Abstract
We investigated chemical cues among groups of zebrafish (Danio rerio) when communicating information about the risk of predation. We found that visual cues of the predator (tiger Oscar, Astronotus ocellatus) did not increase whole-body cortisol levels in groups of zebrafish but that water conditioned by these (donor) zebrafish stressed (target) conspecifics, thereby increasing whole-body cortisol. This finding was confirmed when these zebrafish groups were in different aquaria and communicated exclusively via water transfer. This result indicates that the stress induced in the target zebrafish does not depend on an increase in whole-body cortisol levels in the donor zebrafish. Because cortisol participation is rejected in this predation-risk communication, other chemicals from the stress systems should be investigated.
In predator–prey interactions, the early detection of the predator by the prey can be considered the first phase of the anti-predator response because it effectively allows the prey to avoid a direct contest with the predator1. In fish, the early detection of predators (prior to physical contact) can be mediated by electrical2, visual3 and/or chemical4,5,6,7,8 stimuli.
When fish of certain species are bitten by a predator, alarm substances released from ruptured epidermal club cells7,8,9, and even the blood10, can alert conspecifics to the presence of a predator. However, non-injured fish also alert conspecifics about the presence of a predator4,11,12,13, an effect attributed to as-yet uncharacterized substances produced by the non-injured fish as a result of the disturbance.
Complete information from a predator is required to produce the necessary defense (flight or fight) by the prey: in this case, a stress response is triggered to physiologically facilitate the prey's response14. However, complete information may not always be available if an individual communicates the presence of a predator to conspecifics, because the conspecific may not be able to actually see the predator. In meerkats, for example, one animal remains in a strategic position to better examine approaching predators while the conspecifics forage; however, one alarm signal (sound) emitted by the vigilant animal is sufficient to elicit a complete defense or flight reaction in the conspecifics15. In fish, the release of an alarm substance upon the rupture of epidermal club cells9 or fin-flicking behavior16 can inform conspecifics of predation risk, thus demonstrating that incomplete information (e.g., chemical or visual cues) can trigger a complete defense reaction in conspecifics.
In a previous study, we demonstrated that physically-stressed jundiá or Nile tilapia (Oreochromis niloticus) chemically alerts conspecifics, which increases whole-body cortisol17. In that study17, both the donor and the target fish showed increased cortisol. This suggests that the stimulation of the hyphothalamic-pituitary-interrenal axis (HPI-axis) coulde be an important element in the mechanism of this type of communication in fish. Considering that stressed fish release cortisol and their metabolites into the water18, we hypothesized that the classical cortisol increase of fish under predation risk is involved to chemically stress conspecifics that lack any other information about the predator. In four experiments we tested predictions of this hypothesis in the zebrafish, Danio rerio: a) zebrafish viewing a predator have a higher cortisol levels than controls; b) zebrafish receiving water from conspecifics viewing a predator have cortisol increased; c) Water and sound from the predator should not mask the effects reported in the previous predictions. We found that only the first prediction (a) was not supported, indicating that the classical cortisol peak is not necessary to chemically induce cortisol increase in conspecifics. This conclusion drives future research on intraspecific communication of predation risk into physiological pathways that do not necessarily involve cortisol release.
Results
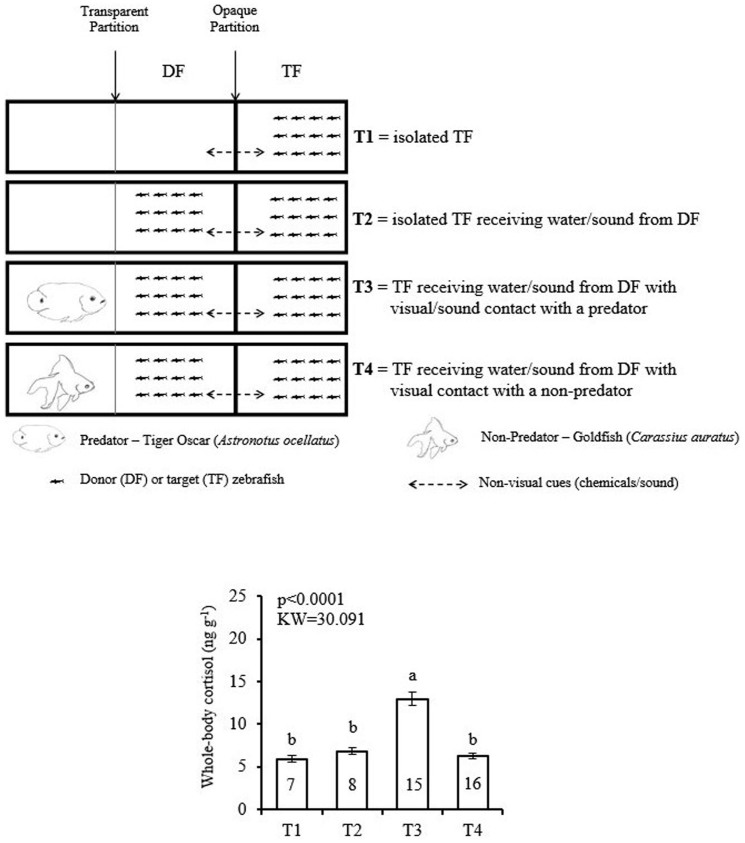
In the first experiment (Fig. 1), we tested whether zebrafish perceiving the predator could induce cortisol changes in target conspecifics not directly perceiving the predator. We found that perception (viewing or hearing) of the predator was not a sufficient stimulus to increase cortisol in the prey zebrafish. However, zebrafish conspecifics receiving non-visual cues from these conspecifics perceiving the predator had whole-body cortisol increased. This effect did not occur when the donor zebrafish faced the non-predator heterospecific.
Figure 1. Study 1.
Effect of zebrafish perceiving a predator fish on conspecific's cortisol. Experimental design and whole-body cortisol response of the target zebrafish. Mean values (±S.E.M.) compared by Kruskal-Wallis complemented by a Dunn's Multiple Comparisons Test. Different letters above means indicate statistical difference (P indicated in the graph). Numbers of repetitions obtained for each treatment (T1 to T4) are inside each column. The fish drawings in the graphics were drawn by LB.
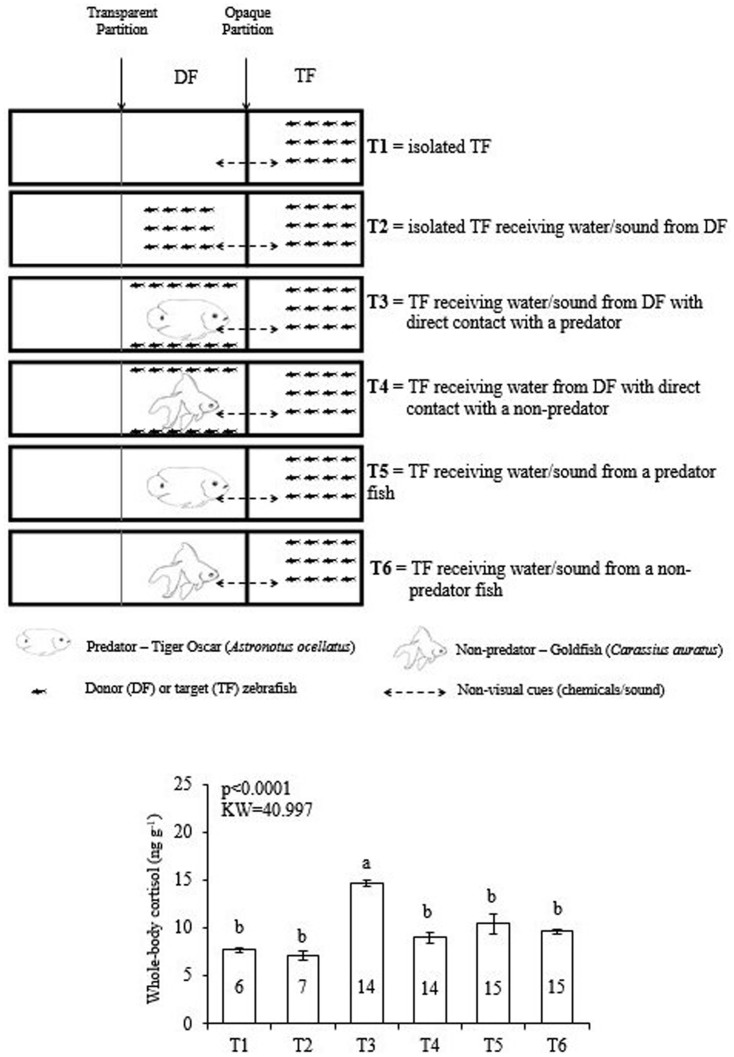
In the second experiment, we investigated the role of the interaction of zebrafish with the predator to induce the cortisol increase in the target zebrafish. In fact, the target zebrafish increased whole-body cortisol levels only if the predator interacted directly with the donor conspecifics (Fig. 2).
Figure 2. Study 2.
Role of zebrafish-predator interaction to induce cortisol increase in zebrafish conspecifics. Experimental design and whole-body cortisol response of the target zebrafish. Mean values (±S.E.M.) compared by Kruskal-Wallis complemented by a Dunn's Multiple Comparisons Test. Different letters above means indicate statistical difference (P indicated in the graph). Numbers of repetitions obtained for each treatment (T1 to T6) are inside each column. The fish drawings in the graphics were drawn by LB.
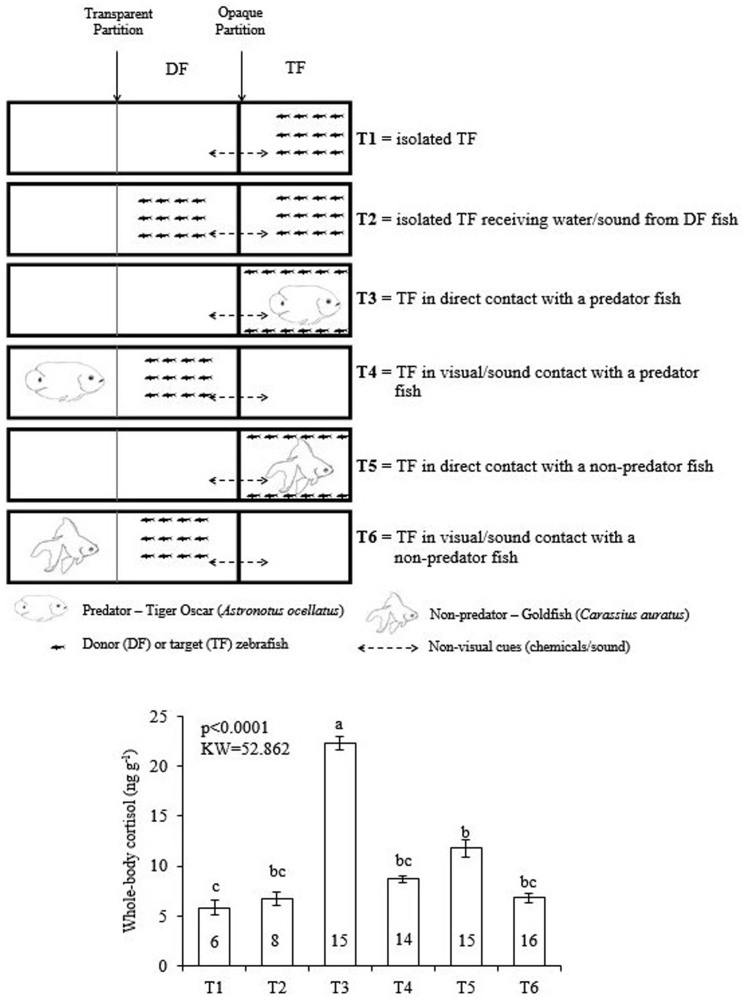
We then performed a third experiment to test the effect of the predator on cortisol level of zebrafish (Fig. 3). We found that direct contact of the zebrafish with the predator markedly increased the whole-body cortisol levels of the fish facing the predator, even when compared with the significant increases reported in experiments 1 and 2. A more discrete cortisol increase occurred in the zebrafish housed in the same aquarium as non-predator goldfish. In this case, however, the response was lower than that induced by the predator and higher than the basal levels of undisturbed zebrafish. This experiment also revealed that visual contact with a predator was not sufficient to increase the whole-body cortisol level of the target zebrafish.
Figure 3. Study 3.
Effect of the predator on cortisol level of zebrafish. Experimental design and whole-body cortisol response of the target zebrafish. Mean values (±S.E.M.) compared by Kruskal-Wallis complemented by a Dunn's Multiple Comparisons Test. Means with at least a same letter above mean are not statistically different from each other (P indicated in the graph). Numbers of repetitions obtained for each treatment (T1 to T6) are inside each column. The fish drawings in the graphics were drawn by LB.
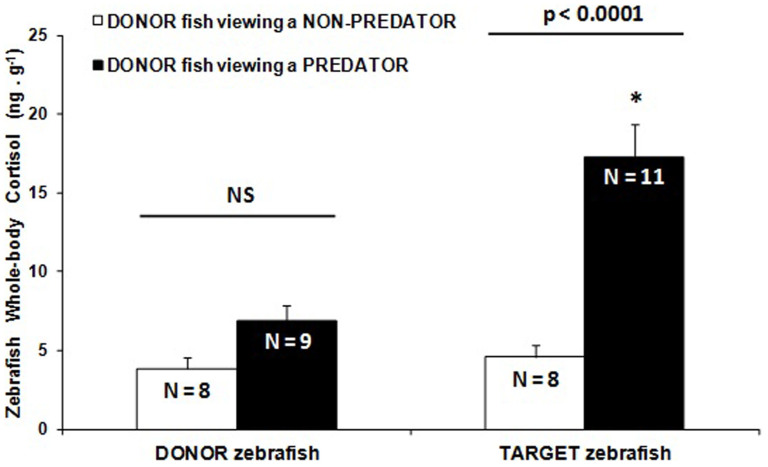
The fourth experiment eliminated confounding effects of sound by using completely separate aquaria for each compartments, with no possibility of any other interferential cue (e.g., sound or electric waves). As detected in the previous experiments, here zebrafish only viewing a predator fish had no change in whole-body cortisol, but induced, by only chemical cues, cortisol increase in conspecifics (Fig. 4). This effect on zebrafish conspecifics did not appear when the donor fish is viewing a non-predator heterospecific.
Figure 4. Study 4.
Stress imposed on zebrafish group (TARGET) by water-conditioned from group of zebrafish DONOR viewing a predator fish. Water was transferred from donor zebrafish to target zebrafish conditioned in different aquaria. The stimulus fish (predator = tiger oscar, Astronotus ocellatus; non-predator = goldfish, Carassius auratus) was in a separated aquaria that could be viewed only by the donor zebrafish. Mean values (±S.E.M.) from repetitions shown inside bars (N). NS = not significant difference between donor condition; * difference in target zebrafish according to condition of the conspecific donors. Statistics: Kruskal-Wallis followed by Dunn's multiple comparison test; KW = 20.716, p = 0.0001.
Discussion
Trying to understand how zebrafish can communicate predation risk to conspecifics, we found that they perceive a predator and release chemicals into the water, a cue that induces cortisol increase in conspecifics – a hormonal reaction that prepares these conspecifics to cope with environmental challenges. Surprisingly, this hormonal preparation did not occur in the donor zebrafish facing the predator, indicating a different way to deal with the predator and communicate with conspecifics. This is the first report to show that chemical communication of predation risk does not depend exclusively on the classical cortisol axis of the stress response, but still activates this axis in conspecifics.
We found that zebrafish viewing/hearing a predator do not have whole-body cortisol changed, but they induced by chemical or sound cues conspecifics to increase this hormone (Fig. 1). A second experiment indicated that the presence of conspecifics with the predator was necessary to zebrafish have cortisol increased (Fig. 2). Moreover, in the third experiment zebrafish facing the predator increased cortisol only when they shared the same space (Fig. 3). All together, these experiments support that unstressed zebrafish facing a predator release some non-visual cues (chemicals or sound) that stress conspecifics. In a further control experiment (Fig. 4), we demonstrate that this intraspecific communication is mediated by chemical cues released by the donor fish into the water.
We have previously demonstrated that zebrafish can recognize potential predator characteristics, such as length, shape and color19. In terms of behavior, movement characteristics appear to be the primary visual cues that allow prey to differentiate between relevant and irrelevant predation threats20. The predator, A. ocellatus, behaved aggressively and performed attack movements against the glass walls, and these movements could have facilitated its recognition as a predator by the zebrafish. This behavior is in accordance with findings that zebrafish recognize a predator primarily through visual cues.
The mechanism by which unstressed zebrafish elicited stress in conspecifics should be investigated in future research. An explanation should be based on the assumption that the donor zebrafish receives incomplete information about the predator; that is, they see but do not smell the predator (an unexpected situation in nature but feasible experimentally). This condition was, however, sufficient to cause the release of complete cues (referred to as disturbance pheromones in crustaceans21 and fish22) that should nonspecifically inform conspecifics of danger. Under these circumstances, fish are more likely to respond to chemical alarm cues if they lack visual information23.
If the donor fish's hypothalamus receives information about the danger through insufficient cues (the fish see the predator, but the information is not complete because chemical cues are absent), a less dramatic hormonal cascade in the hypothalamic-pituitary-interrenal axis can occur. We hypothesize that only the rapid response (i.e., the catecholamine release) should have occurred, as cortisol did not increase in the donor zebrafish facing the predator (Fig. 3) and the sympathetic nervous system rapidly releases catecholamines into the blood24. Thus, hypothetically, other mechanisms involved in the stress response might be invoked to stimulate conspecifics that lack visual cues from the predator and produce a complete stress response with an increase in cortisol levels. Because cortisol can be released in the surrounding water18, but in the present study whole-body cortisol was not increased, other hormones from the HPI axis, or even from the brain-sympathetic-chromaffin axis, should be investigated in this experimental paradigm. The biological significance of such an explanation is that the fish might not respond promptly if the visual and non-visual cues are not consistent with each other, whereas only chemical cues are useful for preparing conspecifics that lack complete information about predation risk.
Early non-visual communication about the presence of a predator is not hampered by physical barriers and can be received by targets that visual cues cannot reach. Therefore, this chemically mediated communication provides additional environmental information to a fish that is unable to detect a potential danger visually, thereby eliciting a stress response that allows fish to prepare for defensive behavior. However, it should be noted that in our experimental design, the putative substance released by the zebrafish could have accumulated in the donor/target fish compartments because the water was continuously recirculating among the compartments. A dose-response study should determine the possibility of this response in nature.
Methods
In a first experiment, we tested whether perception of a predator (the tiger Oscar Astronotus) induces group of zebrafish (Danio rerio) (donor fish) to release non-visual cues that triggers cortisol increase in a group of conspecifics (target fish) lacking any other information about the predator. The non-predator goldfish, Carassius auratus, was an additional control for presence of heterospecific fish. As we found the target fish increased cortisol, in a second experiment we investigated the cortisol of target fish receiving non-visual cues from donor fish sharing compartment with the predator. Then, a third experiment tested the direct effect of the predator on target fish (sharing compartment with the predator). All these experiments revealed that the predator induced cortisol increase only when conspecifics were present (likely mediating the information transfer). However, our set up was not completely clear to avoid other non-chemical effects. Thus, we setup a fourth experiment focused on the main results obtained in the previous three experiments, but each compartment consisted of a glass aquarium (30 × 30 × 30 cm) separated ~0.4 cm from each other, thus assuring exclusive participation of chemical cues in the communication.
Zebrafish and housing conditions
A population of 2000 mixed-sex, adult wild-type zebrafish (Danio rerio) was maintained under a natural photoperiod (~14 h L/10 h D) in indoor tanks (2 fish L−1). The water was maintained as follows: 28.0 ± 2.0°C; pH of 7.0 ± 0.6 units; dissolved oxygen at 6.8 ± 0.4 mg L−1; total ammonia at <0.01 mg L−1; total hardness at 6 mg L−1; and alkalinity at 22 mg L−1 of CaCO3. The fish were fed commercial flakes (TetraMin®, Tetra, Melle, Germany) ad libitum once a day.
Aquaria setup
We divided glass aquaria (120 cm × 40 cm × 40 cm) in their longest dimension into three compartments of the same size (the aquaria set-up is shown in the top panels of Figs. 1, 2 and 3). The left and middle compartments were divided with a transparent glass partition, thus avoiding chemical cues and permitting mostly visual stimuli. The right and middle compartments were separated by an opaque partition to prevent visual cues and allow chemical cues between zebrafish groups.
Chemical communication between the middle and right compartments was ensured through continuous water circulation between these two compartments (~3 L min−1) propelled by a submersed water pump installed in a 1.5-cm hole near the bottom. The efficiency of the water circulation was confirmed by transferring water mixed with methylene blue from one compartment to the other before starting the experiments. The pumps were previously shown to have no effect on zebrafish whole-body cortisol25.
Experimental procedures
Prior to each trial, the zebrafish groups were acclimated in the experimental aquaria for 3 days under a 14/10-h light/dark cycle and fed 3 times a day. The predator and non-predator fish were introduced when the experiment was initiated (pumps were turned on); 1 h later, the zebrafish were immediately captured (netted) and frozen in liquid nitrogen for 10 s to 30 s. To prevent any handling-induced stress response, the time period between their capture and killing was <30 s. The dead fish were stored in liquid nitrogen at −20°C prior to cortisol extraction. In treatment in which zebrafish groups communicated with each other by chemical cues (top panels in Figs. 1, 2 and 3), the same 1-h interval was maintained after the pump was turned on.
Between replicates, when the same aquaria were used, they were completely cleaned before a new replicate was set up. During the experiments, the aquaria were not cleaned, the water was not changed, and the fish were not fed to avoid the effects of undesirable chemical factors.
We ran three trials to obtain 2 to 3 fish samples from each zebrafish group to measure the whole-body cortisol. We ran new sets of three trials to obtain the number of repetitions (N value) shown inside the bars of figures in the result section. Because some samples were lost, treatments were not balanced.
Cortisol extraction and determination
Whole-body cortisol was extracted as described in Barcellos et al.19. Each fish was weighed, minced and placed in a disposable stomacher bag with 2 mL of phosphate-buffered saline (PBS, pH 7.4) for 6 min. The contents were then transferred to a 10-mL screw top disposable test tube to which 5 mL of laboratory-grade ethyl ether was added. The tube was vortexed for 1 min and centrifuged for 10 min at 3000 rpm, and the sample was immediately frozen in liquid nitrogen. The unfrozen portion (the ethyl ether fraction containing cortisol) was transferred to a new tube and completely evaporated under a gentle stream of nitrogen gas for 2 h, yielding a lipid extract containing the cortisol, which was stored at −20°C.
The accuracy of cortisol detection was tested by calculating the recovery from samples spiked with known amounts of cortisol (50, 25 and 12.5 ng mL−1). The mean detection accuracy of the spiked samples was 94.3%. All cortisol values were adjusted for recovery using the following equation: cortisol value = measured value × 1.0604.
The tissue extracts were re-suspended in 1 mL PBS, and the whole-body cortisol levels were measured in duplicate samples of each extract using a commercially available enzyme-linked immunosorbent assay kit (EIAgen™CORTISOL test, BioChem ImmunoSystems). This kit was fully validated for zebrafish tissue extracts using the methodology proposed by Sink et al.26. Precision was tested by performing 12 repeated assays on seven randomly chosen samples in the same plate and calculating the intra-assay coefficient of variation (CV). Reproducibility was tested by assaying the same samples in different plates and calculating the inter-assay CV. To test for linearity and parallelism, the tissue extracts were serially diluted in the buffer provided with the kit. A strong positive correlation between the curves was observed (R2 = 0.8918), and the samples had low inter- and intra-assay CV values (7–10% and 5–9%, respectively).
Statistics
A Kolmogorov-Smirnov test showed that the samples were derived from populations that did not follow normal distributions. A Bartlett test indicated that the SDs of the samples in the same experiment were statistically indistinguishable, except in experiment 4 [Bartlett test (corrected) = 9.9000; P = 0.0194]. Therefore, we applied the Kruskal-Wallis test (Nonparametric ANOVA) followed by Dunn's multiple comparison test to compare the means in each experiment. Significant differences were set at α = 0.05.
Ethical note
Methods were carried out in accordance with the guidelines of the Brazilian College for Animal Experimentation (COBEA; http://www.cobea.org.br) and was approved by the Ethics Commission for Animal Use (CEUA) of Universidade de Passo Fundo, UPF, Passo Fundo, RS, Brazil (Protocol#3/2011-CEUA, July 2009).
Author Contributions
L.J.G.B. and G.L.V. conceptualized the study. L.J.G.B., G.L.V., R.E.B. and P.C.G. interpreted data. G.K., J.G.S.R. and D.F. collected and analyzed data. G.L.V. wrote the paper.
Acknowledgments
This study was funded by the Universidade de Passo Fundo. L.J.G.B. and G.L.V. hold CNPq research fellowships (302073/2011-6 and 307380/2009-2, respectively).
References
- Kelly J. L. & Brown C. Predation risk and decision-making in poeciliid prey. In: Ecology and Evolution of Poeciliid Fishes (Evans, J. P., Pilastro, A. & Schlupp, I. eds), pp. 174–184. Chicago, IL: University of Chicago Press. 2011. [Google Scholar]
- Franchina C. R. & Stoddard P. K. Plasticity of the electric organ discharge waveform of the electric fish Brachyhypopomus pinnicaudatus - I. Quantification of day-night changes. J. Comp. Physiol. A 183, 759–768 (1998). [DOI] [PubMed] [Google Scholar]
- Barreto R. E., Luchiari A. C. & Marcondes A. L. Ventilatory frequency indicates visual recognition of an allopatric predator in naïve Nile tilapia. Behav. Proces. 60, 235–239 (2003). [DOI] [PubMed] [Google Scholar]
- Jordão L. C. Disturbance chemical cues determine changes in spatial occupation by the convict cichlid Archocentrus nigrofasciatus. Behav. Proces. 67, 453–459 (2004). [DOI] [PubMed] [Google Scholar]
- Giaquinto P. C. & Volpato G. L. Hunger suppresses the onset and the freezing component of the antipredator response to conspecific skin extract in pintado catfish. Behaviour 138, 1205–1214 (2001). [Google Scholar]
- Ide L. M., Urbinati E. C. & Hoffmann A. The role of olfaction in the behavioural and physiological responses to conspecific skin extract in Brycon cephalus. J. Fish Biol. 63, 332–343 (2003). [Google Scholar]
- Barreto R. E., Barbosa-Junior A., Giassi A. C. C. & Hoffmann A. The club cell and behavioural physiological responses to chemical alarm cues in the Nile tilapia. Mar. Fresh. Behav. Physiol. 43, 75–81 (2010). [Google Scholar]
- Barbosa Júnior A., Magalhães E. J., Hoffmann A. & Ide L. M. Conspecific and heterospecific alarm substance induces behavioral responses in piau fish Leporinus piau. Acta Ethol. 13, 119–126 (2010). [Google Scholar]
- Chivers D. P. & Smith R. J. F. Chemical alarm signalling in aquatic predator–prey systems: a review and prospectus. Ecoscience 5, 338–352 (1998). [Google Scholar]
- Barreto R. E. et al. Blood Cues induce antipredator behavior in Nile tilapia conspecifics. PLoS ONE 8, e546 42.10.1371/journal.pone.0054642 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisenden B. D., Chivers D. P. & Smith R. J. F. Early warning in the predation sequence: a disturbance pheromone in Iowa darters (Etheostoma exile). J. Chem. Ecol. 21, 1469–1480 (1995). [DOI] [PubMed] [Google Scholar]
- Jordão L. C. & Volpato G. L. Chemical transfer of warning information in non-injured fish. Behaviour 137, 681–690 (2000). [Google Scholar]
- Bryer P. J., Mirza R. S. & Chivers D. P. Chemosensory assessment of predation risk by slimy sculpins (Cottus cognatus): responses to alarm, disturbance, and predator cues. J. Chem. Ecol. 27, 533–546 (2001). [DOI] [PubMed] [Google Scholar]
- Bell A. M., Backstrom T., Huntingford F. A., Pottinger T. G. & Winberg S. Variable neuroendocrine responses to ecologically-relevant challenges in stickelebacks. Physiol. Behav. 91, 15–25 (2007). [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T. H. et al. Selfish sentinels in cooperative mammnas. Science 284, 1640–1644 (1999). [DOI] [PubMed] [Google Scholar]
- Brown G. E., Godin J.-G. J. & Pedersen J. Fin-flicking behavior: a visual antipredator alarm signal in a characin fish, Hemigrammus erythrozonus. Anim. Behav. 58, 469–475 (1999). [DOI] [PubMed] [Google Scholar]
- Barcellos L. J. G., Volpato G. L., Barreto R. E. B., Coldebella I. & Ferreira D. Chemical communication of handling stress in fish. Phys. Behav. 103, 372–375 (2011). [DOI] [PubMed] [Google Scholar]
- Zuberi A., Ali S. & Brown C. A non-invasive assay for monitoring stress response: A comparison between wild and captive-reared rainbowfish (Melanoteania duboulayi). Aquaculture 321, 267–272 (2011). [Google Scholar]
- Barcellos L. J. G. et al. Whole-body cortisol increases after direct and visual contact with a predator in zebrafish Danio rerio. Aquaculture 272, 774–778 (2007). [Google Scholar]
- Brown G. E. Learning about danger: chemical alarm cues and local risk assessment in prey fishes. Fish Fish. 4, 227–234 (2003). [Google Scholar]
- Hazlett B. A. Disturbance pheromones in the crayfish Orconectes virilis. J. Chem. Ecol. 11, 1695–1711 (1985). [DOI] [PubMed] [Google Scholar]
- Wisenden B. D., Chivers D. P. & Smith R. J. F. Early warning in the predation sequence: a disturbance pheromone in iowa darters (Etheostoma exile). J. Chem. Ecol. 21, 1469–1480 (1995). [DOI] [PubMed] [Google Scholar]
- Hartman E. J. & Abrahams M. V. Sensory compensation and the detection of predators: the interaction between chemical and visual information. Proc. R. Soc. Lond. 267, 571–575 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendelaar Bonga S. E. The stress response in fish. Physiol. Rev. 77, 591–625 (1997). [DOI] [PubMed] [Google Scholar]
- Oliveira T. A. et al. Alcohol Impairs Predation Risk Response and Communication in Zebrafish. PLoS ONE 8, e75780; 10.1371/journal.pone.0075780 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink T. D., Lochmann R. T. & Fecteau K. A. Validation, use, and disadvantages of enzyme-linked immunosorbent assay kits for detection of cortisol in channel catfish, largemouth bass, red pacu and golden shiners. Fish Physiol. Biochem. 75, 165–171 (2007). [DOI] [PubMed] [Google Scholar]