Abstract
Background
McCune Albright syndrome (MAS), a disorder caused by somatic activating mutations in the GNAS gene, usually presents with cutaneous, skeletal, and endocrine manifestations. While focal lesions involving multiple tissues have been identified in MAS, almost nothing is known about gastrointestinal lesions in this disease.
Methods
Two MAS patients with perioral freckling, resembling Peutz–Jeghers syndrome (PJS), and two MAS patients without similar pigmentation underwent gastrointestinal endoscopy to establish if they had coexisting hamartomatous polyposis. Three of 4 subjects had documented GNAS mutations in peripheral blood. Genetic testing for STK11 and PRKAR1A genes was performed to exclude presence of coexistent PJS and Carney complex. Genetic testing of biopsy material was also performed.
Results
Hamartomatous gastrointestinal polyps with histological features similar to those in PJS were observed in all 4 subjects, only in the stomach and/or upper duodenum. Activating GNAS mutations were found in the polyps or adjacent mucosa in 3 of 4 subjects. One patient each had mutation only in the blood or tissue, while 2 patients had both. No subject harboured any detectable PRKARIA or STK11 mutation as determined by direct DNA sequencing and copy number variation analysis.
Conclusions
These findings confirm that gastrointestinal polyps are a common manifestation of MAS, indicate an overlap between MAS and PJS, and point towards a putative interaction between the GNAS and STK11 genes in the pathogenesis of these two disorders. The findings suggest a need for routine gastrointestinal endoscopy in patients with MAS, to establish the true incidence of polyps in these patients.
INTRODUCTION
McCune Albright syndrome (MAS) is a rare sporadic disorder caused by postzygotic activating mutations in the GNAS gene1 encoding the α subunit of the stimulatory G protein (Gsα), resulting in decreased GTPase activity of Gsα. Constitutive activation of Gsα persistently elevates cyclic adenosine monophosphate (cAMP) levels. Somatic manifestations are dependent on the distribution of GNAS mutations, with café-au-lait spots, polyostotic fibrous dysplasia, and endocrine excesses including precocious puberty, growth hormone (GH) excess, hypercortisolism, hyperthyroidism, and phosphate wasting.2
Carney complex, caused by mutations in the PRKARIA gene (or less commonly PDE11A or PDE8B genes), encoding type 1 regulatory subunit of protein kinase A, an important effector molecule in many endocrine signalling pathways, can display some features similar to MAS. Peutz–Jeghers Syndrome (PJS) is a result of germline mutations in the tumour suppressor gene STK11. PJS is typified by the diagnostic features of hamartomatous polyposis and perioral freckling.
Following the observation of perioral freckling, four patients with MAS, all with severe clinical features and three with documented GNAS mutations in peripheral blood, underwent gastrointestinal endoscopy to establish if they had coexisting hamartomatous polyposis. Genetic testing for STK11 and PRKAR1A genes excluded coexistent PJS and Carney complex. Hamartomatous, solely upper gastrointestinal polyps, histologically similar to PJS, were observed in all four patients, with activating GNAS mutations in polyps or adjacent mucosa in three of them. This new finding broadens the spectrum of MAS and indicates the need for routine gastrointestinal endoscopy in MAS, to establish the true incidence of polyps. It also suggests a possible interaction between the STK11 and GNAS gene pathways in terms of possible tumorigenesis.
PATIENTS AND METHODS
Informed consent was obtained from all subjects for genetic analysis and all clinical studies.
No patient had any symptoms related to the gastrointestinal tract or any family history of polyps. All four patients had typical clinical features of MAS, with extensive polyostotic fibrous dysplasia of all long bones, large café-au-lait lesions with a typical coast of Maine outline, and three patients had endocrine manifestations. Peripheral blood and biopsy material from all subjects was examined for GNAS mutations.
Patient 1
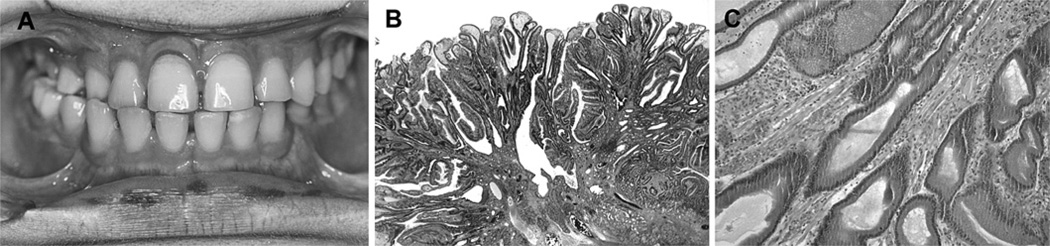
A boy diagnosed at age 10 months later developed endocrinopathies of slowly progressive precocious puberty not requiring treatment, T3 thyrotoxicosis, and acromegaly. At age 15 years, dark brown macular lesions were noted in the lips and oral cavity (figure 1A) reminiscent of perioral freckling described in PJS, suggesting a possible association with gastrointestinal polyps. Gastroscopy and ileo-colonoscopy showed multiple gastric and duodenal polyps with no large bowel polyps. One large duodenal polyp (2×2 cm) was resected with superficial biopsies from other polyps. Subsequent wireless capsule endoscopy demonstrated gastric nodularity and polyps with no small bowel polyps. Repeat endoscopy 1 year later showed a recurrent 1.5 cm duodenal polyp and 10 new small duodenal polyps.
Figure 1.
(A) Clinical photograph of case, showing perioral pigmentary lesions. (B) Duodenal polyp of patient 1 showing fronds with prominent branching covered by well differentiated gastric and duodenal epithelium (haematoxylin and eosin (H&E), low power). (C) Same polyp showing prominent bundles of smooth muscle fibres in the stromal cores (H&E, medium power).
Patient 2
A boy diagnosed at age 2 years later required flutamide and aromatase inhibitors for precocious puberty and somatostatin LAR and cabergoline for GH excess3 Pigmentary lesions of lips, oral cavity and perianal area were noted at age 14 years. Capsule endoscopy showed a large duodenal polyp and multiple small polyps. Ileo-colonoscopy was normal. Repeat endoscopy 1 year later demonstrated a new small duodenal polyp.
Patient 3
A boy diagnosed at age 3 underwent radioactive iodine thyroid ablation for T3 thyrotoxicosis at age 14 years. There was no perioral freckling. Capsule endoscopy was performed at age 23 years following identification of gastrointestinal polyps in patients 1 and 2.This showed two small pedunculated duodenal polyps (<5 mm). Ileo-colonoscopy was normal. Repeat endoscopy 2 years later demonstrated a ‘carpet’ of multiple small gastric polyps.
Patient 4
This boy was diagnosed at age 3 years. He did not have endocrinopathies or perioral freckling. Capsule endoscopy, performed at age 20 years for screening, revealed five small gastric polyps (<5 mm). Gastroscopy showed a single sessile polyp (5 mm) in the first part of the duodenum.
Pathology
Lesion histology was best demonstrated in specimens from patient 1, because sessile polyps up to 12 mm were removed intact. This showed a prominent arborising pattern with prominent bundles of smooth muscle fibres in the lamina propria (figure 1B,C). Gastric pits were elongated. Some cells in the superficial gastric epithelium contained large supranuclear globules of mucin. In the same polyp were seen fundal and pyloric glands, with Brunner’s glands in the submucosa. Oedema with round cells and dilated vessels was seen in some but there was no undue proliferative activity, nuclear atypia or tumour. The features were very similar to the hamartomatous polyps in PJS. In the other patients, specimens were small biopsies taken from polyp surfaces. Not all the changes were present in any one, but elongation of gastric foveolae, enlarged mucin globules in superficial gastric epithelium, and focal prominent bundles of smooth muscle fibres in the lamina propria were present in every patient.
DNA extraction
All germline genetic testing was performed on genomic DNA extracted from blood lymphocytes. Using the method described by Malchoff et al,4 without modification. DNA isolated from formalin fixed tissue was performed according to the method of Gross-Bellard et al5 without modification.
DNA Sequencing
STK11, PRKARIA, PDE11A and PDE8B were screened by direct DNA sequencing.6,7 Dideoxy sequencing of PCR products used a BIGDYE dideoxy sequencing Ready Reaction kit (PE Foster City, California, USA), analysed on an ABI 3730 automated sequencer (PE Foster City). The reference sequence used for this analysis was NM_000516.4.
GNAS was screened by direct sequencing of leucocyte genomic DNA extracted from peripheral blood samples, polyps and adjacent normal looking mucosa, using a PCR based technique.8
Multiplex ligation dependant probe amplification reaction
A multiplex ligation dependant probe amplification (MLPA) assay (MRC-Holland, Amsterdam, Netherlands) was performed according to the manufacturer’s instructions, as previously described.6
RESULTS
Mutational analysis that included the intron exon boundaries for STK11, PRKARIA, PDE11A and PDE8B for all four subjects failed to reveal mutations in any of these genes. In addition, since exon deletions are common in PJS, MLPA analysis was undertaken. This failed to reveal any loss or gain of exons in STK11. Genetic testing of peripheral blood revealed the following.
Patients 1 and 4 were heterozygous for the common c.393C → T (I131I) variant. Patients 2 and 3 were homozygous for the wild type allele (393C).
In peripheral blood DNA, patients 1 and 3 harboured an activating GNAS mutation c.602 G → A (G201H). Patient 2 had an activating GNAS mutation in the c.601C → T (R201C). No mutation was found in peripheral blood DNA for patient 4.
DNA derived from paraffin embedded tissue block of the polyp and adjacent normal mucosa snared by gastroscopy revealed no GNAS mutation in patient 1. Patient 2 harboured c.601C → T only in the block from normal mucosa, suggesting a loss of heterozygosity at this locus. Patient 3 harboured the 602G → A mutation in both polyp and normal mucosa. Patient 4 harboured c.602G → A only in the polyp (table 1).
Table 1.
Clinical and laboratory features
| Feature | Case 1 | Case 2 | Case 3 | Case 4 |
|---|---|---|---|---|
| Age at endoscopy | 15 years | 14 years | 23 years | 20 years |
| Clinical features | ||||
| Fibrous dysplasia | Polyostotic | Polyostotic | Polyostotic | Polyostotic |
| Café-au-lait spots | Multiple | Multiple | Multiple | Multiple |
| Endocrinopathies | Early puberty | GH excess precocious puberty | T3 toxicosis | Absent |
| Oral freckling | Present | Present | Absent | Absent |
| Polyp | ||||
| Oesophageal | Absent | Absent | Absent | Absent |
| Gastric | 2 small, 4 on repeat | Absent | Absent, multiple on repeat | 5 small |
| Duodenal | 1 large, multiple small, 7 on repeat | 1 large, multiple small, +1 large on repeat | 2 small | 1 small |
| Colon | Absent | Absent | Absent | Absent |
| Mutations in blood | 602G → A heterozygous | 601C → T heterozygous | 602G → A heterozygous | |
| Mutations in mucosa | 601C → T heterozygous | 602G → A heterozygous | ||
| Mutations in polyp | 602G → A heterozygous | 602G → A heterozygous |
DISCUSSION
This is the first report of a consistent finding of gastrointestinal polyps in a series of patients with MAS. Confirmation of the link with MAS was substantiated by identification of GNAS mutations in peripheral blood, polyps or adjacent mucosa. A single previous autopsy reported very similar features to those seen in PJS hamartomatous polyps and identical to those in our patients.9
The common 393C → T variant (rs7121) identified in all four subjects is not considered pathogenic as it also occurs in the general population at a frequency of 46.3%, thereby indicating its neutral effect. Both the R201C and the R201H changes have been associated with a change in function of the Gsα. Examination of the respective tumours from the four patients revealed the presence of activating mutations in three of the four cases, confirming the notion that this is a postzygotic event. In one case, however, the R201C alteration present in DNA derived from peripheral blood was absent in DNA obtained from a gastrointestinal polyp, reflecting a loss of heterozygosity.
Hamartomatous gastrointestinal polyps have been associated with inactivating germline mutations in tumour suppressor genes which result in increased cellular growth: SMAD4 and BMPR1A in juvenile polyposis syndrome, PTEN in Cowden syndrome, and STK11 in PJS.10 Those in the first three conditions usually show the histology of juvenile polyps. Only those in PJS consistently show the typical branching pattern with prominent cores of smooth muscle fibres, similar to those reported here.
cAMP is a key mediator of signal transduction of multiple hormones and growth factors. Constitutionally elevated intracellular cAMP levels in MAS suggest an important role of the GNAS-Gsα-cAMP system in regulation of cellular growth, with focal pituitary,11 thyroid12 adrenal13 and testicular14 lesions in this disease. Moreover, isolated gain of function somatic GNAS mutations without features of MAS have been identified in pituitary15 adrenal,16 thyroid,17 and ovarian lesions.18
Gastrointestinal polyps have not been reported in Carney complex but the protein kinase A regulatory unit is found immediately downstream of Gsα. Gastrointestinal tract surveillance may also be indicated in that condition.
PJS is due to inactivating mutations in the STK11 gene coding for a multifunctional serine-threonine kinase, an important tumour suppressor gene modulating cellular growth and polarity. PJS is associated with a significant risk of gastrointestinal and extraintestinal malignancies. The hamartomatous polyps in our patients, together with cutaneous manifestations, indicate an overlap between MAS and PJS. This suggests a possible interaction between STK11 and GNAS, possibly via the cAMP response element-binding protein, a transcription factor activated in response to elevated intracellular cAMP levels, with an important role in the regulation of cellular growth.19
Identification of hamartomatous polyps in MAS may have significant clinical implications. Blood loss, anaemia, intussusception, and malignancy risk are well known complications of hamartomatous polyps in PJS. Repeat endoscopy in two of our patients showed progressive appearance of multiple new gastric and duodenal polyps, emphasising the need for endoscopic surveillance for gastrointestinal tumours in MAS. There are no reports of gastrointestinal malignancy in MAS, perhaps due to a low risk of malignant transformation of gastrointestinal polyps or to a lack of information about long term follow-up. GH excess in MAS, as observed in two of our patients, may alter the risk of gastrointestinal malignancy.20
This report demonstrates a clear association of MAS and gastrointestinal polyps that display distinct histopathological features in common with PJS hamartomatous polyps. Prospective studies involving larger series of patients will better define the association and ascertain the natural history of gastrointestinal polyps in MAS. New mechanisms of tumorigenesis may be identified.
Footnotes
Competing interests None.
Patient consent All patients and parents where the boys were under 18, gave written informed consent for the study, for genetic testing and for publication of findings.
Ethics approval Ethics committee approval was not required as this was not a specific study but a report of clinical investigations undertaken for 4 patients, as part of their standard clinical care.
Contributors Dr Anurag Bajpai wrote the first draft of the paper. Dr Chow performed all pathology and reviewed the paper extensively. Professor Catto-Smith performed the endoscopies and reviewed the paper. Professor Stratakis performed the STK, PDE 11 assays and reviewed the paper. Professor Scott and Michelle Wong performed all the GNAS mutations and Professor Scott reviewed the paper. A/Prof Zacharin rewrote the final paper, collated all data and produced the final document.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991;325:1688–1695. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]
- 2.Zacharin M. The spectrum of McCune Albright syndrome. Pediatr Endocrinol Rev. 2007;(4) Suppl 4:412–418. [PubMed] [Google Scholar]
- 3.Zacharin M. Paediatric management of endocrine complications in McCune-Albright syndrome. J Pediatr Endocrinol Metab. 2005;18:33–41. doi: 10.1515/jpem.2005.18.1.33. [DOI] [PubMed] [Google Scholar]
- 4.Malchoff CD, Reardon G, MacGillivray DC, Yamase H, Rogol AD, Malchoff DM. An unusual presentation of McCune-Albright syndrome confirmed by an activating mutation of the Gs alpha-subunit from a bone lesion. J Clin Endocrinol Metab. 1994;78:803–806. doi: 10.1210/jcem.78.3.8126161. [DOI] [PubMed] [Google Scholar]
- 5.Gross-Bellard M, Oudet P, Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973;36:32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- 6.Chow E, Meldrum CJ, Crooks R, Macrae F, Spigelman AD, Scott RJ. An updated mutation spectrum in an Australian series of PJS patients provides further evidence for only one gene locus. Clin Genet. 2006;70:409–414. doi: 10.1111/j.1399-0004.2006.00704.x. [DOI] [PubMed] [Google Scholar]
- 7.Jenne DE, Reimann H, Nezu J, Friedel W, Loff S, Jeschke R, Müller O, Back W, Zimmer M. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet. 1998;18:38–43. doi: 10.1038/ng0198-38. [DOI] [PubMed] [Google Scholar]
- 8.Candeliere GA, Roughley PJ, Glorieux FH. Polymerase chain reaction-based technique for the selective enrichment and analysis of mosaic arg201 mutations in G alpha s from patients with fibrous dysplasia of bone. Bone. 1997;21:201–206. doi: 10.1016/s8756-3282(97)00107-5. [DOI] [PubMed] [Google Scholar]
- 9.MacMahon HE. Albright’ s syndrome–thirty years later. (Polyostotic fibrous dysplasia) Pathol Annu. 1971;6:81–146. [PubMed] [Google Scholar]
- 10.Zbuk KM, Eng C. Hamartomatous polyposis syndromes. Nat Clin Pract Gastroenterol Hepatol. 2007;4:492–502. doi: 10.1038/ncpgasthep0902. [DOI] [PubMed] [Google Scholar]
- 11.Chen CJ, Liu JY, Cheng SN, Chang FW, Yuh YS. McCune-Albright syndrome associated with pituitary microadenoma: patient report. J Pediatr Endocrinol Metab. 2004;17:365–369. doi: 10.1515/jpem.2004.17.3.365. [DOI] [PubMed] [Google Scholar]
- 12.Mastorakos G, Mitsiades NS, Doufas AG, Koutras DA. Hyperthyroidism in McCune-Albright syndrome with a review of thyroid abnormalities sixty years after the first report. Thyroid. 1997;7:433–439. doi: 10.1089/thy.1997.7.433. [DOI] [PubMed] [Google Scholar]
- 13.Kirk JM, Brain CE, Carson DJ, Hyde JC, Grant DB. Cushing’s syndrome caused by nodular adrenal hyperplasia in children with McCune-Albright syndrome. J Pediatr. 1999;134:789–792. doi: 10.1016/s0022-3476(99)70302-1. [DOI] [PubMed] [Google Scholar]
- 14.Wasniewska M, De Luca F, Bertelloni S, Matarazzo P, Weber g, Crisafulli g, Valenzise M, Lala R. Testicular microlithiasis: an unreported feature of McCune-Albright syndrome in males. J Pediatr. 2004;145:670–672. doi: 10.1016/j.jpeds.2004.06.083. [DOI] [PubMed] [Google Scholar]
- 15.Landis CA, Masters SB, Spada A, Pace AM, Bourne HR, Vallar L. GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature. 1989;340:692–696. doi: 10.1038/340692a0. [DOI] [PubMed] [Google Scholar]
- 16.Fragoso MC, Domenice S, Latronico AC, Martin RM, Pereira MA, Zerbini MC, Lucon AM, Mendonca BB. Cushing’s syndrome secondary to adrenocorticotropin-independent macronodular adrenocortical hyperplasia due to activating mutations of GNAS1 gene. J Clin Endocrinol Metab. 2003;88:2147–2151. doi: 10.1210/jc.2002-021362. [DOI] [PubMed] [Google Scholar]
- 17.Collins MT, Sarlis NJ, Merino MJ, Monroe J, Crawford SE, Krakoff JA, Guthrie LC, Bonat S, Robey PG, Shenker A. Thyroid carcinoma in the McCune-Albright syndrome: contributory role of activating Gs alpha mutations. J Clin Endocrinol Metab. 2003;88:4413–4417. doi: 10.1210/jc.2002-021642. [DOI] [PubMed] [Google Scholar]
- 18.Pienkowski C, Lumbroso S, Bieth E, Sultan C, Rochiccioli P, Tauber M. Recurrent ovarian cyst and mutation of the Gs alpha gene in ovarian cyst fluid cells: what is the link with McCune-Albright syndrome? Acta Paediatr. 1997;86:1019–1021. doi: 10.1111/j.1651-2227.1997.tb15194.x. [DOI] [PubMed] [Google Scholar]
- 19.Siu YT, Jin DY. CREB–a real culprit in oncogenesis. FEBS J. 2007;274:3224–3232. doi: 10.1111/j.1742-4658.2007.05884.x. [DOI] [PubMed] [Google Scholar]
- 20.Matano Y, Okada T, Suzuki A, Yoneda T, Takeda Y, Mabuchi H. Risk of colorectal neoplasm in patients with acromegaly and its relationship with serum growth hormone levels. Am J Gastroenterol. 2005;100:1154–1160. doi: 10.1111/j.1572-0241.2005.40808.x. [DOI] [PubMed] [Google Scholar]