Abstract
This functional magnetic resonance imaging (fMRI) pilot study identified whether breakfast consumption would alter the neural activity in brain regions associated with food motivation and reward in overweight “breakfast skipping” (BS) adolescent girls and examined whether increased protein at breakfast would lead to additional alterations. Ten girls (Age: 15 ± 1 years; BMI percentile 93 ± 1%; BS 5 ± 1×/week) completed 3 testing days. Following the BS day, the participants were provided with, in randomized order, normal protein (NP; 18 ± 1 g protein) or higher protein (HP; 50 ± 1 g protein) breakfast meals to consume at home for 6 days. On day 7 of each pattern, the participants came to the laboratory to consume their respective breakfast followed by appetite questionnaires and an fMRI brain scan to identify brain activation responses to viewing food vs. nonfood images prior to lunch. Breakfast consumption led to enduring (i.e., 3-h post breakfast) reductions in neural activation in the hippocampus, amygdala, cingulate, and parahippocampus vs. BS. HP led to enduring reductions in insula and middle prefrontal cortex activation vs. NP. Hippocampal, amygdala, cingulate, and insular activations were correlated with appetite and inversely correlated with satiety. In summary, the addition of breakfast led to alterations in brain activation in regions previously associated with food motivation and reward with additional alterations following the higher-protein breakfast. These data suggest that increased dietary protein at breakfast might be a beneficial strategy to reduce reward-driven eating behavior in overweight teen girls. Due to the small sample size, caution is warranted when interpreting these preliminary findings.
INTRODUCTION
The obesity epidemic, currently affecting nearly 25 million young people in the United States alone, is considered to be the greatest threat to public health this century (1,2). Recent evidence suggests that several environmental factors, particularly increased availability of highly palatable, energy dense food, and increased presence of powerful food stimuli (i.e., bill-boards, commercials, vending machines) contribute substantially to the shift away from eating according to physiological need towards reward-driven eating, the latter of which leads to positive energy balance and obesity (3,4).
Overweight and obese young people appear to be at an increased risk to these environmental factors. Specifically, they are more likely to consume sugar sweetened drinks (5), less likely to compensate for “fast food” calories (6), and consume more energy-density foods (e.g., sweets, chocolate, and chips) following exposure to food advertisements (7). In addition to the unhealthy eating behaviors arising from the exposure to the current food environment, adolescents also partake in the unhealthy practice of skipping breakfast (8). Although the relationship between breakfast skipping (BS) and obesity is well-established (9,10), a more novel, unexplored association exists between BS and reward-driven eating behavior (11).
Recent data from our lab indicate that the addition of breakfast leads to reductions in physiologic markers of appetite, increases in physiologic markers of satiety, and reductions in energy intake at the next eating occasion in adolescents who habitually skip the breakfast meal (12). Furthermore, additional benefits were observed when the breakfast was higher in dietary protein (12).
While a myriad of studies exist examining the effects of various dietary interventions on the homeostatic, hormonal signals of energy regulation (13), no studies to date have assessed whether the nonhomeostatic, neural mechanisms underlying ingestive behavior are also modulated. This approach may have dramatic implications in terms of developing effective dietary recommendations which target the modern food, obesogenic environment to combat obesity in young people.
The purpose of this study was threefold: (i) to determine whether the daily addition of breakfast, regardless of macro-nutrient composition, influences the activation of specific brain regions associated with food motivation and reward in “breakfast skipping” adolescent girls; (ii) to determine whether the macronutrient composition of the breakfast meal (i.e., increased dietary protein) further impacts this response; and (iii) to identify whether the brain activation responses are associated with indexes of perceived appetite and satiety.
METHODS AND PROCEDURES
Participants
Adolescent girls were recruited from the Kansas City, KS area through advertisements, flyers, and email listserves to participate in the study. Eligibility was determined through the following inclusion criteria: (i) age range 13–18 years; (ii) overweight to obese (BMI: 25–34.9 kg/ m2; 85–99th percentile for BMI for age); (iii) no metabolic or neurological diseases or other health complications; (iv) not been clinically diagnosed with an eating disorder; (v) not currently or previously on a weight loss or other special diet in the past 6 months; (vi) documented regular menstrual cycles between 21–36 days in duration for the past 6 months; (vii) frequently eats lunch (i.e., ≥5 eating occasions/week); (viii) infrequently eats breakfast (i.e., ≤2 breakfast occasions/week); and (ix) right-handed.
Fifty-one volunteers were interested in participating in the study. Twelve met the screening criteria and began the study, and 10 completed all study procedures. The two participants who did not complete the study procedures were unable to stay awake during the functional magnetic resonance imaging (fMRI) brain scan and were thus excluded from all analyses. Subject characteristics of those who completed the study are presented in Table 1.
Table 1.
Subject characteristics of the 10 “breakfast skipping” adolescent girls who completed all study procedures
| Subject characteristics | Mean ± s.e.m. |
|---|---|
| Age (years) | 15 ± 1 |
| Height (cm) | 165 ± 2 |
| Weight (kg) | 79.1 ± 3.3 |
| BMI | |
| Actual (kg/m2) | 29.0 ± 1.0 |
| Percentile (%) | 93.1 ± 1.4 |
| Breakfast Skipping (# times/week) | 5 ± 1 |
| Reasons for skipping (% of participants) | |
| Not hungry | 80% |
| Too early to eat | 50% |
| Strategy to lose weight | 20% |
| No breakfast foods in the house | 10% |
| No one home to prepare breakfast | 10% |
All participants and their parents (or legal guardians) were informed of the study purpose, procedures, and risks and signed the consent/assent forms. The study procedures were approved by the University of Kansas Medical Center Human Subjects Committee and the General Clinical Research Center Advisory Committee. The subjects received $120 for completing all study procedures.
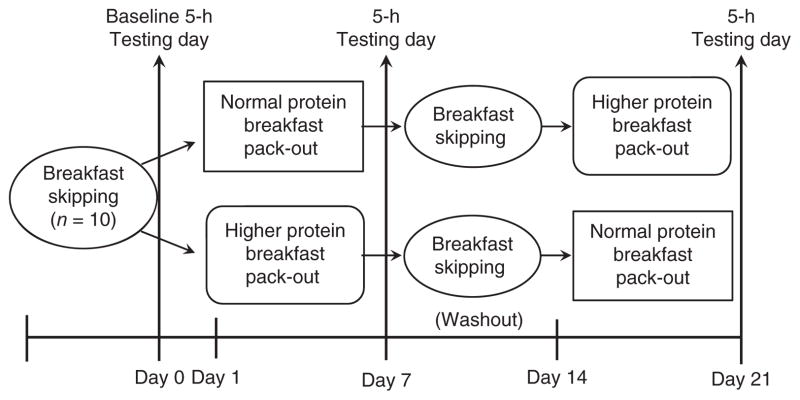
Experimental design
This fMRI pilot study was performed in conjunction with another study examining the effects of increased dietary protein at breakfast on appetite control and the regulation of food intake with respect to the physiological, energy regulatory hormonal signals. The current study utilized a semi-randomized, crossover design consisting of 3 separate testing days (Figure 1). Following the baseline “breakfast skipping” testing day, the participants were randomly provided with a normal protein breakfast (NP; 18 ± 1 g protein/meal) or a higher protein breakfast (HP; 50 ± 1 g protein/meal) to consume at home for 6 days. On the 7th day of each pattern, the participants completed the respective testing day. Each participant performed both breakfast patterns, with a 1 week washout (i.e., breakfast-skipping) period between treatments.
Figure 1.
Study design.
During each testing day, the participants arrived at the General Clinical Research Center (GCRC) following an overnight fast. At time +0 min, they were provided with the respective breakfast meal (i.e., NP or HP) or continued to skip breakfast (i.e., BS). Perceived appetite, satiety, and brain activation responses were measured 3 hour postbreakfast.
Breakfast patterns and respective testing day meals
Following the baseline, BS testing day, the participants randomly completed the NP and HP breakfast patterns. For each pattern, the participants were provided with the respective breakfast meals and asked to consume these at home between 7:00 and 9:00 AM for 6 consecutive days. On day 7 of each pattern, the participants returned to the GCRC to complete the respective testing day. Each breakfast pattern consisted of two types of meals which were provided throughout the 7 days in an alternating style. The NP meals included two types of breakfast cereals (i.e., crispy squares or crispy rice) and milk, whereas the HP meals consisted of baked items (i.e., oatmeal bars or waffles with syrup) and yogurt. Each of the breakfast meals within each treatment were matched for protein, carbohydrates, fat, sugar, fiber, energy content, and palatability.
The dietary characteristics and palatability of the testing day breakfast meals are shown in Table 2. The meals contained ~24% of estimated energy needs (490 ± 10 kcal) for normal to overweight adolescents (14). The NP meal was comprised of 15% protein (18 ± 1 g protein), 65% carbohydrates and 20% fat, whereas, the HP meal included 40% protein (50 ± 1.1 g protein), 40% carbohydrates, and 20% fat. Both meals were served with 237 ml of water. Total energy content, fat, sugar, fiber, energy density, and palatability were similar between the breakfast meals. During the baseline, BS day, the participants were only provided with the 237 ml of water. This day served as the subjects’ normal eating pattern and was used to identify the normal (baseline) responses.
Table 2.
Dietary characteristics of the testing day breakfast meals
| Dietary characteristicsa | Normal-protein (NP) breakfast | Higher-protein (HP) breakfast |
|---|---|---|
| Energy content (kcal) | 490 ± 10 | 490 ± 10 |
| PRO (g) | 18.3 ± 0.3 | 49.9 ± 0.1 |
| CHO (g) | 77.2 ± 1.6 | 51 ± 1.4 |
| Sugar (g) | 12.6 ± 0.3 | 12.8 ± 0.2 |
| Fiber (g) | 6.8 ± 0.1 | 7.1 ± 0.1 |
| Fat (g) | 10.7 ± 0.2 | 10.9 ± 0.2 |
| Testing day menu: | Crispy square cereal with milk Toasted rice cereal 32 g Toasted wheat cereal 62 g Whole milk 32 g Reduced sugar 2% milk 235 g |
Waffle with syrup 1% fat cottage cheese 85 g Liquid egg substitute 80 g Margarine 10.8 g Unbleached flour 32.2 g Lavash bread 47 g Maple syrup 3.2 g Sugar free maple syrup 44 g Yogurt Whipped 1% cottage cheese 85 g Raspberries 20 g |
| Palatability (mm)b | 64 ± 9 | 58 ± 7 |
PRO, protein, CHO, carbohydrate.
Data presented as mean ± s.e.m.
Palatability assessed from a questionnaire completed during breakfast (+15 min) (scale = 1–100 mm).
fMRI
Brain activation responses were assessed prior to lunch (i.e., 3 h after the start of the testing day) in each of the testing days. During the fMRI procedure, the participants laid down in a supine position on the sliding MRI table and focused on the photographs which were projected onto a screen and easily viewed through a mirror.
The fMRI paradigm has been previously published and incorporated stimuli from three categories of pictures including food, non-food (animals), and blurred baseline images (15,16). The pictures from each category were presented in blocks of images. Ten photographs (of the same type of stimuli) were presented per block. The scan involved three repetitions of each block of stimulus-producing images(i.e., food, animal), alternated with blocks of randomized blurred images. Each photograph was projected for 2.5 seconds, with an interstimulus interval of 0.5 seconds. There was a total of 13 blocks of stimuli presented. Individual pictures were randomly assigned to appropriate blocks and were never repeated. Animal pictures were used to control for visual richness and general interest (i.e., appealing but not appetizing). Food and animal image sets were selected in pilot studies to be matched on measures of valence and arousal based on previously validated measures (17), yet maximally different in ratings of perceived appetitive value. The functional scan lasted approximately 7 min and was performed in duplicate. Scanning was performed at the Hoglund Brain Imaging Center at the University of Kansas Medical Center on a 3 Tesla Allegra scanner (Siemens Medical Solutions, Erlangen, Germany).
Recognition memory for the presented images was examined following the scanning session to identify whether the participants were attentive throughout the scans (See ref. 16 for further detail). Functional scans were excluded if the participants scored less than chance (i.e., <50%). Removal of both functional scans on any one testing day led to the exclusion of the subject.
Questionnaires
Perceived indexes of appetite (i.e., hunger, desire to eat, prospective food consumption), and perceived satiety (i.e., fullness) were assessed at the beginning of each testing day (+0 min) and prior to fMRI (+180 min) on each the testing day. The questionnaires contained validated visual analog scales incorporating a 100 mm horizontal line rating scale for each response (18). The questions are worded in the following manner: “how strong is your feeling of” with the anchors of “not at all” to “extremely”.
Data and statistical analyses
The brain activation responses were analyzed using the Brain Voyager QX statistical package and random effects (Brain Innovation, Maastricht, Netherlands, 2004). Preprocessing steps included trilinear three-dimensional motion correction, sinc-interpolated slice scan time correction, two-dimensional spatial smoothing with 4-mm Gaussian filter, and high-pass filter temporal smoothing (16). Functional images were realigned to the anatomic images obtained within each session and standardized using Brain Voyager Talairach transformation, which conforms to the space defined by the Talairach and Tournoux’s stereotaxic atlas (19). Functional scans were discarded if head movement >3 mm along any axis (x, y, or z).
To determine the effects of breakfast on neural activity associated with food motivation, a repeated measures ANOVA was performed on the brain activation maps within the Brain Voyager software using Stimulus (food (i.e., appetizing and appealing) vs. nonfood (i.e., animal, non-appetizing but appealing) × Breakfast (BS vs. NP; BS vs. HP) comparisons. To identify whether the macronutrient composition of the breakfast meal would differentially effect the neural responses, a repeated measures ANOVA was again performed using stimulus (i.e., food vs. nonfood) × Breakfast (NP vs. HP).
Variables representing the experimental conditions were modeled with a hemodynamic response filter and entered into the model using random effects. Contrast between conditions was assessed with t-statistics using random effects. Based on previous research with this paradigm a priori regions of interest included the amygdala, hippocampal formation (hippocampus and parahippocampal cortex), cingulate, insula, striatum, orbitofrontal cortex, and prefrontal cortex (15,16). To identify significant activations in a priori regions, cluster-level statistical threshold was applied to correct for multiple comparisons (20,21). Using this approach, significance was set at P = 0.01, with a cluster-level false-positive rate of α = 0.05. Cluster threshold correction relies on the idea that true activations are likely to extend spatially across multiple voxels within a region. By combining information about the data, including the initial voxelwise significance threshold and data smoothness, a distribution of probable cluster sizes can be obtained, and a separate false-positive threshold for cluster size can be applied. This method allows for a reduction in Type I errors while allowing for the detection of true activations at an appropriate level of correction. Regarding the regions of interest data analysis, follow-up analyses of a priori regions of interest were conducted in regions noted above that achieved statistical significance in the breakfast pattern analyses. Mean percent signal change (food vs. baseline) in the maximum voxel within each region for each individual was exported to the latest version of the Statistical Package for the Social Sciences (SPSS 17.0; SPSS, Chicago, IL).
Pearson’s correlations were computed between the percent signal change in the a priori regions reaching significance and perceived appetite and satiety. Data are expressed as mean ± s.e.m. A P < 0.05 was considered statistically significant. Analyses were conducted using the Statistical Package for the Social Sciences (SPSS 17.0).
RESULTS
BS vs. breakfast
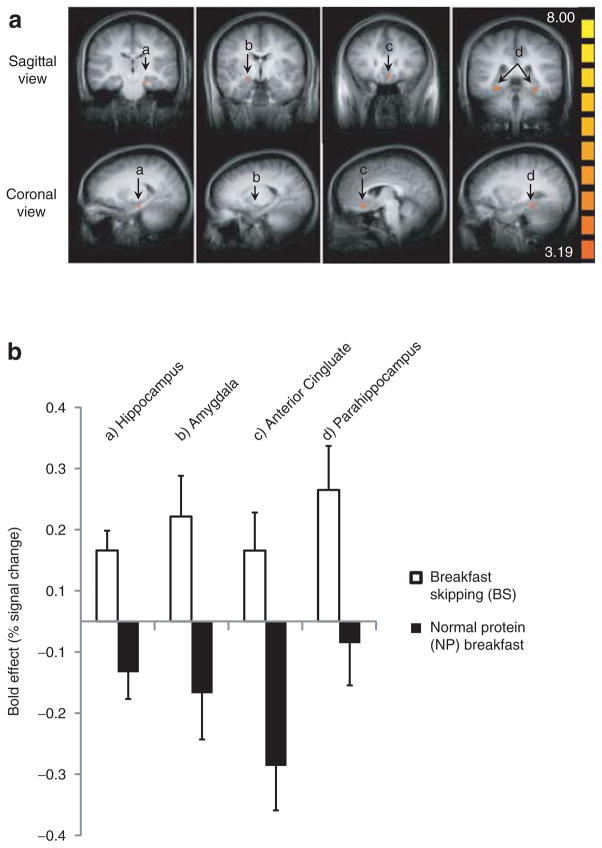
All brain regions significantly affected by breakfast consumption are presented in Table 3. Prelunch neural activity in response to visual food cues (i.e., food > nonfood) was greater in the hippocampus (x, y, z = −19, −20, −15), amygdala (x, y, z = 20, −5, −9), subgenual anterior cingulate (x, y, z = −4, 25, −9; −4, 40, −3), and parahippocampus (x, y, z = 26, −14, −12; −16, 1, −15; −28, −35, −12; −28, 4, −21) regions when breakfast was skipped (i.e., BS) compared to consuming the NP breakfast (Table 3; Figure 2a, b (A–D)). Similar activation responses were also observed when comparing BS vs. HP (data not shown).
Table 3.
Brain regions reaching significance (Food >nonfood) at +180 min (i.e., Prelunch) during the breakfast skipping (BS), normal protein (NP), and higher protein (HP) testing days
| Contrast and region | Coordinates
|
t | Number of voxels | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| BS > NP | |||||
| Hippocampus | −19 | −20 | −15 | 10.32 | 233a |
| Amygdala | 20 | −5 | −9 | 5.49 | 35a |
| Anterior Cingulate | −4 | 25 | −9 | 4.84 | 87a |
| −4 | 40 | −3 | 4.62 | 32 | |
| Parahippocampus | 26 | −14 | −12 | 5.00 | 260 |
| −16 | 1 | −15 | 5.68 | 159 | |
| −28 | −35 | −12 | 6.25 | 246a | |
| −28 | 4 | −21 | 5.60 | 47 | |
| NP > HP | |||||
| Insula | 32 | 16 | 12 | 5.79 | 135b |
| −31 | 10 | 12 | 4.62 | 52 | |
| Middle prefrontal | 29 | 40 | 24 | 4.43 | 51 |
| −37 | 31 | 39 | 4.43 | 163b | |
Figure 2.
Prelunch a priori brain activation following the addition of breakfast; all comparisons food > nonfood (animal). (a) Averaged functional magnetic resonance imaging (fMRI) brain activation contrast map; *greater activation following breakfast skipping vs. normal protein breakfast in the (A) hippocampus, (B) amygdala, (C) subgenual anterior cingulate, and (D) parahippocampus, *Cluster-level statistical threshold adjustment for multiple comparisons; P = 0.01; α = 0.05. (b) Bold effect depicting treatment differences (breakfast skipping vs. normal protein breakfast) in average percent signal change (food vs. nonfood) from the maximum voxel of the activations in a.
NP vs. HP breakfast meals
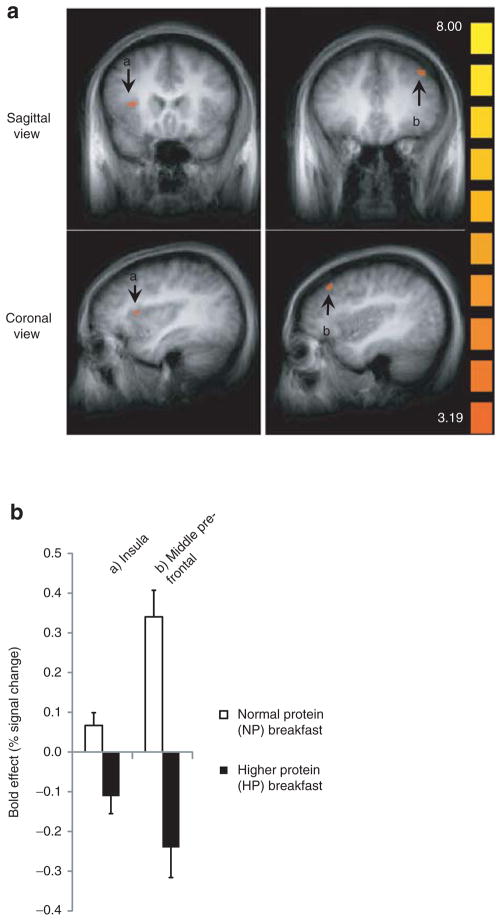
Brain activations significantly modulated by increased dietary protein at breakfast (i.e., HP vs. NP) are also shown in Table 3. In comparing breakfast type, consumption of the NP breakfast resulted in greater activation in the anterior insula (x, y, z = 32, 16, 12; −31, 10, 12) and the middle prefrontal cortex (x, y, z = 29, 40, 24; −37, 31, 39) regions vs. the HP breakfast (Table 3; Figure 3a, b (A, B)).
Figure 3.
Prelunch a priori brain activation following the normal protein (NP) vs. higher protein (HP) breakfast treatments; all comparisons food > nonfood (animal). (a) Averaged functional magnetic resonance imaging (fMRI) brain activation contrast map; *greater activation following the normal protein vs. higher protein breakfast in the (A) insula and (B) middle prefrontal cortex. Cluster-level statistical threshold adjustment for multiple comparisons; P = 0.01; α = 0.05. (b) Bold effect depicting treatment differences (normal protein vs. higher protein breakfast) in average percent signal change (food vs. nonfood) from the maximum voxel of the activations in Figure 3a.
Correlations between the brain activation patterns and perceived appetite and satiety
As shown in Table 4, percent signal change in the hippocampus, amygdala, cingulate, and insula were positively correlated with indexes of appetite including perceived sensations of hunger, desire to eat, and prospective food consumption.
Table 4.
Correlations between prelunch neural activity and perceived appetite and satiety
| Variable | Hippocampus
|
Amygdala
|
Cingulate
|
Insula
|
||||
|---|---|---|---|---|---|---|---|---|
| R | P value | R | P value | R | P value | R | P value | |
| Perceived appetite | ||||||||
| Hunger | 0.578 | 0.01 | 0.554 | 0.01 | 0.429 | 0.05 | 0.346 | NS |
| Desire to eat | 0.584 | 0.01 | 0.572 | 0.01 | 0.484 | 0.05 | 0.473 | 0.05 |
| Prospective food consumption | 0.558 | 0.01 | 0.478 | 0.05 | 0.327 | NS | 0.384 | 0.09 |
| Perceived satiety | ||||||||
| Fullness | −0.421 | 0.07 | −0.464 | 0.05 | −0.368 | NS | −0.334 | NS |
NS, nonsignificant (P > 0.1).
DISCUSSION
The present study was conducted to examine the sustained effects of normal vs. higher-protein breakfast meals on neural activity (prior to lunch) in overweight/obese “breakfast skipping” adolescents. The addition of breakfast resulted in reductions in brain activation responses to food stimuli in the hippocampus, amygdala, anterior cingulate, and parahippocampus prior to lunch. With respect to whether additional alterations were observed with increased dietary protein, the higher protein breakfast meal led to reduced activation in the anterior insula and the middle prefrontal cortex compared to the NP breakfast. Activations in many of the a priori, limbic regions were associated with perceived hunger, desire to eat, and food motivation. These data indicate that the daily addition of a protein-rich breakfast leads to sustained reductions in neural activity in brain regions associate with food motivation and reward. This is the first, but vital step to identify whether modest dietary interventions alter the neural responses surrounding food motivation and reward. Future studies are needed to identify whether these alterations lead to reduced reward-driven eating behavior and over-eating later in the day.
Previous fMRI studies have examined the neural responses involved with acute (premeal (fasting) vs. postmeal (fed)) and chronic (lean vs. obese) energy regulation (15,16,22–26). The primary targets have been key regions of the limbic system which control various aspects of hedonic, reward-driven eating. These include the hippocampus, parahippocampus, and amygdala which are involved with the development of short and long term memories, including memories of food (27); the anterior cingulate known for its role in reward; and the insula which is associated with gustatory processing, reward, desire, and cravings (27). Other medial and prefrontal regions have also been examined due to their integration of physiologic and reward signals and overall executive control of ingestive behavior (27).
Chronic energy imbalance, as observed in obesity, has been shown to impact neural activation in many of the previously mentioned regions. In a recent study (16), pre-and postmeal neural responses were compared between 20 normal weight and obese volunteers. The obese participants exhibited greater premeal activation in the anterior cingulate, medial prefrontal cortex and greater postmeal activation in the medial prefrontal cortex and hippocampus compared to the normal weight participants (16). Several other studies comparing obese and normal weight participants report greater activation in the amygdala, medial prefrontal cortex, insula, anterior cingulate, and hippocampus in obese vs. normal weight (24–26). Stice et al. (24). also reported that individuals exhibiting heightened activity in the anterior insula experienced greater weight gain throughout a 1 year post follow-up period compared to those displaying less insula activation (24).
With respect to acute energy states, Holsen et al. (15). illustrated that the consumption of a standardized meal led to reduced activation in the amygdala, insula, parahippocampus, and anterior cingulate compared to fasting (i.e., pre-meal). Other studies by Fuhrer et al. (22) and Goldstone et al. (23) report similar findings. Specifically, when compared to the fasted state, meal consumption led to reduced brain activation in the amygdala, anterior cingulate, insula, and/or medial frontal cortex (22,23). Collectively, these data illustrate that activation of specific brain regions in the corticolimbic system are responsive to acute and chronic energy status and may play a key role in reward-driven eating behavior and obesity.
The studies mentioned above utilized fMRI technology during fasting or within 30–60 minutes following a standardized meal. Our current study extends these findings to include the assessment of the neural responses 3 h postbreakfast, which is the time in which the next eating occasion would typically be initiated. Using this approach, we are able to identify whether the consumption of normal vs. higher protein breakfast meals have a sustained impact with respect to motivational drive to eat prior to lunch. Breakfast consumption and increased dietary protein were chosen as the dietary interventions due to their established improvements in physiological appetite control and satiety (28) and/or their inverse association with overeating, weight gain, and obesity (8,29,30).
In summary, the findings from this study indicate sustained, reduced activation in key prefrontal and limbic brain regions following the consumption of breakfast, particularly in those meals containing increased dietary protein. In addition, the strong associations between the neural activity of the limbic regions and the perceived hunger, desire, and food motivation responses further support the role of these regions in reward-driven eating behavior.
Limitations
Some of the study limitations are described below. Given that the day-to-day habits of adolescents during the school year are quite different than during the summer months, we sought to test all participants in the same season. In doing so, we were unable to control for menstrual phase for the testing days. According to Barr et al. (31) and Dye et al. (32), hormonal fluctuations throughout the menstrual cycle effect appetite control and energy intake, with greater appetite and daily intake occurring during the luteal vs. follicular menstrual cycle. Based on our study design, we are unable to determine the effects of menstrual phase on our study outcomes. However, menstrual cyclicity was documented and varied between testing days and between participants thus reducing any systematical bias/ effect. Although the current sample size is small with only 10 subjects in a repeated measure design, cluster-level statistical threshold adjustment was applied to correct for multiple comparisons. Regardless, caution is warranted concerning the interpretation and generalizability of the study findings and results should be considered preliminary. Lastly, the current study rationale relies, in part, upon the correlational findings surrounding Breakfast Skipping, reward-driven eating behavior, and obesity and suggests that consuming breakfast, particularly a protein-rich breakfast, will reverse these unwanted outcomes. Even though the relationship between Breakfast Skipping and obesity is strong, limited data exist concerning whether Breakfast Skipping plays a causal role in obesity. Several prospective studies (33–35), ranging from 1 to 10 years, indicate that individuals who skip breakfast on a daily basis have a greater risk of being overweight and/or developing obesity compared to breakfast consumers. While untested, it is also plausible that overweight and obese adolescents begin to skip breakfast as a means to reduce daily energy intake to lose weight. Although this has been frequently cited as the primary reason for Breakfast Skipping, several studies have shown that most adolescents report skipping breakfast mainly due to “lack of time,” “not feeling hungry,” and/or “not liking to eat that early” as opposed to using it as a strategy to lose weight (36,37). We are currently completing a long term (i.e., 12-week) randomized controlled breakfast trial to provide support for the causal role of Breakfast Skipping.
Adolescents in the United States are exposed to limitless food stimuli, on a daily basis, that lead to overeating (38–40). Further, Breakfast Skipping is a common dietary practice that is strongly associated with overweight/obesity in adolescents and could be adversely contributing to the susceptibility to these detrimental environmental stimuli. Dietary recommendations to combat reward-driven eating are lacking, especially in adolescents. In the current study, we demonstrate that the addition of breakfast in overweight/obese “Breakfast Skipping” adolescents leads to reductions in brain activation in specific regions involved with food motivation and reward. In addition, a breakfast higher in dietary protein leads to additional improvements.
Acknowledgments
We thank the study participants for their dedication and compliance; KUMC-GCRC Administrative, Nursing, Bionutrition, and other support staff for assisting with catheter insertions, blood collections and processing, and/or other screening/testing-day procedures and activities. This study was funded through the Building Interdisciplinary Research Careers in Women’s Health 5 K12 HD052027-04 Award.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
References
- 1.Dietary Guidelines Advisory Committee. 2010 Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans. US Department of Agriculture, Agricultural Research Service; Washington, DC: 2010. [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 3.Daniels SR, Arnett DK, Eckel RH, et al. Overweight in children and adolescents: pathophysiology, consequences, prevention, and treatment. Circulation. 2005;111:1999–2012. doi: 10.1161/01.CIR.0000161369.71722.10. [DOI] [PubMed] [Google Scholar]
- 4.Le J, Zhang D, Menees S, Chen J, Raghuveer G. “Vascular age” is advanced in children with atherosclerosis-promoting risk factors. Circ Cardiovasc Imaging. 2010;3:8–14. doi: 10.1161/CIRCIMAGING.109.880070. [DOI] [PubMed] [Google Scholar]
- 5.Berkey CS, Rockett HR, Field AE, Gillman MW, Colditz GA. Sugar-added beverages and adolescent weight change. Obes Res. 2004;12:778–788. doi: 10.1038/oby.2004.94. [DOI] [PubMed] [Google Scholar]
- 6.Ebbeling CB, Sinclair KB, Pereira MA, et al. Compensation for energy intake from fast food among overweight and lean adolescents. JAMA. 2004;291:2828–2833. doi: 10.1001/jama.291.23.2828. [DOI] [PubMed] [Google Scholar]
- 7.Halford JC, Gillespie J, Brown V, Pontin EE, Dovey TM. Effect of television advertisements for foods on food consumption in children. Appetite. 2004;42:221–225. doi: 10.1016/j.appet.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Siega-Riz AM, Popkin BM, Carson T. Trends in breakfast consumption for children in the United States from 1965–1991. Am J Clin Nutr. 1998;67:748S–756S. doi: 10.1093/ajcn/67.4.748S. [DOI] [PubMed] [Google Scholar]
- 9.Deshmukh-Taskar PR, Nicklas TA, O’Neil CE, et al. The relationship of breakfast skipping and type of breakfast consumption with nutrient intake and weight status in children and adolescents: The National Health and Nutrition Examination Survey 1999–2006. J Am Diet Assoc. 2010;110:869–878. doi: 10.1016/j.jada.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 10.Davies JA, Collins PW, Hathaway LS, Bowen DJ. Effect of von Willebrand factor Y/C1584 on in vivo protein level and function and interaction with ABO blood group. Blood. 2007;109:2840–2846. doi: 10.1182/blood-2006-07-035105. [DOI] [PubMed] [Google Scholar]
- 11.Sjöberg A, Hallberg L, Höglund D, Hulthén L. Meal pattern, food choice, nutrient intake and lifestyle factors in The Göteborg Adolescence Study. Eur J Clin Nutr. 2003;57:1569–1578. doi: 10.1038/sj.ejcn.1601726. [DOI] [PubMed] [Google Scholar]
- 12.Leidy HJ, Racki EM. The addition of a protein-rich breakfast and its effects on acute appetite control and food intake in ‘breakfast-skipping’ adolescents. Int J Obes. 2010;34:1125–1133. doi: 10.1038/ijo.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy K, Dhillo WS, Bloom S. Gut peptides in the regulation of food intake and energy homeostasis. Endocr Rev. 2006;27:719–727. doi: 10.1210/er.2006-0028. [DOI] [PubMed] [Google Scholar]
- 14.Food and Nutrition Board. Dietary Reference Intakes for Energy, Carbohydrates, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Vol. 5. National Academy Press; Washington, DC: 2002. pp. 589–786. [DOI] [PubMed] [Google Scholar]
- 15.Holsen LM, Zarcone JR, Thompson TI, et al. Neural mechanisms underlying food motivation in children and adolescents. Neuroimage. 2005;27:669–676. doi: 10.1016/j.neuroimage.2005.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin LE, Holsen LM, Chambers RJ, et al. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity (Silver Spring) 2010;18:254–260. doi: 10.1038/oby.2009.220. [DOI] [PubMed] [Google Scholar]
- 17.Bradley MM, Greenwald MK, Petry MC, Lang PJ. Remembering pictures: pleasure and arousal in memory. J Exp Psychol Learn Mem Cogn. 1992;18:379–390. doi: 10.1037//0278-7393.18.2.379. [DOI] [PubMed] [Google Scholar]
- 18.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 19.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers; New York: 1988. [Google Scholar]
- 20.Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum Brain Mapp. 2006;27:392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forman SD, Cohen JD, Fitzgerald M, et al. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 22.Führer D, Zysset S, Stumvoll M. Brain activity in hunger and satiety: an exploratory visually stimulated FMRI study. Obesity (Silver Spring) 2008;16:945–950. doi: 10.1038/oby.2008.33. [DOI] [PubMed] [Google Scholar]
- 23.Goldstone AP, Prechtl de Hernandez CG, Beaver JD, et al. Fasting biases brain reward systems towards high-calorie foods. Eur J Neurosci. 2009;30:1625–1635. doi: 10.1111/j.1460-9568.2009.06949.x. [DOI] [PubMed] [Google Scholar]
- 24.Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117:924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rothemund Y, Preuschhof C, Bohner G, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37:410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Stoeckel LE, Weller RE, Cook EW, 3rd, et al. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41:636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 27.Van Vugt DA. Brain imaging studies of appetite in the context of obesity and the menstrual cycle. Hum Reprod Update. 2010;16:276–292. doi: 10.1093/humupd/dmp051. [DOI] [PubMed] [Google Scholar]
- 28.Leidy HJ, Racki EM. The addition of a protein-rich breakfast and its effects on acute appetite control and food intake in ‘breakfast-skipping’ adolescents. Int J Obes (Lond) 2010;34:1125–1133. doi: 10.1038/ijo.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westerterp-Plantenga MS, Nieuwenhuizen A, Tomé D, Soenen S, Westerterp KR. Dietary protein, weight loss, and weight maintenance. Annu Rev Nutr. 2009;29:21–41. doi: 10.1146/annurev-nutr-080508-141056. [DOI] [PubMed] [Google Scholar]
- 30.de Castro JM. The time of day of food intake influences overall intake in humans. J Nutr. 2004;134:104–111. doi: 10.1093/jn/134.1.104. [DOI] [PubMed] [Google Scholar]
- 31.Leidy HJ, Dougherty KA, Frye BR, Duke KM, Williams NI. Twenty-four-hour ghrelin is elevated after calorie restriction and exercise training in non-obese women. Obesity (Silver Spring) 2007;15:446–455. doi: 10.1038/oby.2007.542. [DOI] [PubMed] [Google Scholar]
- 32.Leidy HJ, Carnell NS, Mattes RD, Campbell WW. Higher protein intake preserves lean mass and satiety with weight loss in pre-obese and obese women. Obesity (Silver Spring) 2007;15:421–429. doi: 10.1038/oby.2007.531. [DOI] [PubMed] [Google Scholar]
- 33.Ma Y, Bertone ER, Stanek EJ, 3rd, et al. Association between eating patterns and obesity in a free-living US adult population. Am J Epidemiol. 2003;158:85–92. doi: 10.1093/aje/kwg117. [DOI] [PubMed] [Google Scholar]
- 34.van der Heijden AA, Hu FB, Rimm EB, van Dam RM. A prospective study of breakfast consumption and weight gain among U.S. men. Obesity (Silver Spring) 2007;15:2463–2469. doi: 10.1038/oby.2007.292. [DOI] [PubMed] [Google Scholar]
- 35.Bazzano LA, Song Y, Bubes V, et al. Dietary intake of whole and refined grain breakfast cereals and weight gain in men. Obes Res. 2005;13:1952–1960. doi: 10.1038/oby.2005.240. [DOI] [PubMed] [Google Scholar]
- 36.Shaw ME. Adolescent breakfast skipping: an Australian study. Adolescence. 1998;33:851–861. [PubMed] [Google Scholar]
- 37.Sweeney NM, Horishita N. The breakfast-eating habits of inner city high school students. J Sch Nurs. 2005;21:100–105. doi: 10.1177/10598405050210020701. [DOI] [PubMed] [Google Scholar]
- 38.Halford JC, Boyland EJ, Hughes GM, et al. Beyond-brand effect of television food advertisements on food choice in children: the effects of weight status. Public Health Nutr. 2008;11:897–904. doi: 10.1017/S1368980007001231. [DOI] [PubMed] [Google Scholar]
- 39.Jansen A, Theunissen N, Slechten K, et al. Overweight children overeat after exposure to food cues. Eat Behav. 2003;4:197–209. doi: 10.1016/S1471-0153(03)00011-4. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Monteiro C, Popkin BM. Trends of obesity and underweight in older children and adolescents in the United States, Brazil, China, and Russia. Am J Clin Nutr. 2002;75:971–977. doi: 10.1093/ajcn/75.6.971. [DOI] [PubMed] [Google Scholar]