Abstract
Objective
To investigate whether attention deficit hyperactivity disorder (ADHD) may serve as a marker of neuropsychiatric disease and as a target for N-acetylcysteine (NAC) treatment in patients with systemic lupus erythematosus (SLE).
Methods
The ADHD Self-Report Scale (ASRS) was used to assess 49 patients with SLE and 46 matched healthy control subjects. Twenty-four of the patients with SLE were randomized to receive either placebo, NAC at a dosage of 2.4 gm/day, or NAC at a dosage of 4.8 gm/day. Disease activity was evaluated monthly using the British Isles Lupus Assessment Group (BILAG) index, the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), the Fatigue Assessment Scale (FAS), and the ASRS, before and during the 3-month treatment period and after a 1-month washout period.
Results
The cognitive/inattentive (ASRS part A), hyperactivity/impulsive (ASRS part B), and combined (total) ASRS scores were increased in patients with SLE compared with control subjects (mean ± SEM 17.37 ± 1.03 [P = 3 × 10−7], 14.51 ± 0.89 [P = 2 × 10−4], and 31.92 ± 1.74 [P = 8 × 10−7], respectively, versus 10.41 ± 1.02, 9.61 ± 1.21, and 20.02 ± 1.98, respectively. ASRS part A scores correlated with SLEDAI (r = 0.53, P < 0.0001) and BILAG scores (r = 0.36, P = 0.011). ASRS total scores also correlated with SLEDAI (r = 0.45, P = 0.0009) and BILAG scores (r = 0.31, P = 0.025). ASRS part A (r = 0.73, P < 0.0001), ASRS part B (r = 0.47, P = 0.0006), and ASRS total scores (r = 0.67, P < 0.0001) correlated with the FAS score. Relative to the scores in placebo-treated patients, ASRS total scores were reduced in SLE patients treated with NAC dosages of 2.4 gm/day and 4.8 gm/day combined (P = 0.037). ASRS part A scores were reduced by NAC dosages of 2.4 gm/day (P = 0.001) and 4.8 gm/day (P < 0.0001) as well as by NAC at dosages of 2.4 gm/day and 4.8 gm/day combined (P = 0.001).
Conclusion
In patients with SLE, elevated ASRS scores reveal previously unrecognized and clinically significant symptoms of ADHD that respond to NAC treatment.
Systemic lupus erythematosus (SLE) is a chronic inflammatory disease that often has debilitating and potentially life-threatening consequences. The cause of SLE is unknown, and current therapies lack specificity and are associated with significant side effects (1). Existing data in the literature provide evidence that a natural antioxidant, glutathione, is depleted in patients with SLE (which may be a key factor underlying abnormal activation of T lymphocytes) and contributes to self-destructive autoimmunity (2). N-acetylcysteine (NAC), which serves as a precursor of glutathione, is widely available, and large doses can be safely administered intravenously to humans. However, NAC is currently unavailable as an oral medication. Therefore, we initiated a double-blind, placebo-controlled, randomized phase I/II study to evaluate the safety and tolerance as well as the metabolic, immunologic, and therapeutic impact of orally formulated NAC in SLE (3). The results of that recently completed pilot study showed NAC to be safe and capable of improving disease activity and fatigue by inhibiting the autoimmune inflammatory process in 36 patients over a 3-month treatment period (3).
Neuropsychiatric manifestations are a significant cause of morbidity in SLE. The American College of Rheumatology formulated case definitions for neuropsychiatric SLE syndromes (4) that include cognitive dysfunction in patients who have difficulty with attention, concentration, memory, and word-finding. NAC has been shown in controlled clinical trials to improve memory and cognitive function (5), bipolar disease (6), and schizophrenia (7). Attention deficit and hyperactivity disorder (ADHD) may be an early sign of cognitive impairment and progressive mania or depression (8).
In the current study, we documented that ADHD Self-Report Scale (ASRS) scores (9) are elevated in patients with SLE relative to healthy subjects matched for sex, ethnicity, and age within 10 years. Moreover, the cognitive/inattentive components (ASRS part A scores) rather than the hyperactivity/impulsive components (ASRS part B scores) were markedly improved by treatment with NAC. These results suggest that the ASRS can be used to detect cognitive dysfunction, and that early detection of ADHD symptoms may predict and potentially prevent serious neuropsychiatric complications in patients with SLE.
PATIENTS AND METHODS
Human subjects
The validated ASRS Symptom Checklist (9) was used to assess 49 patients with SLE. A first cohort of 24 patients were enrolled in a trial of treatment with NAC (hereafter referred to as the NAC trial) (FDA Investigational New Drug application no. 101,320; clinicaltrials.gov identifier NCT00775476). A second cohort of 25 patients were not enrolled in the NAC trial. As controls, 46 healthy subjects matched with SLE patients for ethnicity, sex, and age within 10 years were also asked to complete the ASRS checklist when they were donating blood for immunobiologic studies (3). In the SLE group, 44 of the 49 patients were white, 4 were African American, and 1 was Asian. In the control group, 45 of the 46 subjects were white, and 1 was African American. The mean ± SEM age of the patients with SLE was 45.9 ± 1.8 years (range 20–67 years), and the mean ± SEM age of subjects in the control group was 48.0 ± 1.5 years (range 26–64 years). Three patients with SLE and 2 healthy control subjects were white men, and all of the other subjects were women.
Clinical study design
The clinical trial design, eligibility criteria, randomization, blinding, monitoring of safety, tolerance, and efficacy of NAC in patients with SLE was recently described in detail (3). As an addendum to that study (3), the ASRS was evaluated as an exploratory outcome in 24 patients with SLE randomized to receive oral treatment with either placebo or NAC in 1 of 2 treatment arms: 1,200 mg or 2,400 mg twice daily for 3 months. Twelve patients were enrolled in each dosing group; 9 received NAC, and 3 received placebo. Because of missing data, only 6 patients enrolled in the placebo arm and 8 patients enrolled in each of the 2 NAC treatment arms could be evaluated.
The ASRS is an 18-item scale that is used to assess the current status of the 18 Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)—defined symptoms of ADHD in adults (9). Symptoms are rated based on frequency, where 0 = never, 1 = rarely, 2 = sometimes, 3 = often, and 4 = very often. In the ASRS, 9 items assess inattention, and 9 items assess hyperactivity/impulsivity. The 9 symptoms of inattentiveness are summed to determine the ASRS part A score, and the 9 hyperactive/impulsive symptoms are summed to determine the ASRS part B score. These 2 scores are summed to compute the total score. For all scales, higher scores indicate more symptoms. The ASRS has high concurrent validity with a rater-administered ADHD symptom scale (10). SLE disease activity was assessed using the British Isles Lupus Assessment Group (BILAG) index (11) and the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) (12). Fatigue was estimated using the Fatigue Assessment Scale (FAS) (13).
Statistical analysis
The overall clinical effectiveness of NAC relative to placebo was analyzed with multilevel modeling as implemented in the Stata routine xtmixed (StataCorp), with the 3 nested levels being drug group, subject within drug group, and study visit within subject. All models included fixed effects for drug group, study visit, and the drug group–by–study visit interaction, along with random intercepts at each design level. Our test for efficacy was the fixed effect for the drug group–by–study visit interaction, which, if significant, indicated that the change in outcome scores over time was significantly different among treatment groups. A paired 2-tailed t-test was used to assess the effects of placebo, of each NAC dose, and of both NAC doses combined on the clinical indices recorded at visits 2–5 relative to those recorded at visit 1. P values less than 0.05 were considered significant. Patients and control subjects were compared by an unpaired 2-tailed t-test. Correlations and t-tests were performed with GraphPad Prism software.
RESULTS
Correlation of ASRS scores with disease activity in patients with SLE
The mean ± SEM scores for the cognitive/inattentive component of the ASRS (part A), the hyperactivity/impulsive component of the ASRS (part B), and ASRS total were 10.41 ± 1.02, 9.61 ± 1.21, and 20.02 ± 1.98, respectively, in the control group of 46 healthy subjects (Figure 1). In a first cohort of 24 patients with SLE who were enrolled in the NAC trial, the mean ± SEM scores for ASRS part A, ASRS part B, and ASRS total were increased (17.23 ± 1.55 [P = 0.0001], 14.36 ± 1.32 [P = 0.004], and 31.59 ± 2.75 [P = 0.0004], respectively) compared with scores in the control group. In a second cohort of 25 patients not enrolled in the NAC trial, the mean ± SEM scores for ASRS part A, ASRS part B, and ASRS total were also increased (18.24 ± 0.90 [P = 0.00003], 14.0 ± 1.02 [P = 0.034], and 32.35 ± 1.62 [P = 0.0004], respectively). In the combined group of all 49 SLE patients, the mean ± SEM scores for ASRS part A, ASRS part B, and ASRS total were also increased (17.37 ± 1.03 [P = 3 × 10−7], 14.51 ± 0.89 [P = 2 × 10−4], and 31.92 ± 1.74 [P = 8 × 10−7], respectively).
Figure 1.

Attention Deficit Hyperactivity Disorder Self-Report Scale (ASRS) part A (cognitive/inattentive), ASRS part B (hyperactivity/impulsive), and total ASRS scores in patients with systemic lupus erythematosus (SLE) and healthy control subjects matched for age within 10 years, sex, and ethnicity. Left, Analysis of cohort 1, comprising 24 patients with SLE and 22 healthy control subjects. Middle, Analysis of cohort 2, comprising 25 patients with SLE and 24 healthy control subjects. Right, Analysis of cohorts 1 and 2 combined. Bars show the mean ± SEM. * = P < 0.05 by unpaired 2-tailed t-test.
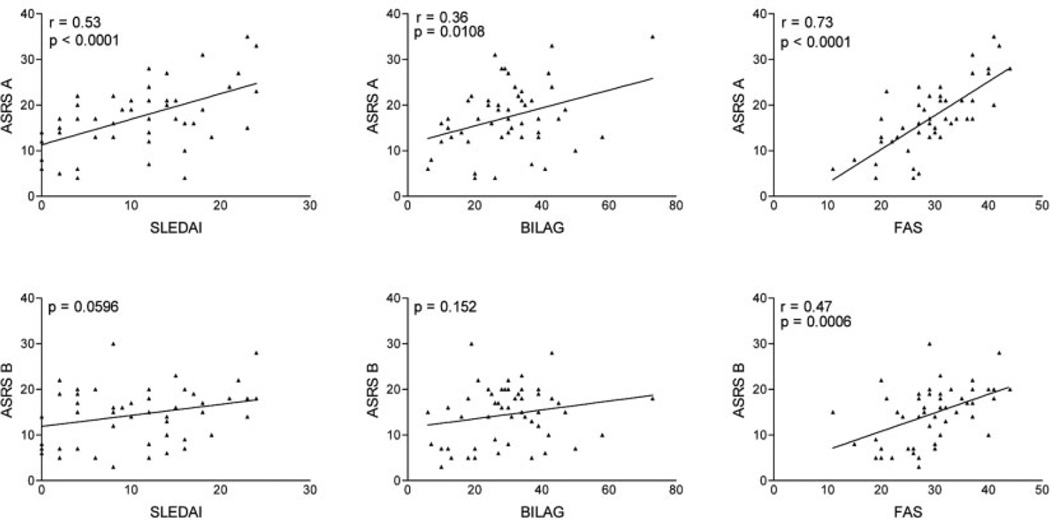
Analysis of the data demonstrated that FAS scores in the patients with SLE correlated with ASRS part A (r = 0.73, P < 0.0001), ASRS part B (r = 0.47, P = 0.0006) (Figure 2), and ASRS total scores (r = 0.67, P < 0.0001). The SLEDAI scores in the patients with SLE correlated with ASRS part A scores (r = 0.53, P < 0.0001) (Figure 2) and ASRS total scores (r = 0.45, P = 0.0009). The BILAG scores in these patients also correlated with ASRS part A scores (r = 0.36, P = 0.0108) (Figure 2) and ASRS total scores (r = 0.31, P = 0.025). There were also significant correlations between the SLEDAI and BILAG scores (r = 0.51, P = 0.0002), the BILAG score and the FAS score (r = 0.40, P = 0.0043), and the SLEDAI and FAS scores (r = 0.24, P = 0.042) (results not shown).
Figure 2.
Correlation of ASRS part A and ASRS part B scores with the SLE Disease Activity Index (SLEDAI), the British Isles Lupus Assessment Group (BILAG) index, and the Fatigue Assessment Scale (FAS) in 49 patients with SLE. Pearson’s correlation coefficients are shown for correlations with P values less than 0.05. See Figure 1 for other definitions.
Twenty-six of the 49 patients had fibromyalgia (FM) and exhibited elevated FAS scores relative to the 23 patients without FM (mean scores 31.5 and 26.6, respectively; P = 0.027). Among SLE patients with FM and SLE patients without FM, 70.8% and 30.2%, respectively, were unemployed (P = 0.007). Twenty-three (88.5%) of 26 SLE patients with FM and none of the SLE patients without FM had major neuropsychiatric manifestations, such as depression, stroke, or a history of seizures (P = 3.2 × 10−18). Among the 26 unemployed patients, the prevalence of FM (68%) and the mean FAS score (31.4) were increased relative to the prevalence of FM (32%; P = 0.007) and the mean FAS score (26.9; P = 0.022) among the 22 employed patients. ASRS part A, ASRS part B, ASRS total, SLEDAI, and BILAG scores were not elevated in SLE patients with FM, depression, antidepressant treatment, or lack of employment compared with patients without these conditions (data not shown).
Effect of NAC treatment on ADHD scores in patients with SLE
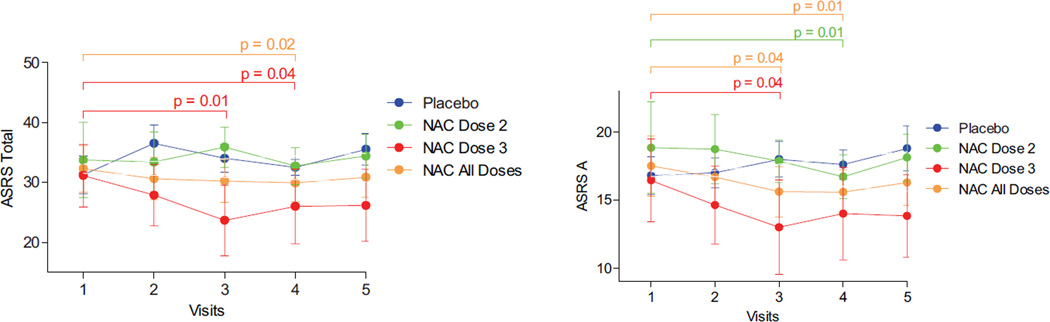
As reported recently, NAC significantly improved lupus disease activity, as measured by the SLEDAI, BILAG, and FAS scores observed in a double-blind, placebo-controlled, randomized pilot study of 36 patients with SLE (3). Here, we investigated the effect of NAC at dosages of 2.4 gm/day (dose 2) and 4.8 gm/day (dose 3) relative to placebo on the ASRS scores in patients with SLE. At visit 1, ASRS part A, ASRS part B, and ASRS total scores were similar between the placebo and NAC dosing groups. Patients exposed to NAC dose 3 had diminished ASRS total scores after 2 months and 3 months of treatment, while ASRS total scores were reduced in patients treated with NAC doses 2 and 3 combined after 3 months (Figure 3).
Figure 3.
Effect of N-acetylcysteine (NAC) and placebo on ASRS total scores and ASRS part A scores in 24 patients with SLE treated with placebo (n = 6), NAC dose 2 (2.4 gm/day; n = 9), NAC dose 3 (4.8 gm/day; n = 9), or NAC all doses (doses 2 and 3 combined; n = 18). Values are the mean ± SEM. P values indicate comparisons of pretreatment values (visit 1) with values after 1 month (visit 2), 2 months (visit 3), 3 months (visit 4), or 4 months (visit 5 [3 months of treatment followed by a 1-month washout period]), determined by paired 2-tailed t-test. See Figure 1 for other definitions.
Using multilevel modeling, the reduction in ASRS total scores was greater in the NAC group (doses 2 and 3 combined) than in the placebo group, as indicated by a significant visit-by-drug interaction (xtmixed z = −2.09, P = 0.037). ASRS part A scores (the cognitive/inattentive component of the ASRS) were influenced by NAC treatment. NAC dose 2 (z = −3.31, P = 0.001) and dose 3 (z = −4.04, P < 0.0001) as well as NAC doses 2 and 3 combined reduced ASRS part A scores (z = −3.41, P = 0.001). The hyperactivity/impulsive components of the ASRS (ASRS part B scores) were not affected by NAC doses 2 and 3, alone or in combination (results not shown). At the end of the 3-month treatment period, the standardized mean difference in effect size for NAC dose 3 (Cohen’s d) was 0.72 for ASRS total scores, 0.71 for ASRS part A scores, and 0.44 for ASRS part B scores.
DISCUSSION
SLE is a chronic, systemic, autoimmune inflammatory disease characterized by a predisposition to proinflammatory T cell death by necrosis due to mitochondrial dysfunction, oxidative stress, and depletion of intracellular glutathione. The potential benefit of reversing glutathione depletion has been suggested by a recently completed double-blind, placebo-controlled trial of NAC treatment in patients with SLE (3). Because lupus disease activity may involve neuropsychiatric disorders, and because NAC has been shown to improve memory in animals (5), it is conceivable that ADHD represents a manifestation of disease that may be responsive to treatment with NAC.
The elevated ASRS scores observed in this study indicate the presence of previously unrecognized and clinically significant symptoms of ADHD in patients with SLE relative to healthy controls matched for age, sex, and ethnic background. Elevated ASRS scores were noted in 2 independent SLE cohorts, as evaluated both separately and together. Importantly, ASRS scores correlated with SLEDAI, BILAG, and FAS scores but not with FM, employment, or an existing diagnosis of depression. FAS scores positively correlated with FM, unemployment, and depression. Nevertheless, ADHD symptoms may be a source of cognitive impairment in SLE, which could lead to functional disability.
Recent studies revealed an increased frequency of ADHD symptoms in patients with tuberous sclerosis, a genetic disease associated with mammalian target of rapamycin (mTOR) activation resulting from genetic mutations of its natural inhibitors tuberous sclerosis proteins 1 and 2 (14). Therefore, it is conceivable that ADHD in patients with SLE may result from the activation of mTOR, which is inhibited, along with disease activity, by NAC (3). Because 44 of 49 patients were white, elevated ASRS scores may not be readily generalized to other ethnicities.
Although based on a relatively small sample size, our treatment results are promising. The effect size (Cohen’s d) of 0.72 for ASRS total scores is similar to the effect size of FDA-approved nonstimulant treatments for ADHD (15). The scores for cognitive/inattentive ADHD symptoms (ASRS part A) but not those for hyperactivity/impulsive symptoms (ASRS part B) showed improvement following treatment with NAC; nevertheless, further work is needed to better quantify that difference.
Our results indicate that among patients with SLE, the rates of both hyperactive/impulsive and cognitive/inattentive symptoms of ADHD are increased; however, we did not confirm the onset of symptoms in childhood as required for an ADHD diagnosis by the DSM-IV (9). Although our data suggest that FM, depression, and antidepressant treatment may not account for ADHD symptoms among patients with SLE, this subject is particularly complex given that SLE is associated with several neuropsychiatric manifestations (4) and, in the absence of SLE, ADHD is associated with depression and other neuropsychiatric conditions (8). Although additional work is needed to clarify these issues, our data do address the severity of ADHD symptoms among patients with SLE. If a symptom is defined as present if it occurs often or very often, then the mean total ASRS score of 32 observed in both SLE cohorts would be achieved by a patient showing as many as 10 symptoms; this number of symptoms is high considering that the diagnostic criteria for ADHD require 6 symptoms (9).
The results of this study suggest that ASRS may be a useful instrument for detecting cognitive dysfunction, which is an important neuropsychiatric manifestation of SLE (4). Longitudinal studies indicate that ADHD symptoms predict the subsequent onset of severe neuropsychiatric disorders that frequently follow the onset of idiopathic ADHD in children (8). The early detection of attention deficit disorder and its responsiveness to NAC may help to prevent the development of neuropsychiatric complications, along with an overall reduction in disease activity and fatigue in patients with SLE (3). Based on the detection of increased ADHD symptoms in patients with SLE, their correlation with lupus disease activity, and the responsiveness of attention deficit disorder to NAC, additional followup studies with larger cohorts and a longer duration are clearly warranted.
ACKNOWLEDGMENT
We thank Dr. Paul Phillips (State University of New York Upstate Medical University, Syracuse, NY) for continued encouragement and support.
Supported in part by the NIH (grant AT-004332), the Alliance for Lupus Research, and the Central New York Community Foundation.
Footnotes
Presented in part at the 74th Annual Scientific Meeting of the American College of Rheumatology, Atlanta, GA, 2010.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Perl had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Garcia, Francis, Dawood, Lai, Faraone, Perl.
Acquisition of data. Garcia, Francis, Dawood, Lai, Faraone, Perl.
Analysis and interpretation of data. Garcia, Francis, Dawood, Lai, Faraone, Perl.
REFERENCES
- 1.Francis L, Perl A. Pharmacotherapy of systemic lupus erythematosus. Expert Opin Pharmacother. 2009;10:1481–1494. doi: 10.1517/14656560902971003. [DOI] [PubMed] [Google Scholar]
- 2.Gergely PJ, Jr, Grossman C, Niland B, Puskas F, Neupane H, Allam F, et al. Mitochondrial hyperpolarization and ATP depletion in patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46:175–190. doi: 10.1002/1529-0131(200201)46:1<175::AID-ART10015>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai ZW, Hanczko R, Bonilla E, Caza TN, Clair B, Bartos A, et al. N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2012;64:2937–2946. doi: 10.1002/art.34502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ACR Ad Hoc Committee on Neuropsychiatric Lupus Nomenclature. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42:599–608. doi: 10.1002/1529-0131(199904)42:4<599::AID-ANR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 5.Martinez M, Hernandez AI, Martinez N. N-acetylcysteine delays age-associated memory impairment in mice: role in synaptic mitochondria. Brain Res. 2000;855:100–106. doi: 10.1016/s0006-8993(99)02349-5. [DOI] [PubMed] [Google Scholar]
- 6.Bernardo M, Dodd S, Gama CS, Copolov DL, Dean O, Kohlmann K, et al. Effects of N-acetylcysteine on substance use in bipolar disorder: a randomised placebo-controlled clinical trial. Acta Neuropsychiatr. 2009;21:239–245. doi: 10.1111/j.1601-5215.2009.00415.x. [DOI] [PubMed] [Google Scholar]
- 7.Berk M, Copolov D, Dean O, Lu K, Jeavons S, Schapkaitz I, et al. N-acetyl cysteine as a glutathione precursor for schizophrenia: a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64:361–368. doi: 10.1016/j.biopsych.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Biederman J, Petty CR, Monuteaux MC, Fried R, Byrne D, Mirto T, et al. Adult psychiatric outcomes of girls with attention deficit hyperactivity disorder: 11-year follow-up in a longitudinal case-control study. Am J Psychiatry. 2010;167:409–417. doi: 10.1176/appi.ajp.2009.09050736. [DOI] [PubMed] [Google Scholar]
- 9.Kessler RC, Adler L, Ames M, Demler O, Faraone S, Hiripi E, et al. The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med. 2005;35:245–256. doi: 10.1017/s0033291704002892. [DOI] [PubMed] [Google Scholar]
- 10.Adler LA, Spencer T, Faraone SV, Kessler RC, Howes MJ, Biederman J, et al. Validity of pilot Adult ADHD Self-Report Scale (ASRS) to rate adult ADHD symptoms. Ann Clin Psychiatry. 2006;18:145–148. doi: 10.1080/10401230600801077. [DOI] [PubMed] [Google Scholar]
- 11.Isenberg DA, Rahman A, Allen E, Farewell V, Akil M, Bruce IN, et al. BILAG 2004: development and initial validation of an updated version of the British Isles Lupus Assessment Group’s disease activity index for patients with systemic lupus erythematosus. Rheumatology (Oxford) 2005;44:902–906. doi: 10.1093/rheumatology/keh624. [DOI] [PubMed] [Google Scholar]
- 12.Hochberg MC for the Diagnostic and Therapeutic Criteria Committee of the American College of Rheumatology. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus [letter] Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 13.Michielsen HJ, De Vries J, Van Heck GL. Psychometric qualities of a brief self-rated fatigue measure: the Fatigue Assessment Scale. J Psychosom Res. 2003;54:345–352. doi: 10.1016/s0022-3999(02)00392-6. [DOI] [PubMed] [Google Scholar]
- 14.Muzykewicz DA, Newberry P, Danforth N, Halpern EF, Thiele EA. Psychiatric comorbid conditions in a clinic population of 241 patients with tuberous sclerosis complex. Epilepsy Behav. 2007;11:506–513. doi: 10.1016/j.yebeh.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Faraone SV, Glatt SJ. A comparison of the efficacy of medications for adult attention-deficit/hyperactivity disorder using meta-analysis of effect sizes. J Clin Psychiatry. 2010;71:754–763. doi: 10.4088/JCP.08m04902pur. [DOI] [PubMed] [Google Scholar]