Abstract
Emerging understanding about interactions between stem cells, scaffolds and morphogenic factors has accelerated translational research in the field of dental pulp tissue engineering. Dental pulp stem cells constitute a sub-population of cells endowed with self-renewal and multipotency. Dental pulp stem cells seeded in biodegradable scaffolds and exposed to dentin-derived morphogenic signals give rise to a pulp-like tissue capable of generating new dentin. Notably, dentin-derived proteins are sufficient to induce dental pulp stem cell differentiation into odontoblasts. Ongoing work is focused on developing ways of mobilizing dentin-derived proteins and disinfecting the root canal of necrotic teeth without compromising the morphogenic potential of these signaling molecules. On the other hand, dentin by itself does not appear to be capable of inducing endothelial differentiation of dental pulp stem cells, despite the well known presence of angiogenic factors in dentin. This is particularly relevant in the context of dental pulp tissue engineering in full root canals, where access to blood supply is limited to the apical foramina. To address this challenge, scientists are looking at ways to use the scaffold as a controlled release device for angiogenic factors. The aim of this manuscript is to present and discuss current strategies to functionalize injectable scaffolds and customize them for dental pulp tissue engineering. The long-term goal of this work is to develop stem cell-based therapies that enable the engineering of functional dental pulps capable of generating new tubular dentin in humans.
Keywords: Tissue engineering, Regenerative Endodontics, Pulp biology, Dental pulp stem cells, Morphogenic signals, Angiogenesis, Dentin
A major goal of the health sciences in the 21st century is to develop clinically relevant strategies for tissue regeneration. The reasoning for this goal comes from the realization that the best substitute of an organ/tissue lost due to disease or trauma is the actual organ/tissue. Broadly speaking, this can be achieved either by transplantation or regeneration. Transplantation-based strategies have been successfully used for decades. However, organ/tissue rejection is a major threat that has been addressed with the prolonged used of immunesuppressive drugs, which carry intrinsic risks for the patient. On the other hand, tissue regeneration mediated by targeted activation of host stem cells, or delivery of autologous stem cells, may allow for similar results as transplantation-based strategies without the need for chronic immunesuppressive therapies. However, tissue regeneration is certainly not devoid of significant challenges. They involve the development of strategies for recruitment or isolation of appropriate stem cells and the generation of a suitable microenvironment that enables the stem cells to differentiate, proliferate and give rise to a fully functional organ/tissue in the correct shape and size. While these are rather substantial challenges, it is becoming increasingly evident that the successful development of tissue regeneration strategies utilizing autologous cells might have long lasting benefits that surpass potential risks. This review will be focused in one aspect of tissue regeneration, i.e. the development of functionalized scaffolds that provide a conducive microenvironment for controlled differentiation of stem cells and generation of a new dental pulp for treatment of necrotic immature permanent teeth.
Tissue engineering
Tissue engineering is a multidisciplinary science that aims the development of clinically relevant strategies for the regeneration of a tissue or organ (1). It involves the identification of progenitor cells capable of tissue regeneration when seeded in biodegradable scaffolds and exposed to morphogenic signals (1–3). Scaffolds must be uniquely developed for the regeneration of each specific tissue or organ. Nevertheless, they share common features such as allowing cell attachment, diffusion of nutrients and oxygen, being biodegradable, and having physical properties aligned with those of the tissue/organ to be regenerated (3). In addition, the scaffolds can be functionalized by enhancing conditions for cell attachment and survival, as well as by providing morphogenic signals that supplement those coming from the host and enable guidance of stem cell differentiation (3).
Broadly speaking, scaffolds can be divided in: A) Casted, i.e. fairly rigid and custom-made for specific purposes; or B) Injectable, i.e. low viscosity gels that can be delivered and “molded” at the site that requires tissue regeneration. Both types of scaffolds can be functionalized with morphogenic signals. Notably, these signals are typically proteins with short half-life. Therefore, the development of a strategy for controlled release of these proteins is critical to maximize their effects for pre-determined time periods. Morphogenic signals can be incorporated both into casted scaffolds using copolymers such as poly(lactic-co-glycolic acid) (PLGA) and gas foaming approaches (4, 5). They can also be mixed with injectable scaffolds such Collagen or the self-assembling hydrogel Puramatrix™, but in this case it is very difficult to slowdown the degradation rate of the proteins. To address this issue, it has been suggested that natural polymers derived from brown algae (e.g. alginates), that are biocompatible and present low immunogenicity, can be used in combination with injectable scaffolds to serve as a “slow release device” for morphogenic signals (6, 7). Notably, the gelation process in presence of divalent ions at physiological levels is a very simple way to incorporate, protect, and release in a controllable rate morphogenic factors from alginate microspheres (8, 9).
The biological standard for the controlled release of morphogenic factors in dental tissue engineering is the microenvironment observed during the tooth development (10, 11). Investigators have attempted to understand this environment as a means to create ideal conditions for guided determination of stem cell fate and dental tissue regeneration (11, 12). The work of many investigators throughout the world identified morphogenic signals that play major roles during tooth development, and that can potentially be used therapeutically in tooth regeneration (13–15). Indeed, knockout of genes such as DSPP (16), DMP1 (17), Msx homeobox family (18, 19), or Amelogenin (20, 21) revealed major tooth developmental defects, indicating that these morphogenic signals are critically involved in these processes. Such findings suggest candidate morphogenic signals that can be either recruited from the surrounding environment, or delivered locally with the use of the scaffold, to direct stem cell fate and optimize guided pulp tissue regeneration.
In addition to the need for guided differentiation of stem cells into odontoblasts, there is also an important need for their differentiation into supporting cells (e.g. vascular endothelial cells, neural cells). Tissue innervation is critical for the functional regulation of the cells involved in pulp regeneration. In addition to the protective effect of the pulp innervation, it also plays important roles in inflammation and tissue repair (22). The rapid induction of a pro-angiogenic response is crucial not only as a means to provide necessary influx of the oxygen and nutrients required by the high metabolic demands of cells engaged in tissue regeneration, but also to enable immunologic responses necessary to protect the emerging tissues from bacterial contamination typically associated with the clinical handling of necrotic teeth. Immune cells such as tissue-infiltrating macrophages require the presence of a functional vascular network to access the regenerated pulp tissue and protect it against bacteria that could possibly remain viable after treatment of necrotic teeth. Indeed, it is plausible to speculate that access of immune cells to the pulp might play a major role in the successful outcome of necrotic teeth treated with Regenerative Endodontics-based approaches.
In addition, it is through the blood vessels that substrates required for dentin mineralization (e.g. calcium, phosphate) are made available to odontoblasts, which perhaps explains the frequent presence of blood vessels in close proximity to the odontoblastic layer particularly in pulps actively engaged in regenerative processes. Furthermore, vascularization is a key determinant of mesenchymal cell heterogeneity in dental tissue engineering, presumably by enabling the recruitment of circulating cells to the developing tooth (11). Later in this review, we will discuss potential strategies proposed for the rapid vascularization of engineered dental pulps.
Tooth-related stem cells
The cells that define the pulp tissue are the odontoblasts, terminally differentiated cells that do not proliferate and that are endowed with the capacity of generating new tubular dentin. Lost odontoblasts can be replaced in normal pulps by resident multipotent stem cells (23, 24) found in both permanent teeth (25) or primary teeth (26). They can differentiate into odontoblasts and also into other cell lineages such as osteoblasts, chondrocytes, and neuronal progenitor cells (25–29). Stem cells have also been identified in other oral tissues, such as the apical papilla (30), mesenchymal follicle (31), periodontal ligament (32), and gingivae (33). It is speculated that stem cells from each tissue are somewhat “primed” to regenerate that same tissue, and therefore is likely that the best stem cells for dental pulp tissue engineering are the pulp stem cells. However, it is rather unclear at this time what is the relative potential of each one of these oral stem cells for dental pulp tissue engineering.
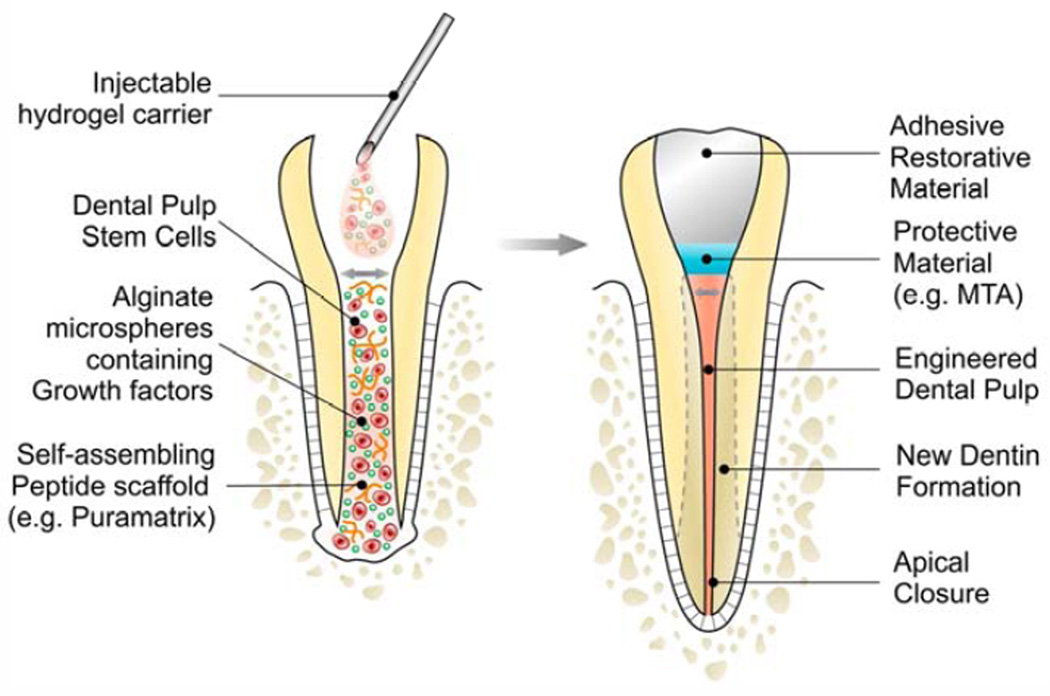
Dental pulp stem cells (DPSC) are relatively easy to be obtained from exfoliated primary teeth or from permanent teeth extracted for orthodontic reasons. Considered a relatively rich source of mesenchymal stem cells, the interest in dental pulp stem cell isolation and banking has increased substantially in recent years. More importantly, exfoliating primary teeth and permanent teeth extracted for orthodontic reasons overlap temporally with immature permanent teeth of adolescents that are relatively prone to trauma-induced pulp necrosis. Therefore, it is suggested that these teeth are an ideal source of stem cells for dental pulp tissue engineering of necrotic immature permanent teeth. In these cases, the goal is to regenerate a functional dental pulp capable of completing vertical and horizontal root formation (Fig. 1).
Figure 1.
Schematic representation of a strategy for dental pulp tissue engineering that is based on the use of a functionalized injectable scaffold and transplantation of dental pulp stem cells.
In proof-of-principle experiments using the permanent tooth slice/scaffold model (34), we observed that stem cells from exfoliated deciduous teeth (SHED) differentiate into functional odontoblasts and also into vascular endothelial cells (35–38). Notably, fluorescent lines created by tetracycline staining of newly formed tubular dentin confirmed that the SHED cells indeed differentiated into mature odontoblasts (38). These experiments suggested the possibility of isolating stem cells from exfoliating primary teeth and transplanting them back in the same patient (autologous transplantation) in clinical scenarios involving pulp necrosis of an immature permanent tooth during the mixed dentition phase.
A critical challenge of the clinical scenario described above is the need for quick vascularization of the engineered tissue to enable the maintenance of viability of transplanted cells (39). Indeed, the anatomy of the dental root is a major limiting factor in regards to access to vascularization, considering that all blood vessels have to come necessarily through a system of narrow foramina located exclusively in one end of the tooth. In young, immature teeth, the apical opening of the root is relatively wide. However, in people aged 21 or more the dimensions of apical foramen are very narrow (40) and tends to decreases progressively over time. It appears that necrotic immature teeth with open apex are the prime candidates for dental pulp tissue engineering at this stage of development of the technique. Even in these cases, we believe that the success rate of such therapy would benefit from the delivery of a pro-angiogenic stimulus.
The recent discovery that dental pulp stem cells differentiate into vascular endothelial cells in addition to differentiating into functional odontoblasts (36, 38, 41), suggests that these cells can serve as a single cellular source for dental pulp tissue engineering. However, a key observation of these studies is that while dentin-derived morphogenic signals are sufficient to induce odontoblastic differentiation, they are not sufficient to induce endothelial differentiation in vivo. This is despite the well-known presence of pro-angiogenic factors in the dentin (42, 43). Therefore, it appears imperative that strategies aiming at dental pulp tissue engineering incorporate pro-angiogenic signals, likely delivered locally with sustained release carriers.
Morphogenic factors in pulp regeneration
The observation that dentin is a reservoir of bioactive morphogenic signals that can be recruited upon demand (44) constitutes a major enabling discovery in the field of dental pulp tissue regeneration. Indeed, this discovery represents a true paradigm shift in the field, since it elevated the dentin to the status of a morphogenic source that enables and guides regenerative processes and tissue repair, rather than being simply an inert and passive tissue. Multiple lines of investigation have demonstrated that dentin-derived proteins are sufficient for odontoblastic differentiation (36, 45, 46). Notably, intentional degradation of dentin-derived proteins with sodium hypochlorite eliminated its inductive potential (36). This finding corroborated previous observations of the critical role of dentin-derived morphogenic signals in odontoblastic differentiation, and raised the possibility that sodium hypochlorite might not be the ideal solution for root canal irrigation in Regenerative Endodontics. In search for a mechanistic explanation for these findings, we and others observed that Bone Morphogenetic Protein (BMP)-2 is a key mediator of dentin-induced odontoblastic differentiation of dental pulp stem cells (36, 45).
Another important dentin-derived morphogenic signal is the Transforming Growth Factor (TGF)-β1 (45). TGF-β1 is present in sound dentin (47) and can be released by acidic activity of cariogenic bacteria (48) or when EDTA is applied over sound dentin (49). The release of TGF-β1 has also been identified after application of calcium hydroxide-containing materials (50), mineral trioxide aggregate (MTA) (51), tricalcium silicate-based cement (52) and self-etching dental adhesives as well (53). TGF-β1 is a very complex molecule with multiple effects. Its full implications in pulp biology and pulp regeneration are yet to be determined.
In addition to the dentin, there are several other sources of morphogenic signals that include, but are not limited to, resident pulp cells (e.g. fibroblasts, neural cells, endothelial cells), circulating cells (e.g. circulating progenitor cells, inflammatory cells), and the pulp extracellular matrix itself. The understanding of the role of these morphogenic signals in the maintenance of dental pulp homeostasis and in the processes that lead to pulp regeneration is emerging. An in depth discussion of the function of each one of these morphogenic factors is beyond the scope of this review. However, we included here a table that summarizes some of the functions of the key morphogenic signals that may play a role in pulp regeneration (Table 1).
Table 1.
Morphogenic factors and their function in dental development, regeneration, and in tissue engineering.
| Protein (Class) | Symbol | Potential role in odontogenesis and tissue regeneration |
Potential role in dental pulp engineering |
|---|---|---|---|
| Bone Morphogenetic Protein 2, 4, 7 (Growth factor) |
BMP-2,-4,-7 | BMP7 mediates epithelial-mesenchymal interactions during the initiation phase of odontogenesis and morphogenesis (81). Experiments using BMP7 (82, 83), BMP2 and BMP4 (84, 85) showed to induce dentin regeneration in vivo. | BMP2 induces differentiation of SHED into dental pulp cells (36), and mineralized tissue formation using DPSC (86) in vivo. BMP7 induces upregulation mineralization on Dental Pulp Stem cells (87). |
| Bone Sialoprotein (SIBLING) |
BSP | BSP stimulates the differentiation of dental pulp cells into odontoblast-like cells and induces regenerative dentin in vivo (88, 89). | Unclear |
| Core-binding Factor subunit Alpha-1 (Transcription factor) |
Cbfa1 | Regulates epithelial-mesenchymal interactions in during morphogenesis and histodifferentiation of the epithelial enamel organ (90), but it plays a stage-specific role in the lineage determination and terminal differentiation of odontoblasts (91). | Unclear |
| Dentin matrix Protein 1 (SIBLING) |
DMP-1 | DMP1 is expressed in mature odontoblasts (92), plays an essential role in tooth mineralization (17), and appears to have a regulatory function in the nucleus (93). Besides, it activates the synthesis of IL-6 and IL-8 from pulp fibroblasts (94). | Induces an organized matrix formation similar to that of pulpal tissue, which might lead to hard tissue formation in vivo (95). |
| Dentin sialophosphoprotein: Dentin sialoprotein, dentin phosphoprotein (SIBLING) |
DSPP: DSP DPP |
DSPP is expressed by odontoblasts and is cleaved into two smaller polypeptides with unique physico-chemical characteristics (DSP, DPP) (96). DSPP may regulate the effect of DMP1 in dentinogenesis (97). DSP is involved in epithelial-mesenchymal interactions that are crucial to later stages of tooth development (98). It promotes growth, migration, and odontoblastic differentiation of HDPC in vitro (100). DPP binds to collagen and initiates formation of apatite crystals in dentin (99). |
Unclear |
| Fibroblast growth Factor-2 (Growth factor) |
FGF2 | Induces differentiation of HDPC in mineralized tissue and upregulates chemokines in vitro (101). | Induces cellularization and revascularization of human teeth implanted into the dorsum of rats (84), and dental tissue mineralization (102). |
| Canonical Wnt pathway (Signal transduction) |
Wnt/β-catenin | Canonical Wnt appears to mediate the downstream events of TGF-β1 during pulp regeneration (103). | Regulates the differentiation of DPSC into odontoblast-like cells (104). |
| Transforming Growth Factor-β1 (Growth factor) |
TGF-β1 | Induces upregulation of dentin matrix by odontoblasts (57). TGF-β is a physiological regulator of osteoblast differentiation (105). | Induces odontoblast-like cell differentiation in vitro (57), and DPSC-mediated mineralization (106). |
| Twist-related protein 1 (Transcription factor) |
TWIST1 | Appears to be involved in the development the supernumerary teeth (107). | Induces differentiation of DPSC into odontoblast-like cells in vitro (108). |
| Vascular Endothelial Growth Factor (Growth factor) |
VEGF | Induces endothelial cell survival and differentiation of new blood vessels, and may be used therapeutically to induce tissue neovascularization (109). | Induces differentiation of SHED into endothelial cells (38). |
Functionalized injectable hydrogels in dental pulp tissue engineering
The discovery that dentin-derived proteins are sufficient to induce full differentiation of dental pulp stem cells into odontoblasts has an important implication for pulp tissue engineering. It indicates that one does not need to provide additional morphogenic signals to achieve odontoblastic differentiation of stem cells transplanted or recruited into the root canal. The focus could be simply to protect these dentin-derived factors from degradation (e.g. avoid exposure to sodium hypochlorite) and to enhance their mobilization by, perhaps, treating the dentin surface with mild organic acids (e.g. EDTA), as we showed (36). However, more complex issues need to be addressed, as follows: A) One needs to provide adequate attachment and prevent anoikis of stem cells transplanted or recruited into the pulp chamber, and; B) One needs to quickly vascularize the regenerating pulp to enable oxygen and nutrient influx, as well as to allow for the arrival of circulating progenitor cells that will complement the cellular heterogeneity of the engineered pulp, as demonstrated by Keller and colleagues (11). Investigators have attempted to address these two issues by functionalizing the scaffolds used in dental pulp tissue engineering with moieties that enable better cell attachment (e.g. RGD) and by incorporating angiogenic factors (e.g. VEGF) (54, 55).
It is becoming increasingly evident that the ideal scaffold for dental pulp tissue engineering will be injectable, not casted. This is due to the narrow spaces within the root canal as well as the complexity of its anatomy, particularly in the apical region. In addition, there are concerns related to the use of solvents (e.g. chloroform, dichloromethane, acetone) that are typically used to solubilize casted scaffold. For example, solvent-casted PLGA requires more than two days for significant volatization (56) and residual levels of solvents may be toxic to cells. On the other hand, hydrogels can be injectable and therefore penetrate throughout the root canal system. Also, they typically undergo setting reaction at physiological pH.
Alginates are versatile natural polymers that have been used extensively as drug and growth factor carriers (57–63). The water-holding capillarity of hydrogels defines the release kinetics of alginates that can be affected by pH, temperature, level of crosslinking, viscosity and stability (58). Alginates act as mechanical barriers, decreasing the diffusivity of low molecular weight chemical compounds or proteins entrapped after their gelation. Substances such as phosphate saline buffer (PBS) that are used as carriers for growth factors can be encapsulated in high viscosity clinical grade alginates (59). On the other hand, due to their hydrophilic nature, alginates present low absorption of serum proteins and consequently low level of adhesion and cellular interaction (7, 60). Consequently, no cell proliferation is typically observed when alginate is used as synthetic extracellular matrix (7). This observation led to the possibility of using alginate microspheres (as slow release device for morphogenic signals), combined with an injectable scaffold that is more conducive to stem cell survival and proliferation. The association between alginate hydrogels and other materials such as nanofibers (61), or modification of the alginate’s structure (e.g. by peptide functionalization) (62) have been investigated to improve the attachment of cells. To address this issue, the Galler and colleagues has recently demonstrated the development of a customized self-assembling peptide hydrogel specifically designed specifically for dental pulp tissue engineering (54). The advantage of this innovative system is the possibility of incorporating signaling molecules and the RGDS sequence for cell adhesion to the structure of the scaffold. Alternatively, it has been suggested an incorporation strategy involving adsorption of growth factors in porous microspheres with posterior encapsulation with alginate (63).
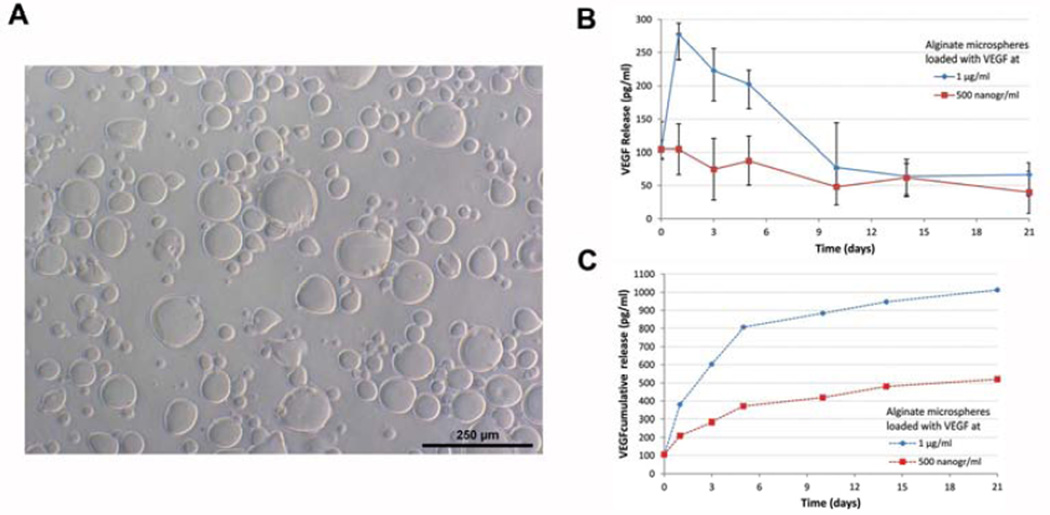
A commercially available self-assembling injectable hydrogels (i.e. Puramatrix™) present favorable viscosity for use as injectable scaffold. Its mixture with a sucrose-based solution and/or cell culture medium triggers fast self-assembling leading to its gelification and generation of a tridimensional environment that provides cell adhesion and enables cell proliferation. We have recently shown that Puramatrix allowed for odontoblastic differentiation of dental pulp stem cells in vitro (64). Ongoing work in our laboratory is attempting to combine the cell-friendly environment provided by Puramatrix™ with the controlled release of morphogenic factors provided by alginate microspheres in the context of dental pulp tissue engineering (Figure 2).
Figure 2.
Characterization of VEGF-containing alginate microspheres. (A) Alginate microspheres loaded with VEGF. (B,C) Graphs depicting release kinetics of VEFG from alginate microspheres (500 or 1,000 ng/ml VEGF) dispersed in a self-assembling hydrogel (Puramatrix™) and PBS. Supernatant was collected and VEGF165 concentration was determined by ELISA, and shown either at indicated time periods (B) or cumulative over the duration of the experiment (C).
Challenges ahead in dental pulp tissue regeneration
The optimization of the ideal release kinetics of morphogenic factors for use in Regenerative Endodontics is a major challenge. The adsorption of proteins has been broadly used for delivery of growth factors in tissue engineering, but there are issues associated with this approach. This method relies on a physical retention and release via the natural porosity of the biodegradable material. Chemical binding with di-sulfite bonds or Heparin have also been used (54, 65). Notably, heparin has natural affinity with growth factors such as Vascular Endothelial Growth Factor (VEGF) (66, 67). We recently observed sustained release of VEGF from alginate microspheres (<275 µm in diameter) for 21 days (Figure 2). However, while appropriate levels of VEGF mediate induction of angiogenesis, excessive VEGF can promote vascular leakage leading to edema and increased interstitial pressure (68). This is a critical challenge in dental pulp tissue engineering since the pulp is encapsulated within non-expanding dentin walls. In this case, excessive interstitial pressure may result in cell death, as was shown in the context of the brain following a stroke (68).
The determination of how much morphogenic factor is ideal goes hand-and-hand with the definition of how quickly it should be released, and for how long. There are many ways to control release kinetics of growth factors. They include structural modifications of alginate by the addition of dextran in semi-interpenetrated networks (69), heparin (70), chitosan (67), alginate-sulfatated (71, 72), and alginate photocrosslinking (73). Ultraviolet (UV) light-initiator systems have been used to promote photo-polymerization of alginate while maintaining it biocompatible (74, 75). These are examples of the complexities involved with the local delivery of morphogenic factors, and demonstrate that this is an area that will require much attention as Regenerative Endodontics moves towards clinical application.
It is possible that multiple morphogenic factors combined with other agents (e.g. antibiotics) will have to be used for ideal dental pulp regeneration. Layering approaches have been proposed for controlled release of multiple growth factors, inhibitors of inflammation and/or antibiotics from microspheres (76, 77). The combination of growth factor and drug delivery has attracted attention due to the potential benefits of antibacterial compounds in the treatment of necrotic root canals (3). In addition, it will be important to find the ideal material to be used to seal the newly regenerated pulp tissue. This material should be biocompatible to maintain cell viability of the regenerated pulp. At the same time, it should provide a good interfacial seal that minimizes microleakage and also provides adequate adhesion to the overlaying restorative material.
It is becoming apparent that revascularization alone without cell transplantation in necrotic teeth is accompanied by resolution of periapical lesion and partial apical closure, but it does not enable the generation of a fully functional dental pulp tissue throughout the full length of the root canal (78, 79). On the other hand, transplantation of human stem cells generates a dental pulp throughout the entire length of human premolars transplanted in the subcutaneous space of immunodeficient mice (80). However, the translation of stem cell-based dental pulp tissue regeneration into routine clinical use faces significant challenges. For example, it is still unclear what is the ideal the source of multipotent stem cells for pulp regeneration. We do not know if dental pulp stem cells are necessarily better than gingival stem cells or bone marrow-derived mesenchymal stem cells in Regenerative Endodontics. An additional challenge is that one would have to establish cell handling protocols that follow Good Manufacturing Practice (GMP) standards, defined by the Food and Drug Administration (FDA) as ex-vivo manipulation of clinical-grade cells that are safe for the patient while being effective therapeutically, in dental clinics and supporting laboratories.
It is becoming increasingly evident that while there are many aspects that can be learned from the broad literature on tissue regeneration, there are questions that are unique to the field of Regenerative Endodontics. They include, but are not limited to, the development of strategies to mobilize while protecting dentin-derived morphogenic signals, the development of functionalized injectable scaffolds that allow for cell attachment/survival and can be used within root canals as a slow release device for angiogenic factors, the development of strategies that eliminate bacterial contamination in necrotic teeth, and the definition of the sources of multipotent stem cells that can regenerate a fully functional dental pulp tissue. It is unquestionable that the most effective way to address such challenges is through integrated work of multidisciplinary research teams that bring together experts in cell and molecular biology, dental clinicians and material scientists.
Acknowledgments
Funded by the Brazilian Federal Agency for the Improvement of Higher Education (CAPES, BEX # 5493/12-9 and # 5279/12-7) and grant R01-DE21410 from the NIH/NIDCR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Yang S, Leong KF, Du Z, Chua CK. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2001;7:679–689. doi: 10.1089/107632701753337645. [DOI] [PubMed] [Google Scholar]
- 3.Lavik E, Langer R. Tissue engineering: current state and perspectives. Appl Microbiol Biotechnol. 2004;65:1–8. doi: 10.1007/s00253-004-1580-z. [DOI] [PubMed] [Google Scholar]
- 4.Sheridan MH, Shea LD, Peters MC, Mooney DJ. Bioabsorbable polymer scaffolds for tissue engineering capable of sustained growth factor delivery. J Control Rel. 2000;64:91–102. doi: 10.1016/s0168-3659(99)00138-8. [DOI] [PubMed] [Google Scholar]
- 5.Golub JS, Kim YT, Duvall CL, Bellamkonda RV, Gupta D, Lin AS, et al. Sustained VEGF delivery via PLGA nanoparticles promotes vascular growth. Am J Physiol. 2010;298:H1959–H1965. doi: 10.1152/ajpheart.00199.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujiwara S, Kumabe S, Iwai Y. Isolated rat dental pulp cell culture and transplantation with an alginate scaffold. Okajimas Folia Anat Jpn. 2006;83:15–24. doi: 10.2535/ofaj.83.15. [DOI] [PubMed] [Google Scholar]
- 7.Bohl KS, Shon J, Rutherford B, Mooney DJ. Role of synthetic extracellular matrix in development of engineered dental pulp. J Biomater Sci Polym Ed. 1998;9:749–764. doi: 10.1163/156856298x00127. [DOI] [PubMed] [Google Scholar]
- 8.Moshaverinia A, Chen C, Akiyama K, Ansari S, Xu X, Chee WW, et al. Alginate hydrogel as a promising scaffold for dental-derived stem cells: an in vitro study. J Mater Sci Mater Med. 2012;23:3041–3051. doi: 10.1007/s10856-012-4759-3. [DOI] [PubMed] [Google Scholar]
- 9.Moshaverinia A, Ansari S, Chen C, Xu X, Akiyama K, Snead ML, et al. Co-encapsulation of anti-BMP2 monoclonal antibody and mesenchymal stem cells in alginate microspheres for bone tissue engineering. Biomaterials. 2013;34:6572–6579. doi: 10.1016/j.biomaterials.2013.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith AJ, Lesot H. Induction and regulation of crown dentinogenesis: embryonic events as a template for dental tissue repair? Crit Rev Oral Biol Med. 2001;12:425–437. doi: 10.1177/10454411010120050501. [DOI] [PubMed] [Google Scholar]
- 11.Keller LV, Kuchler-Bopp S, Lesot H. Restoring physiological cell heterogeneity in the mesenchyme during tooth engineering. Int J Dev Biol. 2012;56:737–746. doi: 10.1387/ijdb.120076hl. [DOI] [PubMed] [Google Scholar]
- 12.Nait Lechguer A, Couble ML, Labert N, Kuchler-Bopp S, Keller L, Magloire H, et al. Cell differentiation and matrix organization in engineered teeth. J Dent Res. 2011;90:583–589. doi: 10.1177/0022034510391796. [DOI] [PubMed] [Google Scholar]
- 13.Thesleff I, Sharpe P. Signalling networks regulating dental development. Mech Dev. 1997;67:111–123. doi: 10.1016/s0925-4773(97)00115-9. [DOI] [PubMed] [Google Scholar]
- 14.Chai Y, Slavkin HC. Prospects for tooth regeneration in the 21st century: a perspective. Microsc Res Tech. 2003;60:469–479. doi: 10.1002/jemt.10287. [DOI] [PubMed] [Google Scholar]
- 15.Sharpe PT, Young CS. Test-tube teeth. Sci Am. 2005;293:34–41. doi: 10.1038/scientificamerican0805-34. [DOI] [PubMed] [Google Scholar]
- 16.Sreenath T, Thyagarajan T, Hall B, Longenecker G, D'Souza R, Hong S, et al. Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J Biol Chem. 2003;278:24874–2480. doi: 10.1074/jbc.M303908200. [DOI] [PubMed] [Google Scholar]
- 17.Sun Y, Lu Y, Chen L, Gao T, D'Souza R, Feng JQ, et al. DMP1 processing is essential to dentin and jaw formation. J Dent Res. 2011;90:619–624. doi: 10.1177/0022034510397839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maas R, Bei M. The genetic control of early tooth development. Crit Rev Oral Biol Med. 1997;8:4–39. doi: 10.1177/10454411970080010101. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Bei M, Woo I, Satokata I, Maas R. Msx1 controls inductive signaling in mammalian tooth morphogenesis. Development. 1996;122:3035–3044. doi: 10.1242/dev.122.10.3035. [DOI] [PubMed] [Google Scholar]
- 20.Gibson CW, Yuan ZA, Hall B, Longenecker G, Chen E, Thyagarajan T, et al. Amelogenin-deficient mice display an amelogenesis imperfecta phenotype. J Biol Chem. 2001;276:31871–31875. doi: 10.1074/jbc.M104624200. [DOI] [PubMed] [Google Scholar]
- 21.Zeichner-David M, Diekwisch T, Fincham A, Lau E, MacDougall M, Moradian-Oldak J, et al. Control of ameloblast differentiation. Int J Dev Biol. 1995;39:69–92. [PubMed] [Google Scholar]
- 22.Chiego DJ, Jr, Avery JK, Klein RM. Neuroregulation of protein synthesis in odontoblasts of the first molar of the rat after wounding. Cell Tissue Res. 1987;248:119–123. doi: 10.1007/BF01239971. [DOI] [PubMed] [Google Scholar]
- 23.Tziafas D, Smith AJ, Lesot H. Designing new treatment strategies in vital pulp therapy. J Dent. 2000;28:77–92. doi: 10.1016/s0300-5712(99)00047-0. [DOI] [PubMed] [Google Scholar]
- 24.Fitzgerald M, Chiego DJ, Jr, Heys DR. Autoradiographic analysis of odontoblast replacement following pulp exposure in primate teeth. Arch Oral Biol. 1990;35:707–715. doi: 10.1016/0003-9969(90)90093-p. [DOI] [PubMed] [Google Scholar]
- 25.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alge DL, Zhou D, Adams LL, Wyss BK, Shadday MD, Woods EJ, et al. Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model. J Tissue Eng Regen Med. 2010;4:73–81. doi: 10.1002/term.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang W, Walboomers XF, Shi S, Fan M, Jansen JA. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. 2006;12:2813–2823. doi: 10.1089/ten.2006.12.2813. [DOI] [PubMed] [Google Scholar]
- 29.Sakai K, Yamamoto A, Matsubara K, Nakamura S, Naruse M, Yamagata M, et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest. 2012;122:80–90. doi: 10.1172/JCI59251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166–171. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morsczeck C, Gotz W, Schierholz J, Zeilhofer F, Kuhn U, Mohl C, et al. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24:155–165. doi: 10.1016/j.matbio.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 33.Xu X, Chen C, Akiyama K, Chai Y, Le AD, Wang Z, et al. Gingivae Contain Neural-crest- and Mesoderm-derived Mesenchymal Stem Cells. J Dent Res. 2013 Jul 18; doi: 10.1177/0022034513497961. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakai VT, Cordeiro MM, Dong Z, Zhang Z, Zeitlin BD, Nor JE. Tooth slice/scaffold model of dental pulp tissue engineering. Adv Dent Res. 2011;23:325–332. doi: 10.1177/0022034511405325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, et al. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod. 2008;34:962–969. doi: 10.1016/j.joen.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Casagrande L, Demarco FF, Zhang Z, Araujo FB, Shi S, Nor JE. Dentin-derived BMP-2 and odontoblast differentiation. J Dent Res. 2010;89:603–608. doi: 10.1177/0022034510364487. [DOI] [PubMed] [Google Scholar]
- 37.Bento LW, Zhang Z, Imai A, Nor F, Dong Z, Shi S, et al. Endothelial Differentiation of SHED Requires MEK1/ERK Signaling. J Dent Res. 2013;92:51–57. doi: 10.1177/0022034512466263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakai VT, Zhang Z, Dong Z, Neiva KG, Machado MA, Shi S, et al. SHED differentiate into functional odontoblasts and endothelium. J Dent Res. 2010;89:791–796. doi: 10.1177/0022034510368647. [DOI] [PubMed] [Google Scholar]
- 39.Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: a review of current status and a call for action. J Endod. 2007;33:377–390. doi: 10.1016/j.joen.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 40.Wu YN, Shi JN, Huang LZ, Xu YY. Variables affecting electronic root canal measurement. Int Endod J. 1992;25:88–92. doi: 10.1111/j.1365-2591.1992.tb00741.x. [DOI] [PubMed] [Google Scholar]
- 41.Bento LW, Zhang Z, Imai A, Nor F, Dong Z, Shi S, et al. Endothelial differentiation of SHED requires MEK1/ERK signaling. J Dent Res. 2013;92:51–57. doi: 10.1177/0022034512466263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts-Clark DJ, Smith AJ. Angiogenic growth factors in human dentine matrix. Arch Oral Biol. 2000;45:1013–1016. doi: 10.1016/s0003-9969(00)00075-3. [DOI] [PubMed] [Google Scholar]
- 43.Zhang R, Cooper PR, Smith G, Nor JE, Smith AJ. Angiogenic activity of dentin matrix components. J Endod. 2011;37:26–30. doi: 10.1016/j.joen.2010.08.042. [DOI] [PubMed] [Google Scholar]
- 44.Smith AJ, Tobias RS, Plant CG, Browne RM, Lesot H, Ruch JV. In vivo morphogenetic activity of dentine matrix proteins. J Biol Buccale. 1990;18:123–129. [PubMed] [Google Scholar]
- 45.Begue-Kirn C, Smith AJ, Ruch JV, Wozney JM, Purchio A, Hartmann D, et al. Effects of dentin proteins, transforming growth factor beta 1 (TGF beta 1) and bone morphogenetic protein 2 (BMP2) on the differentiation of odontoblast in vitro. Int J Dev Biol. 1992;36:491–503. [PubMed] [Google Scholar]
- 46.Tziafas D, Alvanou A, Panagiotakopoulos N, Smith AJ, Lesot H, Komnenou A, et al. Induction of odontoblast-like cell differentiation in dog dental pulps after in vivo implantation of dentine matrix components. Arch Oral Biol. 1995;40:883–893. doi: 10.1016/0003-9969(95)00069-2. [DOI] [PubMed] [Google Scholar]
- 47.Zhao S, Sloan AJ, Murray PE, Lumley PJ, Smith AJ. Ultrastructural localisation of TGF-beta exposure in dentine by chemical treatment. Histochem J. 2000;32:489–494. doi: 10.1023/a:1004100518245. [DOI] [PubMed] [Google Scholar]
- 48.Farges JC, Romeas A, Melin M, Pin JJ, Lebecque S, Lucchini M, et al. TGF-beta1 induces accumulation of dendritic cells in the odontoblast layer. J Dent Res. 2003;82:652–656. doi: 10.1177/154405910308200816. [DOI] [PubMed] [Google Scholar]
- 49.Baker SM, Sugars RV, Wendel M, Smith AJ, Waddington RJ, Cooper PR, et al. TGF-beta/extracellular matrix interactions in dentin matrix: a role in regulating sequestration and protection of bioactivity. Calcif Tissue Int. 2009;85:66–74. doi: 10.1007/s00223-009-9248-4. [DOI] [PubMed] [Google Scholar]
- 50.Graham L, Cooper PR, Cassidy N, Nor JE, Sloan AJ, Smith AJ. The effect of calcium hydroxide on solubilisation of bio-active dentine matrix components. Biomaterials. 2006;27:2865–2873. doi: 10.1016/j.biomaterials.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 51.Tomson PL, Grover LM, Lumley PJ, Sloan AJ, Smith AJ, Cooper PR. Dissolution of bio-active dentine matrix components by mineral trioxide aggregate. J Dent. 2007;35:636–642. doi: 10.1016/j.jdent.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 52.Laurent P, Camps J, About I. Biodentine(TM) induces TGF-beta1 release from human pulp cells and early dental pulp mineralization. Int Endod J. 2012;45:439–448. doi: 10.1111/j.1365-2591.2011.01995.x. [DOI] [PubMed] [Google Scholar]
- 53.Ferracane JL, Cooper PR, Smith AJ. Dentin Matrix Component Solubilization by Solutions of pH Relevant to Self-etching Dental Adhesives. J Adhes Dent. 2013 doi: 10.3290/j.jad.a29536. [DOI] [PubMed] [Google Scholar]
- 54.Galler KM, Hartgerink JD, Cavender AC, Schmalz G, D'Souza RN. A customized self-assembling peptide hydrogel for dental pulp tissue engineering. Tissue Eng Part A. 2012;18:176–184. doi: 10.1089/ten.tea.2011.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zieris A, Prokoph S, Levental KR, Welzel PB, Grimmer M, Freudenberg U, et al. FGF-2 and VEGF functionalization of starPEG-heparin hydrogels to modulate biomolecular and physical cues of angiogenesis. Biomaterials. 2010;31:7985–7994. doi: 10.1016/j.biomaterials.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 56.Manson J, Dixon D. The influence of solvent processing on polyester bioabsorbable polymers. J Biomater Appl. 2012;26:623–634. doi: 10.1177/0885328210376997. [DOI] [PubMed] [Google Scholar]
- 57.Dobie K, Smith G, Sloan AJ, Smith AJ. Effects of alginate hydrogels and TGF-beta 1 on human dental pulp repair in vitro. Connect Tissue Res. 2002;43:387–390. doi: 10.1080/03008200290000574. [DOI] [PubMed] [Google Scholar]
- 58.Tonnesen HH, Karlsen J. Alginate in drug delivery systems. Drug Dev Ind Pharm. 2002;28:621–630. doi: 10.1081/ddc-120003853. [DOI] [PubMed] [Google Scholar]
- 59.Zimmermann H, Zimmermann D, Reuss R, Feilen PJ, Manz B, Katsen A, et al. Towards a medically approved technology for alginate-based microcapsules allowing long-term immunoisolated transplantation. J Mater Sci Mater Med. 2005;16:491–501. doi: 10.1007/s10856-005-0523-2. [DOI] [PubMed] [Google Scholar]
- 60.Jeong SI, Krebs MD, Bonino CA, Khan SA, Alsberg E. Electrospun alginate nanofibers with controlled cell adhesion for tissue engineering. Macromol Biosci. 2010;10:934–943. doi: 10.1002/mabi.201000046. [DOI] [PubMed] [Google Scholar]
- 61.Palma Santana B, Nedel F, Piva E, Varella de Carvalho R, Fernando Demarco F, Lenin Villarreal Carreno N. Preparation, modification, and characterization of alginate hydrogel with nano-/microfibers: a new perspective for tissue engineering. Biomed Res Int. 2013;2013:307602. doi: 10.1155/2013/307602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kolambkar YM, Dupont KM, Boerckel JD, Huebsch N, Mooney DJ, Hutmacher DW, et al. An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects. Biomaterials. 2011;32:65–74. doi: 10.1016/j.biomaterials.2010.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chung HJ, Kim HK, Yoon JJ, Park TG. Heparin immobilized porous PLGA microspheres for angiogenic growth factor delivery. Pharm Res. 2006;23:1835–1841. doi: 10.1007/s11095-006-9039-9. [DOI] [PubMed] [Google Scholar]
- 64.Cavalcanti BN, Zeitlin BD, Nor JE. A hydrogel scaffold that maintains viability and supports differentiation of dental pulp stem cells. Dent Mater. 2013;29:97–102. doi: 10.1016/j.dental.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao Y, Gao S, Zhao S, Li Y, Cheng L, Li J, et al. Synthesis and characterization of disulfide-crosslinked alginate hydrogel scaffolds. Mater Science Engin. 2012;32:2153–2162. [Google Scholar]
- 66.Zieris A, Chwalek K, Prokoph S, Levental KR, Welzel PB, Freudenberg U, et al. Dual independent delivery of pro-angiogenic growth factors from starPEG-heparin hydrogels. J Control Release. 2011;156:28–36. doi: 10.1016/j.jconrel.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 67.Lee KW, Yoon JJ, Lee JH, Kim SY, Jung HJ, Kim SJ, et al. Sustained release of vascular endothelial growth factor from calcium-induced alginate hydrogels reinforced by heparin and chitosan. Transplant Proc. 2004;36:2464–2465. doi: 10.1016/j.transproceed.2004.08.078. [DOI] [PubMed] [Google Scholar]
- 68.Paul R, Zhang ZG, Eliceiri BP, Jiang Q, Boccia AD, Zhang RL, Chopp M, Cheresh DA. Src deficiency or blockade of Src activity in mice provides cerebral protection following stroke. Nat Med. 2001;7:222–227. doi: 10.1038/84675. [DOI] [PubMed] [Google Scholar]
- 69.Matricardi P, Pontoriero M, Coviello T, Casadei MA, Alhaique F. In situ cross-linkable novel alginate-dextran methacrylate IPN hydrogels for biomedical applications: mechanical characterization and drug delivery properties. Biomacromolecules. 2008;9:2014–2020. doi: 10.1021/bm800252c. [DOI] [PubMed] [Google Scholar]
- 70.McLennan G, Johnson MS, Stookey KR, Zhang Z, Fife WK. Kinetics of release of heparin from alginate hydrogel. J Vasc Interv Radiol. 2000;11:1087–1094. doi: 10.1016/s1051-0443(07)61344-x. [DOI] [PubMed] [Google Scholar]
- 71.Freeman I, Cohen S. The influence of the sequential delivery of angiogenic factors from affinity-binding alginate scaffolds on vascularization. Biomaterials. 2009;30:2122–2131. doi: 10.1016/j.biomaterials.2008.12.057. [DOI] [PubMed] [Google Scholar]
- 72.Freeman I, Kedem A, Cohen S. The effect of sulfation of alginate hydrogels on the specific binding and controlled release of heparin-binding proteins. Biomaterials. 2008;29:3260–3268. doi: 10.1016/j.biomaterials.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 73.Jeon O, Powell C, Solorio LD, Krebs MD, Alsberg E. Affinity-based growth factor delivery using biodegradable, photocrosslinked heparin-alginate hydrogels. J Control Release. 2011;154:258–266. doi: 10.1016/j.jconrel.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rondeau E, Cooper-White JJ. Biopolymer microparticle and nanoparticle formation within a microfluidic device. Langmuir. 2008;24:6937–6945. doi: 10.1021/la703339u. [DOI] [PubMed] [Google Scholar]
- 75.Buwalda SJ, Perez LB, Teixeira S, Calucci L, Forte C, Feijen J, et al. Self-assembly and photo-cross-linking of eight-armed PEG-PTMC star block copolymers. Biomacromolecules. 2011;12:2746–2754. doi: 10.1021/bm200515h. [DOI] [PubMed] [Google Scholar]
- 76.Khanna O, Larson JC, Moya ML, Opara EC, Brey EM. Generation of alginate microspheres for biomedical applications. J Vis Exp. 2012 doi: 10.3791/3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khanna O, Moya ML, Opara EC, Brey EM. Synthesis of multilayered alginate microcapsules for the sustained release of fibroblast growth factor-1. J Biomed Mater Res A. 2010;95:632–640. doi: 10.1002/jbm.a.32883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tawfik H, Abu-Seida AM, Hashem AA, Nagy MM. Regenerative potential following revascularization of immature permanent teeth with necrotic pulps. Int Endod J. 2013;46:910–922. doi: 10.1111/iej.12079. [DOI] [PubMed] [Google Scholar]
- 79.Wang X, Thibodeau B, Trope M, Lin LM, Huang GT. Histologic characterization of regenerated tissues in canal space after the revitalization/revascularization procedure of immature dog teeth with apical periodontitis. J Endod. 2010;36:56–63. doi: 10.1016/j.joen.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 80.Rosa V, Zhang Z, Grande RH, Nör JE. Dental pulp tissue engineering in full-length human root canals. J Dent Res. 2013;92:970–975. doi: 10.1177/0022034513505772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang YH, Rutherford B, Upholt WB, Mina M. Effects of BMP-7 on mouse tooth mesenchyme and chick mandibular mesenchyme. Dev Dyn. 1999;216:320–335. doi: 10.1002/(SICI)1097-0177(199912)216:4/5<320::AID-DVDY2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 82.Rutherford RB, Wahle J, Tucker M, Rueger D, Charette M. Induction of reparative dentine formation in monkeys by recombinant human osteogenic protein-1. Arch Oral Biol. 1993;38:571–576. doi: 10.1016/0003-9969(93)90121-2. [DOI] [PubMed] [Google Scholar]
- 83.Jepsen S, Albers HK, Fleiner B, Tucker M, Rueger D. Recombinant human osteogenic protein-1 induces dentin formation: an experimental study in miniature swine. J Endod. 1997;23:378–382. doi: 10.1016/S0099-2399(97)80187-2. [DOI] [PubMed] [Google Scholar]
- 84.Nakashima M. Induction of dentin formation on canine amputated pulp by recombinant human bone morphogenetic proteins (BMP)-2 and-4. J Dent Res. 1994;73:1515–1522. doi: 10.1177/00220345940730090601. [DOI] [PubMed] [Google Scholar]
- 85.Nakashima M. Induction of dentine in amputated pulp of dogs by recombinant human bone morphogenetic proteins-2 and -4 with collagen matrix. Arch Oral Biol. 1994;39:1085–1089. doi: 10.1016/0003-9969(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 86.Yang X, van der Kraan PM, Bian Z, Fan M, Walboomers XF, Jansen JA. Mineralized tissue formation by BMP2-transfected pulp stem cells. J Dent Res. 2009;88:1020–1025. doi: 10.1177/0022034509346258. [DOI] [PubMed] [Google Scholar]
- 87.Suzuki T, Lee CH, Chen M, Zhao W, Fu SY, Qi JJ, et al. Induced migration of dental pulp stem cells for in vivo pulp regeneration. J Dent Res. 2011;90:1013–1018. doi: 10.1177/0022034511408426. [DOI] [PubMed] [Google Scholar]
- 88.Decup F, Six N, Palmier B, Buch D, Lasfargues JJ, Salih E, et al. Bone sialoprotein-induced reparative dentinogenesis in the pulp of rat's molar. Clin Oral Investig. 2000;4:110–119. doi: 10.1007/s007840050126. [DOI] [PubMed] [Google Scholar]
- 89.Six N, Decup F, Lasfargues JJ, Salih E, Goldberg M. Osteogenic proteins (bone sialoprotein and bone morphogenetic protein-7) and dental pulp mineralization. J Mater Sci Mater Med. 2002;13:225–232. doi: 10.1023/a:1013846516693. [DOI] [PubMed] [Google Scholar]
- 90.D'Souza RN, Aberg T, Gaikwad J, Cavender A, Owen M, Karsenty G, et al. Cbfa1 is required for epithelial-mesenchymal interactions regulating tooth development in mice. Development. 1999;126:2911–2920. doi: 10.1242/dev.126.13.2911. [DOI] [PubMed] [Google Scholar]
- 91.Gaikwad JS, Hoffmann M, Cavender A, Bronckers AL, D'Souza RN. Molecular insights into the lineage-specific determination of odontoblasts: the role of Cbfa1. Adv Dent Res. 2001;15:19–24. doi: 10.1177/08959374010150010501. [DOI] [PubMed] [Google Scholar]
- 92.George A, Silberstein R, Veis A. In situ hybridization shows Dmp1 (AG1) to be a developmentally regulated dentin-specific protein produced by mature odontoblasts. Connect Tissue Res. 1995;33:67–72. doi: 10.3109/03008209509016984. [DOI] [PubMed] [Google Scholar]
- 93.Siyam A, Wang S, Qin C, Mues G, Stevens R, D'Souza RN, et al. Nuclear localization of DMP1 proteins suggests a role in intracellular signaling. Biochem Biophys Res Commun. 2012;424:641–646. doi: 10.1016/j.bbrc.2012.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Abd-Elmeguid A, Yu DC, Kline LW, Moqbel R, Vliagoftis H. Dentin matrix protein-1 activates dental pulp fibroblasts. J Endod. 2012;38:75–80. doi: 10.1016/j.joen.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 95.Prescott RS, Alsanea R, Fayad MI, Johnson BR, Wenckus CS, Hao J, et al. In vivo generation of dental pulp-like tissue by using dental pulp stem cells, a collagen scaffold, and dentin matrix protein 1 after subcutaneous transplantation in mice. J Endod. 2008;34:421–426. doi: 10.1016/j.joen.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.MacDougall M, Simmons D, Luan X, Nydegger J, Feng J, Gu TT. Dentin phosphoprotein and dentin sialoprotein are cleavage products expressed from a single transcript coded by a gene on human chromosome 4. Dentin phosphoprotein DNA sequence determination. J Biol Chem. 1997;272:835–842. doi: 10.1074/jbc.272.2.835. [DOI] [PubMed] [Google Scholar]
- 97.Gibson MP, Zhu Q, Wang S, Liu Q, Liu Y, Wang X, et al. The rescue of dentin matrix protein 1 (DMP1)-deficient tooth defects by the transgenic expression of dentin sialophosphoprotein (DSPP) indicates that DSPP is a downstream effector molecule of DMP1 in dentinogenesis. J Biol Chem. 2013;288:7204–7214. doi: 10.1074/jbc.M112.445775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ritchie HH, Berry JE, Somerman MJ, Hanks CT, Bronckers AL, Hotton D, et al. Dentin sialoprotein (DSP) transcripts: developmentally-sustained expression in odontoblasts and transient expression in pre-ameloblasts. Eur J Oral Sci. 1997;105:405–413. doi: 10.1111/j.1600-0722.1997.tb02137.x. [DOI] [PubMed] [Google Scholar]
- 99.Butler WT, Ritchie H. The nature and functional significance of dentin extracellular matrix proteins. Int J Dev Biol. 1995;39:169–179. [PubMed] [Google Scholar]
- 100.Lee SY, Kim SY, Park SH, Kim JJ, Jang JH, Kim EC. Effects of recombinant dentin sialoprotein in dental pulp cells. J Dent Res. 2012;91:407–412. doi: 10.1177/0022034511436113. [DOI] [PubMed] [Google Scholar]
- 101.Kim YS, Min KS, Jeong DH, Jang JH, Kim HW, Kim EC. Effects of fibroblast growth factor-2 on the expression and regulation of chemokines in human dental pulp cells. J Endod. 2010;36:1824–1830. doi: 10.1016/j.joen.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 102.Ishimatsu H, Kitamura C, Morotomi T, Tabata Y, Nishihara T, Chen KK, et al. Formation of dentinal bridge on surface of regenerated dental pulp in dentin defects by controlled release of fibroblast growth factor-2 from gelatin hydrogels. J Endod. 2009;35:858–865. doi: 10.1016/j.joen.2009.03.049. [DOI] [PubMed] [Google Scholar]
- 103.Shao MY, Cheng R, Wang FM, Yang H, Cheng L, Hu T. beta-Catenin and Rho GTPases as downstream targets of TGF-beta1 during pulp repair. Cell Biol Int. 2011;35:105–109. doi: 10.1042/CBI20100114. [DOI] [PubMed] [Google Scholar]
- 104.Scheller EL, Chang J, Wang CY. Wnt/beta-catenin inhibits dental pulp stem cell differentiation. J Dent Res. 2008;87:126–130. doi: 10.1177/154405910808700206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Erlebacher A, Filvaroff EH, Ye JQ, Derynck R. Osteoblastic responses to TGF-beta during bone remodeling. Mol Biol Cell. 1998;9:1903–1918. doi: 10.1091/mbc.9.7.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu J, Jin T, Chang S, Ritchie HH, Smith AJ, Clarkson BH. Matrix and TGF-beta-related gene expression during human dental pulp stem cell (DPSC) mineralization. In Vitro Cell Dev Biol Anim. 2007;43:120–128. doi: 10.1007/s11626-007-9022-8. [DOI] [PubMed] [Google Scholar]
- 107.Lu Y, Li Y, Cavender AC, Wang S, Mansukhani A, D'Souza RN. Molecular studies on the roles of Runx2 and Twist1 in regulating FGF signaling. Dev Dyn. 2012;241:1708–1715. doi: 10.1002/dvdy.23858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li Y, Lu Y, Maciejewska I, Galler KM, Cavender A, D'Souza RN. TWIST1 promotes the odontoblast-like differentiation of dental stem cells. Adv Dent Res. 2011;23:280–224. doi: 10.1177/0022034511405387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ferrara N. VEGF: an update on biological and therapeutic aspects. Curr Opin Biotechnol. 2000;11:617–624. doi: 10.1016/s0958-1669(00)00153-1. [DOI] [PubMed] [Google Scholar]