Abstract
Uncontrolled neovascularization occurs in several angiogenesis-dependent diseases, including cancer. Neovascularization is tightly controlled by the balance between angiogenic growth factors and antiangiogenic agents. The various natural angiogenesis inhibitors identified so far affect neovascularization by different mechanisms of action. Thrombospondin-1 (TSP-1) is a matricellular modular glycoprotein that acts as a powerful endogenous inhibitor of angiogenesis. It acts both indirectly, by sequestering angiogenic growth factors and effectors in the extracellular environment, and directly, by inducing an antiangiogenic program in endothelial cells following engagement of specific receptors including CD36, CD47, integrins and proteoglycans (all involved in angiogenesis ). In view of its central, multifaceted role in angiogenesis, TSP-1 has served as a source of antiangiogenic tools, including TSP-1 fragments, synthetic peptides and peptidomimetics, gene therapy strategies, and agents that up-regulate TSP-1 expression. This review discusses TSP-1-based inhibitors of angiogenesis, their mechanisms of action and therapeutic potential, drawing our experience with angiogenic growth factor-interacting TSP-1 peptides, and the possibility of exploiting them to design novel antiangiogenic agents.
Keywords: angiogenesis, tumor, integrins, interactions, thrombospondin-1
1. Neovascularization
Angiogenesis is the process of new blood vessel formation from existing ones. It takes place in embryonic development and inflammation [1] and during angiogenesis-dependent diseases, including cancer [2]. In view of its essential contribution to physiological processes and major pathologies, angiogenesis is tightly controlled, mainly through the balance between the production and release of pro-angiogenic and antiangiogenic molecules (Figure 1).
Figure 1.
The balance between the production and release of pro- and antiangiogenic molecules regulates neovascularization. For a more exhaustive list of antiangiogenic compounds and abbreviations, see Table 1 and Table 2.
Pro-angiogenic molecules are a heterogeneous group of proteins that include the vascular endothelial growth factors (VEGFs) and fibroblast growth factors (FGFs). They induce angiogenesis by interacting with tyrosine kinase receptors (TKRs) expressed on the endothelial cell (EC) surface. A common theme among angiogenic growth factors (AGFs) is their interaction with (co)receptors other than TKRs (e.g. heparan sulfate proteoglycans (HSPGs) and integrins [3]). This complex pattern of extracellular interactions is mirrored by the intricacy of the signal transduction pathway(s) triggered by AGFs in ECs [4]. Once stimulated, ECs acquire the “angiogenic phenotype”, namely the ability to execute all the different steps of the angiogenic process, including extracellular matrix (ECM) degradation, a change of surface expression of adhesion molecules, proliferation, and chemotactic migration [4].
2. Antiangiogenic Compounds
Antiangiogenic compounds are a heterogeneous group of molecules that includes proteins, polysaccharides and glycosphingolipids found in the body fluids and ECM. A common theme among antiangiogenic compounds is their ability to directly bind and sequester AGFs in the extracellular environment, thus hampering their interaction with ECs (Table 1).
Table 1.
Endogenous antiangiogenic molecules that bind and sequester AGFs in the extracellular environment. TSP-1 is highlighted in grey.
| Molecule | AGF bound | Reference |
|---|---|---|
| TSP-1 | FGF2, VEGF, HGF, HIV-1 Tat, TGF-β1 | [5,6,7,8], [9,10], [11], [12], [13] |
| α2-macroglobulin | FGF2, VEGF, TGF-β, IL8, TNF | [14], [15], [16], [17], [18] |
| heparin | FGF2, VEGF, HIV-1 Tat, HGF | [19], [20], [21], [22] |
| pentraxin-3 (PTX3) | FGF2, FGF8 | [23] |
| factor VII-activating protease | FGF2, PDGF | [24], [25] |
| platelet factor 4 (PF-4) | FGF2, VEGF | [26], [27] |
| SPARC | VEGF, PDGF | [28], [29] |
| CXCL13 | FGF2 | [30] |
| gangliosides | FGF2 | [31] |
| fibstatin (fibronectin fragment) | FGF2 | [32] |
| vitronectin | FGF2 | [33] |
| soluble VEGF receptor (VEGFR)-1 | VEGF | [34] |
| ADAMTS1 | VEGF | [35] |
| heparin affin regulatory peptide (HARP) | VEGF | [36] |
| connective tissue growth factor (CTGF) | VEGF | [37] |
| soluble endoglin | TGF-β1 | [38] |
| decorin | TGF-β1 | [39] |
| secretory component | IL8 | [40] |
However, under certain conditions, the direct interaction with a given ligand may lead to oligomerization of the AGF, its protection from proteolytic degradation and a rise in local concentration. This, in turn, will lead to proangiogenic rather than antiangiogenic effects, as already demonstrated for AGF binding to heparin, collagen and fibrinogen/fibrin (see [3] and references therein).
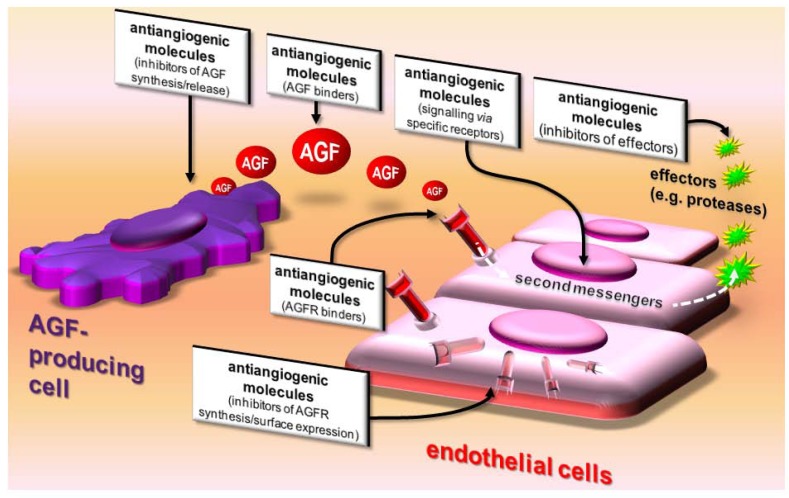
Besides binding AGFs, antiangiogenic molecules can inhibit angiogenesis by: i) inhibiting AGF production by tumor cells; ii) inhibiting surface expression of AGF-receptors on ECs; iii) binding (and masking) AGF receptors; iv) reducing EC responsiveness to AGFs (usually by engaging specific receptors that modify the EC phenotype); v) inhibiting effectors of angiogenesis produced by ECs (i.e., proteases required for ECM degradation) (Figure 2 and Table 2).
Figure 2.
Action on angiogenesis. Antiangiogenic molecules affect AGFs by acting on AGF-producing cells, AGFs themselves, AGF receptors (AGFR), ECs, and angiogenesis effectors produced by activated ECs.
Table 2.
Endogenous antiangiogenic molecules and their mechanisms of action. TSP-1 is evidenced in grey.
| MOLECULE | MECHANISM OF ACTION |
|---|---|
| inhibition of AGF expression/release by producing cells | |
| homocysteine | lowering FGF2 levels [43] |
| interleukin (IL)-12 | lowering FGF2 levels [44] |
| TSP-1 | lowering FGF2 levels [45] |
| inhibition/interference with AGF receptors on ECs | |
| IL-1, IFN-γ | TK- FGF receptors (TK-FGFR) down-regulation [46] |
| anosmin-1 | TK-FGFR occupancy [47] |
| thromboxane | inhibition of TK-FGFR1 internalization [48] |
| soluble form of TKR | formation of heterodimers with TK-FGFR1 [49] |
| antithrombin | HSPG down-regulation [50] |
| PF4 | HSPG occupancy [51], unknown [52] |
| MOLECULE | MECHANISM OF ACTION |
| endostatin | HSPG occupancy [53] |
| kallistatin | HSPG occupancy [54] |
| histidine-rich glycoprotein | HSPG occupancy [51] |
| endosulfatases | HSPG desulfation [55,56] |
| heparinase | HSPG degradation [57] |
| TSP-1 | HSPG occupancy [9], integrin occupancy [48] |
| inhibition/interference with AGF-activated second messengers in ECs | |
| heat-shock proteins 70 and 90 | down-regulation of pAkt, c-Raf-1 and ERK1/2 [58] |
| sprouty proteins | inhibition of TK-FGFR signalling [59] |
| homeobox gene GAX | inhibition of NF-kB signalling [60] |
| semaphorin-3F | inhibition of ERK1/2 signalling [61] |
| angiostatin [a plasminogen (Plg) fragment] | inhibition of ERK1/2 signalling [62] |
| ghrelin | inhibition of TKR/MAPK signalling [63] |
| lysophosphatidylcholine | inhibition of ras/ERK1/2 signalling [64] |
| pigment epithelium-derived factor | inhibition of Fyn signalling [65] |
| TSP-1 | inhibition of VEGF-mediated Akt signalling [66] |
| modification of EC apoptosis, phenotype, responsiveness to AGFs | |
| cleaved HMW kininogen | tropomyosin engagement [67] |
| IL-4 | alteration of cell cycle [68] |
| kininostatin (kininogen fragment) | inhibition of cyclin D1 expression [69] |
| vitamin D3-binding protein | CD36 engagement [70] |
| endostatin | Shb activation [71] |
| histidine-rich glycoprotein | tropomyosin engagement [72] |
| endostatin | cytoskeleton organization [73], Shb activation [71] |
| TSP-1 | TNF-α over-expression [74], CD36 engagement [66,75], apoptosis [45,66,74], ECM modification [76] |
| inhibition/interference with angiogenesis effectors | |
| IL-12 | inhibition of FGF-induced proteases [44] |
| tissue inhibitor metalloproteinase (TIMP)-2, 4 | inhibition of FGF-induced proteases [77] |
| kallistatin | inhibition of FGF-induced proteases [54] |
| TSP-1 | inhibition of FGF-induced proteases [78], binding to matrix metalloproteinase-2 (MMP-2) [79] |
| unknown mechanism of action | |
| collagen I [80], alphastatin (fibrinogen fragment) [81], CXCL14 [82], IL-12 [83], IP-10 [84], vasostatin [85], vasculostatin (fragment of brain angiogenesis inhibitor-1) [86], TGF-β1 [87], TNFs [88], somatostatin [89], retinoids [90], apolipoprotein(a) [91], prolactin (16 kDa fragment) [92] | |
In view of the extensive literature, here we only report inhibitors of FGF2 as a prototypic AGF.
Two main considerations emerge from Table 1 and Table 2: i) some antiangiogenic molecules (including TSP-1) target different AGFs simultaneously; ii) some antiangiogenic molecules (including TSP-1) inhibit the angiogenic process through multiple mechanisms. These indications may provide useful suggestions for designing therapeutic strategies to inhibit angiogenesis, since pathological neovascularization is often the result of the simultaneous, non-redundant actions of various AGFs [41,42]. Inhibiting neovascularization by drugs directed against a single AGF/TKR presents several limitations [41]. Moreover, developing drugs acting on multiple targets/mechanisms may limit the insurgence of drug resistance, which is a major problem with conventional antineoplastic therapies.
TSP-1 interferes simultaneously with several AGFs (Table 1) through different mechanisms of action (Table 2), thus offering a paradigm for the development of antiangiogenic drugs. This review discusses TSP-1 and TSP-1-based inhibitors of angiogenesis, their mechanisms of action and therapeutic potential.
3. Structure and Biological Activity of TSP
The mammalian family of TSPs comprises five members, including TSP-1 and TSP-2, which form group A, homotrimeric TSPs. They are very similar in structure and can all inhibit angiogenesis, although they are expressed differently in various tissues during development and adulthood.
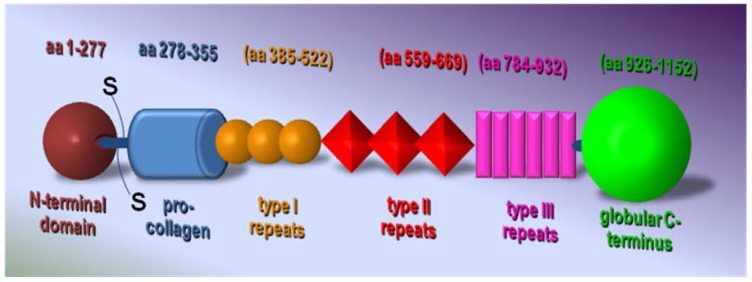
TSP-1 was the first endogenous inhibitor of angiogenesis to be identified [30,93]. Each TSP-1 monomer is formed by an N-terminal globular domain, followed by the coiled-coil oligomerization domain, a von Willebrand Factor type C procollagen domain, three properdin-like type I repeats, two epidermal growth factor-like type II repeats and a signature domain comprising a third type II repeat, the calcium-binding wire-type III repeats, and the lectin-like C-terminal globular domain [14] (Figure 3).
Figure 3.
Schematic representation of TSP-1 structure.
Type I repeats is a relatively small region commonly considered the main antiangiogenic site of TSP-1. Interestingly, this domain is also present in several other proteins, often giving them significant antiangiogenic activity [94,95].
The region comprising the third type II repeats, the type III repeats and the C-terminal globular end is the most conserved region in the TSP family [96]. Its properties are affected by calcium. The cooperative binding of calcium ions is the main feature of type III repeats and profoundly affects the structure/availability of active sequences in the whole cassette. The type III repeats contain a cryptic integrin recognition motif RGD [97], two sequences that bind cathepsin G and neutrophil elastase [98], and binding sites for collagen V and FGF2. These binding sites become exposed only after drastic structural changes induced by a low calcium concentration or disulfide bond reduction, illustrating the importance of environmental conditions for the bioavailability and activity of these sequences [7,97,98,99]. TSP-1 is active both as a whole molecule and as fragments [100], a property shared by the matricellular PTX3 [23,101], whereas most endogenous angiogenesis inhibitors are fragments of larger molecules with no intrinsic antiangiogenic activity (such as fibronectin, kininogen and plasminogen (Table 1 and Table 2).
TSP-1 has an extremely complex, context-dependent effect on angiogenesis, reflecting the heterogeneity of its functional domains, each interacting with selected cell receptors, AGFs, ECM components, and proteases [100]. Thus, in a given biological setting, the local/temporal expression of these ligands and the availability of each TSP-1 domain drive the pattern of molecular interactions which, in turn, dictate the biological effects of TSP-1 [78]. As a consequence, TSP-1 exerts both pro- and antiangiogenic effects in vitro and in vivo depending on its concentration [102], free or ECM-associated status [103,104,105] and oligomerization [106,107,108].
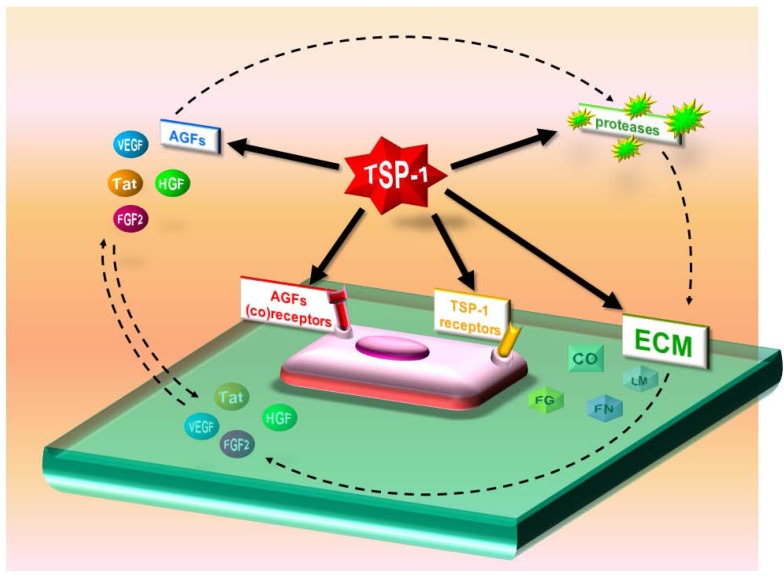
TSP-1 affects angiogenesis both directly and indirectly. As a direct inhibitor, it interacts with specific receptors (i.e., CD36 and CD47 [109]) on ECs to affect apoptosis and functions related to angiogenesis. As an indirect inhibitor, it binds to and influences the activity/bioavailability of various mediators of angiogenesis, including AGFs, cytokines and proteases (Table 1 and Table 2, Figure 4).
Figure 4.
Direct and indirect antiangiogenic actions of TSP-1. TSP-1 sequesters AGFs in the extracellular environment and masks various AGF receptors. TSP-1 also reduces EC responsiveness to AGFs and induces apoptosis by activating CD36. It binds matrix metalloproteinase-2 (MMP-2), favoring its clearance. Finally, it inhibits AGF production by tumor cells.
3.1. Direct effects of TSP-1
TSP directly affects ECs and tumor cells by interacting with specific receptors. CD36 was the first TSP-1 receptor identified [110]. It is an 88-kDa glycoprotein expressed by many cell types including ECs [111]. TSP-1 and CD36 interact through the CLESH-1 domain in CD36 and the type I repeats in TSP-1 [112]. CD36 constitutively associates with β1 integrins and VEGF receptor 2 (VEGFR-2) in ECs [113,114], with interesting implications for the cross-talk among TSP-1, CD36, VEGFR-2 and integrins in the antiangiogenic action of TSP-1. This interaction has manifold consequences: it inhibits FGF2-induced EC migration, morphological organization [112,113], production of nitric oxide (NO) [115] and angiogenesis in vivo [116], induces apoptosis in ECs [116] and tumor cells [117,118] and down-regulates the expression and phosphorylation of VEGFR-2 on EC surface [113,114].
CD47 was originally identified as the receptor that mediates cell adhesion and spreading to TSP-1 [119,120]. CD47 also forms a signalling complex with integrins [121]. By binding to CD47, TSP-1 inhibits NO-cGMP signalling, hence also neovascularization [115,122].
TSP-1 binds heparin and HSPGs (syndecan-1 and 4, perlecan, decorin) through its N-terminal domain (Table 3). This interaction can have antiangiogenic effects. TSP-1 displaces VEGF from EC HSPGs, inhibiting angiogenesis [9]. However, by binding to syndecan-4, TSP-1 can also exert pro-angiogenic effects, protecting ECs from apoptosis and stimulating tubulogenesis [123]. The heparin binding motif Hep II also comprises the binding sequence for α6 integrin, pointing to cooperation between HSPGs and integrins, as already demonstrated for CD36 and CD47 (see above).
Table 3.
TSP-1 ligands and their binding domains in the TSP-1 structure.
| Ligand | Binding domain in TSP-1 | Reference | ||
|---|---|---|---|---|
| free molecules (body fluids) | AGFs | FGF2 | • type III repeats | [7] |
| VEGF | • type I repeats | [37] | ||
| HGF | • 3D conformation | [11] | ||
| HIV-1 Tat | N.D. | [12] | ||
| TGF-β | • 2nd type I repeats (RFK sequence) | [132,133,134] | ||
| • type I repeats (WSXW sequence) | [133,134] | |||
| PDGF-BB | • 3D conformation | [135] | ||
| proteases and regulators | MMP-2 | • type I repeats | [136] | |
| Plg/plasmin | N.D. | [137,138,139] | ||
| tissue Plg activator | N.D. | [140] | ||
| urokinase Plg activator | N.D. | [141] | ||
| neutrophil elastase | • type III repeats | [142] | ||
| cathepsin G | • type III repeats | [142,143] | ||
| tissue factor inhibitor | N.D. | [144] | ||
| others | heparin | • N-ter domain [motifs Hep I (aa 17-35) & Hep II (aa 78-94)] | [103,145] | |
| • type I repeats | [146,147] | |||
| • signature domain | [148] | |||
| histidine-rich glycoprotein | N.D. | [149] | ||
| factor V | N.D. | [150] | ||
| angiocidin | • 2nd and 3rd type I repeats (CSVTCG sequence) | [151] | ||
| calumenin | • N-ter domain (aa 21- 228) | [152] | ||
| endostatin | N.D. | [153] | ||
| cell surface receptors | CD36 | • type I repeats | [112] | |
| CD47 | • C-ter domain | [118,122,154] | ||
| HSPGs | • N-ter domain [motifs Hep I (aa 17-35) & Hep II (aa 78-94)] | [103,145] | ||
| • signature domain | [148] | |||
| sulfated glycolipids | • N-ter domain | [155] | ||
| • 3D conformation | [155] | |||
| LRP | • N-ter domain | [126,155] | ||
| VLDL receptor | • N-ter domain | [156,157] | ||
| calreticulin | • N-ter domain (aa 17-35) | [126,158] | ||
| integrins | • N-ter domain | [107,129,159,160,161] | ||
| • type I repeats | [128,130,162] | |||
| • type III repeats (RGD sequence) | [118,163] | |||
| ECM | collagen I | N.D. | [164] | |
| collagen V | • procollagen domain + type I & II repeats | [165,166] | ||
| fibronectin | • N-ter domain + type I & II repeats | [167,168] | ||
| laminin | N.D. | [165] | ||
| fibrinogen/fibrin | • N-ter domain | [157] | ||
| • procollagen domain | [146] | |||
| • type I repeats | [169,170] | |||
| von Willebrand factor | • signature domain | [171] | ||
| dermatan sulfate | • N-ter domain (KKTR sequence) | [172] | ||
| chondroitin sulfate | • N-ter domain | [155] | ||
| IGF-binding protein-5 | • N.D. | [173] | ||
N.D., not determined. HSPGs, reported here as cell surface receptors, are also constituents of ECM. Conversely, dermatan- and chondroitin-sulfates, reported as ECM components, also exist as saccharidic chains of cell surface proteoglycans.
The low-density lipoprotein receptor-related protein (LRP) acts as a TSP-1 receptor. Like HSPGs, LRP can exert opposite effects on angiogenesis. It mediates endocytosis of the TSP-1/VEGF [124] and TSP-1/MMP-2 [79] complexes, contributing to the CD36-independent inhibition of VEGF angiogenic activity by TSP-1. However, the binding of TSP-1 to calreticulin (Table 3) enhances calreticulin binding to LRP that, in turn, induces EC motility and focal adhesion disassembly [125,126].
TSP interacts with several β1 integrins and with αvβ3, involved in angiogenesis. Different binding sites for integrins have been mapped in TSP-1 (Table 3). Again, the various interactions between the different functional domains of TSP-1 and the different integrins can mediate both pro- [127] and antiangiogenic [128] effects. However, preclinical studies indicate that small TSP-1 peptides containing the integrin binding sites, as well as disintegrins or anti-integrin antibodies, block EC pro-angiogenic functions such as adhesion, proliferation, survival, wound healing, motility and angiogenesis in vivo [127,129,130].
3.2. Indirect effects of TSP-1
Besides cell surface receptors, TSP-1 interacts with several other partners, including AGFs (Table 3). TSP-1 binds FGF2 with affinity similar to the FGF2/HSPG interaction. Accordingly, heparin prevents the TSP-1/FGF2 interaction and TSP-1 prevents the FGF2/HSPG interaction [5]. As a consequence of its interaction with FGF2, TSP-1 inhibits FGF2-triggered proliferation and chemotaxis in ECs [5,6,7]. Finally, TSP-1 prevents FGF2 accumulation in the ECM, favoring its mobilization as inactive TSP-1/FGF2 complexes [6]. These observations suggest that free TSP-1 acts as a scavenger for ECM-associated FGF2, affecting its location, bioavailability and function.
TSP-1 binds both free and cell-associated VEGF [9], suggesting that it regulates the bioavailability of VEGF in the microenvironment and its capacity to bind to its EC receptors during neovascularization. Also, TSP/VEGF complexes are internalized via LRP-1 [131]. This contributes to TSP-1’s ability to inhibit VEGF-induced EC tubulogenesis in vitro and angiogenesis in vivo [9].
TSP-1 binds HGF in a calcium-independent manner. Heat denaturation reduces its binding to HGF, suggesting that a proper 3D conformation is required [11]. Mature two-chain and precursor single-chain HGF both bind to TSP-1. Heparin prevents this interaction but does not disrupt established complexes. At a biological level, TSP-1 inhibits HGF-induced chemotaxis of ECs in vitro and HGF-induced angiogenesis in vivo [11].
TSP-1 binds HIV-1 Tat [12] inhibiting Tat-induced EC migration in vitro and angiogenesis in vivo [12,174]. It also binds and activates transforming growth factor (TGF)-β1 through sequences located in the type I repeats [132,133,134]. TSP-1-associated TGF-β1 is biologically active and protected from inactivation. As a result, the inhibition of ECs by TSP-1 is at least partly mediated by complexed TGF-β1 [13,134]. TSP-1 binds both free or substrate-associated PDGF-BB in a calcium-dependent way [135], but the biological importance of these interactions remains to be clarified.
As a matricellular protein, TSP-1 participates in organizing the ECM, which provides environmental and positional cues to ECs during angiogenesis. ECM components interact to form a complex structural framework, and TSP-1 binds several of them (Table 3), with various consequences on ECM assembly and adherent EC behavior. Also, ECM is continuously remodeled by proteases. TSP-1 binds several proteases, including MMP-2, plasmin, neutrophil elastase and cathepsin G [79,98,136,175,176]. It promotes MMP-2 clearance via endocytosis by LRP [79,175] and suppresses MMP-3-mediated activation of proMMP-9 [177]. Conversely, it stimulates MMP-9 expression in ECs, promoting tubulogenesis [178]. The antiangiogenic 140 kDa TSP-1 fragment induces TIMP-2 over-expression [103], whereas the proangiogenic N-terminal domain of TSP-1 increases MMP-9 and MMP-2 release and reduces TIMP-2 expression by ECs.
Thus many of TSP-1’s effects on neovascularization are due to its ability to bind several molecules present in body fluids, ECMs, and the EC surface. It can therefore be envisaged at the centre of a complex interplay among AGFs, ECM components and their receptors and proteases. Through these multiple interactions, TSP-1 orchestrates their bioavailability, mutual binding and activities, leading to regulation of EC behavior during angiogenesis (Figure 5).
Figure 5.
TSP-1 interactome. AGFs bind receptors inducing proteases that remodel ECM and mobilize AGFs, creating an environment favorable to EC proliferation and migration. TSP-1 binds several of these regulators, orchestrating their interactions/activities and leading to fine tuning of EC behavior during neovascularization.
The “multi-binding” properties of TSP-1 depend on its modular structure in which several binding sequences are in close proximity, a feature that may lead to the formation of large multi-molecular complexes [140,149] in which the activity of each sequence becomes context-dependent, according to the environmental conditions and the predominant ligand. The ”multi-binding” capacity may also favor the coupling of some of its receptors (e.g. CD36, CD47, integrins, HSPGs and VEGFR-2, see above).
4. Therapeutic Exploitation of TSP-1 as an Antiangiogenic Agent
With its multifaceted roles in the control of angiogenesis and oncogenesis, TSP-1 could be exploited therapeutically by different approaches.
4.1. TSP-1 upregulation
This is based on the observation that many antiangiogenic molecules act indirectly by upregulating the production of TSP-1. Thus, the simplest way to exploit TSP-1’s antiangiogenic potential would be to deliver molecules that induce its over-expression in producing cells (Table 4).
Table 4.
Natural and synthetic molecules that induce over-expression of TSP-1.
| Molecule | References |
|---|---|
| glucose | [179] |
| peroxisome proliferator-activated receptor agonist fenofibrate | [180] |
| trichostatin-A | [181] |
| retinoic acid | [182,183] |
| somatostatin receptor subtype 2 | [10] |
| cyclic adenosine 5'-monophosphate-activated guanine nucleotide exchange factor for Rap1 | [184] |
| angiostatin | [185] |
| PHA -665752 (a small molecule, ATP-competitive inhibitor of c-Met receptor) | [186] |
| delta4-tibolone | [187] |
| phorbol 12-myristate 13-acetate | [183] |
| fibulin-5 | [188] |
| angiotensin II and its agonist CGP42112A | [189,190] |
| endostatin | [191] |
| estradiol | [192] |
| progesterone and raloxifene | [193] |
| IL-6 | [183] |
| IL-18 | [194] |
| erythropoietin | [195] |
| epidermal growth factor | [196] |
| TFG-β1, FGF2 | [197] |
| thrombin | [198] |
| inhibitors of DNA methyltransferases and histone deacetylases | [199] |
| CD26-processed chemokines CXCL12 and CCL5 | [200] |
The practical exploitation of this approach might be hampered by the unpredictable effects of non-selective over-expression of such a pleiotropic molecule. Nonetheless, it is interesting to note that the antiangiogenic, antineoplastic activity of metronomic, low-dose cyclophosphamide has been associated with increased levels of TSP-1 [201,202]. Similarly, TSP-1 is induced in colon cancer models after treatment with 5-FU [203], in rat prostate tumors treated with cyclophosphamide, doxorubicin or paclitaxel [204], in head and neck squamous carcinoma cells treated with docetaxel [205], in neuroblastoma cells treated with valproic acid [206], and in HT-29 colon cancer xenografts treated with metronomic irinotecan [207]. Metronomic irinotecan also raises plasma levels and gene expression of TSP-1 in patients with metastatic colorectal cancer [208].
4.2. Gene therapy
More controlled TSP-1 over-expression could be achieved by gene therapy, whose advantages are schematized in Figure 6. TSP-1 over-expression can be obtained by targeting the TSP-1 gene itself or a number of oncogenes/oncosuppressor genes that influence its expression. Adams and co-workers [106] nicely demonstrated the versatility of the gene therapy approach by using different TSP-1 modules differing in their capacity to selectively interact with various ligands.
Figure 6.
Advantages of TSP-1-based gene therapy. By different strategies (1) and by targeting different cell types (4), it is possible to induce directly the expression of the TSP-1 gene, to stimulate or inhibit the expression of TSP-1 enhancers/inhibitors (2) or to express selected TSP-1 modules (3).
The list below describes some TSP-1-based gene therapy strategies:
i) Fibroblasts retrovirally transduced to produce high levels of TSP-1 resulted in high levels of the protein that inhibited angiogenesis and tumor growth in different models [209]; ii) recombinant adeno-associated virus (AAV)-mediated delivery of the three type I repeats (3TSR) resulted in expression of the transgene in normal tissues, reduced VEGF-induced angiogenesis, reduced tumor growth and microvessel density both locally and at distant sites [210]; iii) AAV-mediated gene therapy has also been exploited to express a TSP-1 fragment that inhibits human leukemia xenografts growth in nude mice [211]. iv) expression of 3TSR or of the second type I repeats containing the TGF-β-activating sequences significantly inhibited in vivo tumor angiogenesis and growth in nude mice [212]; v) expression of TSP-1-derived 4N1K peptide-containing proteins in renal cell carcinoma tissues was associated with a decrease in tumor growth and angiogenesis [213]; vi) transfection of a TSP-1 complementary cDNA antisense into glioblastoma cells lines significantly reduced TSP-1 production and cell motility [214]; vii) p53 inactivation lowered TSP-1 production [215,216]. Accordingly, topical delivery of p53 DNA to the lung increased TSP-1 expression, reduced microvessel density and limited lung tumor burden, prolonging the survival of tumor-bearing mice [217]; viii) c-Myc-regulated cluster miRNA-17-92, over-expressed in many human cancers, inhibited TSP-1 expression in cancer cells and in ECs. Inhibition of miR-17-92 by means of microRNAs increased TSP-1 expression and reduced VEGF-induced EC proliferation, migration and morphogenesis [218,219]; ix) transfection of c-Jun and/or RARalpha expression vectors into hepatoma cells and ECs raised mRNA and protein levels of TSP-1 [183]; x) over-expression of connexin-26 in human breast tumor cells up-regulated both the transcription and translation of TSP-1, retarding tumor growth in vivo [220]; xi) knockdown of Her-2/neu expression by siRNA increased the expression of TSP-1 and reduced that of VEGF [221]; xii) silencing CD26 using siRNA increased TSP-1 expression in T cells [200]. As discussed below, over-expression of TSP-1 or related molecules can sometimes lead to an increase in tumor growth, as after over-expression of the TSP-1 fragment 167-569 in C6 glioma cells [222].
4.3. TSP-1-based peptides and peptidomimetics
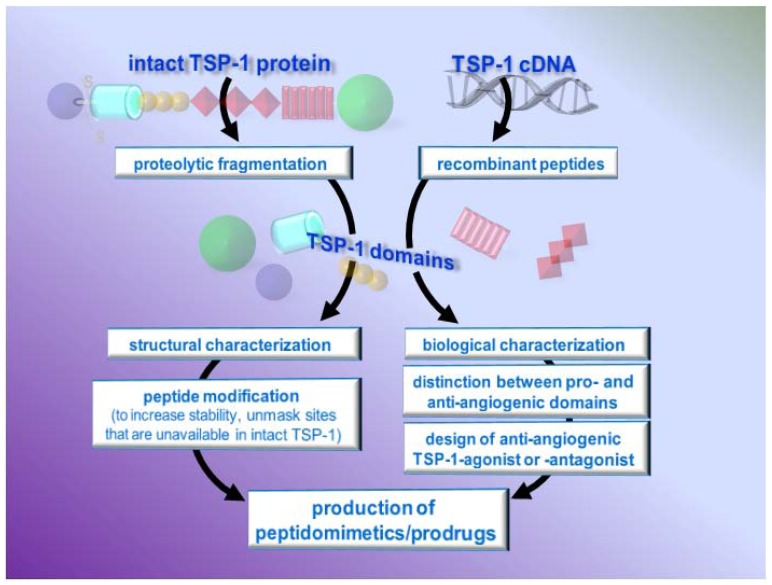
The use of TSP-1-based drugs must deal with the fact that TSP-1, like its related peptides, can elicit both anti- and pro-angiogenic responses. However, this expands, rather than limits, the possibility of developing TSP-1-based antiangiogenic therapies by designing drugs or gene therapies that mimic the antiangiogenic effects of TSP-1 or antagonize the pro-angiogenic ones (Figure 7).
Figure 7.
Design of anti-angiogenic TSP-1 peptides/peptidomimetics
The use of biologically relevant, functional protein sequences as an entry point for the development of novel lead compounds is a powerful tool in drug discovery [223,224,225,226]. The starting sequences can elucidate the roles of key interactions (hot spots) in the regulation of important protein-protein interactions that a drug must agonize or antagonize. This knowledge may then boost our ability to interfere with specific pathological interactions, providing attractive therapeutic opportunities and extending medicinal chemistry to new classes of compounds.
4.3.1. Characterization of TSP-1 active domains and sequences
The design of TSP-1-based drugs started from the identification/characterization of TSP-1 active domains. These studies used antibodies directed against the various portions of TSP-1 or peptides representing various TSP-1 fragments. The latter can be obtained by controlled proteolytic digestion of the intact TSP-1 protein [227] (e.g. by ADAMTS-1 [228] or thrombin [103]) or, more often, by the production of recombinant fragments [100]. Table 5 lists the TSP-1-derived peptides studied so far for their ability to regulate neovascularization. The pro-angiogenic domains of TSP-1 are mostly mapped in its N-terminal domain, while the main antiangiogenic sequences are in the second and third type I repeats.
Table 5.
Pro- and antiangiogenic TSP-1-derived peptides.
| Pro-angiogenic peptides | Mechanism | Reference |
| peptides from the N-ter domain |
|
[78,103,129] |
| antiangiogenic peptides | ||
| integrin–binding sequence of the N-ter domain |
|
[129] |
| sequences in the pro-collagen domain |
|
[227] |
| various peptides from the second and third type I repeats |
|
[228,234,235] |
| peptide from the type III repeats |
|
[8] |
| peptide 4N1 in the C-ter domain |
|
[122] |
Besides ECs, TSP-1-derived peptides also act on tumor cells. A peptide representing the Hep-I sequence induced promyelocytic leukemia cell differentiation and apoptosis [229], while 3TSR inhibited proliferation and induced apoptosis of B16F10 tumor cells in a TGF-β-dependent manner [114] in vitro and reduced tumor growth in orthotopic pancreatic xenografts [230,231] and in a model of polyoma middle T transgenic mice [232]. The peptide GGWSHW, located within the type I repeats, induces promyelocytic leukemia cell differentiation and apoptosis [229]. Retro-inverso peptides of the WSHWSxPWS sequence (aa sequence 438–452) inhibit the growth of MDA-MB-435 carcinoma cells in the mammary fat pad of nude mice [100,233]. WSxW-containing peptides also inhibit TSP-1-mediated activation of the TGF-β latent complex [133] and motility of glioma cells [214].
A fragment spanning the type III repeats and C-terminal domain causes promyelocytic leukemia NB4 cell death through CD47/αϖβ3 [118]. Peptides containing the second type I repeats also inhibit tumor growth by regulating tumor cell proliferation and apoptosis in a TGF-β-dependent manner. Their capacity to inhibit angiogenesis is instead TGΦ−β−independent [134]. Finally, peptide 4N1, interacting with CD47, induces apoptosis in different breast cancer cell lines [236] and sensitizes human prostate tumor cells to taxane cytotoxicity [237], though it protects normal cells from apoptosis [238].
4.3.2. Modifications of TSP-1-derived peptides and generation of peptidomimetics
The therapeutic exploitation of synthetic peptides is limited by their well-known shortcomings: unfavorable pharmacodynamics/pharmacokinetics (poor oral bioavailability and/or short duration of action), lack of receptor selectivity and low affinity (with Kd in the mM-μM range, compared to the pM-nM range of the Kd for parent proteins [239]). Modifying the peptide structure by acylation, PEGylation, fatty acid acylation, unnatural amino acids or restricted conformation can largely overcome these limits [240]. Many TSP-1 sequences exploitable for the design of antiangiogenic drugs are exposed in the TSP-1 molecule only after drastic structural changes induced by a low calcium concentration, different ligands, or reduction of disulfide bonds, indicating the structural modifications that must be introduced (and maintained) in the derived peptides. Guo and coworkers synthesized stereospecific analogs of the KRFKQDGGWSHWSPWSSC peptide from TSP-1 type I repeats that allowed dissection of different biological properties of the peptide enhancing the desiderable ones [233]. Similarly, the peptide D-reverse amKRFKQDGGWSH-WSPWSSac inhibits proliferation of C6 glioma cells in vitro and tumor growth in vivo [241]. Other modified TSP-1 peptides have been described: CVX-22 is a chimera obtained by fusing two mimetic nonamer peptides from TSP-1 type I repeats to the Fab binding site of a humanized scaffold antibody. This chimera selectively induces apoptosis of VEGFR-2-positive ECs in melanoma [242]. A phase I trial for this compound found some possibly drug-related adverse events but no dose-limiting toxicities [243].
ABT-510 is an antiangiogenic TSP-1 modified nonapeptide designed on the 7-mer active sequence GVITRIR of the second type I repeats. Although not active in the native conformation, appropriate modifications gave it strong antiangiogenic activity [75]. In detail, the first L-ile residue of the GVITRIR sequence was substituted with D-ile and the first Arg with the non-natural amino acid norvaline. The resulting D-enantiomer was capped by the addition of sarcosine at the N-terminus and proline ethylamide at the C-terminus, generating peptide ABT-526 [75]. A more soluble version of the peptide, eventually named ABT-510, was obtained by substituting D-ile with D-allo-ile [244,245]. Since its original preparation and description in 1999 [75], the antiangiogenic and antineoplastic activity of ABT-510 has been thoroughly investigated in vitro, in vivo and in humans, demonstrating that it has a favorable potency, solubility and pharmacodynamics/pharmacokinetics profile [245]. In ECs, it inhibits proliferation and migration, induces CD36-dependent apoptosis, and up-regulates CD95L/FasL. Besides acting on ECs, ABT-510 also induces apoptosis of CD36-expressing tumor cells [246], suggesting a double effect on both the vascular and tumor compartments, an important property of TSP-1 and related reagents that has been already mentioned above and that will be discussed further in this review.
In vivo ABT-510 inhibits angiogenesis in different assays [244,245,247,248]. It reduces tumor growth and microvessel density in a ras-dependent/VEGF-independent tumor model [249], in syngeneic and xenograft gliomas [247], in orthotopic bladder cancer [244] and ovarian carcinoma xenografts [246] and in Lewis lung carcinoma [245]. Besides tumor growth, ABT-510 also inhibits metastasis in the B16F10 model [244] and ovarian cancer xenografts [246]. It has shown promising single-agent activity in canine cancer, inducing objective responses and disease stabilization [250].
On the basis of these favorable preclinical data, ABT-510 was tested, as a single agent, in three phase I and 4 phase II clinical trials between 2005 and 2008. Although phase I studies indicated that ABT-510 was safe and had a good toxicity profile even after several months’ use with different schedules [251,252,253], it showed little clinical activity on renal cell carcinoma, soft tissue sarcoma and melanoma [252,253,254,255]. These disappointing results, however, were no different from those already seen with other antiangiogenic agents used as single agents [253], justifying further evaluation in combination therapies [254,255]. Preclinical studies with the combination of ABT-510 and valproic acid [256] or CeeNu [250] gave favorable results and two phase I trials demonstrated the safety of ABT-510 in combination with chemotherapeutics such as gemcitabine, cisplatin and 5-FU/leucovorin [257,258].
A completely different and innovative rational approach that may overcome the limits of peptide-based antiangiogenic therapy is the identification of synthetic, non-peptidic molecules that mimic the hot-spot interactions in macromolecular complexes [224,259,260]. By using a peptide array approach followed by binding assays with synthetic peptides and recombinant proteins, we identified a FGF2 binding sequence of TSP-1 in the 15mer sequence DDDDDNDKIPDDRDN of the type III repeats. The peptide itself did not inhibit FGF2 function but served as a tool to identify the physico-chemical determinants of FGF2 recognition by TSP-1 and to design non-peptidic inhibitors. Nuclear magnetic resonance and molecular dynamics simulations taking into account the full flexibility of the ligand and receptor identified the relevant residues and conformational determinants for the peptide-FGF interaction. This information was translated into a pharmacophore model used to screen the NCI2003 small molecule databases, leading to the identification of three small molecules that bound FGF2 with affinities in the nanomolar range of concentration. These compounds prevented FGF2 binding to ECs, and inhibited FGF2-induced EC proliferation in vitro, and angiogenesis in the CAM assay. Although the lead compounds have still to be derivatized to improve the drug-like properties before they can be considered real drug candidates, our study show that it is feasible to develop small molecule mimics of TSP-1, and more in general of endogenous proteins, as therapeutic agents [8].
5. Conclusions
Among all the endogenous inhibitors of angiogenesis, TSP-1 seems the most promising for the development of efficacious antiangiogenic/antineoplastic therapies. TSP-1 can act with different mechanisms on different targets at cellular (leukocytes, endothelial, tumor and stromal cells) and molecular (AGFs, cell surface receptors, ECM) levels. Thus, TSP-1 can inhibit tumor progression not only through its well-known antiangiogenic action. It can induce an antineoplastic immune response by recruiting macrophages in the tumor and enhancing their cytotoxicity towards the tumor [261]. TSP-1 can also inhibit megakaryocytopoiesis [262] and coagulation [144,150,171], suggesting that its appropriate exploitation might help preventing the thromboembolic disorders that contribute to the morbidity/mortality of oncological patients [263]. Finally, and perhaps most importantly, TSP-1 can act directly on cancer cells, reducing their growth, inducing apoptosis [10,117,134,264,265,266], increasing their sensitivity to chemotherapeutics [237], and preventing metastatic dissemination [244,246]. Thus, TSP-1 can be positioned at the crossroads between tumor growth, angiogenesis, immunity and coagulation (Figure 8), extending its possibilities for therapeutic exploitation.
Figure 8.
TSP-1 interferes with tumor progression at different levels. It blocks neovascularization, thus inhibiting tumor growth and metastasis which are further inhibited by its direct action on tumor cells. By acting on immune cells, TSP-1 may enhance the immune antineoplastic response. Finally, through its action on coagulation, TSP-1 may control the thromboembolic events that afflict oncological patients.
However, TSP-1 can exert opposite effects on the immune response against tumors, as demonstrated by the fact that it inhibited TCR-mediated T lymphocyte early activation [267]. Also, peptide 4N1K induces cell death of monocytes and monocyte-derived DCs [268]. Paradigmatic of these divergent effects on the immune response is the observation that the absence of TSP-1 in “knock-out” mice can either increase [269] or attenuate [270] Th17 response. Also, while the TSP-1-N-terminal domain renders DCs phagocytic, the TSP-1-C-terminal domain causes a tolerizing phenotype in the same cells [271]. Thus, while the intact TSP-1 molecule inhibits phorbol myristate acetate/LPS-induced homotypic aggregation of human monocytes, the 70-kDa fragment of TSP-1 generated by proteolytic cleavage promotes homotypic aggregation [272]. Similarly, TSP-1 or its peptides can enhance thrombosis, instead of inhibiting it [150,273,274]. Finally, TSP-1 and the peptides can actually increase tumorigenicity [102,275,276,277,278] [192,222,279,280].
In conclusion, TSP-1 will remain an interesting source of therapeutic molecules for a variety of different applications, once the limits imposed by its structural and functional complexity are overcome by identification of the specific active sequence(s) and their proper exploitation.
Acknowledgements
Part of the work presented here was supported by grants from: Fondazione CARIPLO (MR, GT, MP), Istituto Superiore di Sanità, Progetto Nazionale AIDS (MR), Ministero dell’Istruzione, Università e Ricerca (MR, MP), Ministero della Salute (GT), Associazione Italiana per la Ricerca sul Cancro (MP, GT), Fondazione Berlucchi (MP).
References
- 1.Carmeliet P., Jain R.K. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 3.Rusnati M., Presta M. Extracellular angiogenic growth factor interactions: an angiogenesis interactome survey. Endothelium. 2006;13:93–111. doi: 10.1080/10623320600698011. [DOI] [PubMed] [Google Scholar]
- 4.Presta M., Dell'Era P., Mitola S., Moroni E., Ronca R., Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005;16:159–178. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Taraboletti G., Belotti D., Borsotti P., Vergani V., Rusnati M., Presta M., Giavazzi R. The 140-kilodalton antiangiogenic fragment of thrombospondin-1 binds to basic fibroblast growth factor. Cell Growth Differ. 1997;8:471–479. [PubMed] [Google Scholar]
- 6.Margosio B., Marchetti D., Vergani V., Giavazzi R., Rusnati M., Presta M., Taraboletti G. Thrombospondin 1 as a scavenger for matrix-associated fibroblast growth factor 2. Blood. 2003;102:4399–4406. doi: 10.1182/blood-2003-03-0893. [DOI] [PubMed] [Google Scholar]
- 7.Margosio B., Rusnati M., Bonezzi K., Cordes B.L., Annis D.S., Urbinati C., Giavazzi R., Presta M., Ribatti D., Mosher D.F., Taraboletti G. Fibroblast growth factor-2 binding to the thrombospondin-1 type III repeats, a novel antiangiogenic domain. Int. J. Biochem. Cell Biol. 2008;40:700–709. doi: 10.1016/j.biocel.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colombo G., Margosio B., Ragona L., Neves M., Bonifacio S., Annis D.S., Stravalaci M., Tomaselli S., Giavazzi R., Rusnati M., Presta M., Zetta L., Mosher D.F., Ribatti D., Gobbi M., Taraboletti G. Non-peptidic thrombospondin-1-mimics as fibroblast growth factor-2 inhibitors: an integrated strategy for the development of new antiangiogenic compounds. J. Biol. Chem. 2010 doi: 10.1074/jbc.M109.085605. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta K., Gupta P., Wild R., Ramakrishnan S., Hebbel R.P. Binding and displacement of vascular endothelial growth factor (VEGF) by thrombospondin: effect on human microvascular endothelial cell proliferation and angiogenesis. Angiogenesis. 1999;3:147–158. doi: 10.1023/a:1009018702832. [DOI] [PubMed] [Google Scholar]
- 10.Laklai H., Laval S., Dumartin L., Rochaix P., Hagedorn M., Bikfalvi A., Le Guellec S., Delisle M.B., Schally A.V., Susini C., Pyronnet S., Bousquet C. Thrombospondin-1 is a critical effector of oncosuppressive activity of sst2 somatostatin receptor on pancreatic cancer. Proc. Natl. Acad. Sci. USA. 2009;106:17769–17774. doi: 10.1073/pnas.0908674106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamszus K., Joseph A., Jin L., Yao Y., Chowdhury S., Fuchs A., Polverini P.J., Goldberg I.D., Rosen E.M. Scatter factor binds to thrombospondin and other extracellular matrix components. Am. J. Pathol. 1996;149:805–819. [PMC free article] [PubMed] [Google Scholar]
- 12.Rusnati M., Taraboletti G., Urbinati C., Tulipano G., Giuliani R., Molinari-Tosatti M.P., Sennino B., Giacca M., Tyagi M., Albini A., Noonan D., Giavazzi R., Presta M. Thrombospondin-1/HIV-1 tat protein interaction: modulation of the biological activity of extracellular Tat. FASEB J. 2000;14:1917–1930. doi: 10.1096/fj.99-0902com. [DOI] [PubMed] [Google Scholar]
- 13.Murphy-Ullrich J.E., Schultz-Cherry S., Hook M. Transforming growth factor-beta complexes with thrombospondin. Mol. Biol. Cell. 1992;3:181–188. doi: 10.1091/mbc.3.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asplin I.R., Wu S.M., Mathew S., Bhattacharjee G., Pizzo S.V. Differential regulation of the fibroblast growth factor (FGF) family by alpha(2)-macroglobulin: evidence for selective modulation of FGF-2-induced angiogenesis. Blood. 2001;97:3450–3457. doi: 10.1182/blood.v97.11.3450. [DOI] [PubMed] [Google Scholar]
- 15.Bhattacharjee G., Asplin I.R., Wu S.M., Gawdi G., Pizzo S.V. The conformation-dependent interaction of alpha 2-macroglobulin with vascular endothelial growth factor. A novel mechanism of alpha 2-macroglobulin/growth factor binding. J. Biol. Chem. 2000;275:26806–26811. doi: 10.1074/jbc.M000156200. [DOI] [PubMed] [Google Scholar]
- 16.Feige J.J., Negoescu A., Keramidas M., Souchelnitskiy S., Chambaz E.M. Alpha 2-macroglobulin: a binding protein for transforming growth factor-beta and various cytokines. Horm. Res. 1996;45:227–232. doi: 10.1159/000184793. [DOI] [PubMed] [Google Scholar]
- 17.Kurdowska A., Alden S.M., Noble J.M., Stevens M.D., Carr F.K. Involvement of alpha-2-macroglobulin receptor in clearance of interleukin 8-alpha-2-macroglobulin complexes by human alveolar macrophages. Cytokine. 2000;12:1046–1053. doi: 10.1006/cyto.1999.0640. [DOI] [PubMed] [Google Scholar]
- 18.LaMarre J., Wollenberg G.K., Gonias S.L., Hayes M.A. Cytokine binding and clearance properties of proteinase-activated alpha 2-macroglobulins. Lab. Invest. 1991;65:3–14. [PubMed] [Google Scholar]
- 19.Rusnati M., Presta M. Interaction of angiogenic basic fibroblast growth factor with endothelial cell heparan sulfate proteoglycans. Biological implications in neovascularization. Int. J. Clin. Lab. Res. 1996;26:15–23. doi: 10.1007/BF02644769. [DOI] [PubMed] [Google Scholar]
- 20.Norrby K. 2.5 kDa and 5.0 kDa heparin fragments specifically inhibit microvessel sprouting and network formation in VEGF165-mediated mammalian angiogenesis. Int. J. Exp. Pathol. 2000;81:191–198. doi: 10.1046/j.1365-2613.2000.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rusnati M., Coltrini D., Oreste P., Zoppetti G., Albini A., Noonan D., d'Adda di Fagagna F., Giacca M., Presta M. Interaction of HIV-1 Tat protein with heparin. Role of the backbone structure, sulfation, and size. J. Biol. Chem. 1997;272:11313–11320. doi: 10.1074/jbc.272.17.11313. [DOI] [PubMed] [Google Scholar]
- 22.Lietha D., Chirgadze D.Y., Mulloy B., Blundell T.L., Gherardi E. Crystal structures of NK1-heparin complexes reveal the basis for NK1 activity and enable engineering of potent agonists of the MET receptor. Embo. J. 2001;20:5543–5555. doi: 10.1093/emboj/20.20.5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rusnati M., Camozzi M., Moroni E., Bottazzi B., Peri G., Indraccolo S., Amadori A., Mantovani A., Presta M. Selective recognition of fibroblast growth factor-2 by the long pentraxin PTX3 inhibits angiogenesis. Blood. 2004;104:92–99. doi: 10.1182/blood-2003-10-3433. [DOI] [PubMed] [Google Scholar]
- 24.Shibamiya A., Muhl L., Tannert-Otto S., Preissner K.T., Kanse S.M. Nucleic acids potentiate Factor VII-activating protease (FSAP)-mediated cleavage of platelet-derived growth factor-BB and inhibition of vascular smooth muscle cell proliferation. Biochem.J. 2007;404:45–50. doi: 10.1042/BJ20070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Etscheid M., Beer N., Kress J.A., Seitz R., Dodt J. Inhibition of bFGF/EGF-dependent endothelial cell proliferation by the hyaluronan-binding protease from human plasma. Eur. J. Cell Biol. 2004;82:597–604. doi: 10.1078/0171-9335-00349. [DOI] [PubMed] [Google Scholar]
- 26.Lozano R.M., Redondo-Horcajo M., Jimenez M.A., Zilberberg L., Cuevas P., Bikfalvi A., Rico M., Gimenez-Gallego G. Solution structure and interaction with basic and acidic fibroblast growth factor of a 3-kDa human platelet factor-4 fragment with antiangiogenic activity. J. Biol. Chem. 2001;276:35723–35734. doi: 10.1074/jbc.M101565200. [DOI] [PubMed] [Google Scholar]
- 27.Bikfalvi A. Platelet factor 4: an inhibitor of angiogenesis. Semin. Thromb. Hemost. 2004;30:379–385. doi: 10.1055/s-2004-831051. [DOI] [PubMed] [Google Scholar]
- 28.Kupprion C., Motamed K., Sage E.H. SPARC (BM-40, osteonectin) inhibits the mitogenic effect of vascular endothelial growth factor on microvascular endothelial cells. J. Biol. Chem. 1998;273:29635–29640. doi: 10.1074/jbc.273.45.29635. [DOI] [PubMed] [Google Scholar]
- 29.Raines E.W., Lane T.F., Iruela-Arispe M.L., Ross R., Sage E.H. The extracellular glycoprotein SPARC interacts with platelet-derived growth factor (PDGF)-AB and -BB and inhibits the binding of PDGF to its receptors. Proc. Natl. Acad. Sci. USA. 1992;89:1281–1285. doi: 10.1073/pnas.89.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spinetti G., Camarda G., Bernardini G., Romano Di Peppe S., Capogrossi M.C., Napolitano M. The chemokine CXCL13 (BCA-1) inhibits FGF-2 effects on endothelial cells. Biochem. Biophys. Res. Commun. 2001;289:19–24. doi: 10.1006/bbrc.2001.5924. [DOI] [PubMed] [Google Scholar]
- 31.Rusnati M., Tanghetti E., Urbinati C., Tulipano G., Marchesini S., Ziche M., Presta M. Interaction of fibroblast growth factor-2 (FGF-2) with free gangliosides: biochemical characterization and biological consequences in endothelial cell cultures. Mol. Biol. Cell. 1999;10:313–327. doi: 10.1091/mbc.10.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bossard C., Van den Berghe L., Laurell H., Castano C., Cerutti M., Prats A.C., Prats H. Antiangiogenic properties of fibstatin, an extracellular FGF-2-binding polypeptide. Cancer Res. 2004;64:7507–7512. doi: 10.1158/0008-5472.CAN-04-0287. [DOI] [PubMed] [Google Scholar]
- 33.Hollier B., Harkin D.G., Leavesley D., Upton Z. Responses of keratinocytes to substrate-bound vitronectin: growth factor complexes. Exp. Cell Res. 2005;305:221–232. doi: 10.1016/j.yexcr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Shibuya M. Vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1): a dual regulator for angiogenesis. Angiogenesis. 2006;9:225–230; discussion 231. doi: 10.1007/s10456-006-9055-8. [DOI] [PubMed] [Google Scholar]
- 35.Luque A., Carpizo D.R., Iruela-Arispe M.L. ADAMTS1/METH1 inhibits endothelial cell proliferation by direct binding and sequestration of VEGF165. J. Biol. Chem. 2003;278:23656–23665. doi: 10.1074/jbc.M212964200. [DOI] [PubMed] [Google Scholar]
- 36.Heroult M., Bernard-Pierrot I., Delbe J., Hamma-Kourbali Y., Katsoris P., Barritault D., Papadimitriou E., Plouet J., Courty J. Heparin affin regulatory peptide binds to vascular endothelial growth factor (VEGF) and inhibits VEGF-induced angiogenesis. Oncogene. 2004;23:1745–1753. doi: 10.1038/sj.onc.1206879. [DOI] [PubMed] [Google Scholar]
- 37.Inoki I., Shiomi T., Hashimoto G., Enomoto H., Nakamura H., Makino K., Ikeda E., Takata S., Kobayashi K., Okada Y. Connective tissue growth factor binds vascular endothelial growth factor (VEGF) and inhibits VEGF-induced angiogenesis. Faseb J. 2002;16:219–221. doi: 10.1096/fj.01-0332fje. [DOI] [PubMed] [Google Scholar]
- 38.Venkatesha S., Toporsian M., Lam C., Hanai J., Mammoto T., Kim Y.M., Bdolah Y., Lim K.H., Yuan H.T., Libermann T.A., Stillman I.E., Roberts D., D'Amore P.A., Epstein F.H., Sellke F.W., Romero R., Sukhatme V.P., Letarte M., Karumanchi S.A. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 39.O'Connor-McCourt M.D., Wakefield L.M. Latent transforming growth factor-beta in serum. A specific complex with alpha 2-macroglobulin. J. Biol. Chem. 1987;262:14090–14099. [PubMed] [Google Scholar]
- 40.Kemeny L., Szolnoky G., Kenderessy A.S., Gyulai R., Kiss M., Michel G., Nagy K., Ruzicka T., Dobozy A. Identification of a soluble interleukin-8 inhibitor in the supernatant of polymorphonuclear leukocytes. Immunol. Lett. 1998;64:23–29. doi: 10.1016/s0165-2478(98)00082-0. [DOI] [PubMed] [Google Scholar]
- 41.Eggert A., Ikegaki N., Kwiatkowski J., Zhao H., Brodeur G.M., Himelstein B.P. High-level expression of angiogenic factors is associated with advanced tumor stage in human neuroblastomas. Clin. Cancer Res. 2000;6:1900–1908. [PubMed] [Google Scholar]
- 42.Barthlen W., Flaadt D., Girgert R., Conzelmann J., Schweizer P., Zugmaier G., Buck M., Knabbe C. Significance of heparin-binding growth factor expression on cells of solid pediatric tumors. J. Pediatr. Surg. 2003;38:1296–1304. doi: 10.1016/s0022-3468(03)00385-3. [DOI] [PubMed] [Google Scholar]
- 43.Chang P.Y., Lu S.C., Lee C.M., Chen Y.J., Dugan T.A., Huang W.H., Chang S.F., Liao W.S., Chen C.H., Lee Y.T. Homocysteine inhibits arterial endothelial cell growth through transcriptional downregulation of fibroblast growth factor-2 involving G protein and DNA methylation. Circ. Res. 2008;102:933–941. doi: 10.1161/CIRCRESAHA.108.171082. [DOI] [PubMed] [Google Scholar]
- 44.Meeran S.M., Katiyar S., Elmets C.A., Katiyar S.K. Interleukin-12 deficiency is permissive for angiogenesis in UV radiation-induced skin tumors. Cancer Res. 2007;67:3785–3793. doi: 10.1158/0008-5472.CAN-06-3134. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Zak S., Treven J., Nash N., Gutierrez L.S. Lack of thrombospondin-1 increases angiogenesis in a model of chronic inflammatory bowel disease. Int. J. Colorectal Dis. 2008;23:297–304. doi: 10.1007/s00384-007-0397-5. [DOI] [PubMed] [Google Scholar]
- 46.Norioka K., Mitaka T., Mochizuki Y., Hara M., Kawagoe M., Nakamura H. Interaction of interleukin-1 and interferon-gamma on fibroblast growth factor-induced angiogenesis. Jpn. J. Cancer Res. 1994;85:522–529. doi: 10.1111/j.1349-7006.1994.tb02390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu Y., Guimond S.E., Travers P., Cadman S., Hohenester E., Turnbull J.E., Kim S.H., Bouloux P.M. Novel mechanisms of fibroblast growth factor receptor 1 regulation by extracellular matrix protein anosmin-1. J. Biol. Chem. 2009;284:29905–29920. doi: 10.1074/jbc.M109.049155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ashton A.W., Cheng Y., Helisch A., Ware J.A. Thromboxane A2 receptor agonists antagonize the proangiogenic effects of fibroblast growth factor-2: role of receptor internalization, thrombospondin-1, and alpha(v)beta3. Circ. Res. 2004;94:735–742. doi: 10.1161/01.RES.0000122043.11286.57. [DOI] [PubMed] [Google Scholar]
- 49.Ueno H., Gunn M., Dell K., Tseng A., Jr., Williams L. A truncated form of fibroblast growth factor receptor 1 inhibits signal transduction by multiple types of fibroblast growth factor receptor. J. Biol. Chem. 1992;267:1470–1476. [PubMed] [Google Scholar]
- 50.Zhang W., Chuang Y.J., Swanson R., Li J., Seo K., Leung L., Lau L.F., Olson S.T. Antiangiogenic antithrombin down-regulates the expression of the proangiogenic heparan sulfate proteoglycan, perlecan, in endothelial cells. Blood. 2004;103:1185–1191. doi: 10.1182/blood-2003-08-2920. [DOI] [PubMed] [Google Scholar]
- 51.Brown K.J., Parish C.R. Histidine-rich glycoprotein and platelet factor 4 mask heparan sulfate proteoglycans recognized by acidic and basic fibroblast growth factor. Biochemistry. 1994;33:13918–13927. doi: 10.1021/bi00250a047. [DOI] [PubMed] [Google Scholar]
- 52.Sulpice E., Bryckaert M., Lacour J., Contreres J.O., Tobelem G. Platelet factor 4 inhibits FGF2-induced endothelial cell proliferation via the extracellular signal-regulated kinase pathway but not by the phosphatidylinositol 3-kinase pathway. Blood. 2002;100:3087–3094. doi: 10.1182/blood.V100.9.3087. [DOI] [PubMed] [Google Scholar]
- 53.Nyberg P., Xie L., Kalluri R. Endogenous inhibitors of angiogenesis. Cancer Res. 2005;65:3967–3979. doi: 10.1158/0008-5472.CAN-04-2427. [DOI] [PubMed] [Google Scholar]
- 54.Miao R.Q., Agata J., Chao L., Chao J. Kallistatin is a new inhibitor of angiogenesis and tumor growth. Blood. 2002;100:3245–3252. doi: 10.1182/blood-2002-01-0185. [DOI] [PubMed] [Google Scholar]
- 55.Wang S., Ai X., Freeman S.D., Pownall M.E., Lu Q., Kessler D.S., Emerson C.P., Jr. QSulf1, a heparan sulfate 6-O-endosulfatase, inhibits fibroblast growth factor signaling in mesoderm induction and angiogenesis. Proc. Natl. Acad. Sci. USA. 2004;101:4833–4838. doi: 10.1073/pnas.0401028101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lai J.P., Sandhu D.S., Shire A.M., Roberts L.R. The tumor suppressor function of human sulfatase 1 (SULF1) in carcinogenesis. J. Gastrointest. Cancer. 2008;39:149–158. doi: 10.1007/s12029-009-9058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chua C.C., Rahimi N., Forsten-Williams K., Nugent M.A. Heparan sulfate proteoglycans function as receptors for fibroblast growth factor-2 activation of extracellular signal-regulated kinases 1 and 2. Circ. Res. 2004;94:316–323. doi: 10.1161/01.RES.0000112965.70691.AC. [DOI] [PubMed] [Google Scholar]
- 58.Kaur G., Belotti D., Burger A.M., Fisher-Nielson K., Borsotti P., Riccardi E., Thillainathan J., Hollingshead M., Sausville E.A., Giavazzi R. Antiangiogenic properties of 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin: an orally bioavailable heat shock protein 90 modulator. Clin. Cancer Res. 2004;10:4813–4821. doi: 10.1158/1078-0432.CCR-03-0795. [DOI] [PubMed] [Google Scholar]
- 59.Hanafusa H., Torii S., Yasunaga T., Nishida E. Sprouty1 and Sprouty2 provide a control mechanism for the Ras/MAPK signalling pathway. Nat. Cell Biol. 2002;4:850–858. doi: 10.1038/ncb867. [DOI] [PubMed] [Google Scholar]
- 60.Patel S., Leal A.D., Gorski D.H. The homeobox gene Gax inhibits angiogenesis through inhibition of nuclear factor-kappaB-dependent endothelial cell gene expression. Cancer Res. 2005;65:1414–1424. doi: 10.1158/0008-5472.CAN-04-3431. [DOI] [PubMed] [Google Scholar]
- 61.Kessler O., Shraga-Heled N., Lange T., Gutmann-Raviv N., Sabo E., Baruch L., Machluf M., Neufeld G. Semaphorin-3F is an inhibitor of tumor angiogenesis. Cancer Res. 2004;64:1008–1015. doi: 10.1158/0008-5472.can-03-3090. [DOI] [PubMed] [Google Scholar]
- 62.Redlitz A., Daum G., Sage E.H. Angiostatin diminishes activation of the mitogen-activated protein kinases ERK-1 and ERK-2 in human dermal microvascular endothelial cells. J. Vasc. Res. 1999;36:28–34. doi: 10.1159/000025623. [DOI] [PubMed] [Google Scholar]
- 63.Baiguera S., Conconi M.T., Guidolin D., Mazzocchi G., Malendowicz L.K., Parnigotto P.P., Spinazzi R., Nussdorfer G.G. Ghrelin inhibits in vitro angiogenic activity of rat brain microvascular endothelial cells. Int. J. Mol. Med. 2004;14:849–854. [PubMed] [Google Scholar]
- 64.Rikitake Y., Kawashima S., Yamashita T., Ueyama T., Ishido S., Hotta H., Hirata K., Yokoyama M. Lysophosphatidylcholine inhibits endothelial cell migration and proliferation via inhibition of the extracellular signal-regulated kinase pathway. Arterioscler. Thromb. Vasc. Biol. 2000;20:1006–1012. doi: 10.1161/01.ATV.20.4.1006. [DOI] [PubMed] [Google Scholar]
- 65.Kanda S., Mochizuki Y., Nakamura T., Miyata Y., Matsuyama T., Kanetake H. Pigment epithelium-derived factor inhibits fibroblast-growth-factor-2-induced capillary morphogenesis of endothelial cells through Fyn. J. Cell Sci. 2005;118:961–970. doi: 10.1242/jcs.01686. [DOI] [PubMed] [Google Scholar]
- 66.Sun J., Hopkins B.D., Tsujikawa K., Perruzzi C., Adini I., Swerlick R., Bornstein P., Lawler J., Benjamin L.E. Thrombospondin-1 modulates VEGF-A-mediated Akt signaling and capillary survival in the developing retina. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H1344–H1351. doi: 10.1152/ajpheart.01246.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang J.C., Donate F., Qi X., Ziats N.P., Juarez J.C., Mazar A.P., Pang Y.P., McCrae K.R. The antiangiogenic activity of cleaved high molecular weight kininogen is mediated through binding to endothelial cell tropomyosin. Proc. Natl. Acad. Sci. USA. 2002;99:12224–12229. doi: 10.1073/pnas.192668299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim J., Cheon I.S., Won Y.J., Na H.J., Kim Y.M., Choe J. IL-4 inhibits cell cycle progression of human umbilical vein endothelial cells by affecting p53, p21(Waf1), cyclin D1, and cyclin E expression. Mol. Cells. 2003;16:92–96. [PubMed] [Google Scholar]
- 69.Guo Y.L., Wang S., Colman R.W. Kininostatin, an angiogenic inhibitor, inhibits proliferation and induces apoptosis of human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2001;21:1427–1433. doi: 10.1161/hq0901.095277. [DOI] [PubMed] [Google Scholar]
- 70.Kanda S., Mochizuki Y., Miyata Y., Kanetake H., Yamamoto N. Effects of vitamin D(3)-binding protein-derived macrophage activating factor (GcMAF) on angiogenesis. J. Natl. Cancer Inst. 2002;94:1311–1319. doi: 10.1093/jnci/94.17.1311. [DOI] [PubMed] [Google Scholar]
- 71.Dixelius J., Larsson H., Sasaki T., Holmqvist K., Lu L., Engstrom A., Timpl R., Welsh M., Claesson-Welsh L. Endostatin-induced tyrosine kinase signaling through the Shb adaptor protein regulates endothelial cell apoptosis. Blood. 2000;95:3403–3411. [PubMed] [Google Scholar]
- 72.Guan X., Juarez J.C., Qi X., Shipulina N.V., Shaw D.E., Morgan W.T., McCrae K.R., Mazar A.P., Donate F. Histidine-proline rich glycoprotein (HPRG) binds and transduces anti-angiogenic signals through cell surface tropomyosin on endothelial cells. Thromb. Haemost. 2004;92:403–412. doi: 10.1160/TH04-02-0073. [DOI] [PubMed] [Google Scholar]
- 73.Dixelius J., Cross M., Matsumoto T., Sasaki T., Timpl R., Claesson-Welsh L. Endostatin regulates endothelial cell adhesion and cytoskeletal organization. Cancer Res. 2002;62:1944–1947. [PubMed] [Google Scholar]
- 74.Rege T.A., Stewart J., Jr., Dranka B., Benveniste E.N., Silverstein R.L., Gladson C.L. Thrombospondin-1-induced apoptosis of brain microvascular endothelial cells can be mediated by TNF-R1. J. Cell Physiol. 2009;218:94–103. doi: 10.1002/jcp.21570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dawson D.W., Volpert O.V., Pearce S.F., Schneider A.J., Silverstein R.L., Henkin J., Bouck N.P. Three distinct D-amino acid substitutions confer potent antiangiogenic activity on an inactive peptide derived from a thrombospondin-1 type 1 repeat. Mol. Pharmacol. 1999;55:332–338. doi: 10.1124/mol.55.2.332. [DOI] [PubMed] [Google Scholar]
- 76.Zhou L., Isenberg J.S., Cao Z., Roberts D.D. Type I collagen is a molecular target for inhibition of angiogenesis by endogenous thrombospondin-1. Oncogene. 2006;25:536–545. doi: 10.1038/sj.onc.1209069. [DOI] [PubMed] [Google Scholar]
- 77.Lafleur M.A., Handsley M.M., Knauper V., Murphy G., Edwards D.R. Endothelial tubulogenesis within fibrin gels specifically requires the activity of membrane-type-matrix metalloproteinases (MT-MMPs) J. Cell Sci. 2002;115:3427–3438. doi: 10.1242/jcs.115.17.3427. [DOI] [PubMed] [Google Scholar]
- 78.Donnini S., Morbidelli L., Taraboletti G., Ziche M. ERK1-2 and p38 MAPK regulate MMP/TIMP balance and function in response to thrombospondin-1 fragments in the microvascular endothelium. Life Sci. 2004;74:2975–2985. doi: 10.1016/j.lfs.2003.09.075. [DOI] [PubMed] [Google Scholar]
- 79.Fears C.Y., Grammer J.R., Stewart J.E., Jr., Annis D.S., Mosher D.F., Bornstein P., Gladson C.L. Low-density lipoprotein receptor-related protein contributes to the antiangiogenic activity of thrombospondin-2 in a murine glioma model. Cancer Res. 2005;65:9338–9346. doi: 10.1158/0008-5472.CAN-05-1560. [DOI] [PubMed] [Google Scholar]
- 80.Kroon M.E., van Schie M.L., van der Vecht B., van Hinsbergh V.W., Koolwijk P. Collagen type 1 retards tube formation by human microvascular endothelial cells in a fibrin matrix. Angiogenesis. 2002;5:257–265. doi: 10.1023/a:1024540701634. [DOI] [PubMed] [Google Scholar]
- 81.Staton C.A., Brown N.J., Rodgers G.R., Corke K.P., Tazzyman S., Underwood J.C., Lewis C.E. Alphastatin, a 24-amino acid fragment of human fibrinogen, is a potent new inhibitor of activated endothelial cells in vitro and in vivo. Blood. 2004;103:601–606. doi: 10.1182/blood-2003-07-2192. [DOI] [PubMed] [Google Scholar]
- 82.Shellenberger T.D., Wang M., Gujrati M., Jayakumar A., Strieter R.M., Burdick M.D., Ioannides C.G., Efferson C.L., El-Naggar A.K., Roberts D., Clayman G.L., Frederick M.J. BRAK/CXCL14 is a potent inhibitor of angiogenesis and a chemotactic factor for immature dendritic cells. Cancer Res. 2004;64:8262–8270. doi: 10.1158/0008-5472.CAN-04-2056. [DOI] [PubMed] [Google Scholar]
- 83.Sgadari C., Angiolillo A.L., Tosato G. Inhibition of angiogenesis by interleukin-12 is mediated by the interferon-inducible protein 10. Blood. 1996;87:3877–3882. [PubMed] [Google Scholar]
- 84.Angiolillo A.L., Sgadari C., Taub D.D., Liao F., Farber J.M., Maheshwari S., Kleinman H.K., Reaman G.H., Tosato G. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J. Exp. Med. 1995;182:155–162. doi: 10.1084/jem.182.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pike S.E., Yao L., Jones K.D., Cherney B., Appella E., Sakaguchi K., Nakhasi H., Teruya-Feldstein J., Wirth P., Gupta G., Tosato G. Vasostatin, a calreticulin fragment, inhibits angiogenesis and suppresses tumor growth. J. Exp. Med. 1998;188:2349–2356. doi: 10.1084/jem.188.12.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaur B., Brat D.J., Devi N.S., Van Meir E.G. Vasculostatin, a proteolytic fragment of Brain Angiogenesis Inhibitor 1, is an antiangiogenic and antitumorigenic factor. Oncogene. :2005. doi: 10.1038/sj.onc.1208317. [DOI] [PubMed] [Google Scholar]
- 87.Pepper M.S., Belin D., Montesano R., Orci L., Vassalli J.D. Transforming growth factor-beta 1 modulates basic fibroblast growth factor-induced proteolytic and angiogenic properties of endothelial cells in vitro. J. Cell Biol. 1990;111:743–755. doi: 10.1083/jcb.111.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sato N., Nariuchi H., Tsuruoka N., Nishihara T., Beitz J.G., Calabresi P., Frackelton A.R., Jr. Actions of TNF and IFN-gamma on angiogenesis in vitro. J. Invest. Dermatol. 1990;95:85S–89S. doi: 10.1111/1523-1747.ep12874809. [DOI] [PubMed] [Google Scholar]
- 89.Grant M.B., Caballero S., Millard W.J. Inhibition of IGF-I and b-FGF stimulated growth of human retinal endothelial cells by the somatostatin analogue, octreotide: a potential treatment for ocular neovascularization. Regul. Pept. 1993;48:267–278. doi: 10.1016/0167-0115(93)90356-d. [DOI] [PubMed] [Google Scholar]
- 90.Ribatti D., Alessandri G., Baronio M., Raffaghello L., Cosimo E., Marimpietri D., Montaldo P.G., De Falco G., Caruso A., Vacca A., Ponzoni M. Inhibition of neuroblastoma-induced angiogenesis by fenretinide. Int. J. Cancer. 2001;94:314–321. doi: 10.1002/ijc.1441. [DOI] [PubMed] [Google Scholar]
- 91.Schulter V., Koolwijk P., Peters E., Frank S., Hrzenjak A., Graier W.F., van Hinsbergh V.W., Kostner G.M. Impact of apolipoprotein(a) on in vitro angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2001;21:433–438. doi: 10.1161/01.ATV.21.3.433. [DOI] [PubMed] [Google Scholar]
- 92.Duenas Z., Torner L., Corbacho A.M., Ochoa A., Gutierrez-Ospina G., Lopez-Barrera F., Barrios F.A., Berger P., Martinez de la Escalera G., Clapp C. Inhibition of rat corneal angiogenesis by 16-kDa prolactin and by endogenous prolactin-like molecules. Invest. Ophthalmol. Vis. Sci. 1999;40:2498–2505. [PubMed] [Google Scholar]
- 93.Russo K., Ragone R., Facchiano A.M., Capogrossi M.C., Facchiano A. Platelet-derived growth factor-BB and basic fibroblast growth factor directly interact in vitro with high affinity. J. Biol. Chem. 2002;277:1284–1291. doi: 10.1074/jbc.M108858200. [DOI] [PubMed] [Google Scholar]
- 94.Tucker R.P. The thrombospondin type 1 repeat superfamily. Int. J. Biochem. Cell Biol. 2004;36:969–974. doi: 10.1016/j.biocel.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 95.Karagiannis E.D., Popel A.S. A systematic methodology for proteome-wide identification of peptides inhibiting the proliferation and migration of endothelial cells. Proc. Natl. Acad. Sci. USA. 2008;105:13775–13780. doi: 10.1073/pnas.0803241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Adams J.C. Functions of the conserved thrombospondin carboxy-terminal cassette in cell-extracellular matrix interactions and signaling. Int. J. Biochem. Cell Biol. 2004;36:1102–1114. doi: 10.1016/j.biocel.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 97.Kvansakul M., Adams J.C., Hohenester E. Structure of a thrombospondin C-terminal fragment reveals a novel calcium core in the type 3 repeats. Embo. J. 2004;23:1223–1233. doi: 10.1038/sj.emboj.7600166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hogg P.J. Thrombospondin 1 as an enzyme inhibitor. Thromb. Haemost. 1994;72:787–792. [PubMed] [Google Scholar]
- 99.Carlson C.B., Bernstein D.A., Annis D.S., Misenheimer T.M., Hannah B.L., Mosher D.F., Keck J.L. Structure of the calcium-rich signature domain of human thrombospondin-2. Nat. Struct. Mol Biol. 2005;12:910–914. doi: 10.1038/nsmb997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Iruela-Arispe M.L., Lombardo M., Krutzsch H.C., Lawler J., Roberts D.D. Inhibition of angiogenesis by thrombospondin-1 is mediated by 2 independent regions within the type 1 repeats. Circulation. 1999;100:1423–1431. doi: 10.1161/01.cir.100.13.1423. [DOI] [PubMed] [Google Scholar]
- 101.Camozzi M., Rusnati M., Bugatti A., Bottazzi B., Mantovani A., Bastone A., Inforzato A., Vincenti S., Bracci L., Mastroianni D., Presta M. Identification of an antiangiogenic FGF2-binding site in the N terminus of the soluble pattern recognition receptor PTX3. J. Biol. Chem. 2006;281:22605–22613. doi: 10.1074/jbc.M601023200. [DOI] [PubMed] [Google Scholar]
- 102.Motegi K., Harada K., Ohe G., Jones S.J., Ellis I.R., Crouch D.H., Schor S.L., Schor A.M. Differential involvement of TGF-beta1 in mediating the motogenic effects of TSP-1 on endothelial cells, fibroblasts and oral tumour cells. Exp. Cell Res. 2008;314:2323–2333. doi: 10.1016/j.yexcr.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 103.Taraboletti G., Morbidelli L., Donnini S., Parenti A., Granger H.J., Giavazzi R., Ziche M. The heparin binding 25 kDa fragment of thrombospondin-1 promotes angiogenesis and modulates gelatinase and TIMP-2 production in endothelial cells. Faseb J. 2000;14:1674–1676. doi: 10.1096/fj.99-0931fje. [DOI] [PubMed] [Google Scholar]
- 104.Ferrari do Outeiro-Bernstein M.A., Nunes S.S., Andrade A.C., Alves T.R., Legrand C., Morandi V. A recombinant NH(2)-terminal heparin-binding domain of the adhesive glycoprotein, thrombospondin-1, promotes endothelial tube formation and cell survival: a possible role for syndecan-4 proteoglycan. Matrix Biol. 2002;21:311–324. doi: 10.1016/S0945-053X(02)00010-0. [DOI] [PubMed] [Google Scholar]
- 105.Murphy-Ullrich J.E., Gurusiddappa S., Frazier W.A., Hook M. Heparin-binding peptides from thrombospondins 1 and 2 contain focal adhesion-labilizing activity. J. Biol. Chem. 1993;268:26784–26789. [PubMed] [Google Scholar]
- 106.Adams J.C., Bentley A.A., Kvansakul M., Hatherley D., Hohenester E. Extracellular matrix retention of thrombospondin 1 is controlled by its conserved C-terminal region. J. Cell Sci. 2008;121:784–795. doi: 10.1242/jcs.021006. [DOI] [PubMed] [Google Scholar]
- 107.Calzada M.J., Sipes J.M., Krutzsch H.C., Yurchenco P.D., Annis D.S., Mosher D.F., Roberts D.D. Recognition of the N-terminal modules of thrombospondin-1 and thrombospondin-2 by alpha6beta1 integrin. J. Biol. Chem. 2003;278:40679–40687. doi: 10.1074/jbc.M302014200. [DOI] [PubMed] [Google Scholar]
- 108.Vogel T., Guo N.H., Krutzsch H.C., Blake D.A., Hartman J., Mendelovitz S., Panet A., Roberts D.D. Modulation of endothelial cell proliferation, adhesion, and motility by recombinant heparin-binding domain and synthetic peptides from the type I repeats of thrombospondin. J. Cell Biochem. 1993;53:74–84. doi: 10.1002/jcb.240530109. [DOI] [PubMed] [Google Scholar]
- 109.Bornstein P. Thrombospondins function as regulators of angiogenesis. J. Cell Commun. Signal. 2009:189–200. doi: 10.1007/s12079-009-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Asch A.S., Barnwell J., Silverstein R.L., Nachman R.L. Isolation of the thrombospondin membrane receptor. J. Clin. Invest. 1987;79:1054–1061. doi: 10.1172/JCI112918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Swerlick R.A., Lee K.H., Wick T.M., Lawley T.J. Human dermal microvascular endothelial but not human umbilical vein endothelial cells express CD36 in vivo and in vitro. J. Immunol. 1992;148:78–83. [PubMed] [Google Scholar]
- 112.Dawson D.W., Pearce S.F., Zhong R., Silverstein R.L., Frazier W.A., Bouck N.P. CD36 mediates the In vitro inhibitory effects of thrombospondin-1 on endothelial cells. J. Cell Biol. 1997;138:707–717. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Primo L., Ferrandi C., Roca C., Marchio S., di Blasio L., Alessio M., Bussolino F. Identification of CD36 molecular features required for its in vitro angiostatic activity. FASEB J. 2005;19:1713–1715. doi: 10.1096/fj.05-3697fje. [DOI] [PubMed] [Google Scholar]
- 114.Zhang X., Kazerounian S., Duquette M., Perruzzi C., Nagy J.A., Dvorak H.F., Parangi S., Lawler J. Thrombospondin-1 modulates vascular endothelial growth factor activity at the receptor level. FASEB J. 2009;23:3368–3376. doi: 10.1096/fj.09-131649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Isenberg J.S., Martin-Manso G., Maxhimer J.B., Roberts D.D. Regulation of nitric oxide signalling by thrombospondin 1: implications for anti-angiogenic therapies. Nat. Rev. Cancer. 2009;9:182–194. doi: 10.1038/nrc2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jimenez B., Volpert O.V., Crawford S.E., Febbraio M., Silverstein R.L., Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat. Med. 2000;6:41–48. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- 117.Li K., Yang M., Yuen P.M., Chik K.W., Li C.K., Shing M.M., Lam H.K., Fok T.F. Thrombospondin-1 induces apoptosis in primary leukemia and cell lines mediated by CD36 and Caspase-3. Int. J. Mol. Med. 2003;12:995–1001. [PubMed] [Google Scholar]
- 118.Saumet A., Slimane M.B., Lanotte M., Lawler J., Dubernard V. Type 3 repeat/C-terminal domain of thrombospondin-1 triggers caspase-independent cell death through CD47/alphavbeta3 in promyelocytic leukemia NB4 cells. Blood. 2005;106:658–667. doi: 10.1182/blood-2004-09-3585. [DOI] [PubMed] [Google Scholar]
- 119.Gao A.G., Lindberg F.P., Dimitry J.M., Brown E.J., Frazier W.A. Thrombospondin modulates alpha v beta 3 function through integrin-associated protein. J. Cell Biol. 1996;135:533–544. doi: 10.1083/jcb.135.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gao A.G., Lindberg F.P., Finn M.B., Blystone S.D., Brown E.J., Frazier W.A. Integrin-associated protein is a receptor for the C-terminal domain of thrombospondin. J. Biol. Chem. 1996;271:21–24. doi: 10.1074/jbc.271.1.21. [DOI] [PubMed] [Google Scholar]
- 121.Brown E.J., Frazier W.A. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130–135. doi: 10.1016/s0962-8924(00)01906-1. [DOI] [PubMed] [Google Scholar]
- 122.Kanda S., Shono T., Tomasini-Johansson B., Klint P., Saito Y. Role of thrombospondin-1-derived peptide, 4N1K, in FGF-2-induced angiogenesis. Exp. Cell Res. 1999;252:262–272. doi: 10.1006/excr.1999.4622. [DOI] [PubMed] [Google Scholar]
- 123.Nunes S.S., Outeiro-Bernstein M.A., Juliano L., Vardiero F., Nader H.B., Woods A., Legrand C., Morandi V. Syndecan-4 contributes to endothelial tubulogenesis through interactions with two motifs inside the pro-angiogenic N-terminal domain of thrombospondin-1. J Cell Physiol. 2008;214:828–837. doi: 10.1002/jcp.21281. [DOI] [PubMed] [Google Scholar]
- 124.Oganesian A., Armstrong L.C., Migliorini M.M., Strickland D.K., Bornstein P. Thrombospondins use the VLDL receptor and a nonapoptotic pathway to inhibit cell division in microvascular endothelial cells. Mol. Biol. Cell. 2008;19:563–571. doi: 10.1091/mbc.E07-07-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Orr A.W., Pedraza C.E., Pallero M.A., Elzie C.A., Goicoechea S., Strickland D.K., Murphy-Ullrich J.E. Low density lipoprotein receptor-related protein is a calreticulin coreceptor that signals focal adhesion disassembly. J. Cell Biol. 2003;161:1179–1189. doi: 10.1083/jcb.200302069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Orr A.W., Elzie C.A., Kucik D.F., Murphy-Ullrich J.E. Thrombospondin signaling through the calreticulin/LDL receptor-related protein co-complex stimulates random and directed cell migration. J. Cell Sci. 2003;116:2917–2927. doi: 10.1242/jcs.00600. [DOI] [PubMed] [Google Scholar]
- 127.Staniszewska I., Zaveri S., Del Valle L., Oliva I., Rothman V.L., Croul S.E., Roberts D.D., Mosher D.F., Tuszynski G.P., Marcinkiewicz C. Interaction of alpha9beta1 integrin with thrombospondin-1 promotes angiogenesis. Circ. Res. 2007;100:1308–1316. doi: 10.1161/01.RES.0000266662.98355.66. [DOI] [PubMed] [Google Scholar]
- 128.Short S.M., Derrien A., Narsimhan R.P., Lawler J., Ingber D.E., Zetter B.R. Inhibition of endothelial cell migration by thrombospondin-1 type-1 repeats is mediated by beta1 integrins. J. Cell Biol. 2005;168:643–653. doi: 10.1083/jcb.200407060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chandrasekaran L., He C.Z., Al-Barazi H., Krutzsch H.C., Iruela-Arispe M.L., Roberts D.D. Cell contact-dependent activation of alpha3beta1 integrin modulates endothelial cell responses to thrombospondin-1. Mol. Biol. Cell. 2000;11:2885–2900. doi: 10.1091/mbc.11.9.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Calzada M.J., Zhou L., Sipes J.M., Zhang J., Krutzsch H.C., Iruela-Arispe M.L., Annis D.S., Mosher D.F., Roberts D.D. Alpha4beta1 integrin mediates selective endothelial cell responses to thrombospondins 1 and 2 in vitro and modulates angiogenesis in vivo. Circ. Res. 2004;94:462–470. doi: 10.1161/01.RES.0000115555.05668.93. [DOI] [PubMed] [Google Scholar]
- 131.Greenaway J., Lawler J., Moorehead R., Bornstein P., Lamarre J., Petrik J. Thrombospondin-1 inhibits VEGF levels in the ovary directly by binding and internalization via the low density lipoprotein receptor-related protein-1 (LRP-1) J. Cell Physiol. 2007;210:807–818. doi: 10.1002/jcp.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schultz-Cherry S., Chen H., Mosher D.F., Misenheimer T.M., Krutzsch H.C., Roberts D.D., Murphy-Ullrich J.E. Regulation of transforming growth factor-beta activation by discrete sequences of thrombospondin 1. J. Biol. Chem. 1995;270:7304–7310. doi: 10.1074/jbc.270.13.7304. [DOI] [PubMed] [Google Scholar]
- 133.Young G.D., Murphy-Ullrich J.E. The tryptophan-rich motifs of the thrombospondin type 1 repeats bind VLAL motifs in the latent transforming growth factor-beta complex. J. Biol. Chem. 2004;279:47633–47642. doi: 10.1074/jbc.M404918200. [DOI] [PubMed] [Google Scholar]
- 134.Miao W.M., Seng W.L., Duquette M., Lawler P., Laus C., Lawler J. Thrombospondin-1 type 1 repeat recombinant proteins inhibit tumor growth through transforming growth factor-beta-dependent and -independent mechanisms. Cancer Res. 2001;61:7830–7839. [PubMed] [Google Scholar]
- 135.Hogg P.J., Hotchkiss K.A., Jimenez B.M., Stathakis P., Chesterman C.N. Interaction of platelet-derived growth factor with thrombospondin 1. Biochem. J. 1997;326:709–716. doi: 10.1042/bj3260709. (Pt 3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bein K., Simons M. Thrombospondin type 1 repeats interact with matrix metalloproteinase 2. Regulation of metalloproteinase activity. J. Biol. Chem. 2000;275:32167–32173. doi: 10.1074/jbc.M003834200. [DOI] [PubMed] [Google Scholar]
- 137.Rabhi-Sabile S., de Romeuf C., Pidard D. On the mechanism of plasmin-induced aggregation of human platelets: implication of secreted von Willebrand factor. Thromb. Haemost. 1998;79:1191–1198. [PubMed] [Google Scholar]
- 138.Anonick P.K., Yoo J.K., Webb D.J., Gonias S.L. Characterization of the antiplasmin activity of human thrombospondin-1 in solution. Biochem. J. 1993;289:903–909. doi: 10.1042/bj2890903. (Pt 3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Silverstein R.L., Leung L.L., Harpel P.C., Nachman R.L. Complex formation of platelet thrombospondin with plasminogen. Modulation of activation by tissue activator. J. Clin. Invest. 1984;74:1625–1633. doi: 10.1172/JCI111578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Silverstein R.L., Nachman R.L., Leung L.L., Harpel P.C. Activation of immobilized plasminogen by tissue activator. Multimolecular complex formation. J. Biol. Chem. 1985;260:10346–10352. [PubMed] [Google Scholar]
- 141.Silverstein R.L., Nachman R.L., Pannell R., Gurewich V., Harpel P.C. Thrombospondin forms complexes with single-chain and two-chain forms of urokinase. J. Biol. Chem. 1990;265:11289–11294. [PubMed] [Google Scholar]
- 142.Hogg P.J., Jimenez B.M., Chesterman C.N. Identification of possible inhibitory reactive centers in thrombospondin 1 that may bind cathepsin G and neutrophil elastase. Biochemistry. 1994;33:6531–6537. doi: 10.1021/bi00187a021. [DOI] [PubMed] [Google Scholar]
- 143.Hogg P.J., Owensby D.A., Chesterman C.N. Thrombospondin 1 is a tight-binding competitive inhibitor of neutrophil cathepsin G. Determination of the kinetic mechanism of inhibition and localization of cathepsin G binding to the thrombospondin 1 type 3 repeats. J. Biol. Chem. 1993;268:21811–21818. [PubMed] [Google Scholar]
- 144.Mast A.E., Stadanlick J.E., Lockett J.M., Dietzen D.J., Hasty K.A., Hall C.L. Tissue factor pathway inhibitor binds to platelet thrombospondin-1. J. Biol. Chem. 2000;275:31715–31721. doi: 10.1074/jbc.M006595200. [DOI] [PubMed] [Google Scholar]
- 145.Feitsma K., Hausser H., Robenek H., Kresse H., Vischer P. Interaction of thrombospondin-1 and heparan sulfate from endothelial cells. Structural requirements of heparan sulfate. J. Biol. Chem. 2000;275:9396–9402. doi: 10.1074/jbc.275.13.9396. [DOI] [PubMed] [Google Scholar]
- 146.Panetti T.S., Kudryk B.J., Mosher D.F. Interaction of recombinant procollagen and properdin modules of thrombospondin-1 with heparin and fibrinogen/fibrin. J. Biol. Chem. 1999;274:430–437. doi: 10.1074/jbc.274.1.430. [DOI] [PubMed] [Google Scholar]
- 147.Yu H., Tyrrell D., Cashel J., Guo N.H., Vogel T., Sipes J.M., Lam L., Fillit H.M., Hartman J., Mendelovitz S., Panel A., Roberts D.D. Specificities of heparin-binding sites from the amino-terminus and type 1 repeats of thrombospondin-1. Arch. Biochem. Biophys. 2000;374:13–23. doi: 10.1006/abbi.1999.1597. [DOI] [PubMed] [Google Scholar]
- 148.Lawler J., Ferro P., Duquette M. Expression and mutagenesis of thrombospondin. Biochemistry. 1992;31:1173–1180. doi: 10.1021/bi00119a029. [DOI] [PubMed] [Google Scholar]
- 149.Silverstein R.L., Leung L.L., Harpel P.C., Nachman R.L. Platelet thrombospondin forms a trimolecular complex with plasminogen and histidine-rich glycoprotein. J. Clin. Invest. 1985;75:2065–2073. doi: 10.1172/JCI111926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Isordia-Salas I., Manns J.M., Sainz I., Parekh H., DeLa Cadena R.A. Thromsbospondin-1 binds to the heavy chain of elastase activated coagulation factor V (FVaHNE) and enhances thrombin generation on the surface of a promyelocytic cell line. Thromb. Res. 2005;116:533–543. doi: 10.1016/j.thromres.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 151.Zhou J., Rothman V.L., Sargiannidou I., Dimitrov S., Qiu C., Smith E., Sheffield J., Sharma M., Tuszynski G.P. Cloning and characterization of angiocidin, a tumor cell binding protein for thrombospondin-1. J. Cell Biochem. 2004;92:125–146. doi: 10.1002/jcb.20076. [DOI] [PubMed] [Google Scholar]
- 152.Hansen G.A., Vorum H., Jacobsen C., Honore B. Calumenin but not reticulocalbin forms a Ca2+-dependent complex with thrombospondin-1. A potential role in haemostasis and thrombosis. Mol. Cell Biochem. 2009;320:25–33. doi: 10.1007/s11010-008-9895-1. [DOI] [PubMed] [Google Scholar]
- 153.Faye C., Moreau C., Chautard E., Jetne R., Fukai N., Ruggiero F., Humphries M.J., Olsen B.R., Ricard-Blum S. Molecular interplay between endostatin, integrins, and heparan sulfate. J. Biol. Chem. 2009;284:22029–22040. doi: 10.1074/jbc.M109.002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Floquet N., Dedieu S., Martiny L., Dauchez M., Perahia D. Human thrombospondin's (TSP-1) C-terminal domain opens to interact with the CD-47 receptor: a molecular modeling study. Arch. Biochem. Biophys. 2008;478:103–109. doi: 10.1016/j.abb.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 155.Elzie C.A., Murphy-Ullrich J.E. The N-terminus of thrombospondin: the domain stands apart. Int. J. Biochem. Cell Biol. 2004;36:1090–1101. doi: 10.1016/j.biocel.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 156.Mikhailenko I., Krylov D., Argraves K.M., Roberts D.D., Liau G., Strickland D.K. Cellular internalization and degradation of thrombospondin-1 is mediated by the amino-terminal heparin binding domain (HBD). High affinity interaction of dimeric HBD with the low density lipoprotein receptor-related protein. J. Biol. Chem. 1997;272:6784–6791. doi: 10.1074/jbc.272.10.6784. [DOI] [PubMed] [Google Scholar]
- 157.Tan K., Duquette M., Liu J.H., Zhang R., Joachimiak A., Wang J.H., Lawler J. The structures of the thrombospondin-1 N-terminal domain and its complex with a synthetic pentameric heparin. Structure. 2006;14:33–42. doi: 10.1016/j.str.2005.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Michalak M., Corbett E.F., Mesaeli N., Nakamura K., Opas M. Calreticulin: one protein, one gene, many functions. Biochem. J. 1999;344:281–292. doi: 10.1042/0264-6021:3440281. (Pt 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li Z., Calzada M.J., Sipes J.M., Cashel J.A., Krutzsch H.C., Annis D.S., Mosher D.F., Roberts D.D. Interactions of thrombospondins with alpha4beta1 integrin and CD47 differentially modulate T cell behavior. J. Cell Biol. 2002;157:509–519. doi: 10.1083/jcb.200109098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Calzada M.J., Roberts D.D. Novel integrin antagonists derived from thrombospondins. Curr. Pharm. Des. 2005;11:849–866. doi: 10.2174/1381612053381792. [DOI] [PubMed] [Google Scholar]
- 161.Furrer J., Luy B., Basrur V., Roberts D.D., Barchi J.J., Jr. Conformational analysis of an alpha3beta1 integrin-binding peptide from thrombospondin-1: implications for antiangiogenic drug design. J. Med. Chem. 2006;49:6324–6333. doi: 10.1021/jm060833l. [DOI] [PubMed] [Google Scholar]
- 162.Krutzsch H.C., Choe B.J., Sipes J.M., Guo N., Roberts D.D. Identification of an alpha(3)beta(1) integrin recognition sequence in thrombospondin-1. J. Biol. Chem. 1999;274:24080–24086. doi: 10.1074/jbc.274.34.24080. [DOI] [PubMed] [Google Scholar]
- 163.Calzada M.J., Annis D.S., Zeng B., Marcinkiewicz C., Banas B., Lawler J., Mosher D.F., Roberts D.D. Identification of novel beta1 integrin binding sites in the type 1 and type 2 repeats of thrombospondin-1. J. Biol. Chem. 2004;279:41734–41743. doi: 10.1074/jbc.M406267200. [DOI] [PubMed] [Google Scholar]
- 164.Lahav J., Schwartz M.A., Hynes R.O. Analysis of platelet adhesion with a radioactive chemical crosslinking reagent: interaction of thrombospondin with fibronectin and collagen. Cell. 1982;31:253–262. doi: 10.1016/0092-8674(82)90425-1. [DOI] [PubMed] [Google Scholar]
- 165.Mumby S.M., Raugi G.J., Bornstein P. Interactions of thrombospondin with extracellular matrix proteins: selective binding to type V collagen. J. Cell Biol. 1984;98:646–652. doi: 10.1083/jcb.98.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Galvin N.J., Vance P.M., Dixit V.M., Fink B., Frazier W.A. Interaction of human thrombospondin with types I-V collagen: direct binding and electron microscopy. J. Cell Biol. 1987;104:1413–1422. doi: 10.1083/jcb.104.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sottile J., Hocking D.C. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol. Biol. Cell. 2002;13:3546–3559. doi: 10.1091/mbc.E02-01-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lahav J., Lawler J., Gimbrone M.A. Thrombospondin interactions with fibronectin and fibrinogen. Mutual inhibition in binding. Eur. J. Biochem. 1984;145:151–156. doi: 10.1111/j.1432-1033.1984.tb08534.x. [DOI] [PubMed] [Google Scholar]
- 169.Bale M.D., Mosher D.F. Thrombospondin is a substrate for blood coagulation factor XIIIa. Biochemistry. 1986;25:5667–5673. doi: 10.1021/bi00367a048. [DOI] [PubMed] [Google Scholar]
- 170.Bale M.D., Mosher D.F. Effects of thrombospondin on fibrin polymerization and structure. J. Biol. Chem. 1986;261:862–868. [PubMed] [Google Scholar]
- 171.Pimanda J.E., Annis D.S., Raftery M., Mosher D.F., Chesterman C.N., Hogg P.J. The von Willebrand factor-reducing activity of thrombospondin-1 is located in the calcium-binding/C-terminal sequence and requires a free thiol at position 974. Blood. 2002;100:2832–2838. doi: 10.1182/blood-2002-03-0770. [DOI] [PubMed] [Google Scholar]
- 172.Merle B., Malaval L., Lawler J., Delmas P., Clezardin P. Decorin inhibits cell attachment to thrombospondin-1 by binding to a KKTR-dependent cell adhesive site present within the N-terminal domain of thrombospondin-1. J. Cell Biochem. 1997;67:75–83. [PubMed] [Google Scholar]
- 173.Moralez A.M., Maile L.A., Clarke J., Busby W.H., Jr., Clemmons D.R. Insulin-like growth factor binding protein-5 (IGFBP-5) interacts with thrombospondin-1 to induce negative regulatory effects on IGF-I actions. J. Cell Physiol. 2005;203:328–334. doi: 10.1002/jcp.20343. [DOI] [PubMed] [Google Scholar]
- 174.Taraboletti G., Benelli R., Borsotti P., Rusnati M., Presta M., Giavazzi R., Ruco L., Albini A. Thrombospondin-1 inhibits Kaposi's sarcoma (KS) cell and HIV-1 Tat-induced angiogenesis and is poorly expressed in KS lesions. J. Pathol. 1999;188:76–81. doi: 10.1002/(SICI)1096-9896(199905)188:1<76::AID-PATH312>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 175.Yang Z., Strickland D.K., Bornstein P. Extracellular matrix metalloproteinase 2 levels are regulated by the low density lipoprotein-related scavenger receptor and thrombospondin 2. J. Biol. Chem. 2001;276:8403–8408. doi: 10.1074/jbc.M008925200. [DOI] [PubMed] [Google Scholar]
- 176.Iruela-Arispe M.L., Luque A., Lee N. Thrombospondin modules and angiogenesis. Int. J. Biochem. Cell Biol. 2004;36:1070–1078. doi: 10.1016/j.biocel.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 177.Rodriguez-Manzaneque J.C., Lane T.F., Ortega M.A., Hynes R.O., Lawler J., Iruela-Arispe M.L. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc. Natl. Acad. Sci. USA. 2001;98:12485–12490. doi: 10.1073/pnas.171460498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Qian X., Wang T.N., Rothman V.L., Nicosia R.F., Tuszynski G.P. Thrombospondin-1 modulates angiogenesis in vitro by up-regulation of matrix metalloproteinase-9 in endothelial cells. Exp. Cell Res. 1997;235:403–412. doi: 10.1006/excr.1997.3681. [DOI] [PubMed] [Google Scholar]
- 179.Wang S., Skorczewski J., Feng X., Mei L., Murphy-Ullrich J.E. Glucose up-regulates thrombospondin 1 gene transcription and transforming growth factor-beta activity through antagonism of cGMP-dependent protein kinase repression via upstream stimulatory factor 2. J. Biol. Chem. 2004;279:34311–34322. doi: 10.1074/jbc.M401629200. [DOI] [PubMed] [Google Scholar]
- 180.Panigrahy D., Kaipainen A., Huang S., Butterfield C.E., Barnes C.M., Fannon M., Laforme A.M., Chaponis D.M., Folkman J., Kieran M.W. PPARalpha agonist fenofibrate suppresses tumor growth through direct and indirect angiogenesis inhibition. Proc. Natl. Acad. Sci. USA. 2008;105:985–990. doi: 10.1073/pnas.0711281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kang J.H., Kim S.A., Chang S.Y., Hong S., Hong K.J. Inhibition of trichostatin A-induced antiangiogenesis by small-interfering RNA for thrombospondin-1. Exp. Mol. Med. 2007;39:402–411. doi: 10.1038/emm.2007.45. [DOI] [PubMed] [Google Scholar]
- 182.Castle V.P., Ou X., O'Shea S., Dixit V.M. Induction of thrombospondin 1 by retinoic acid is important during differentiation of neuroblastoma cells. J. Clin. Invest. 1992;90:1857–1863. doi: 10.1172/JCI116062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kim S.A., Kang J.H., Cho I., Bae S.W., Hong K.J. Cell-type specific regulation of thrombospondin-1 expression and its promoter activity by regulatory agents. Exp. Mol. Med. 2001;33:117–123. doi: 10.1038/emm.2001.21. [DOI] [PubMed] [Google Scholar]
- 184.Doebele R.C., Schulze-Hoepfner F.T., Hong J., Chlenski A., Zeitlin B.D., Goel K., Gomes S., Liu Y., Abe M.K., Nor J.E., Lingen M.W., Rosner M.R. A novel interplay between Epac/Rap1 and mitogen-activated protein kinase kinase 5/extracellular signal-regulated kinase 5 (MEK5/ERK5) regulates thrombospondin to control angiogenesis. Blood. 2009;114:4592–4600. doi: 10.1182/blood-2009-04-217042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lee T.Y., Muschal S., Pravda E.A., Folkman J., Abdollahi A., Javaherian K. Angiostatin regulates the expression of antiangiogenic and proapoptotic pathways via targeted inhibition of mitochondrial proteins. Blood. 2009;114:1987–1998. doi: 10.1182/blood-2008-12-197236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Puri N., Khramtsov A., Ahmed S., Nallasura V., Hetzel J.T., Jagadeeswaran R., Karczmar G., Salgia R. A selective small molecule inhibitor of c-Met, PHA665752, inhibits tumorigenicity and angiogenesis in mouse lung cancer xenografts. Cancer Res. 2007;67:3529–3534. doi: 10.1158/0008-5472.CAN-06-4416. [DOI] [PubMed] [Google Scholar]
- 187.Mirkin S., Mahony M.C., Archer D.F. Effect of tibolone and its metabolites on vascular endothelial growth factor isoforms 121 and 165 and thrombospondin-1 mRNA in Ishikawa cells. Menopause. 2004;11:82–88. doi: 10.1097/01.GME.0000074101.35126.7A. [DOI] [PubMed] [Google Scholar]
- 188.Albig A.R., Schiemann W.P. Fibulin-5 antagonizes vascular endothelial growth factor (VEGF) signaling and angiogenic sprouting by endothelial cells. DNA Cell Biol. 2004;23:367–379. doi: 10.1089/104454904323145254. [DOI] [PubMed] [Google Scholar]
- 189.Naito T., Masaki T., Nikolic-Paterson D.J., Tanji C., Yorioka N., Kohno N. Angiotensin II induces thrombospondin-1 production in human mesangial cells via p38 MAPK and JNK: a mechanism for activation of latent TGF-beta1. Am. J. Physiol. Renal Physiol. 2004;286:F278–F287. doi: 10.1152/ajprenal.00139.2003. [DOI] [PubMed] [Google Scholar]
- 190.Fischer J.W., Stoll M., Hahn A.W., Unger T. Differential regulation of thrombospondin-1 and fibronectin by angiotensin II receptor subtypes in cultured endothelial cells. Cardiovasc. Res. 2001;51:784–791. doi: 10.1016/s0008-6363(01)00345-5. [DOI] [PubMed] [Google Scholar]
- 191.Ding I., Sun J.Z., Fenton B., Liu W.M., Kimsely P., Okunieff P., Min W. Intratumoral administration of endostatin plasmid inhibits vascular growth and perfusion in MCa-4 murine mammary carcinomas. Cancer Res. 2001;61:526–531. [PubMed] [Google Scholar]
- 192.Hyder S.M., Liang Y., Wu J. Estrogen regulation of thrombospondin-1 in human breast cancer cells. Int. J. Cancer. 2009;125:1045–1053. doi: 10.1002/ijc.24373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Navarro F.J., Mirkin S., Archer D.F. Effect of raloxifene, 17beta-estradiol, and progesterone on mRNA for vascular endothelial growth factor isoforms 121 and 165 and thrombospondin-1 in Ishikawa cells. Fertil. Steril. 2003;79:1409–1415. doi: 10.1016/s0015-0282(03)00350-9. [DOI] [PubMed] [Google Scholar]
- 194.Kim J., Kim C., Kim T.S., Bang S.I., Yang Y., Park H., Cho D. IL-18 enhances thrombospondin-1 production in human gastric cancer via JNK pathway. Biochem. Biophys. Res. Commun. 2006;344:1284–1289. doi: 10.1016/j.bbrc.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 195.Congote L.F., DiFalco M.R., Gibbs B.F. Thrombospondin 1, produced by endothelial cells under the action of erythropoietin, stimulates thymidine incorporation into erythroid cells and counteracts the inhibitory action of insulin-like growth factor binding protein 3. Cytokine. 2005;30:248–253. doi: 10.1016/j.cyto.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 196.Soula-Rothhut M., Coissard C., Sartelet H., Boudot C., Bellon G., Martiny L., Rothhut B. The tumor suppressor PTEN inhibits EGF-induced TSP-1 and TIMP-1 expression in FTC-133 thyroid carcinoma cells. Exp. Cell Res. 2005;304:187–201. doi: 10.1016/j.yexcr.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 197.Horiguchi H., Jin L., Ruebel K.H., Scheithauer B.W., Lloyd R.V. Regulation of VEGF-A, VEGFR-I, thrombospondin-1, -2, and -3 expression in a human pituitary cell line (HP75) by TGFbeta1, bFGF, and EGF. Endocrine. 2004;24:141–146. doi: 10.1385/ENDO:24:2:141. [DOI] [PubMed] [Google Scholar]
- 198.Martinez-Sales V., Vila V., Ferrando M., Reganon E. Atorvastatin neutralizes the up-regulation of thrombospondin-1 induced by thrombin in human umbilical vein endothelial cells. Endothelium. 2007;14:233–238. doi: 10.1080/10623320701617209. [DOI] [PubMed] [Google Scholar]
- 199.Hellebrekers D.M., Jair K.W., Vire E., Eguchi S., Hoebers N.T., Fraga M.F., Esteller M., Fuks F., Baylin S.B., van Engeland M., Griffioen A.W. Angiostatic activity of DNA methyltransferase inhibitors. Mol. Cancer Ther. 2006;5:467–475. doi: 10.1158/1535-7163.MCT-05-0417. [DOI] [PubMed] [Google Scholar]
- 200.Liu Z., Christensson M., Forslow A., De Meester I., Sundqvist K.G. A CD26-controlled cell surface cascade for regulation of T cell motility and chemokine signals. J. Immunol. 2009;183:3616–3624. doi: 10.4049/jimmunol.0804336. [DOI] [PubMed] [Google Scholar]
- 201.Bocci G., Francia G., Man S., Lawler J., Kerbel R.S. Thrombospondin 1, a mediator of the antiangiogenic effects of low-dose metronomic chemotherapy. Proc. Natl. Acad. Sci. USA. 2003;100:12917–12922. doi: 10.1073/pnas.2135406100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hamano Y., Sugimoto H., Soubasakos M.A., Kieran M., Olsen B.R., Lawler J., Sudhakar A., Kalluri R. Thrombospondin-1 associated with tumor microenvironment contributes to low-dose cyclophosphamide-mediated endothelial cell apoptosis and tumor growth suppression. Cancer Res. 2004;64:1570–1574. doi: 10.1158/0008-5472.can-03-3126. [DOI] [PubMed] [Google Scholar]
- 203.Zhao H.Y., Ooyama A., Yamamoto M., Ikeda R., Haraguchi M., Tabata S., Furukawa T., Che X.F., Iwashita K., Oka T., Fukushima M., Nakagawa M., Ono M., Kuwano M., Akiyama S. Down regulation of c-Myc and induction of an angiogenesis inhibitor, thrombospondin-1, by 5-FU in human colon cancer KM12C cells. Cancer Lett. 2008;270:156–163. doi: 10.1016/j.canlet.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 204.Damber J.E., Vallbo C., Albertsson P., Lennernas B., Norrby K. The anti-tumour effect of low-dose continuous chemotherapy may partly be mediated by thrombospondin. Cancer Chemother. Pharmacol. 2006;58:354–360. doi: 10.1007/s00280-005-0163-8. [DOI] [PubMed] [Google Scholar]
- 205.Yoo G.H., Piechocki M.P., Ensley J.F., Nguyen T., Oliver J., Meng H., Kewson D., Shibuya T.Y., Lonardo F., Tainsky M.A. Docetaxel induced gene expression patterns in head and neck squamous cell carcinoma using cDNA microarray and PowerBlot. Clin. Cancer Res. 2002;8:3910–3921. [PubMed] [Google Scholar]
- 206.Cinatl J., Jr., Kotchetkov R., Blaheta R., Driever P.H., Vogel J.U., Cinatl J. Induction of differentiation and suppression of malignant phenotype of human neuroblastoma BE(2)-C cells by valproic acid: enhancement by combination with interferon-alpha. Int. J. Oncol. 2002;20:97–106. [PubMed] [Google Scholar]
- 207.Bocci G., Falcone A., Fioravanti A., Orlandi P., Di Paolo A., Fanelli G., Viacava P., Naccarato A.G., Kerbel R.S., Danesi R., Del Tacca M., Allegrini G. Antiangiogenic and anticolorectal cancer effects of metronomic irinotecan chemotherapy alone and in combination with semaxinib. Br. J. Cancer. 2008;98:1619–1629. doi: 10.1038/sj.bjc.6604352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Allegrini G., Falcone A., Fioravanti A., Barletta M.T., Orlandi P., Loupakis F., Cerri E., Masi G., Di Paolo A., Kerbel R.S., Danesi R., Del Tacca M., Bocci G. A pharmacokinetic and pharmacodynamic study on metronomic irinotecan in metastatic colorectal cancer patients. Br. J. Cancer. 2008;98:1312–1319. doi: 10.1038/sj.bjc.6604311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Castle V.P., Dixit V.M., Polverini P.J. Thrombospondin-1 suppresses tumorigenesis and angiogenesis in serum- and anchorage-independent NIH 3T3 cells. Lab. Invest. 1997;77:51–61. [PubMed] [Google Scholar]
- 210.Zhang X., Xu J., Lawler J., Terwilliger E., Parangi S. Adeno-associated virus-mediated antiangiogenic gene therapy with thrombospondin-1 type 1 repeats and endostatin. Clin. Cancer Res. 2007;13:3968–3976. doi: 10.1158/1078-0432.CCR-07-0245. [DOI] [PubMed] [Google Scholar]
- 211.Liu P., Wang Y., Li Y.H., Yang C., Zhou Y.L., Li B., Lu S.H., Yang R.C., Cai Y.L., Tobelem G., Caen J., Han Z.C. Adenovirus-mediated gene therapy with an antiangiogenic fragment of thrombospondin-1 inhibits human leukemia xenograft growth in nude mice. Leuk. Res. 2003;27:701–708. doi: 10.1016/s0145-2126(02)00346-6. [DOI] [PubMed] [Google Scholar]
- 212.Yee K.O., Streit M., Hawighorst T., Detmar M., Lawler J. Expression of the type-1 repeats of thrombospondin-1 inhibits tumor growth through activation of transforming growth factor-beta. Am. J. Pathol. 2004;165:541–552. doi: 10.1016/s0002-9440(10)63319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Miyata Y., Koga S., Takehara K., Kanetake H., Kanda S. Expression of thrombospondin-derived 4N1K peptide-containing proteins in renal cell carcinoma tissues is associated with a decrease in tumor growth and angiogenesis. Clin. Cancer Res. 2003;9:1734–1740. [PubMed] [Google Scholar]
- 214.Amagasaki K., Sasaki A., Kato G., Maeda S., Nukui H., Naganuma H. Antisense-mediated reduction in thrombospondin-1 expression reduces cell motility in malignant glioma cells. Int. J. Cancer. 2001;94:508–512. doi: 10.1002/ijc.1497. [DOI] [PubMed] [Google Scholar]
- 215.Dameron K.M., Volpert O.V., Tainsky M.A., Bouck N. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science. 1994;265:1582–1584. doi: 10.1126/science.7521539. [DOI] [PubMed] [Google Scholar]
- 216.Dameron K.M., Volpert O.V., Tainsky M.A., Bouck N. The p53 tumor suppressor gene inhibits angiogenesis by stimulating the production of thrombospondin. Cold Spring Harb. Symp. Quant. Biol. 1994;59:483–489. doi: 10.1101/sqb.1994.059.01.053. [DOI] [PubMed] [Google Scholar]
- 217.Gautam A., Densmore C.L., Melton S., Golunski E., Waldrep J.C. Aerosol delivery of PEI-p53 complexes inhibits B16-F10 lung metastases through regulation of angiogenesis. Cancer Gene Ther. 2002;9:28–36. doi: 10.1038/sj.cgt.7700405. [DOI] [PubMed] [Google Scholar]
- 218.Suarez Y., Fernandez-Hernando C., Yu J., Gerber S.A., Harrison K.D., Pober J.S., Iruela-Arispe M.L., Merkenschlager M., Sessa W.C. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc. Natl. Acad. Sci. USA. 2008;105:14082–14087. doi: 10.1073/pnas.0804597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Dews M., Homayouni A., Yu D., Murphy D., Sevignani C., Wentzel E., Furth E.E., Lee W.M., Enders G.H., Mendell J.T., Thomas-Tikhonenko A. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat. Genet. 2006;38:1060–1065. doi: 10.1038/ng1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Qin H., Shao Q., Thomas T., Kalra J., Alaoui-Jamali M.A., Laird D.W. Connexin26 regulates the expression of angiogenesis-related genes in human breast tumor cells by both GJIC-dependent and -independent mechanisms. Cell Commun. Adhes. 2003;10:387–393. doi: 10.1080/cac.10.4-6.387.393. [DOI] [PubMed] [Google Scholar]
- 221.Yang G., Cai K.Q., Thompson-Lanza J.A., Bast R.C., Jr., Liu J. Inhibition of breast and ovarian tumor growth through multiple signaling pathways by using retrovirus-mediated small interfering RNA against Her-2/neu gene expression. J. Biol. Chem. 2004;279:4339–4345. doi: 10.1074/jbc.M311153200. [DOI] [PubMed] [Google Scholar]
- 222.de Fraipont F., Keramidas M., El Atifi M., Chambaz E.M., Berger F., Feige J.J. Expression of the thrombospondin 1 fragment 167-569 in C6 glioma cells stimulates tumorigenicity despite reduced neovascularization. Oncogene. 2004;23:3642–3649. doi: 10.1038/sj.onc.1207438. [DOI] [PubMed] [Google Scholar]
- 223.Wells J.A., McClendon C.L. Reaching for high-hanging fruit in drug discovery at protein-protein interfaces. Nature. 2007;450:1001–1009. doi: 10.1038/nature06526. [DOI] [PubMed] [Google Scholar]
- 224.Robinson J.A., Demarco S., Gombert F., Moehle K., Obrecht D. The design, structures and therapeutic potential of protein epitope mimetics. Drug Discov. Today. 2008;13:944–951. doi: 10.1016/j.drudis.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 225.Murray J.K., Gellman S.H. Targeting protein-protein interactions: lessons from p53/MDM2. Biopolymers. 2007;88:657–686. doi: 10.1002/bip.20741. [DOI] [PubMed] [Google Scholar]
- 226.Sulochana K.N., Ge R. Developing antiangiogenic peptide drugs for angiogenesis-related diseases. Curr. Pharm. Des. 2007;13:2074–2086. doi: 10.2174/138161207781039715. [DOI] [PubMed] [Google Scholar]
- 227.Tolsma S.S., Volpert O.V., Good D.J., Frazier W.A., Polverini P.J., Bouck N. Peptides derived from two separate domains of the matrix protein thrombospondin-1 have anti-angiogenic activity. J. Cell Biol. 1993;122:497–511. doi: 10.1083/jcb.122.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lee N.V., Sato M., Annis D.S., Loo J.A., Wu L., Mosher D.F., Iruela-Arispe M.L. ADAMTS1 mediates the release of antiangiogenic polypeptides from TSP1 and 2. Embo. J. 2006;25:5270–5283. doi: 10.1038/sj.emboj.7601400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Bruel A., Touhami-Carrier M., Thomaidis A., Legrand C. Thrombospondin-1 (TSP-1) and TSP-1-derived heparin-binding peptides induce promyelocytic leukemia cell differentiation and apoptosis. Anticancer Res. 2005;25:757–764. [PubMed] [Google Scholar]
- 230.Zhang X., Galardi E., Duquette M., Delic M., Lawler J., Parangi S. Antiangiogenic treatment with the three thrombospondin-1 type 1 repeats recombinant protein in an orthotopic human pancreatic cancer model. Clin. Cancer Res. 2005;11:2337–2344. doi: 10.1158/1078-0432.CCR-04-1900. [DOI] [PubMed] [Google Scholar]
- 231.Zhang X., Connolly C., Duquette M., Lawler J., Parangi S. Continuous administration of the three thrombospondin-1 type 1 repeats recombinant protein improves the potency of therapy in an orthotopic human pancreatic cancer model. Cancer Lett. 2007;247:143–149. doi: 10.1016/j.canlet.2006.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Yee K.O., Connolly C.M., Duquette M., Kazerounian S., Washington R., Lawler J. The effect of thrombospondin-1 on breast cancer metastasis. Breast Cancer Res. Treat. 2009;114:85–96. doi: 10.1007/s10549-008-9992-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Guo N.H., Krutzsch H.C., Inman J.K., Shannon C.S., Roberts D.D. Antiproliferative and antitumor activities of D-reverse peptides derived from the second type-1 repeat of thrombospondin-1. J. Pept. Res. 1997;50:210–221. doi: 10.1111/j.1399-3011.1997.tb01187.x. [DOI] [PubMed] [Google Scholar]
- 234.Jimenez B., Volpert O.V., Reiher F., Chang L., Munoz A., Karin M., Bouck N. c-Jun N-terminal kinase activation is required for the inhibition of neovascularization by thrombospondin-1. Oncogene. 2001;20:3443–3448. doi: 10.1038/sj.onc.1204464. [DOI] [PubMed] [Google Scholar]
- 235.Guo N., Krutzsch H.C., Inman J.K., Roberts D.D. Thrombospondin 1 and type I repeat peptides of thrombospondin 1 specifically induce apoptosis of endothelial cells. Cancer Res. 1997;57:1735–1742. [PubMed] [Google Scholar]
- 236.Manna P.P., Frazier W.A. CD47 mediates killing of breast tumor cells via Gi-dependent inhibition of protein kinase A. Cancer Res. 2004;64:1026–1036. doi: 10.1158/0008-5472.CAN-03-1708. [DOI] [PubMed] [Google Scholar]
- 237.Lih C.J., Wei W., Cohen S.N. Txr1: a transcriptional regulator of thrombospondin-1 that modulates cellular sensitivity to taxanes. Genes Dev. 2006;20:2082–2095. doi: 10.1101/gad.1441306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Rath G.M., Schneider C., Dedieu S., Sartelet H., Morjani H., Martiny L., El Btaouri H. Thrombospondin-1 C-terminal-derived peptide protects thyroid cells from ceramide-induced apoptosis through the adenylyl cyclase pathway. Int. J. Biochem. Cell Biol. 2006;38:2219–2228. doi: 10.1016/j.biocel.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 239.Goldfarb D.S., Gariepy J., Schoolnik G., Kornberg R.D. Synthetic peptides as nuclear localization signals. Nature. 1986;322:641–644. doi: 10.1038/322641a0. [DOI] [PubMed] [Google Scholar]
- 240.Nestor J.J., Jr. The medicinal chemistry of peptides. Curr Med Chem. 2009;16:4399–4418. doi: 10.2174/092986709789712907. [DOI] [PubMed] [Google Scholar]
- 241.Bogdanov A., Jr., Marecos E., Cheng H.C., Chandrasekaran L., Krutzsch H.C., Roberts D.D., Weissleder R. Treatment of experimental brain tumors with trombospondin-1 derived peptides: an in vivo imaging study. Neoplasia. 1999;1:438–445. doi: 10.1038/sj.neo.7900044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Coronella J., Li L., Johnson K., Pirie-Shepherd S., Roxas G., Levin N. Selective activity against proliferating tumor endothelial cells by CVX-22, a thrombospondin-1 mimetic CovX-Body. Anticancer Res. 2009;29:2243–2252. [PubMed] [Google Scholar]
- 243.Molckovsky A., Siu L.L. First-in-class, first-in-human phase I results of targeted agents: Highlights of the 2008 American Society of Clinical Oncology meeting. J. Hematol. Oncol. 2008;1:20. doi: 10.1186/1756-8722-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Reiher F.K., Volpert O.V., Jimenez B., Crawford S.E., Dinney C.P., Henkin J., Haviv F., Bouck N.P., Campbell S.C. Inhibition of tumor growth by systemic treatment with thrombospondin-1 peptide mimetics. Int. J. Cancer. 2002;98:682–689. doi: 10.1002/ijc.10247. [DOI] [PubMed] [Google Scholar]
- 245.Haviv F., Bradley M.F., Kalvin D.M., Schneider A.J., Davidson D.J., Majest S.M., McKay L.M., Haskell C.J., Bell R.L., Nguyen B., Marsh K.C., Surber B.W., Uchic J.T., Ferrero J., Wang Y.C., Leal J., Record R.D., Hodde J., Badylak S.F., Lesniewski R.R., Henkin J. Thrombospondin-1 mimetic peptide inhibitors of angiogenesis and tumor growth: design, synthesis, and optimization of pharmacokinetics and biological activities. J. Med. Chem. 2005;48:2838–2846. doi: 10.1021/jm0401560. [DOI] [PubMed] [Google Scholar]
- 246.Greenaway J., Henkin J., Lawler J., Moorehead R., Petrik J. ABT-510 induces tumor cell apoptosis and inhibits ovarian tumor growth in an orthotopic, syngeneic model of epithelial ovarian cancer. Mol. Cancer Ther. 2009;8:64–74. doi: 10.1158/1535-7163.MCT-08-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Anderson J.C., Grammer J.R., Wang W., Nabors L.B., Henkin J., Stewart J.E., Jr., Gladson C.L. ABT-510, a modified type 1 repeat peptide of thrombospondin, inhibits malignant glioma growth in vivo by inhibiting angiogenesis. Cancer Biol. Ther. 2007;6:454–462. doi: 10.4161/cbt.6.3.3630. [DOI] [PubMed] [Google Scholar]
- 248.Quesada A.J., Nelius T., Yap R., Zaichuk T.A., Alfranca A., Filleur S., Volpert O.V., Redondo J.M. In vivo upregulation of CD95 and CD95L causes synergistic inhibition of angiogenesis by TSP1 peptide and metronomic doxorubicin treatment. Cell Death Differ. 2005;12:649–658. doi: 10.1038/sj.cdd.4401615. [DOI] [PubMed] [Google Scholar]
- 249.Viloria-Petit A., Miquerol L., Yu J.L., Gertsenstein M., Sheehan C., May L., Henkin J., Lobe C., Nagy A., Kerbel R.S., Rak J. Contrasting effects of VEGF gene disruption in embryonic stem cell-derived versus oncogene-induced tumors. EMBO. J. 2003;22:4091–4102. doi: 10.1093/emboj/cdg408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Rusk A., Cozzi E., Stebbins M., Vail D., Graham J., Valli V., Henkin J., Sharpee R., Khanna C. Cooperative activity of cytotoxic chemotherapy with antiangiogenic thrombospondin-I peptides, ABT-526 in pet dogs with relapsed lymphoma. Clin. Cancer Res. 2006;12:7456–7464. doi: 10.1158/1078-0432.CCR-06-0110. [DOI] [PubMed] [Google Scholar]
- 251.Hoekstra R., de Vos F.Y., Eskens F.A., Gietema J.A., van der Gaast A., Groen H.J., Knight R.A., Carr R.A., Humerickhouse R.A., Verweij J., de Vries E.G. Phase I safety, pharmacokinetic, and pharmacodynamic study of the thrombospondin-1-mimetic angiogenesis inhibitor ABT-510 in patients with advanced cancer. J. Clin. Oncol. 2005;23:5188–5197. doi: 10.1200/JCO.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 252.Baker L.H., Rowinsky E.K., Mendelson D., Humerickhouse R.A., Knight R.A., Qian J., Carr R.A., Gordon G.B., Demetri G.D. Randomized, phase II study of the thrombospondin-1-mimetic angiogenesis inhibitor ABT-510 in patients with advanced soft tissue sarcoma. J. Clin. Oncol. 2008;26:5583–5588. doi: 10.1200/JCO.2008.17.4706. [DOI] [PubMed] [Google Scholar]
- 253.Gordon M.S., Mendelson D., Carr R., Knight R.A., Humerickhouse R.A., Iannone M., Stopeck A.T. A phase 1 trial of 2 dose schedules of ABT-510, an antiangiogenic, thrombospondin-1-mimetic peptide, in patients with advanced cancer. Cancer. 2008;113:3420–3429. doi: 10.1002/cncr.23953. [DOI] [PubMed] [Google Scholar]
- 254.Ebbinghaus S., Hussain M., Tannir N., Gordon M., Desai A.A., Knight R.A., Humerickhouse R.A., Qian J., Gordon G.B., Figlin R. Phase 2 study of ABT-510 in patients with previously untreated advanced renal cell carcinoma. Clin. Cancer Res. 2007;13:6689–6695. doi: 10.1158/1078-0432.CCR-07-1477. [DOI] [PubMed] [Google Scholar]
- 255.Markovic S.N., Suman V.J., Rao R.A., Ingle J.N., Kaur J.S., Erickson L.A., Pitot H.C., Croghan G.A., McWilliams R.R., Merchan J., Kottschade L.A., Nevala W.K., Uhl C.B., Allred J., Creagan E.T. A phase II study of ABT-510 (thrombospondin-1 analog) for the treatment of metastatic melanoma. Am. J. Clin. Oncol. 2007;30:303–309. doi: 10.1097/01.coc.0000256104.80089.35. [DOI] [PubMed] [Google Scholar]
- 256.Yang Q., Tian Y., Liu S., Zeine R., Chlenski A., Salwen H.R., Henkin J., Cohn S.L. Thrombospondin-1 peptide ABT-510 combined with valproic acid is an effective antiangiogenesis strategy in neuroblastoma. Cancer Res. 2007;67:1716–1724. doi: 10.1158/0008-5472.CAN-06-2595. [DOI] [PubMed] [Google Scholar]
- 257.Gietema J.A., Hoekstra R., de Vos F.Y., Uges D.R., van der Gaast A., Groen H.J., Loos W.J., Knight R.A., Carr R.A., Humerickhouse R.A., Eskens F.A. A phase I study assessing the safety and pharmacokinetics of the thrombospondin-1-mimetic angiogenesis inhibitor ABT-510 with gemcitabine and cisplatin in patients with solid tumors. Ann. Oncol. 2006;17:1320–1327. doi: 10.1093/annonc/mdl102. [DOI] [PubMed] [Google Scholar]
- 258.Hoekstra R., de Vos F.Y., Eskens F.A., de Vries E.G., Uges D.R., Knight R., Carr R.A., Humerickhouse R., Verweij J., Gietema J.A. Phase I study of the thrombospondin-1-mimetic angiogenesis inhibitor ABT-510 with 5-fluorouracil and leucovorin: a safe combination. Eur. J. Cancer. 2006;42:467–472. doi: 10.1016/j.ejca.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 259.Clackson T., Wells J.A. A hot spot of binding energy in a hormone-receptor interface. Science. 1995;267:383–386. doi: 10.1126/science.7529940. [DOI] [PubMed] [Google Scholar]
- 260.Thanos C.D., DeLano W.L., Wells J.A. Hot-spot mimicry of a cytokine receptor by a small molecule. Proc. Natl. Acad. Sci. USA. 2006;103:15422–15427. doi: 10.1073/pnas.0607058103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Martin-Manso G., Galli S., Ridnour L.A., Tsokos M., Wink D.A., Roberts D.D. Thrombospondin 1 promotes tumor macrophage recruitment and enhances tumor cell cytotoxicity of differentiated U937 cells. Cancer Res. 2008;68:7090–7099. doi: 10.1158/0008-5472.CAN-08-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Yang M., Li K., Ng M.H., Yuen P.M., Fok T.F., Li C.K., Hogg P.J., Chong B.H. Thrombospondin-1 inhibits in vitro megakaryocytopoiesis via CD36. Thromb. Res. 2003;109:47–54. doi: 10.1016/S0049-3848(03)00142-7. [DOI] [PubMed] [Google Scholar]
- 263.Castelli R., Porro F., Tarsia P. The heparins and cancer: review of clinical trials and biological properties. Vasc. Med. 2004;9:205–213. doi: 10.1191/1358863x04vm566ra. [DOI] [PubMed] [Google Scholar]
- 264.John A.S., Hu X., Rothman V.L., Tuszynski G.P. Thrombospondin-1 (TSP-1) up-regulates tissue inhibitor of metalloproteinase-1 (TIMP-1) production in human tumor cells: exploring the functional significance in tumor cell invasion. Exp. Mol. Pathol. 2009;87:184–188. doi: 10.1016/j.yexmp.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kang S.Y., Halvorsen O.J., Gravdal K., Bhattacharya N., Lee J.M., Liu N.W., Johnston B.T., Johnston A.B., Haukaas S.A., Aamodt K., Yoo S., Akslen L.A., Watnick R.S. Prosaposin inhibits tumor metastasis via paracrine and endocrine stimulation of stromal p53 and Tsp-1. Proc. Natl. Acad. Sci. USA. 2009;106:12115–12120. doi: 10.1073/pnas.0903120106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Koskimaki J.E., Karagiannis E.D., Rosca E.V., Vesuna F., Winnard P.T., Jr., Raman V., Bhujwalla Z.M., Popel A.S. Peptides derived from type IV collagen, CXC chemokines, and thrombospondin-1 domain-containing proteins inhibit neovascularization and suppress tumor growth in MDA-MB-231 breast cancer xenografts. Neoplasia. 2009;11:1285–1291. doi: 10.1593/neo.09620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Li Z., He L., Wilson K., Roberts D. Thrombospondin-1 inhibits TCR-mediated T lymphocyte early activation. J. Immunol. 2001;166:2427–2436. doi: 10.4049/jimmunol.166.4.2427. [DOI] [PubMed] [Google Scholar]
- 268.Johansson U., Higginbottom K., Londei M. CD47 ligation induces a rapid caspase-independent apoptosis-like cell death in human monocytes and dendritic cells. Scand. J. Immunol. 2004;59:40–49. doi: 10.1111/j.0300-9475.2004.01355.x. [DOI] [PubMed] [Google Scholar]
- 269.Turpie B., Yoshimura T., Gulati A., Rios J.D., Dartt D.A., Masli S. Sjogren's syndrome-like ocular surface disease in thrombospondin-1 deficient mice. Am. J. Pathol. 2009;175:1136–1147. doi: 10.2353/ajpath.2009.081058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Yang K., Vega J.L., Hadzipasic M., Schatzmann Peron J.P., Zhu B., Carrier Y., Masli S., Rizzo L.V., Weiner H.L. Deficiency of thrombospondin-1 reduces Th17 differentiation and attenuates experimental autoimmune encephalomyelitis. J. Autoimmun. 2009;32:94–103. doi: 10.1016/j.jaut.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Tabib A., Krispin A., Trahtemberg U., Verbovetski I., Lebendiker M., Danieli T., Mevorach D. Thrombospondin-1-N-terminal domain induces a phagocytic state and thrombospondin-1-C-terminal domain induces a tolerizing phenotype in dendritic cells. PLoS One. 2009;4:e6840. doi: 10.1371/journal.pone.0006840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Yamauchi Y., Kuroki M., Imakiire T., Uno K., Abe H., Beppu R., Yamashita Y., Shirakusa T. Opposite effects of thrombospondin-1 via CD36 and CD47 on homotypic aggregation of monocytic cells. Matrix Biol. 2002;21:441–448. doi: 10.1016/S0945-053X(02)00036-7. [DOI] [PubMed] [Google Scholar]
- 273.Bonnefoy A., Daenens K., Feys H.B., De Vos R., Vandervoort P., Vermylen J., Lawler J., Hoylaerts M.F. Thrombospondin-1 controls vascular platelet recruitment and thrombus adherence in mice by protecting (sub)endothelial VWF from cleavage by ADAMTS13. Blood. 2006;107:955–964. doi: 10.1182/blood-2004-12-4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Voit S., Udelhoven M., Lill G., Aktas B., Nieswandt B., Schror K., Weber A.A. The C-terminal peptide of thrombospondin-1 stimulates distinct signaling pathways but induces an activation-independent agglutination of platelets and other cells. FEBS Lett. 2003;544:240–245. doi: 10.1016/s0014-5793(03)00472-1. [DOI] [PubMed] [Google Scholar]
- 275.Farrow B., Berger D.H., Rowley D. Tumor-derived pancreatic stellate cells promote pancreatic cancer cell invasion through release of thrombospondin-2. J. Surg. Res. 2009;156:155–160. doi: 10.1016/j.jss.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 276.Ghoneim C., Soula-Rothhut M., Rothhut B. Thrombospondin-1 in differentiated thyroid cancer: Dr. Jekyll and Mr. Hyde. Connect. Tissue Res. 2008;49:257–260. doi: 10.1080/03008200802147795. [DOI] [PubMed] [Google Scholar]
- 277.Sid B., Langlois B., Sartelet H., Bellon G., Dedieu S., Martiny L. Thrombospondin-1 enhances human thyroid carcinoma cell invasion through urokinase activity. Int. J. Biochem. Cell Biol. 2008;40:1890–1900. doi: 10.1016/j.biocel.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 278.Albo D., Berger D.H., Rothman V.L., Tuszynski G.P. Role of urokinase plasminogen activator receptor in thrombospondin 1-mediated tumor cell invasion. J. Surg. Res. 1999;82:331–338. doi: 10.1006/jsre.1998.5578. [DOI] [PubMed] [Google Scholar]
- 279.Robinet A., Emonard H., Banyai L., Laronze J.Y., Patthy L., Hornebeck W., Bellon G. Collagen-binding domains of gelatinase A and thrombospondin-derived peptides impede endocytic clearance of active gelatinase A and promote HT1080 fibrosarcoma cell invasion. Life Sci. 2008;82:376–382. doi: 10.1016/j.lfs.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 280.Rath G.M., Schneider C., Dedieu S., Rothhut B., Soula-Rothhut M., Ghoneim C., Sid B., Morjani H., El Btaouri H., Martiny L. The C-terminal CD47/IAP-binding domain of thrombospondin-1 prevents camptothecin- and doxorubicin-induced apoptosis in human thyroid carcinoma cells. Biochim. Biophys. Acta. 2006;1763:1125–1134. doi: 10.1016/j.bbamcr.2006.08.001. [DOI] [PubMed] [Google Scholar]