Abstract
MicroRNAs have been involved in the pathogenesis of different types of cancer, however their function in pituitary tumorigenesis remains poorly understood. Cyclic-AMP (cAMP)-dependent protein kinase (PKA)-defective pituitaries occasionally form aggressive growth-hormone (GH)-producing pituitary tumors in the background of hyperplasia caused by haploinsufficiency of the PKA’s main regulatory subunit, PRKAR1A. The molecular basis for this development remains unknown. We have identified a 17-microRNA signature of pituitary tumors formed in the background of hyperplasia (caused in half of the cases by PRKAR1A-mutations). We selected two microRNAs on the basis of their functional screen analysis: inhibition of miR-26b expression and up-regulation of miR-128 suppressed the colony formation ability and invasiveness of pituitary tumor cells. Furthermore, we identified that miR-26b and miR-128 affected pituitary tumor cell behavior through regulation of their direct targets, PTEN and BMI1, respectively. In addition, we found that miR-128 through BMI1 direct binding on the PTEN promoter affected PTEN expression levels and AKT activity in the pituitary tumor cells. Taken together, we have identified a microRNA signature for GH-producing pituitary tumors and found that miR-26b and miR-128 regulate the activity of the PTEN-AKT pathway in these tumors. This is the first suggestion of the possible involvement of microRNAs regulating the PTEN-AKT pathway in GH-producing pituitary tumor formation in the context of hyperplasia or due to germline PRKAR1A defects.
Keywords: Growth-hormone producing adenomas, Carney complex, acromegaly, pituitary hyperplasia, protein kinase A, protein kinase B
Introduction
MicroRNAs (miRNAs) are small non coding RNAs of 19 to 25 nucleotides that negatively regulate gene expression at the post-transcriptional level. They target messenger RNA (mRNA) in a sequence-specific manner, inducing translational repression or mRNA degradation, depending on the degree of complementarity between miRNAs and 3`UTR region of their targets (1). Increasing evidence indicates that miRNAs have distinct expression patterns among tissues and cells at different differentiation stages. Furthermore, several studies have shown that miRNAs are involved in different processes including differentiation metabolism cell growth and apoptosis (2), both in animals and plants. Increasing evidence indicates that several miRNAs are directly involved in the pathogenesis of several human cancers, including lung cancer (3), breast cancer (4), colon cancer (5), Burkitt lymphoma (6) and thyroid cancer (7). Moreover, recent studies revealed that deregulation of miRNAs were responsible for endocrine carcinogenesis, including pancreatic, parathyroid and pituitary tumors (8, 9).
Most of the pituitary tumors are benign neoplasms; they account for 10%–15% of all intracranial tumors (10). These tumors often release pituitary hormones such as the growth hormone (GH), prolactin (PRL), adrenocorticotropic hormone (ACTH), and β-subunit derivatives such as thyroid stimulating hormone (TSH), follicle-stimulating hormone (FSH) and luteinizing hormone (LH). What starts the formation of these tumors remains unknown; equally unclear is what makes these benign lesions acquire an aggressive behavior and spread through local invasion, despite the fact that they technically remain “benign” as they almost never develop distal metastases (11).
Several deregulated miRNAs have been involved not only in pituitary cell proliferation and apoptosis, but also in neoplastic transformation (11). Recently, Bottoni et al., showed that miR-15a and miR-16 were expressed at lower levels in GH-and PRL-secreting pituitary adenomas compared with normal pituitary tissue, were inversely correlated with tumor diameter, and were directly correlated with secretion of the antineoplastic cytokine p43 (12). In addition, miR-132, miR-128a, miR-16 and let-7 expression have been found to be down-regulated in pituitary adenomas relative to normal tissues (13). Amaral et al. identified that miR-145, miR-21, miR-141, let-7a, miR-150, miR-15a, miR-16 and miR-143 levels were suppressed in ACTH-secreting pituitary tumors relative to normal pituitary tissues (14). Collectively, all these data strongly suggest that miRNAs could potentially play an essential role as central regulators of pituitary tumorigenesis and could be potentially useful as clinical markers
We studied a relatively unique setting in pituitary tumor formation, GH-producing tumors that develop in the setting of somatomammotroph hyperplasia. Mostly this happens in the context cyclic-AMP (cAMP)-dependent protein kinase (PKA)-defective glands but not exclusively. Patients with a germline mutation that leads to haploinsufficiency of the PKA’s type 1A regulatory subunit, PRKAR1A, develop invariably GH-producing cell hyperplasia (15). However, only 15% or less of these patients form an adenoma after a series of genomic events involving chromosomal translocations, deletions and amplifications that are unusual for benign tumors (15, 16). Consequently, these tumors like others developing in the context of GH-producing cell hyperplasia (i.e. in McCune-Albright syndrome and in other sporadic cases) tend to be aggressive and relatively resistant to medical therapies (17, 18). Cells from PRKAR1A-defective tumors cultured in vitro are relatively dedifferentiated and pluripotential, expressing both somatomammotroph features (GH and PRL) and β-subunit and its mature derivative hormones (TSH, LH and FSH) (18, 19).
In our patients, we identified a novel microRNA-gene network regulating their tumor formation. Specifically, we found 17 microRNAs to be differentially expressed between their pituitary tumors and normal tissues. MicroRNA screen analysis revealed that suppression of miR-26b, which was the top up-regulated microRNA in pituitary tumors, and up-regulation of miR-128, which was the top down-regulated microRNA in pituitary tumors, suppressed the colony formation ability and invasiveness of pituitary tumor cells. We identified that miR-26b and miR-128 controlled the pituitary cell properties through regulation of their direct targets, PTEN and BMI1, respectively. Interestingly, miR-128 through BMI1 binding on the PTEN promoter affected PTEN expression levels in pituitary tumor cells. This is the first demonstration of the involvement of microRNAs and the PTEN-AKT pathway in GH-producing pituitary tumor formation in the context of hyperplasia or due to germline PRKAR1A defects.
Results
MicroRNA signature of GH-producing pituitary tumors
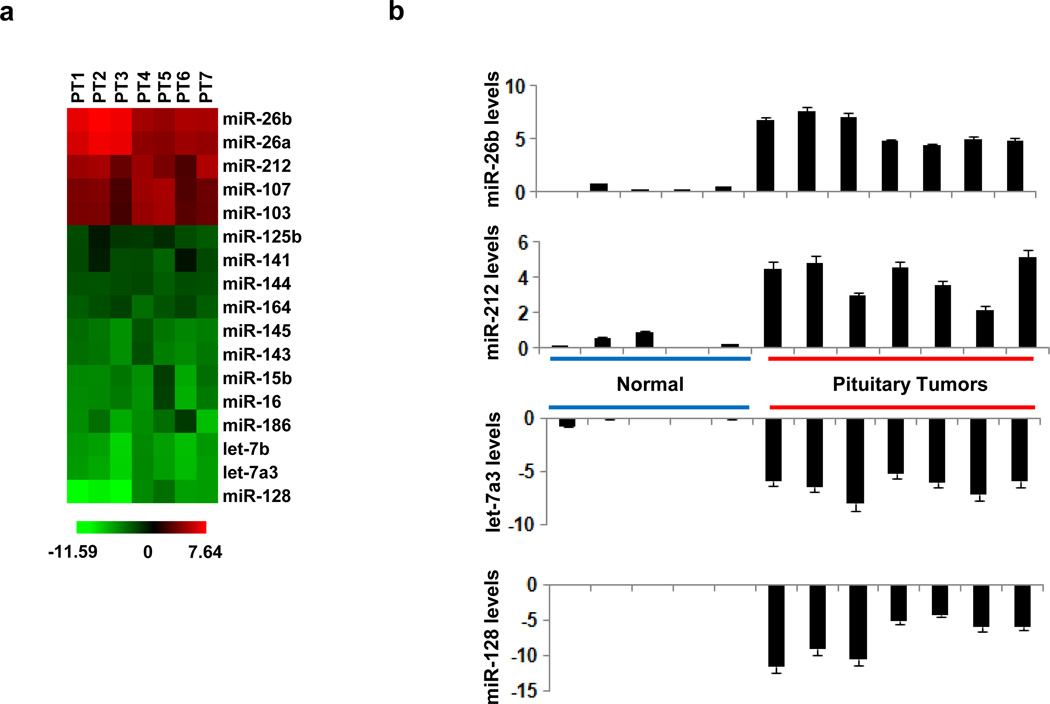
We identified 17 microRNAs to be differentially expressed between tumors and normal pituitary tissue (Figure 1a). Specifically, we identified 5 microRNAs (miR-26b, miR-26a, miR-212, miR-107, miR-103) to be up-regulated and 12 microRNAs (miR-125b, miR-141, miR-144, miR-164, miR-145, miR-143, miR-15b, miR-16, let-7b, let-7a3, miR-128) to be down-regulated in pituitary tumors relative to normal tissues. The microRNA array data were validated by performing microRNA real-time PCR analysis (Figure 1b). Both analyses revealed that miR-26b (~6-fold) and miR-212 (~4-fold) were the top two up-regulated microRNAs while let-7a3 (~6-fold) and miR-128 (~7.5-fold) were the top two down-regulated microRNAs in tumors relative to control tissues, suggesting their potential role in pituitary tumor formation
Figure 1.
MicroRNA signature of GH-producing pituitary tumors. (a) Heatmap representation of differentially expressed microRNAs identified by microRNA array analysis between normal and cancer (patients 1–7) tissues. (b) MiR-26b, miR-212, let-7a3 and miR-128 expression levels assessed by real-time PCR analysis in 5 normal and 7 cancer pituitary tissues. The experiment has been performed in triplicate and data are shown as mean ± SD.
MiR-26b and miR-128 regulate the tumorigenicity and invasiveness of pituitary cells
To assess the functional role of these differentially expressed microRNAs, we tested whether inhibition of up-regulated microRNAs or overexpression of down-regulated microRNAs affect the tumorigenicity and invasiveness of pituitary cancer cells. Specifically, AtT-20 pituitary cancer cells were transfected with antisense microRNAs for the five up-regulated microRNAs and with microRNA mimics for the twelve down-regulated microRNAs and tested their ability to form colonies in soft agar (Figure 2a). We found that the inhibitors against miR-26b and miR-26a and the overexpression of miR-128 inhibited >60% the ability of AtT-20 cells to form colonies in soft agar. In addition, we examined the effects of the differentially expressed microRNAs on pituitary cancer cell invasiveness. We transfected AtT-20 cells with antisense microRNAs for the five up-regulated microRNAs and with microRNA mimics for the twelve down-regulated microRNAs for 24h and then cells were seeded in matrigel invasion plates. After 16h (total 40h from the initiation of the experiment), we examined the invasive ability of these transfected cells (Figure 2b). We identified that inhibition of miR-26b and miR-26a and overexpression of miR-128 and miR-186 suppressed >60% the invasive ability of AtT-20 cells (Figure 2c). These data revealed that inhibition of miR-26b and overexpression of miR-128 control more efficiently the tumorigenicity and invasiveness of AtT-20 pituitary tumor cells. Interestingly, miR-26b is the highest up-regulated microRNA while miR-128 is the top down-regulated microRNA in human pituitary tumors relative to normal tissues
Figure 2.
MiR-26b and miR-128 regulate the colony formation ability and invasiveness of pituitary tumor cells. (a) Number of colonies (mean ± SD) of AtT-20 pituitary cells untreated or treated with 50nM microRNAs (miR-125b, miR-141, miR-144, miR-164, miR-145, miR-143, miR-15b, miR-16, miR-186, let-7b, let-7a3, miR-128) or anti-sense microRNAs (as-miR-26b, as-miR-26a, miR-128, as-miR-212, as-miR-107, as-miR-103) for 48h. (b) Schematic of microRNA library screen in AtT-20 cells in order to assess which microRNAs affect their invasiveness. (c) Number of invading cells (mean ± SD) of AtT-20 pituitary cells untreated or treated with 50nM microRNAs (miR-125b, miR-141, miR-144, miR-164, miR-145, miR-143, miR-15b, miR-16, miR-186, let-7b, let-7a3, miR-128) or anti-sense microRNAs (as-miR-26b, as-miR-26a, miR-128, as-miR-212, as-miR-107, as-miR-103).
Identification of miR-26b and miR-128 direct gene targets
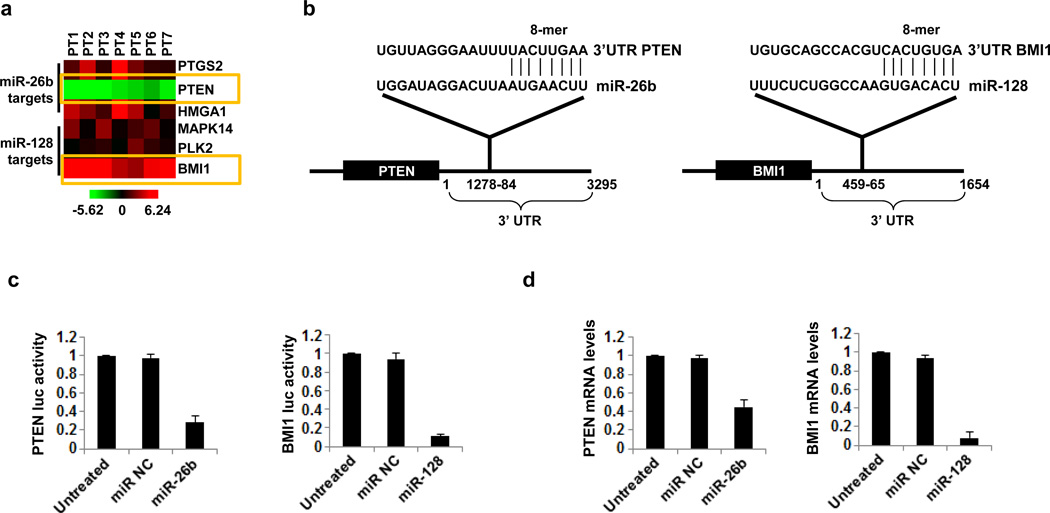
We were interested in identifying miR-26b and miR-128 down-stream direct targets. Bioinformatic analysis revealed that the top three identified direct gene targets according different criteria (described in materials and methods) were PTGS2, PTEN and HMGA1 for miR-26b and MAPK14, PLK2 and BMI1 for miR-128 (Table 1). Due to the fact that microRNAs frequently act as negative regulators of gene expression, we were interested in identifying if there is an inverse correlation between the microRNAs and these predicted direct gene targets. Thus, we tested the mRNA expression levels of these potential gene targets and identified an inverse correlation between miR-26b and PTEN (down-regulated) and between miR-128 and BMI1 (up-regulated) in our pituitary tumors (Figure 3a).
Table 1.
Top 3 microRNA predicted direct targets
| MicroRNA | Symbol | Gene Description |
|---|---|---|
| miR-26b | PTGS2 | prostaglandin-endoperoxide synthase 2 |
| PTEN | phosphatase and tensin homolog | |
| HMGA1 | high mobility group AT-hook 1 | |
| miR-128 | MAPK14 | mitogen-activated protein kinase 14 |
| PLK2 | polo-like kinase 2 | |
| BMI1 | BMI1 polycomb ring finger oncogene 1 |
Figure 3.
MiR-26b regulates PTEN expression and miR-128 regulates BMI1 expression in pituitary cancers. (a) Heatmap representation of PTGS2, PTEN, HMGA1, MAPK14, PLK2 and BMI1 mRNA expression levels in 7 pituitary tumors assessed by real-time PCR analysis. (b) Sequence complementarity between miR-26b and the 3’UTR of PTEN gene and between miR-128 and the 3’UTR of BMI1 gene. (c) PTEN 3’UTR luciferase activity (mean ± SD) and (d) mRNA expression levels (mean ± SD) in untreated or miR negative control (miR-NC) and miR-26b treated HEK293 cells for 24h.
To identify whether miR-26b and miR-128 regulate directly PTEN and BMI1, respectively, through binding in their 3’UTRs (Figure 3b), we performed luciferase assay. Specifically the 3’UTRs of PTEN and BMI1 were cloned under luciferase, and luciferase activity was measured after overexpression of miR-26b and miR-128, respectively. We found that miR-26b overexpression inhibited >60% PTEN luciferase activity while miR-128 overexpression inhibited 85% of BMI1 luciferase activity, validating the direct interactions between miR-26b and PTEN and miR-128 and BMI1 (Figure 3c). Furthermore, overexpression of miR-26b blocked >50% PTEN mRNA levels while miR-128 overexpression blocked >90% BMI1 mRNA levels (Figure 3d). All these data suggest that miR-26b regulates directly PTEN expression levels while miR-128 regulates directly BMI1 expression levels in pituitary tumor cells.
MiR-26b and miR-128 control pituitary tumor formation and cell invasiveness through regulation of their direct targets
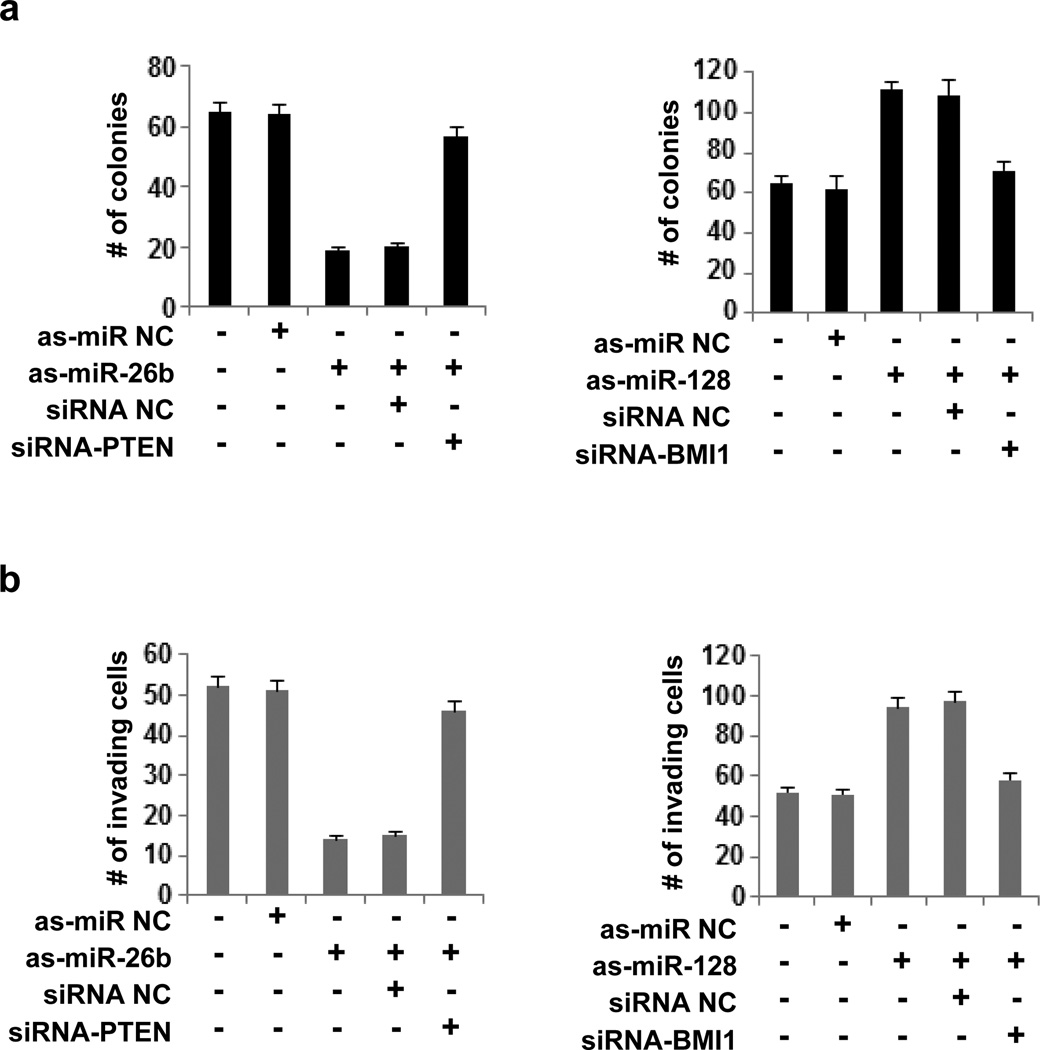
We were interested in studying if the effects of miR-26b and miR-128 on the ability of pituitary cells to form colonies in soft agar and to invade were mediated by PTEN and BMI1, respectively. Inhibition of miR-26b resulted in suppression of colony growth; this suppression was blocked when PTEN was knocked down by siRNA against PTEN (Figure 4a). In addition, inhibition of miR-128 increased the ability of pituitary cells to form colonies in soft agar; inhibition of BMI1 by siRNA blocked this effect. We got similar data when we tested the invasiveness of these cells in the same experimental conditions (Figure 4b), suggesting that miR-26b and miR-128 regulate the invasiveness and colony formation of AtT-20 cells through regulation of their direct downstream targets.
Figure 4.
MiR-26b and miR-128 control the tumorigenicity and invasiveness of pituitary tumors cell through regulation of PTEN and BMI1, respectively. (a) Number of colonies (mean ± SD) and (b) invading AtT-20 cells untreated or treated with 50nM antisense-microRNA negative control (as-miR-NC), antisense-microRNA-26b (as-miR-26b), antisense-microRNA-128 (asmiR-128), siRNA negative control (siRNA NC) and siRNA against PTEN (siRNA-PTEN).
MiR-26b and miR-128 regulate PTEN-AKT pathway in pituitary AtT-20 cells
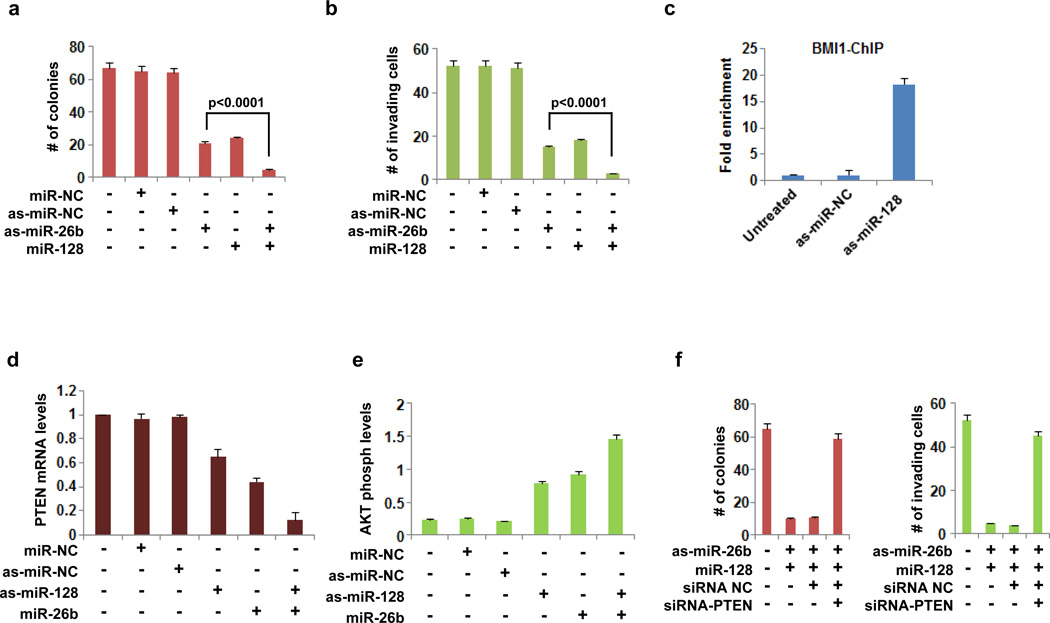
We were interested in testing if there is any synergy between miR-26b and miR-128 affecting pituitary tumor formation and/or progression. We found that the combination of inhibition of miR-26b and up-regulation of miR-128 had a more potent effect on suppressing colony formation and invasiveness of AtT-20 cells (Figures 5a, b).
Figure 5.
MiR-26 and miR-128 regulate the PTEN-AKT pathway in AtT-20 pituitary cells. (a) Number of colonies and (b) invading AtT-20 cells, untreated or treated with 50nM miR-NC, asmiR-NC, as-miR-26b and miR-128. (c) Fold enrichment of BMI1 in the promoter area of PTEN in AtT-20 cells treated with 50NM as-miR-NC or as-miR-128, assessed by chromatin immunoprecipitation followed by real-time PCR analysis. (d) PTEN mRNA expression levels (mean ± SD) assessed by real-time PCR analysis and E, AKT phosphorylation levels (S473) in AtT-20 cells treated for 48h with 50nM as-miR-NC, as-miR-128, miR-26b and their combinations. (f) Number of colonies and invading AtT-20 cells, untreated or treated with 50nM as-miR-26b and miR-128 or combination of as-miR-26b, miR-128 and siRNA NC or combination of as-miR-26b, miR-128 and siRNA-PTEN. The experiments have been performed in triplicate and data are shown as mean ± SD.
Due to the fact that inhibition of miR-26b and up-regulation of miR-128 had very similar effects on AtT-20 cells, we examined if there was a molecular link between miR-26/PTEN and miR-128/BMI1 signaling pathways. Interestingly, a recent study has shown that BMI1 is a transcriptional suppressor of PTEN expression levels in nasopharyngeal carcinomas (20). Thus, we examined if BMI1 regulated the expression of PTEN in pituitary AtT-20 cells by performing chromatin immunoprecipitation analysis with an antibody against BMI1 followed by PCR analysis in the promoter region of PTEN in AtT-20 cells (Figure 5c). We found that inhibition of miR-128 resulted in increased expression of BMI1 in AtT-20 cells. Then, we tested PTEN mRNA levels by real-time PCR analysis. We identified that inhibition of miR-128 resulted in suppression of PTEN mRNA levels in AtT-20 cells (Figure 5d). The combination of inhibition of miR-128 and overexpression of miR-26b blocked even more effectively PTEN mRNA levels. Overall, these data suggested that both miR-128 (through BMI1 binding) and miR-26b (directly) regulated PTEN expression levels in pituitary AtT-20 cells. It is well known that PTEN is a negative regulator of the AKT signaling pathway in multiple cancers. We found that inhibition of miR-128 and overexpression of miR-26b resulted in increased AKT activity (S473 phosphorylation levels) in AtT-20 cells (Figure 5e). These data suggested that miR-128 and miR-26b regulate PTEN-AKT pathway in pituitary cancer. To examine the role of the miR-128/miR-26/PTEN-AKT axis in our pituitary tumors, we studied the effects of miR-128 and/or miR-26b in the presence or absence of PTEN. We identified that suppression of PTEN expression by siRNA, blocked the inhibitory effects on colony formation and invasiveness of miR-26b down-regulation and miR-128 up-regulation in AtT-20 cells (Figure 5f), suggesting that the miR-26b and miR-128 function in these cells depended on PTEN-AKT pathway activation.
The miR-26b/miR-128/PTEN pathway in the GH-producing pituitary tumors
We finally tested in the human tumors we studied here the expression levels of the different members of the signaling pathway that we identified above. We found an inverse correlation between miR-26b and PTEN (r=−0.8431), miR-128 and BMI1 (r=−0.8725) and miR-128 and PTEN (r=−0.7236) (Figure 6a).
Figure 6.
MicroRNA-gene networks regulating pituitary oncogenesis. (a) Correlation (r represents the correlation coefficient) in the expression levels of miR-26b, miR-128, PTEN and BMI1 in human pituitary tumors. (b) Schematic of miR-26b and miR-128 signaling pathway in pituitary oncogenesis.
Thus, overall, in this study we have identified a novel signaling pathway (Figure 6b). Specifically, we found that miR-26b is highly up-regulated while miR-128 is highly down-regulated in GH-producing pituitary tumors developing from somatomammotroph hyperplasia. MiR-26b increased levels suppressed directly PTEN expression while miR-128 low levels resulted in the up-regulation of its direct target BMI1. Furthermore, we identified that BMI1 binds in the promoter area of PTEN and suppresses its expression resulting in activation of the AKT kinase.
Discussion
In the past few years, several miRNAs have been implicated in various human cancers. Both losses and gains of miRNAs function have been shown to contribute to cancer development through different mechanisms. To characterize the microRNAome of pituitary oncogenesis, initially we analyzed microRNA expression levels in a unique setting, that of relatively aggressive GH-producing tumors, developing in the context of pituitary somatomammotroph hyperplasia. We found 17 microRNAs differentially expressed, and among them, two that ended up being involved in the same pathway controlling PTEN/AKT signaling. MiR-26 and miR-212 were the top up-regulated miRNAs while miR-16, let-7a3 and miR-128 were the top down-regulated miRNAs in the studied pituitary tumors relative to normal tissues. Previous studies have linked these microRNAs with the pathogenesis of different types of cancer through regulation of key gene targets. Specifically, miR-15 and miR-16 have been found to act as tumor suppressors through regulation of the anti-apoptotic BCL2 (21). In addition, let-7 family members are down-regulated in different cancer types and directly target several oncogenes such as KRAS, HMGA2 and IL-6 (22–24). Furthermore, miR-26 suppresses tumorigenesis in c-Myc-driven B lymphoma cells (25) and is located on chromosome 3p21.3 which is frequently deleted in small cell lung carcinomas, renal cell carcinomas, and breast carcinomas (26). Recently, miR-212 down-regulation has been found to be involved in lung cancer response to chemotherapy (26). Furthermore, Incoronato et al. suggested that miR-212 acts as a tumor suppressor through direct regulation of anti-apoptotic protein PED (27). All these data suggest that the deregulated microRNAs in pituitary tumors are also involved in the pathogenesis of other cancer types.
Computational and molecular analyses revealed that miR-26b and miR-128 regulate the expression levels of PTEN and BMI1. PTEN is one of the most frequently mutated tumor suppressor genes in human cancers, leading to the activation of the PI3K/AKT signaling pathway and increased cell survival and oncogenesis (28–30). Numerous reports suggest that the transcriptional role of PTEN lies at a key position in a complex network of tumor suppressor and oncogenes that regulate cellular transformation. Specifically, stress kinase pathways such as MEKK4 and JNK promote resistance to apoptosis, suppressing PTEN transcription activity by direct binding of NF-κB to the PTEN promoter (31). Similarly, deregulation of BMI-1 has been found to alter cell proliferation, apoptosis, senescence, and stem cell self-renewal (32–35) and correlated with the invasive and metastatic phenotype of several human cancer types (36–38). Furthermore, BMI-1 is a member of the Polycomb group (PcG) family of proteins (35).The PcG proteins function within distinct multi-subunit complexes and epigenetically regulate gene expression by altering chromatin states at specific promoters (35, 37).
Our analysis identified that miR-128 which targets BMI1 expression also affected PTEN expression levels. Interestingly, we found that BMI1 binds in the promoter region of PTEN, suppressing its expression levels. Consistent with our data, a previous study has shown that BMI1 represses PTEN by binding to the PTEN promoter locus (20).
Overall, microRNAs and transcription factors play a critical regulatory role in tumor formation and/or progression. Like transcription factors, microRNAs are trans-acting molecules that interact with a number of other regulatory elements. They, thus, generate a complex grid of communications between signaling pathways (39) that are uncovered only after studies like the one reported here. In this investigation, the unexpected involvement of the PTEN-AKT pathway in GH-producing pituitary tumors was uncovered. These finding should be followed by additional research and the potential use of PTEN-AKT-targeting molecules in vivo and in vitro that may lead to new therapies for patients with aggressive GH-producing pituitary tumors.
Materials and Methods
Subjects and DNA studies
Seven human pituitary tumors and 5 normal pituitary tissues were collected, the latter from unidentified human cadavers. All normal tissues were examined histologically for any lesions prior to use in this experiment and screened negative for all genes that the 7 tumors were screened for (see below). Tissue from all patients was collected at surgery under research protocols approved by the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). Tissues from these patients and the normal control tissues have been used in our previous studies (15, 16, 18, 19, 40–42) and their histology and clinical cases have been extensively presented (15, 16, 18, 19, 42). All tumors met the criteria for GH-producing tumors surrounded by hyperplasia (15, 18, 43) and also stained positively for both PRL and GH, which identified them of being of the somatomammotroph lineage. All tumors with PRKAR1A mutations displayed loss of heterozygosity (LOH) for the PRKAR1A locus on 17q22-24 (15, 40, 42, 44). All tumor samples were sequenced for activating mutations in the GNAS gene and were found to be negative (40). In addition to PRKAR1A (42, 44), all peripheral DNA samples of the patients were sequenced for the AIP, MEN1, CDKN1B, and CDKN2C genes and were found to be negative for any coding sequence mutations (40). The case of patient #2 has been published as a single patient report (45); he was also sequenced for PTEN mutations with negative results. Cells from patient #5’s tumor were the ones studied in vitro previously (19). Patients #2, #4, #6 and #7 had no PRKAR1A mutations; patients #1, #3, and #5 carried the c.491_492delTG/p.Val164fsX4, c.693insT/p.Arg232X, and c.693insT/p.Arg232X (same as #1, but from unrelated family) mutations, respectively. These mutations lead to nonsense mRNA-mediated decay (NMD) of the mutant allele and, thus, in combination with the LOH of the normal allele lead to no expression of the PRKAR1A gene in pituitary tumor tissues, as we have demonstrated elsewhere (42, 44).
MicroRNA Expression Analysis
The RNA isolation was performed using the mirVana miRNA isolation Kit (Ambion, Inc, TX, USA) according to the manufacturer’s instructions. TaqMan microRNA array assays were used in order to study the expression levels of 365 microRNAs in 7 pituitary tumors and 5 normal pituitary tissues.
MicroRNA Real-Time PCR Analysis
We validated our results with real time (RT) polymerase chain reaction (PCR), using the miRvana qRT-PCR miRNA detection kit and qRT-PCR primer sets, according to the manufacturer’s instructions (Ambion, Inc, TX, USA). We used the U6 small nuclear RNA as internal control. RT PCR experiments were performed in triplicate and the data are presented as mean ± SD.
Colony Formation Assay
AtT-20 pituitary cancer cells (purchased by ATCC) were transfected with 50nM antisense microRNAs (as-miR-26b, as-miR-26a, miR-128, as-miR-212, as-miR-107, as-miR-103) for the five up-regulated microRNAs and with 50nM microRNA mimics (miR-125b, miR-141, miR-144, miR-164, miR-145, miR-143, miR-15b, miR-16, miR-186, let-7b, let-7a3, miR-128) for the twelve down-regulated microRNAs for 48h. Then, samples in triplicate of 105 cells were mixed 4:1 (v/v) with 2.0% agarose in growth medium for a final concentration of 0.4% agarose. The cell mixture was plated on top of a solidified layer of 0.5% agarose in growth medium. Cells were fed every 6 to 7 days with growth medium containing 0.4% agarose. The number of colonies was counted after 20 days. The experiment was repeated thrice and the statistical significance was calculated using Student’s t test.
Invasion Assays
AtT-20 cells were transfected with 50nM antisense microRNAs (as-miR-26b, as-miR-26a, miR-128, as-miR-212, as-miR-107, as-miR-103) for the five up-regulated microRNAs and with 50nM microRNA mimics (miR-125b, miR-141, miR-144, miR-164, miR145, miR-143, miR-15b, miR-16, miR-186, let-7b, let-7a3, miR-128) for the twelve down-regulated microRNAs for 24h. Invasion of matrigel has been conducted by using standardized conditions with BDBioCoat growth factor reduced MATRIGEL invasion chambers (PharMingen). Assays were conducted according to manufacturer’s protocol, by using 10% FBS as chemoattractant. Non-invading cells on the top side of the membrane were removed while invading cells were fixed and stained with 4′-6-diamidino-2-phenylindole (DAPI, Vector Laboratories Inc.), 16h post seeding. In all assays, 10 fields per insert were scored and SD was measured. The experiment was repeated thrice and the statistical significance was calculated using Student’s t test.
MicroRNA Target Prediction Methods
We used three databases to detect the putative microRNA gene-targets: miRBase (http//microrna.sanger.ac.uk), miRanda (http:/www.microrna.org) and Target scan version 4.2 (http://www.targetscan.org/index.html) databases. Then, we selected the commonly predicted microRNA targets from the three databases and those that were conserved in other species, aiming to the higher biological significance of our results. Studies were then planned according to known pathway interactions per micro-RNA family (20–23).
MicroRNA Transfection Experiments
AtT-20 pituitary cells were seeded in 6-well plates and were transfected with 50 nM miR-26b or miR-128 (Ambion, Inc, TX, USA) using siPORT NeoFX transfection kit. siPORT NeoFX is a lipid transfection agent consisting of a mixture of lipid that spontaneously forms a complex of microRNAs and facilitates its transfer to target cells. Transfection with 50nM of scramble negative control microRNA was used as an internal control. No cell toxicity was detected due to the transfection agent (data not shown). RNA was extracted 48 hours after microRNA transfection and RT PCR analysis was performed as described above. RT PCR experiments were performed in triplicate and the data are presented as mean ± SD.
Luciferase reporter assay
HEK293 cells in 24-well plates were transfected using Fugene6 (Roche, Penzberg, Germany). 50nM of miR-26b or miR-128, the firefly luciferase reporter gene construct (PTEN 3’UTR in pEZX-MT01 vector or BMI1 3’UTR in pEZX-MT01 vector) (200 ng) and 1 ng of the pRL-SV40 Renilla luciferase construct (for normalization) were co-transfected per well. Cell extracts were prepared 24h after transfection, and the luciferase activity was measured using the Dual Luciferase Reporter Assay System (Promega, WI, USA). Luciferase reporter assays were performed in triplicate and the data are presented as mean ± SD.
Chromatin Immunoprecipitation
Chromatin immunoprecipitation was carried out as described previously (24, 46). Briefly, the chromatin fragments, derived from untreated, 50nM as-miR-NC or as-miR-128a treated (24h) AtT-20 cells, were immunoprecipitated with 6ug of antibody against BMI1. DNA extraction was performed using Qiagen Purification Kit. Real-time PCR analysis was performed for BMI1 binding site in PTEN promoter area using the following primers: forward 5’-ACCTTTGCCGG GTCTCTCT -3’ and reverse 5’- AGAGTCCCGCCACATCAC-3’ (PCR product: 258bp).
Real-Time PCR Analysis
The oligonucleotide primers used for β-actin forward: 5’- CCTGTACGCCAACACAGTGC-3’ and reverse 5’- ATACTCCTGCTTGCTGATCC-3’; for PTEN forward: 5’-CCGAAAGGTTTTGCTACCATTCT-3’ and reverse 5’-AAAATTA TTTCCTTTCTGAGCATTCC-3’; for BMI1 forward: 5’-AATCTAAGGAGGAGGTGA-3’ and reverse: 5’-AAACAAGA AGAGGTGGA-3’; for HMGA1 forward: 5’- GAAGGAGCCCAGCGAAGTG -3’ and reverse: 5’- TTCTCCAGTTTTTTGGGTCTGC -3’; for PTGS2 forward: 5’- GAATCATTCACCAGGCAAATTG-3’ and reverse 5’- TCTGTAC TGCGGGTGGAACA-3’; for MAPK14 forward: 5’- GCCGAGCTGTTGACTGGAAG-3’ and reverse: 5’-GGAGGTCCCTGCTTTCAAAGG-3’; for PLK2 forward: 5’- AGATCTCGCGGATTATCGTC-3’ and reverse 5’-TCGTAACATTTTGCAAAGCC-3’. All the samples were processed in triplicates. The average value was used for the measurements and the results were normalized using the expression levels of the housekeeping gene β-actin. Real-time PCR experiments were performed in triplicate.
ELISA assay for AKT S473 phosphorylation status
AtT-20 cells were treated with 50nm of miR-NC, as-miR-NC, as-miR-128 and/or miR-26b for 48h, the cells were lysed and AKT S473 phosphorylation status was measured by an ELISA immunoassay kit (cat no. KCB887, R&D Systems).
Statistical analysis
A two-sample t test was done for experiments described above. Experiments were done at least in triplicate, and a mean was calculated. A P value of <0.05 was considered significant.
References
- 1.Bartel DP. MicroRNAs: Genomics,biogenesis,mechanism and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 2.Croce CM, Calin GA. MicroRNAs, cancer and stem cell division. Cell. 2005;122:6–7. doi: 10.1016/j.cell.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 3.Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64:3753–3756. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 4.Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 5.Bandres E, Bitarte N, Arias F, Agorreta J, Fortes P, Agirre X, et al. macrophage migration inhibitory factor production and proliferation of gastrointestinal cancer cells. Clin Cancer Res. 2009;15:2281–2290. doi: 10.1158/1078-0432.CCR-08-1818. [DOI] [PubMed] [Google Scholar]
- 6.Metzler M, Wilda M, Busch K, Viehmann S, Borkhardt A. High expression of precursor microRNA-155-/BIC RNA in children with Burkitt lymphoma. Genes Chromosome Cancer. 2004;39:167–169. doi: 10.1002/gcc.10316. [DOI] [PubMed] [Google Scholar]
- 7.He H, Jazdzewski K, Li W, Liyanarachchi S, Nagy R, Volinia S, et al. The role of microRNA gene in papillary thyroid carcinoma. Pro Natl Acad Sci USA. 2005;102:19075–19080. doi: 10.1073/pnas.0509603102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stilling G, Sun Z, Zhang S, Jin L, Righi A, Kovacs G, et al. MicroRNAs expression in ACTH-producing pituitary tumors up regulation of microRNA-122 and -493 in pituitary carcinoma. Endocrine. 2010;38:67–75. doi: 10.1007/s12020-010-9346-0. [DOI] [PubMed] [Google Scholar]
- 9.Rahbari R, Holloway AK, He M, Khanafshar E, Clark OH, Kebebew E. Identification of differentially expressed microRNA in parathyroid tumors. Ann Surg Oncol. 2011;2:1158–1165. doi: 10.1245/s10434-010-1359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asa SL, Ezzat S. The cytogenesis and pathogenesis of pituitary adenoma. Endocr Rev. 1998;19:798–827. doi: 10.1210/edrv.19.6.0350. [DOI] [PubMed] [Google Scholar]
- 11.Asa SL, Ezzat S. The pathogenesis of pituitary tumors. Nat Rev Cancer. 2001;2:836–849. doi: 10.1038/nrc926. [DOI] [PubMed] [Google Scholar]
- 12.Bottoni A, Piccin D, Tagliati F, Luchin A, Zatelli MC, degli Uberti EC. MiR-15a and miR-16-1 downregulation in pituitary adenomas. J Cell Physiol. 2005;204:280–285. doi: 10.1002/jcp.20282. [DOI] [PubMed] [Google Scholar]
- 13.Bottoni A, Zatelli MC, Ferracin M, Tagliati F, Piccin D, Vignali C, et al. Identification of differentially expressed microRNAs by microarray: a possible role for microRNA genes in pituitary adenomas. Journal Cell Physiol. 2007;210:370–377. doi: 10.1002/jcp.20832. [DOI] [PubMed] [Google Scholar]
- 14.Amaral FC, Torres N, Saggioro F, Neder L, Machado HR, Silva WA, Jr, et al. MicroRNAs differentially expressed in ACTH-secreting pituitary tumors. J Clin Endocrinol Metab. 2009;94:320–323. doi: 10.1210/jc.2008-1451. [DOI] [PubMed] [Google Scholar]
- 15.Pack S, Kirshner LS, Pak E, Carney JA, Zhuang Z, Stratakis CA. Pituitary tumors in patients with the “complex of spotty skin pigmentation, myxomas, endocrine overactivity and schwannomas” (Carney complex): evidence for progression from somatomammotroph hyperplasia to adenoma. J Clin Encocrinol Metab. 2000;85:3860–3865. doi: 10.1210/jcem.85.10.6875. [DOI] [PubMed] [Google Scholar]
- 16.Pack SD, Qin LX, Pak E, Wang Y, Ault DO, Mannan P, et al. Common genetic changes in hereditary and sporadic pituitary adenomas detected by comparative genomic hybridization. Genes, Chromosomes & Cancer. 2005;43:72–82. doi: 10.1002/gcc.20162. [DOI] [PubMed] [Google Scholar]
- 17.Galland F, Kamenicky P, Affres H, Reznik Y, Pontvert D, Le Bouc Y, et al. McCune-Albright syndrome and acromegaly: effects of hypothalamopituitary radiotherapy and/or pegvisomant in somatostatin analog-resistant patients. J Clin Endocrinol Metab. 2006;91:4957–4961. doi: 10.1210/jc.2006-0561. [DOI] [PubMed] [Google Scholar]
- 18.Boikos SA, Stratakis CA. Pituitary pathology in patients with Carney Complex: growth-hormone producing hyperplasia or tumors and their association with other abnormalities. Pituitary. 2006;9:203–209. doi: 10.1007/s11102-006-0265-2. [DOI] [PubMed] [Google Scholar]
- 19.Bossis I, Voutetakis A, Matyakhina L, Pack S, Abu-Asab M, Bourdeau I, et al. A pleiomorphic GH pituitary adenoma from a Carney complex patient displays universal allelic loss at the protein kinase A regulatory subunit 1A (PRKARIA) locus. J Med Genet. 2004;41:596–600. doi: 10.1136/jmg.2004.020214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song LB, Li J, Liao WT, Feng Y, Yu CP, Hu LJ, et al. The polycomb group protein Bmi1 represses the tumor suppressor PTEN and induces epithelial-mesenchymal transition in human nasopharyngeal epithelial cells. J Clin Invest. 2009;119:3626–3636. doi: 10.1172/JCI39374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, et al. MiR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci.U. S. A. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, et al. Ras is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Mayr C, Hemann MT, Bartel DP. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science. 2007;315:1576–1579. doi: 10.1126/science.1137999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin-28, Let-7 microRNA, and IL-6 links inflomation to cell transformation. cell. 2009;139:693–306. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang TC, Yu D, Lee YS, Wentzel EA, Arking DE, West KM, et al. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet. 2008;40:43–50. doi: 10.1038/ng.2007.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kashuba VI, Li J, Wang F, Senchenko VN, Protopopov A, Malyukova A, et al. RBSP3 (HYA22) is a tumor suppressor gene implicated in major epithelial malignancies. Proc Natl Acad Sci U S A. 2004;101:4906–4911. doi: 10.1073/pnas.0401238101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Incoronato M, Garofalo M, Urso L, Romano G, Quintavalle C, Zanca C, et al. MiR-212 increases tumor necrosis factor-related apoptosis-inducing ligand sensitivity in non-small cell lung cancer by targeting the antiapoptotic protein PED. Cancer Res. 2010;70:3638–3646. doi: 10.1158/0008-5472.CAN-09-3341. [DOI] [PubMed] [Google Scholar]
- 28.Di Cristofano A, De Acetis M, Koff A, Cordon-Cardo C, Pandolfi PP. Pten and p27KIP21cooperate in prostate cancer tumor suppression in the mouse. Nat Genet. 2001;27:222–224. doi: 10.1038/84879. [DOI] [PubMed] [Google Scholar]
- 29.Podsypanina K, Ellenson LH. Mutation of PTEN/Mmac1 in mice causes neoplasia inmultiple organ systems. Proc Natl Acad Sci USA. 1999;96:1563–1568. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trotman LC, Niki M, Dotan ZA, Koutcher JA, Di Cristofano A, Xiao A, et al. Pten dose dictates cancer progression in the prostate. Plos Biol. 2003:1E59. doi: 10.1371/journal.pbio.0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia D, Srinivas H, Ahn YH, Sethi G, Sheng X, Yung WK, et al. Mitogen activated protein kinase kinase-4 promotes cell survival by decreasing PTEN expression throught an NF-kappa B-dependent pathway. J Biol Chem. 2007;282:3507–3519. doi: 10.1074/jbc.M610141200. [DOI] [PubMed] [Google Scholar]
- 32.Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobs JJ, Kieboom K, Marino S, DePinho RA, van Lohuizen M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999;397:164–168. doi: 10.1038/16476. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs JJ, Scheijen B, Voncken JW, Kieboom K, Berns A, van Lohuizen M. Bmi-1 collaborates with c-Myc in tumorigenesis by inhibiting c-Myc-induced apoptosis via INK4a/ARF. Genes Dev. 1999;13:2678–2690. doi: 10.1101/gad.13.20.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Lohuizen M, Frasch M, Wientjens E, Berns A. Sequence similarity between the mammalian bmi-1 proto-oncogene and the Drosophila regulatory genes Psc and Su(z)2. Nature. 1991;353:353–355. doi: 10.1038/353353a0. [DOI] [PubMed] [Google Scholar]
- 36.Guo BH, Feng Y, Zhang R, Xu LH, Li MZ, Kung HF, et al. Bmi-1 promotes invasion and metastasis, and its elevated expression is correlated with an advanced stage of breast cancer. Mol Cancer. 2011;10:10. doi: 10.1186/1476-4598-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lukacs RU, Memarzadeh S, Wu H, Witte ON. Bmi-1 is a crucial regulator of prostate stem cell self-renewal and malignant transformation. Cell Stem Cell. 2010;7:682–693. doi: 10.1016/j.stem.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu WL, Guo XZ, Zhang LJ. Prognostic relevance of Bmi -1 expression and autoantibodies in esophageal squamous cell carcinoma. BMC Cancer. 2010;10:467. doi: 10.1186/1471-2407-10-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sinha AU, Kaimal V, Chen J, Jegga AG. Dissecting microregulation of a master regulatory network. BMC Genomics. 2008;9:88. doi: 10.1186/1471-2164-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stratakis CA, Tichomirowa MA, Boikos S, Azevedo MF, Lodish M, Martari M, et al. The role of germlineAIP MEN1, PRKAR1A, CDKN1B and CDKN2C mutations in causing pituitary adenomas in a large cohort of children, adolescents, and patients with genetic syndromes. Clin Genet. 2010;78:457–463. doi: 10.1111/j.1399-0004.2010.01406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farrell WE, Azevedo MF, Batista DL, Smith A, Bourdeau I, Horvath A, et al. Unique gene expression profile associated with an early-onset multiple endocrine neoplasia (MEN1)-associated pituitary adenoma. J Clin Endocrinol Metab. 2011 doi: 10.1210/jc.2011-1127. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, et al. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the carney complex. Nat Genet. 2000;26:89–92. doi: 10.1038/79238. [DOI] [PubMed] [Google Scholar]
- 43.Kovacs K, Horvath E, Thomer MO, Rogol AD. Mammosomatotroph hyperplasia associated with acromegaly and hyperprolactinemia in a patient with the McCune-Albright syndrome. Virch Arch Pathol Anat. 1984;403:77–86. doi: 10.1007/BF00689340. [DOI] [PubMed] [Google Scholar]
- 44.Horvath A, Bertherat J, Groussin L, Guillaud-Bataille M, Tsang K, Cazabat L, et al. Mutations and polymorphisms in the gene encoding regulatory subunit type 1-alpha of protein kinase A (PRKAR1A): an update. Hum Mutat. 2010;31:369–379. doi: 10.1002/humu.21178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mai PL, Korde L, Kramer J, Peters J, Mueller CM, Pfeiffer S, et al. A possible new syndrome with growth-hormone secreting pituitary adenoma, colonic polyposis, lipomatosis, lentigines and renal carcinoma in association with familial testicular germ cell malignancy: A case report. J Med Case Reports (BMC) 2007;1:1–6. doi: 10.1186/1752-1947-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hatziapostolou M, Polytarchou C, Aggelidou E, Drakaki A, Poultsides GA, Jaeger SA, et al. An HNF4α-miRNA Inflammatory Feedback Circuit Regulates Hepatocellular Oncogenesis. Cell. 2011;147:1233–1247. doi: 10.1016/j.cell.2011.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]