Abstract
The purpose of this study was to determine the effects of dietary protein intake and eating frequency on perceived appetite, satiety, and hormonal responses in overweight/obese men. Thirteen men (age 51 ± 4 years; BMI 31.3 ± 0.8 kg/m2) consumed eucaloric diets containing normal protein (79 ± 2 g protein/day; 14% of energy intake as protein) or higher protein (138 ± 3 g protein/day; 25% of energy intake as protein) equally divided among three eating occasions (3-EO; every 4 h) or six eating occasions (6-EO; every 2 h) on four separate days in randomized order. Hunger, fullness, plasma glucose, and hormonal responses were assessed throughout 11 h. No protein × eating frequency interactions were observed for any of the outcomes. Independent of eating frequency, higher protein led to greater daily fullness (P < 0.05) and peptide YY (PYY) concentrations (P < 0.05). In contrast, higher protein led to greater daily ghrelin concentrations (P < 0.05) vs. normal protein. Protein quantity did not influence daily hunger, glucose, or insulin concentrations. Independent of dietary protein, 6-EO led to lower daily fullness (P < 0.05) and PYY concentrations (P < 0.05). The 6-EO also led to lower glucose (P < 0.05) and insulin concentrations (P < 0.05) vs. 3-EO. Although the hunger-related perceived sensations and hormonal responses were conflicting, the fullness-related responses were consistently greater with higher protein intake but lower with increased eating frequency. Collectively, these data suggest that higher protein intake promotes satiety and challenge the concept that increasing the number of eating occasions enhances satiety in overweight and obese men.
INTRODUCTION
Overweight and obesity continues to be a major medical and public health concern affecting the lives of over 144 million (66%) US adults (1). Based on prevalence trends from the National Health and Nutrition Examination Studies, it is estimated that within 30 years, nearly all American adults will be overweight or obese if successful, long-term prevention and/or treatment strategies to combat this epidemic are not implemented (2). This public health concern has prompted numerous diets proposing “optimal weight loss” mediated by decreased appetite and food intake. Two of the more popular dietary approaches promoting better weight management include higher protein intake and greater eating frequency (3).
Accumulating evidence suggests that diets containing higher dietary protein (ranging from 1.1 to 1.6 g protein/kg/day) lead to greater reductions in total energy intake, body weight, and fat mass while preserving lean body mass, compared to diets containing 0.8 g/kg/day (the recommended dietary allowance; RDA) (4-8). One key factor in the efficacy of these diets involves the improvement in appetite control. Single, higher protein meals generally reduce postprandial hunger (9) and increase postprandial satiety (9-12). They do so by reportedly altering hormones associated with appetite regulation such as ghrelin (9) and peptide YY (PYY) (13). Limited data exist as to whether these alterations continue when individuals consume higher protein meals throughout the course of an entire day. Accordingly, the primary aim of this study was to explore the effects of higher protein intake on perceived appetite and hormonal responses throughout the day in overweight and obese men.
Although there is strong scientific support for the incorporation of additional dietary protein for improved appetite control and weight management, the influence of eating frequency on these outcomes is highly conflicting. The majority of studies have focused on whether greater eating frequency leads to increased daily energy expenditure (see Review by Bellisle et al. (14)) yet most have found little, if any, impact on these outcomes (14-16). Researchers are now beginning to focus their attention on the other side of the energy balance equation by examining the impact of eating frequency on appetite control and food intake. In some (15,17) but not all (16,18,19) studies, greater eating frequency has led to reduced hunger, increased satiety, and decreased food intake (15-19). The discrepant findings may stem from the wide range of eating frequencies that have been arbitrarily defined as frequent eating when consisting of 4-17 eating occasions/day and infrequent eating when consisting of one to three meals/day.
Although the overall message in the mainstream media proposes an “eat six times a day” strategy for better appetite control and food intake regulation, very little, if any, scientific evidence exists to evaluate the efficacy of this approach. Thus, a second aim of this study was to compare the effects of eating three times/day, which has been the standard dietary pattern in the past, vs. six times/day on these outcomes. This study design also permitted investigation of a possible synergistic effect of higher protein intake and greater eating frequency on perceived appetite and hormonal responses throughout the day.
METHODS AND PROCEDURES
Subjects
Potential participants were recruited through newspaper advertisements. Eligibility was based on the following criteria: (i) men age ≥21 years; (ii) BMI 25.0-34.9 kg/m2; (iii) percent body fat >25% assessed through skinfold measurements; (iv) not dieting and no weight loss or gain (≥4.5 kg) within the past 6 months; (v) nonsmoking; (vi) nondiabetic; (vii) clinically normal blood profiles (normal liver and kidney function; fasting blood glucose <110 mg/dl); (viii) consistent habitual activity patterns over the past 3 months; and (ix) habitual dietary pattern of consuming three meals/day for the past 3 months. Twenty-five men were screened; 21 gave informed consent; 16 began and 13 completed all study procedures. Reasons for the three dropouts were military duty, relocation, and nonstudy compliance. Participants signed an informed consent form approved by the Purdue University Biomedical Institutional Review Board and received monetary compensation for completing all study procedures. Clinical testing occurred between January 2008 and July 2008. Subject characteristics for the completed participants are displayed in Table 1.
Table 1. Subject characteristics of 13 overweight and obese men.
| Subject characteristics | Mean ± s.e.m. |
|---|---|
| Age (year) | 51 ± 4 |
| Height (cm) | 178 ± 2 |
| Weight (kg) | 99.6 ± 2.4 |
| BMI (kg/m2) | 31.3 ± 0.8 |
| Body fat (%) | 31 ± 3 |
| Fasting glucose (mg/dl) | 97 ± 1 |
| Fasting insulin (pmol/l) | 75 ± 16 |
| Habitual meal pattern (# meals/day) | 2.9 ± 0.3 |
Data expressed as mean ± s.e.m.
Experimental design
This study incorporated a randomized, crossover design consisting of four 11-h trials. On separate days, the participants consumed eucaloric diets containing either normal protein (79 ± 2 g protein/day; 0.8 g/kg/day) or higher protein (138 ± 3 g protein/day; 1.4 g/kg/day) equally divided among three eating occasions (3-EO; provided every 4 h) or six eating occasions (6-EO; provided every 2 h) in a randomized order. Pre- and postprandial hunger, satiety, plasma glucose, and hormonal responses (plasma insulin, ghrelin, and PYY) were assessed throughout each 11-h trial.
Specific testing day procedures
On the evening prior to each trial, participants were provided with a standardized normal protein dinner to be consumed at home between 5 and 7 pm. The participants then fasted until their arrival at the laboratory between 7 and 8 am the following morning. Upon arrival, the participants were placed in a supine position on a bed, and a catheter was inserted in an antecubetal vein of the nondominant arm and kept patent for the remainder of the testing period by saline drip. For the next 30 min, the participants acclimated to the room and were familiarized with the testing day procedures. At time 0, a baseline (fasting) blood sample was taken, questionnaires were completed, and the first eating occasion was provided to the participants. Over the remaining 11 h, blood sampling and questionnaires were repeated every 20 min. The remaining eating occasions were provided to the participants at set times and in specific quantities according to the treatment random ization. For each eating occasion, the participants were required to consume all foods and water provided to them within 15 min. No additional food or drink was provided to the participants. During the testing period, participants remained in a semi-supine position and were permitted to watch television, read, or use computers. At the end of the 11 h, the catheter was removed and the participants were permitted to leave the laboratory. There were 1-2 weeks between each of the four trials.
Eating occasions
The characteristics of the diets are shown in Table 2. The participants were fed according to their daily energy needs. Due to the reduced activity of the volunteers during the 11-h testing days, daily energy need was estimated as resting energy expenditure × 1.0 activity factor using the Harris Benedict equation for men (20). For the 3-EO pattern, energy intake was equally divided among all 3-EO provided every 4 h with 280 ml of water provided with each eating occasion. Thus, each meal in the 3-EO pattern contained one-third of the participant’s daily energy needs. During the 6-EO pattern, energy intake was equally divided among all 6-EO provided every 2 h with 140 ml of water provided with each eating occasion. Thus, each meal in the 6-EO pattern contained one-sixth of the participant’s daily energy needs. Regardless of eating frequency, the normal protein diet contained 14% protein (~0.8 g protein/kg/day), 60% carbohydrate, and 26% fat; the higher protein diet contained 25% protein (~1.4 g protein/kg/day), 49% carbohydrate, and 26% fat. The additional dietary protein in the higher protein diet was primarily from lean pork and egg products (25% and 15% of total protein intake, respectively), whereas the normal protein diet was void of all striated tissue and eggs.
Table 2. Dietary characteristics of the test day diets.
| Normal protein testing day |
Higher protein testing day |
|||||||
|---|---|---|---|---|---|---|---|---|
| 3 Eating occasions (3-EO) |
6 Eating occasions (6-EO) |
3 Eating occasions (3-EO) |
6 Eating occasions (6-EO) |
|||||
| Dietary characteristics | Average eating occasion |
Total (sum) | Average eating occasion |
Total (sum) | Average eating occasion |
Total (sum) | Average eating occasion |
Total (sum) |
| Energy content (kcal) |
710 ± 30a | 2,130 ± 80b | 352 ± 15c | 2,110 ± 90b | 728 ± 28a | 2,180 ± 80b | 360 ± 15b | 2,160 ± 90b |
| PRO (g) | 26 ± 1a | 79 ± 2b | 13 ± 0c | 78 ± 2b | 46 ± 1d | 139 ± 4e | 23 ± 1a | 137 ± 4e |
| CHO (g) | 109 ± 6a | 331 ± 15b | 55 ± 3c | 327 ± 15b | 91 ± 4d | 272 ± 13e | 45 ± 2f | 270 ± 13e |
| Fat (g) | 21 ± 1a | 63 ± 2b | 10 ± 0c | 62 ± 3b | 21 ± 1a | 64 ± 3b | 11 ± 1c | 63 ± 3b |
Data presented as mean ± s.e.m. Different letters denote significance across rows; significance P < 0.05; repeated measures ANOVA within and between treatments. Eating occasion columns include the average for each of the eating occasions consumed during the testing day. Total (sum) columns include the sum of all of the eating occasions consumed during the testing day.
CHO, carbohydrate; PRO, protein.
Appetite questionnaires
Questionnaires assessing perceived hunger and satiety (fullness) were completed every 20 min throughout each trial using a 100-mm visual analog scale presented in paper form. The visual analog scale had end anchors ranging from “not at all” to “extremely” and included validated appetite questions such as, “How strong is your feeling of …right now?”(21).
Hhormonal responses
Blood samples were drawn into tubes containing EDTA (ethylenediaminetetraacetic acid) every 20 min throughout each of the 11-h trials. Samples were centrifuged at −4 °C for 15 min; the plasma was separated and stored in microcentrifuge tubes at −80 °C for future analyses. Protease inhibitors (Pefabloc SC, Roche Applied Science, Indianapolis, IN) were added to reduce protein degradation. Plasma active ghrelin and total PYY were measured with 2-plex Milliplex assay kits and Luminex technologies (Millipore/LINCO Research, St Charles, MO).
Data and statistical analysis
To assess perceived appetite (hunger), satiety (fullness), glucose, and hormonal responses, total 11-h area under the curve (AUC) was calculated from the fasting (baseline) time point and the 31 postprandial time points for each outcome. We further divided the testing day into three segments: period I: time 0-240 min; period II: time 240-480 min; and period III: time 480-620 min and calculated individual AUCs for each outcome with these periods. With all AUC measurements, the trapezoidal rule was utilized (22). Additionally, pre- and postmeal appetitive and hormonal peak responses were also identified for each eating occasion. A two-factor, repeated measures analysis of variance was utilized to identify main effects of dietary protein, eating frequency, and interactions on all study outcomes. Data are expressed as mean ± s.e.m. P < 0.05 was considered statistically significant. The sample size (n = 13) provided >80% observed power to detect main effect differences among dietary protein and eating frequency treatments perceived hunger, satiety, and PYY concentrations. Analyses were conducted using the Statistical Package for the Social Sciences (SPSS; version 16.0; SPSS, Chicago, IL).
RESULTS
As shown in Figures 1-3, the line graphs illustrate the appetitive and hormonal responses completed every 20 min throughout the 11-h testing day, whereas the bar graphs depict the AUC analyses for periods I, II, and III of each testing day.
Figure 1.
Perceived appetite and satiety throughout the 11-h testing days following the dietary protein and eating frequency treatments. *Main effects; P < 0.05. Period I: time 0-240 min; period II: time 240-480 min; period III: time 480-620 min.
Figure 3.
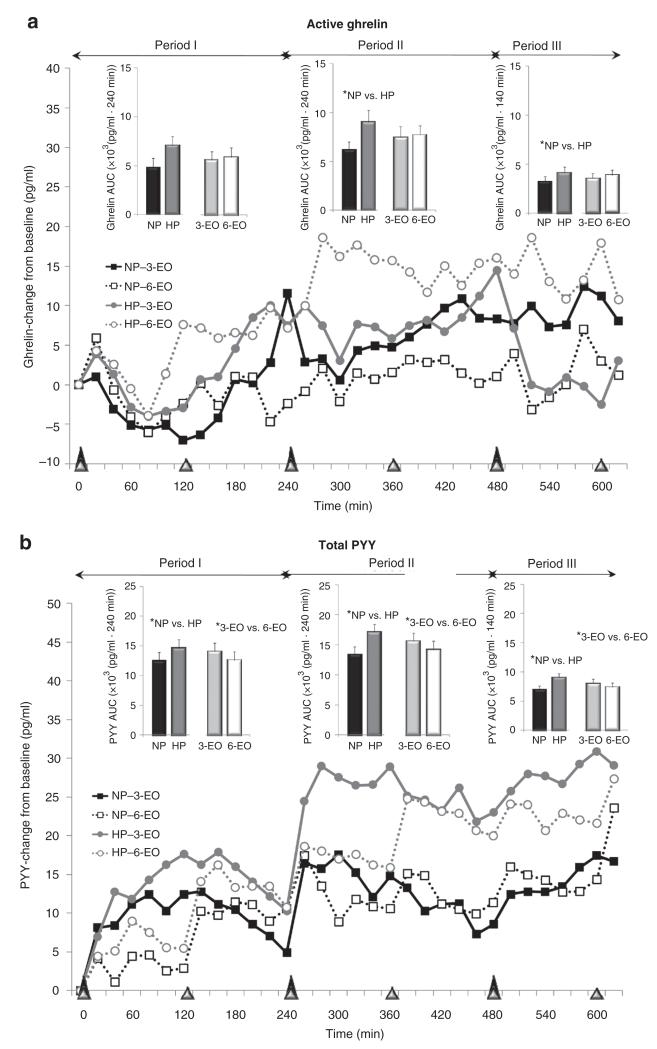
Plasma active ghrelin and total PYY responses throughout the 11-h testing days following the dietary protein and meal frequency treatments. *Main effects; P < 0.05. Period I: time 0-240 min; period II: time 240-480 min; period III: time 480-620 min.
Perceived appetite
Perceived hunger gradually declined throughout each of the 11-h testing days and exhibited eating-occasion oscillations with larger fluctuations observed following the 3-EO vs. 6-EO (Figure 1a). No protein × eating frequency interactions or main effects of dietary protein and eating frequency were observed for total (11 h) perceived hunger AUC (Table 3). When examining the data according to specific time periods across the day (i.e., periods I, II, III), no main effects or interactions for perceived appetite were detected (Figure 1a). Although the overall hunger responses were not different between protein and eating frequency treatments, the average premeal hunger peak prior to each eating occasion was greater in 3-EO (54 ± 6 mm) vs. 6-EO (47 ± 6 mm; P < 0.01) with no difference with respect to dietary protein.
Table 3. Total area under the curve (AUC) assessments for the appetitive and hormonal responses following each study treatment in 13 overweight and obese men.
| Outcomes | NP; 3-EO | NP; 6-EO | HP; 3-EO | HP; 6-EO |
|---|---|---|---|---|
| Perceived sensations | ||||
| Hunger (×103 (mm·620 min)) | 15.3 ± 3.6 | 17.8 ± 7.1 | 13.5 ± 2.7 | 16.2 ± 3.9 |
| Fullness (×103 (mm·620 min))ab | 42.9 ± 3.7 | 38.6 ± 3.6 | 45.0 ± 3.0 | 41.0 ± 3.5 |
| Plasma glucose (×103 (mg/dl·620 min))b | 68.4 ± 27.9 | 67.6 ± 34.3 | 69.1 ± 26.4 | 64.8 ± 23.2 |
| Hormonal responses | ||||
| Insulin (×103 (pmol/l·620 min))b | 248 ± 47 | 205 ± 38 | 250 ± 56c | 191 ± 36 |
| Ghrelin (×103 (pg/ml·620 min))a | 13.3 ± 2.5 | 14.6 ± 3.2 | 19.9 ± 3.0 | 20.3 ± 3.1 |
| PYY (×103 (pg/ml·620 min))ab | 35.1 ± 3.3 | 32.5 ± 3.8 | 42.6 ± 3.6 | 38.5 ± 3.5 |
Data expressed as mean ± s.e.m.
NP, normal protein; PYY, peptide YY; 3-EO, 3 eating occasions; 6-EO, 6 eating occasions.
Main effect of protein; P < 0.05.
Main effect of eating occasion; P < 0.05.
Perceived satiety
Perceived fullness gradually increased throughout each of the 11-h testing days and exhibited eating-occasion oscillations with larger fluctuations observed following the 3-EO vs. 6-EO (Figure 1b). No protein × eating frequency interactions were observed for total (11 h) fullness AUC. Subsequently, main effects of dietary protein (P < 0.05) and eating frequency (P < 0.05) were observed for total (11 h) fullness AUC (Table 3). With both eating frequency patterns combined, the higher protein diet led to a 6% increase in 11-h fullness AUC vs. normal protein (P < 0.05) (Table 3). Alternately, when combining the higher protein and normal protein diets, the 6-EO pattern led to a 10% reduction in 11-h fullness AUC vs. 3-EO pattern (P < 0.05) (Table 3). Similar main effects and post hoc comparison were also observed when examining the data according to specific time periods across the day (i.e., periods I, II, III) (Figure 1b). Additionally, the average postmeal fullness peak was greater in higher protein (87 ± 3.5 mm) vs. normal protein (83 ± 4 mm; P < 0.01) but smaller in 6-EO (79 ± 5 mm) vs. 3-EO (90 ± 3 mm; P < 0.01).
Glucose
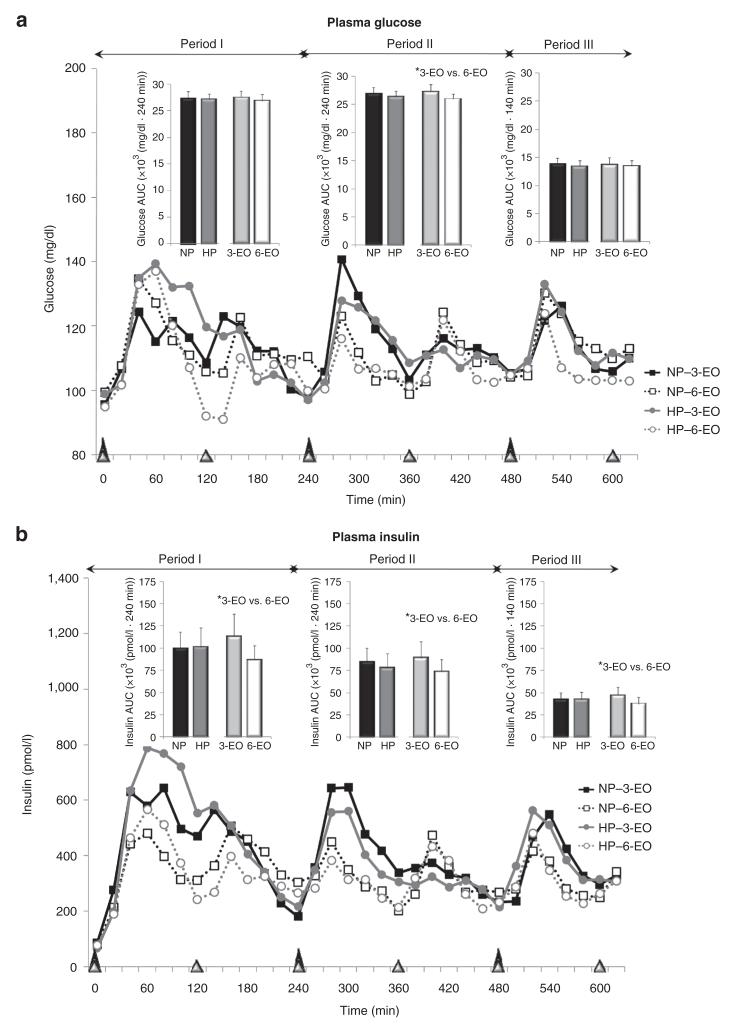
Eating-relating oscillations in plasma glucose concentrations were observed following each eating occasion with larger fluctuations observed following the 3-EO vs. 6-EO (Figure 2a). No protein × eating frequency interactions were observed for total (11 h) glucose AUC. With respect to main effects, although no difference in total (11 h) glucose AUC was observed between normal protein vs. higher protein, a main effect of eating frequency (P < 0.05) was identified (Table 3). Independent of dietary protein, 6-EO led to a 4% reduction in 11-h glucose AUC vs. 3-EO (Table 3). When separating the 11-h testing day into periods, the main effect of eating frequency was only observed during the middle segment (period II) (Figure 2a).
Figure 2.
Plasma glucose and insulin responses throughout the 11-h testing days following the dietary protein and meal frequency treatments. *Main effects; P < 0.05. Period I: time 0-240 min; period II: time 240-480 min; period III: time 480-620 min.
Insulin
Eating-relating oscillations in plasma insulin concentrations were observed following each eating occasion, with larger fluctuations observed following the 3-EO vs. 6-EO (Figure 2b). No protein × eating frequency interactions were observed for total (11 h) insulin AUC. Although no difference in total (11 h) insulin AUC was observed between normal protein vs. higher protein, a main effect of eating frequency (P < 0.05) was identified (Table 3). Independent of dietary protein, 6-EO led to 20% reduction in 11-h insulin vs. 3-EO (Table 3). Main effect of eating frequency was observed during periods I, II, and III (all, P < 0.05) (Figure 2b). There was no effect of dietary protein within any of the time periods (Figure 2b).
Active ghrelin
Eating-relating oscillations in plasma ghrelin concentrations were observed within 3-EO but not 6-EO (Figure 3a). No protein × eating frequency interactions were observed for total 11-h ghrelin AUC. Although no difference in total (11 h) ghrelin AUC was observed between 3-EO vs. 6-EO, a main effect of dietary protein (P < 0.05) was identified (Table 3). With both eating frequencies combined, higher protein diet led to 44% increase in 11-h ghrelin AUC vs. normal protein diet (P < 0.05) (Table 3). When separating the 11-h testing day into periods, the main effect of dietary protein was observed during periods II-III (P < 0.05) (Figure 3a). The average premeal ghrelin peak was greater in higher protein (35.2 ± 4.4 pg/ml) vs. normal protein (25.4 ± 4.0 pg/ml; P < 0.05) treatments with no differences occurring between eating frequency patterns.
Total PYY
Gradual eating-relating oscillations in plasma PYY concentrations were observed following each eating occasion with larger fluctuations observed following the 3-EO vs. 6-EO (Figure 3b). No protein × eating frequency interactions were observed for total (11 h) PYY AUC. Main effects of dietary protein (P < 0.05) and eating frequency (P < 0.05) were observed for total (11 h) PYY AUC (Table 3). With both eating frequency patterns combined, higher protein diet led to a 20% increase in 11-h PYY AUC vs. normal protein (P < 0.05) (Table 3). Alternately, when combining the higher protein and normal protein diets, 6-EO led to a 9% reduction in 11-h PYY AUC vs. 3-EO (P < 0.05) (Table 3). Similar main effects in PYY were observed when examined according to specific time periods across the day (i.e., periods I, II, III) (Figure 3b). The average postmeal PYY peak was greater in higher protein (60.8 ± 6.1 pg/ml) vs. normal protein (50.8 ± 6 pg/ml; P < 0.001) but not different between eating frequency treatments.
DISCUSSION
We sought to provide scientific evidence regarding the effects of higher protein intake and greater eating frequency on perceived appetite and hormonal responses in overweight and obese men. Dietary protein and eating frequency were shown to have no effect on perceived hunger and led to inconsistent and/or conflicting glucose, insulin, and ghrelin responses. However, both of these dietary factors significantly altered satiety. Whereas higher protein intake increased daily perceived fullness, frequent eating led to reductions in daily perceived fullness. These findings were further supported by the elevated PYY concentrations observed with higher vs. normal protein intake and by the reduced PYY concentrations observed with frequent vs. infrequent eating. These data strengthen the current literature indicating that increased dietary protein leads to increased satiety, refute the long-standing assumption that increased eating frequency has beneficial effects, and suggest that overweight and obese men might achieve better appetite control by consuming three higher protein meals per day.
There is convincing evidence that higher protein consumption at single meals and during longer-term dietary interventions leads to increased perceived satiety (6). Many of the studies confirming these findings focused on single meals with large amounts and proportions of dietary protein (i.e., 80-100% of the meal) (5,6). Several recent tightly-controlled respiratory chamber studies have tracked appetitive sensations while administering higher protein diets (~30% of intake as dietary protein) over an entire day of eating. In two specific studies, participants consumed breakfast, lunch, and dinner meals containing either 10% or 30% of total energy intake as dietary protein. The 30% protein diets led to reduced overall hunger and greater overall satiety compared to the 10% protein diets (P < 0.05) (23,24). Our current study further supports the satiating properties of dietary protein when consumed at this quantity but shows very little, if any, influence on hunger.
One potential mechanism contributing to the reported satiety-enhancing and hunger-suppressing properties of dietary protein may involve hormonal responses to specific macronutrients (9). Several researchers report that meals and/or diets containing increased dietary protein lead to initial and sustained increases in postprandial PYY concentrations along with reductions in postprandial ghrelin concentrations (9,13,25). In our current study, overall PYY concentrations were greater following the higher vs. normal protein diet, a finding that is consistent with the current literature. However, the higher ghrelin concentrations following the higher vs. normal protein diet are not consistent with the perceived hunger responses in this study nor the ghrelin responses observed in our previous study (9). The disparate findings between our two studies may be attributable to the gender and/or energy state differences of the participants. In our previous study, overweight and obese women consumed an energy restriction, weight loss diet, whereas the current study was performed in overweight and obese men who consumed a eucaloric diet. Further research is needed to identify whether gender and energy state differences in appetite control exist with respect to how dietary protein is perceived and which mechanisms are altered.
Although many of the protein studies incorporate similar quantities of protein, the eating frequency studies have a myriad of experimental design approaches making it challenging to develop an overall conclusion regarding the influence of eating frequency on appetite control and food intake. For example, of the studies that compare frequent eating occasions (i.e., >3 occasions), several only include part of the day by comparing the influence of a single breakfast meal vs. dividing the energy intake into 5-EO consumed every hour over the course of the morning (18). Thus, it is difficult to ascertain whether the differences in appetite control would be maintained throughout an entire day. Of those that monitor appetitive responses and food intake throughout an entire day (10-24 h), several incorporate eating frequencies beyond what could practically be followed in daily living (i.e., eating frequency of 12-17 eating occasions/day, eating every 30-40 min) (26,27). The experimental designs of these studies limit the ability to develop feasible recommendations for better weight management. Regardless of these varying experimental designs, very little, if any differences have been observed with perceived hunger or satiety, hormonal responses, or subsequent food intake when comparing single (or few) vs. multiple eating occasions. Besides the current study, only one other study has examined the effect of providing 6-EO (eating every 2 h) vs. 2-EO (eating every 8 h). The study indirectly accessed appetite control by incorporating an ad libitum feeding regiment throughout the evening (15). No difference in evening and/or daily energy intake was observed between the 6-EO vs. 2-EO (15). Over the past 10 years, many adult Americans have transitioned from the typical “three-meals/day” dietary pattern to eating more frequently throughout the day—eating ~4.3 times/day (26). Based on the current and previous evidence-based studies, the overall findings suggest that eating beyond the typical three meals/day pattern does not lead to better appetite control in overweight and obese individuals.
Although appetite control, as assessed through perceived hunger, satiety, PYY, and ghrelin responses, was negatively altered with greater eating frequency, frequent eating led to reduced glucose and insulin responses throughout the day. These findings suggest that overweight and obese adults who typically exhibit a higher risk for type 2 diabetes and metabolic syndrome, may experience improved glycemic control, potentially reducing the risk for diabetes, cardiovascular disease, and additional weight gain when smaller, more frequent meals are consumed (28).
Limitations
The current study did not include any acclimation days to the different eating patterns or protein intakes. Thus, it is unclear as to whether any habitualization to these treatments would have led to differential responses. Additionally, this was an acute study with only four testing days and no follow-up or documentation regarding any changes in subsequent daily energy intake. Although these findings are a relevant step to identify the influence of these dietary factors, further research involving a long-term intervention is necessary to confirm the present findings, document changes in chronic food intake, and to identify the long-term implications for appetite control, energy regulation, and body weight.
In summary, the findings that higher protein intake and lower eating frequency independently promote daily perceived satiety in conjunction with comparable differences in the satiety hormone PYY suggest that overweight and obese men might achieve better appetite control by consuming three higher protein meals per day.
ACKNOWLEDGMENTS
The authors thank the study participants for their dedication and compliance during the testing days; Trent Wisehart, Carmen Martin, Matt Greiser, Laura Hass, and Amanda Sands for their efforts in performing the testing day procedures, sample processing, and data entry; Janice Green for preparing all study foods; Arthur Rosen, MD, who provided medical coverage; and Doug Maish, EMT-P, who performed all catheter insertions and provided clinical laboratory services. This study was funded by the National Pork Board and the American Egg Board—Egg Nutrition Center, with additional support provided by the Purdue University Ingestive Behavior Research Center (postdoctoral fellowship to HJL), and the NIH-sponsored Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) NIH-5 K12 HD052027-04.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 2008;16:2323–2330. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- 3.Eat 6 meals a day with increased protein. 2009 http://www.google.com/search?hl=en&q=6+meals+and+high+protein+for+weight+loss.
- 4.Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. National Academy Press; Washington, DC: 2002. [DOI] [PubMed] [Google Scholar]
- 5.Halton TL, Hu FB. The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review. J Am Coll Nutr. 2004;23:373–385. doi: 10.1080/07315724.2004.10719381. [DOI] [PubMed] [Google Scholar]
- 6.Westerterp-Plantenga MS, Nieuwenhuizen A, Tomé D, Soenen S, Westerterp KR. Dietary protein, weight loss, and weight maintenance. Annu Rev Nutr. 2009;29:21–41. doi: 10.1146/annurev-nutr-080508-141056. [DOI] [PubMed] [Google Scholar]
- 7.Layman DK, Evans E, Baum JI, et al. Dietary protein and exercise have additive effects on body composition during weight loss in adult women. J Nutr. 2005;135:1903–1910. doi: 10.1093/jn/135.8.1903. [DOI] [PubMed] [Google Scholar]
- 8.Leidy HJ, Carnell NS, Mattes RD, Campbell WW. Higher protein intake preserves lean mass and satiety with weight loss in pre-obese and obese women. Obesity (Silver Spring) 2007;15:421–429. doi: 10.1038/oby.2007.531. [DOI] [PubMed] [Google Scholar]
- 9.Leidy HJ, Mattes RD, Campbell WW. Effects of acute and chronic protein intake on metabolism, appetite, and ghrelin during weight loss. Obesity (Silver Spring) 2007;15:1215–1225. doi: 10.1038/oby.2007.143. [DOI] [PubMed] [Google Scholar]
- 10.Veldhorst M, Smeets A, Soenen S, et al. Protein-induced satiety: effects and mechanisms of different proteins. Physiol Behav. 2008;94:300–307. doi: 10.1016/j.physbeh.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Smeets AJ, Soenen S, Luscombe-Marsh ND, Ueland Ø , Westerterp-Plantenga MS. Energy expenditure, satiety, and plasma ghrelin, glucagon-like peptide 1, and peptide tyrosine-tyrosine concentrations following a single high-protein lunch. J Nutr. 2008;138:698–702. doi: 10.1093/jn/138.4.698. [DOI] [PubMed] [Google Scholar]
- 12.Leidy HJ, Bossingham MJ, Mattes RD, Campbell WW. Increased dietary protein consumed at breakfast leads to an initial and sustained feeling of fullness during energy restriction compared to other meal times. Br J Nutr. 2009;101:798–803. doi: 10.1017/s0007114508051532. [DOI] [PubMed] [Google Scholar]
- 13.Batterham RL, Heffron H, Kapoor S, et al. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006;4:223–233. doi: 10.1016/j.cmet.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Bellisle F, McDevitt R, Prentice AM. Meal frequency and energy balance. Br J Nutr. 1997;77(Suppl 1):S57–S70. doi: 10.1079/bjn19970104. [DOI] [PubMed] [Google Scholar]
- 15.Taylor MA, Garrow JS. Compared with nibbling, neither gorging nor a morning fast affect short-term energy balance in obese patients in a chamber calorimeter. Int J Obes Relat Metab Disord. 2001;25:519–528. doi: 10.1038/sj.ijo.0801572. [DOI] [PubMed] [Google Scholar]
- 16.Smeets AJ, Westerterp-Plantenga MS. Acute effects on metabolism and appetite profile of one meal difference in the lower range of meal frequency. Br J Nutr. 2008;99:1316–1321. doi: 10.1017/S0007114507877646. [DOI] [PubMed] [Google Scholar]
- 17.Jackson SJ, Leahy FE, Jebb SA, et al. Frequent feeding delays the gastric emptying of a subsequent meal. Appetite. 2007;48:199–205. doi: 10.1016/j.appet.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Speechly DP, Rogers GG, Buffenstein R. Acute appetite reduction associated with an increased frequency of eating in obese males. Int J Obes Relat Metab Disord. 1999;23:1151–1159. doi: 10.1038/sj.ijo.0801046. [DOI] [PubMed] [Google Scholar]
- 19.Stote KS, Baer DJ, Spears K, et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr. 2007;85:981–988. doi: 10.1093/ajcn/85.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris JL, Bargh JA. Television viewing and unhealthy diet: implications for children and media interventions. Health Commun. 2009;24:660–673. doi: 10.1080/10410230903242267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill AJ, Blundell JE. Nutrients and behaviour: research strategies for the investigation of taste characteristics, food preferences, hunger sensations and eating patterns in man. J Psychiatr Res. 1982;17:203–212. doi: 10.1016/0022-3956(82)90023-1. [DOI] [PubMed] [Google Scholar]
- 22.Wolever TM, Bolognesi C. Prediction of glucose and insulin responses of normal subjects after consuming mixed meals varying in energy, protein, fat, carbohydrate and glycemic index. J Nutr. 1996;126:2807–2812. doi: 10.1093/jn/126.11.2807. [DOI] [PubMed] [Google Scholar]
- 23.Lejeune MP, Westerterp KR, Adam TC, Luscombe-Marsh ND, Westerterp-Plantenga MS. Ghrelin and glucagon-like peptide 1 concentrations, 24-h satiety, and energy and substrate metabolism during a high-protein diet and measured in a respiration chamber. Am J Clin Nutr. 2006;83:89–94. doi: 10.1093/ajcn/83.1.89. [DOI] [PubMed] [Google Scholar]
- 24.Westerterp-Plantenga MS, Lejeune MP, Smeets AJ, Luscombe-Marsh ND. Sex differences in energy homeostatis following a diet relatively high in protein exchanged with carbohydrate, assessed in a respiration chamber in humans. Physiol Behav. 2009;97:414–419. doi: 10.1016/j.physbeh.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Diepvens K, Häberer D, Westerterp-Plantenga M. Different proteins and biopeptides differently affect satiety and anorexigenic/orexigenic hormones in healthy humans. Int J Obes (Lond) 2008;32:510–518. doi: 10.1038/sj.ijo.0803758. [DOI] [PubMed] [Google Scholar]
- 26.Solomon TP, Chambers ES, Jeukendrup AE, Toogood AA, Blannin AK. The effect of feeding frequency on insulin and ghrelin responses in human subjects. Br J Nutr. 2008;100:810–819. doi: 10.1017/S000711450896757X. [DOI] [PubMed] [Google Scholar]
- 27.Jenkins DJ, Wolever TM, Vuksan V, et al. Nibbling versus gorging: metabolic advantages of increased meal frequency. N Engl J Med. 1989;321:929–934. doi: 10.1056/NEJM198910053211403. [DOI] [PubMed] [Google Scholar]
- 28.Bloomgarden ZT. Approaches to treatment of pre-diabetes and obesity and promising new approaches to type 2 diabetes. Diabetes Care. 2008;31:1461–1466. doi: 10.2337/dc08-zb07. [DOI] [PMC free article] [PubMed] [Google Scholar]