majeska R.J. Prevention of fracture healing in rats by an inhibitor of angiogenesis. Bone. 2001;29:560–564. doi: 10.1016/s8756-3282(01)00608-1. [DOI] [PubMed] [Google Scholar]
- 98.Katoh H., Hosono K., Ito Y., Suzuki T., Ogawa Y., Kubo H., Kamata H., Mishima T., Tamaki H., Sakagami H., Sugimoto Y., Narumiya S., Watanabe M., Majima M. COX-2 and prostaglandin EP3/EP4 signaling regulate the tumor stromal proangiogenic microenvironment via CXCL12-CXCR4 chemokine systems. Am. J. Pathol. 2010;176:1469–1483. doi: 10.2353/ajpath.2010.090607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xie C., Liang B., Xue M., Lin A.S., Loiselle A., Schwarz E.M., Guldberg R.E., O'Keefe R.J., Zhang X. Rescue of impaired fracture healing in COX-2-/- mice via activation of prostaglandin E2 receptor subtype 4. Am. J. Pathol. 2009;175:772–785. doi: 10.2353/ajpath.2009.081099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cottrell J.A., O'Connor J.P. Pharmacological inhibition of 5-lipoxygenase accelerates and enhances fracture-healing. J. Bone Joint Surg. Am. 2009;91:2653–2665. doi: 10.2106/JBJS.H.01844. [DOI] [PubMed] [Google Scholar]
- 101.Ren W., Dziak R. Effects of leukotrienes on osteoblastic cell proliferation. Calcified Tissue Int. 1991;49:197–201. doi: 10.1007/BF02556118. [DOI] [PubMed] [Google Scholar]
- 102.Gallwitz W.E., Mundy G.R., Lee C.H., Qiao M., Roodman G.D., Raftery M., Gaskell S.J., Bonewald L.F. 5-Lipoxygenase metabolites of arachidonic acid stimulate isolated osteoclasts to resorb calcified matrices. J. Biol. Chem. 1993;268:10087–10094. [PubMed] [Google Scholar]
- 103.Garcia C., Boyce B.F., Gilles J., Dallas M., Qiao M., Mundy G.R., Bonewald L.F. Leukotriene B4 stimulates osteoclastic bone resorption both in vitro and in vivo. J. Bone Miner. Res. 1996;11:1619–1627. doi: 10.1002/jbmr.5650111105. [DOI] [PubMed] [Google Scholar]
- 104.Bonewald L.F., Flynn M., Qiao M., Dallas M.R., Mundy G.R., Boyce B.F. Mice lacking 5-lipoxygenase have increased cortical bone thickness. Adv. Exp. Med. Biol. 1997;433:299–302. doi: 10.1007/978-1-4899-1810-9_63. [DOI] [PubMed] [Google Scholar]
- 105.Traianedes K., Dallas M.R., Garrett I.R., Mundy G.R., Bonewald L.F. 5-Lipoxygenase metabolites inhibit bone formation in vitro. Endocrinology. 1998;139:3178–3184. doi: 10.1210/endo.139.7.6115. [DOI] [PubMed] [Google Scholar]
- 106.Maxis K., Delalandre A., Martel-Pelletier J., Pelletier J.P., Duval N., Lajeunesse D. The shunt from the cyclooxygenase to lipoxygenase pathway in human osteoarthritic subchondral osteoblasts is linked with a variable expression of the 5-lipoxygenase-activating protein. Arthritis Res. Ther. 2006;8:R181. doi: 10.1186/ar2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hogevold H.E., Grogaard B., Reikeras O. Effects of short-term treatment with corticosteroids and indomethacin on bone healing—A mechanical study of osteotomies in rats. Acta Orthop. 1992;63:607–611. doi: 10.1080/17453679209169718. [DOI] [PubMed] [Google Scholar]