Abstract
Background
PRKAR1A codes for the type 1a regulatory subunit (R1α) of the cAMP-dependent protein kinase A (PKA), an enzyme with an important role in cell cycle regulation and proliferation. PKA dyregulation has been found in various tumors and PRKAR1A-inactivating mutations have been reported in mostly endocrine neoplasias. In this study, we investigated PKA activity and the PRKAR1A gene in normal and tumor endometrium.
Methods
Specimens were collected from 31 patients with endometrial cancer. We used as controls 41 samples of endometrium that were collected from surrounding normal tissues or from women undergoing gynecological operations for other reasons. In all samples, we sequenced the PRKAR1A coding sequence and studied PKA subunit expression; we also determined PKA activity and cAMP binding.
Results
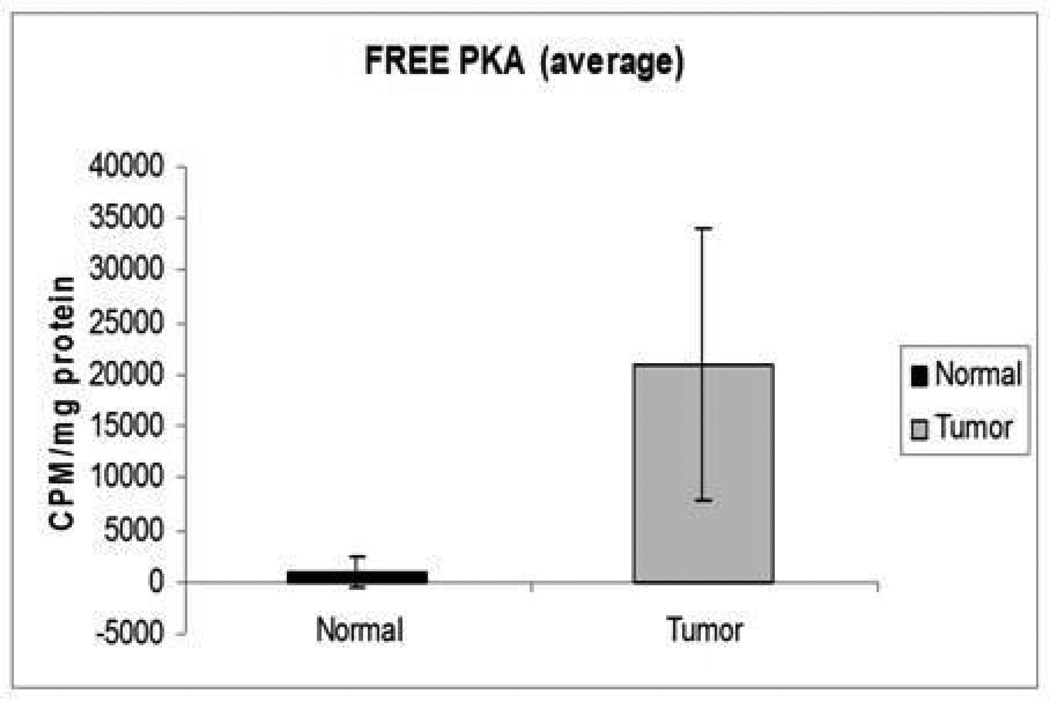
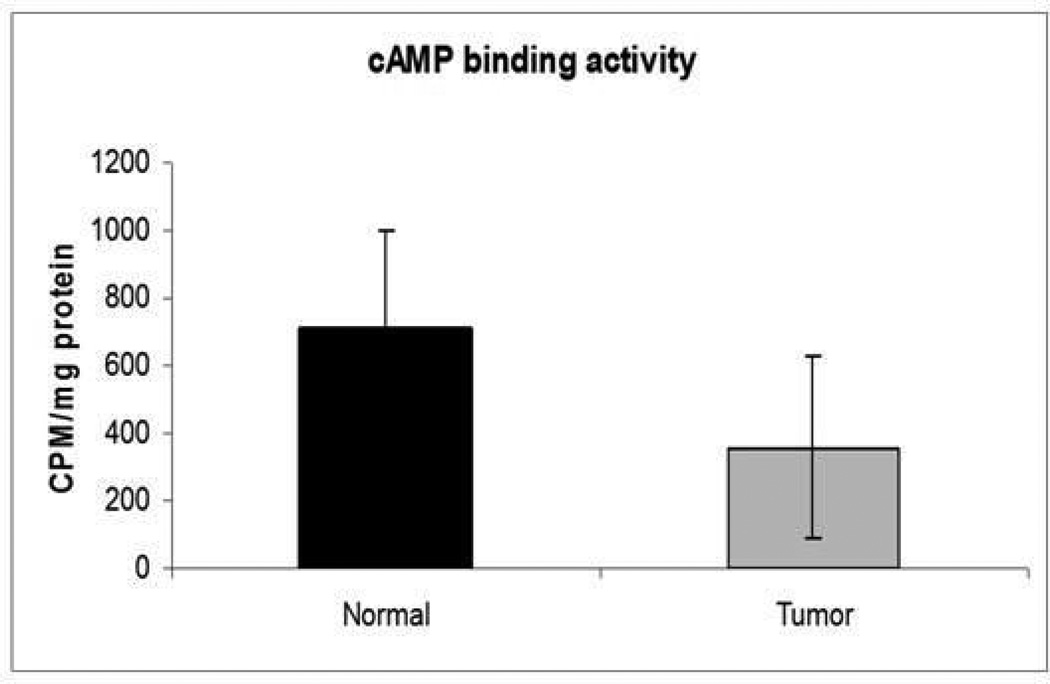
PRKAR1A mutations were not found. However, PKA regulatory subunit protein levels, both R1α and those of regulatory subunit type 2b (R2β) were lower in tumor samples; cAMP binding was also lower in tumors compared to normal endometrium (p<0.01). Free PKA activity was higher in tumor samples compared with control tissue (p<0.01).
Conclusions
There are significant PKA abnormalities in tumors of the endomentrium compared to surrounding normal tissue; since these were not due to PRKAR1A mutations other mechanisms affecting PKA function ought to be explored.
Introduction
Protein kinase A (PKA), a serine-threonine kinase and the main effector of cyclic adenosine monophosphate (cAMP) signaling in most cells, is widely involved in the regulation of cell growth and proliferation [1]. PKA consists of a tetramer of two regulatory (R) and two catalytic (C) subunits; a homodimer consisted of each of the four regulatory subunits (R1α, R1β, R2α and R2β) serves as a binding scaffold for the enzymatically active catalytic subunits and thus the PKA holoenzyme is inactive. Upon binding of the cAMP to the R subunits, the C subunits are released to mediate kinase activity [2]. The most important regulator of PKA activity is its regulatory subunit type 1A (R1α). Mutations of the PRKAR1A gene coding for R1α cause Carney Complex (CNC), a multiple neoplasia syndrome associated with skin, heart, and other myxomas, a variety of other tumors, mostly in endocrine tissues, such as the adrenal, thyroid, and pituitary glands, and a number of skin lesions from common nevi and lentigines, to blue nevi and café-au-lait spots [3]. Ovarian and other gonadal tumors also occur in CNC and changes in R1α protein expression and/or PRKAR1A mutations have been detected in a variety of sporadic (not associated with CNC or any other familial tumor ssyndrome) lesions [4–6].
Endometrial cancer is a hormone-dependent lesion. Although there have been no studies to date of PKA, PRKAR1A or any related molecule in endometrial tumors, several of the hormones that regulate endometrial tissue growth and proliferation exert their effects via cAMP and the PKA signaling pathway [7, 8]. The aim of this study was to investigate any possible PKA or cAMP signaling aberrations in endometrial cancer; since PRKAR1A is the only PKA-associated gene that has been found mutated in sporadic tumors, we also investigated this gene’s coding sequence for any mutations.
Methods
Samples
Thirty-one female patients 27 to 81 years old (mean age 60.1 years), diagnosed with endometrial cancer were included in this study. All patients were treated in the First Department of Obstetrics & Gynecology, Athens University Medical School, Alexandra Hospital, Athens, Greece between years 2006 and 2007. For 27 of the patients, tissue from the surrounding endometrium was available; these samples were histologically normal. Normal endometrial tissue was also collected from an additional 14 patients who underwent hysterectomy for non-oncological indications. These patients had no other cancer diagnoses and their family history was also free of endometrial cancer, None of the patients or the controls had clinical features of CNC [6, 9]. All studies were approved by the institutional ethics review board of Alexandra Hospital.
DNA analysis
Tissues were microdissected and DNA was extracted by normal and cancers samples using standard methods (Qiagen Dneasy blood and tissue kit, Quiagen, Inc., Gaithersburg, MD). Direct bidirectional sequencing was employed to analyze all PRKAR1A-coding sequence (exon 2-exon 10), as previously published [4–6].
Immunocytochemistry and immunoblotting
Formalin-fixed, paraffin-embedded tumor and normal endometrium from all patients were incubated with antibodies against the PKA subunits R1α (Abcam), R2α (Santa Cruz), R2β and Cα (BD Bioscences), as previously described [4–6]. The same antibodies were used for Western blot analysis of PKA subunit expression in frozen tissue extracts. Images captured on film were analyzed using the Image Quant Software and were normalized against the expression of the house-keeping gene GAPDH (Abcam), for quantification of PKA subunit protein expression
PKA and cAMP binding assays
PKA activity was measured in cell extracts following a protocol previously described [10]. Basal PKA activity reflects free enzymatic activity without stimulation with cAMP whereas total PKA activity was measured following the addition of cAMP. All determinations of PKA activity were done at least twice for each sample and an average value was calculated. The Gilman procedure was used in order to measure cAMP binding [11].
Statistics
Tumors (n=31) and controls (n=41) were compared using the t-test; a value of p<0.05 indicated significance in difference for any measured value.
Results
DNA sequencing
No pathogenic PRKAR1A mutations were found in any samples. In 5 controls and in 9 tumor samples, we found a previously described polymorphism IVS3-5dupT; in 6 of the 9 samples where normal tissue was available from the same patient, the IVS3-5dupT polymorphism was determined to be present in the germline DNA. In addition, one synonymous polymorphism (c.87G>A/p.Ala29Ala) was found in one tumor sample, also in the germline state.
PKA subunit expression and cAMP/PKA function
Both PKA R subunits R1α and R2β were significantly under-expressed in tumor, compared to normal endometrial tissue (Figures 1, 2 and 3). PKA assays also showed that both free and basal enzymatic activities were higher in tumor than in normal endometrium. cAMP addition resulted in lower total PKA levels in tumor tissues; thus, the augmented basal activity of the enzyme appeared to be independent of cAMP (Figures 4 and 5). Accordingly, cAMP-binding affinity was significantly lower in tumor compared to normal endometrium (Figure 6).
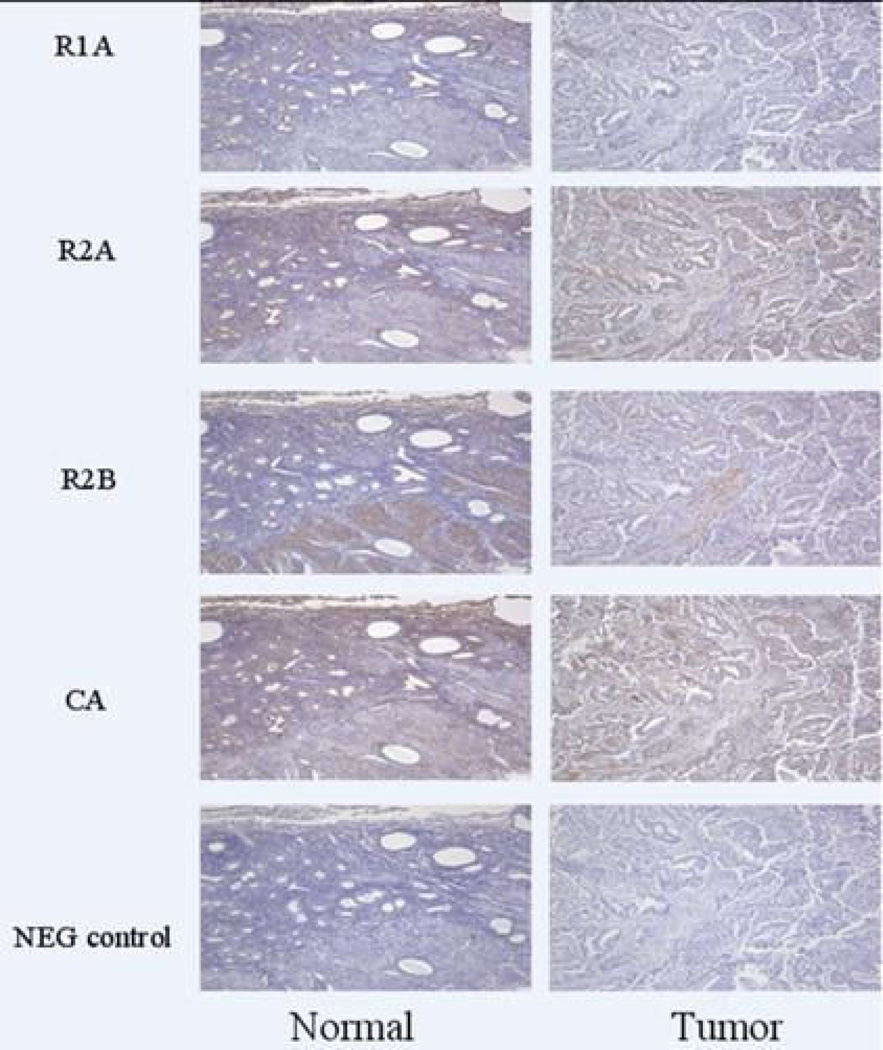
Figure 1.
R1α and R2β expression was relatively decreased in tumor compared to normal endometrium by immunohistochemistry.
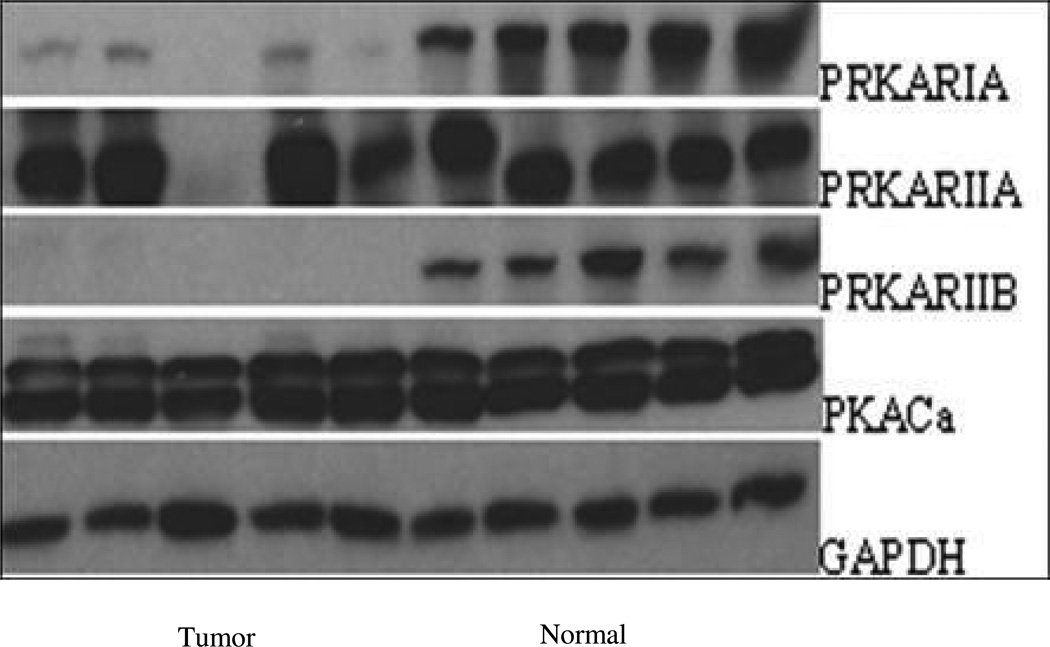
Figure 2.
In WB analysis R1a and R2b proteins was decreased in tumors in comparison to normal tissues
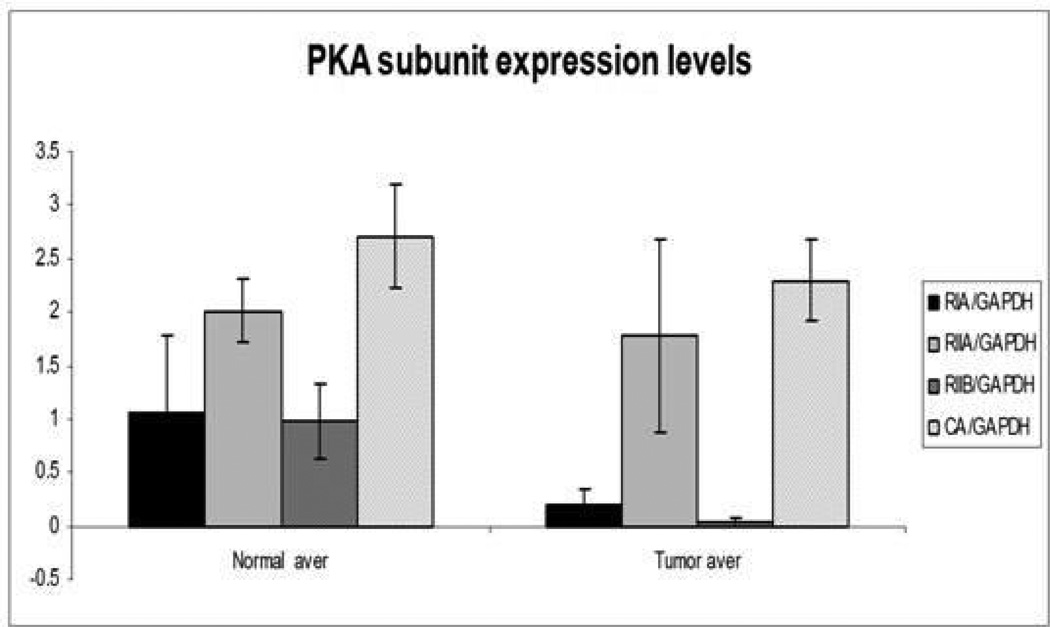
Figure 3.
R1A and R2B expression was significantly decreased in tumor samples in comparison to normal endometrium (P=0.02, P<0.01 respectively)
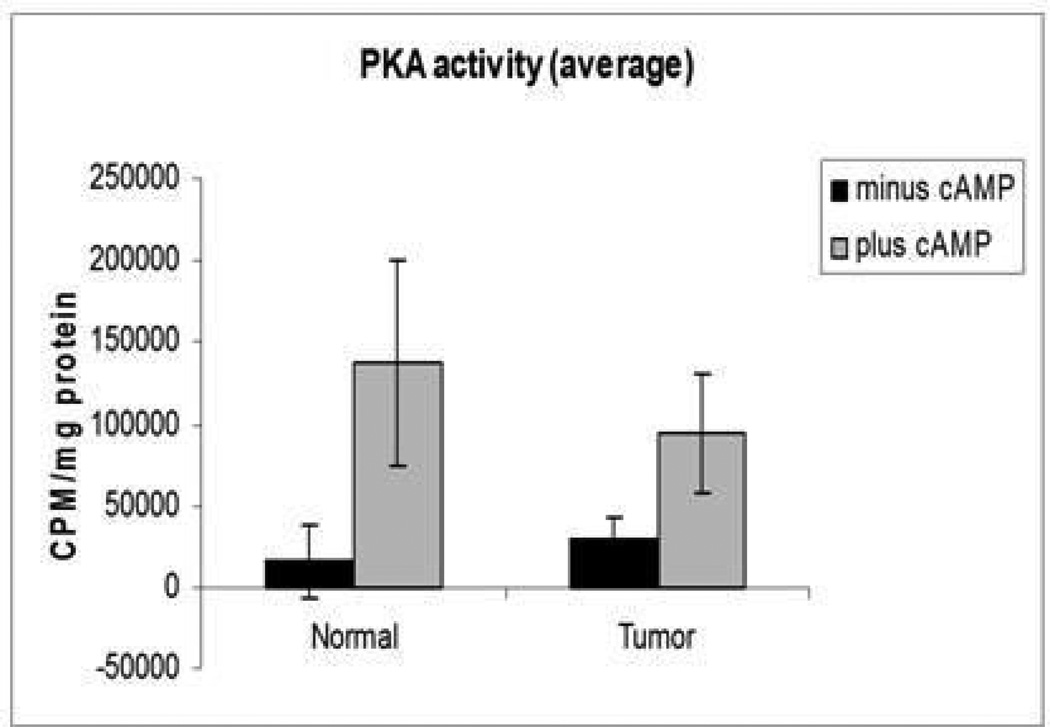
Figure 4.
Basal PKA activity was higher in tumor (P=0.06) in comparison to normal endometrium. After stimulation with cAMP, total PKA levels were lower in tumor in comparison to normal samples (P=0.036).
Figure 5.
Free PKA activity was higher in tumor samples in comparison to normal endometrium (P<0.01).
Figure 6.
cAMP binding affinity was lower in tumor samples in comparison to normal endometrium (P<0.01).
Discussion
Endometrial cancer currently represents the most frequent malignancy of the female reproductive tract. The etiology and progression of these tumors involve hormonal actions, mainly of estradiol and progesterone. Interestingly, these steroid hormones in addition to their many other functions affect PKA signaling in endometrial cells [12]. PKA and cAMP signaling have also been implicated in cell cycle programming, differentiation and even immune regulation of endometrial tissue [13].
In the present study, significant abnormalities of PKA activity and cAMP binding were found in endometrial cancer tissue. These were not associated with PRKAR1A-inactivating gene mutations at the germline or somatic level, indicating that epigenetic, micro-RNA-mediated or other mechanisms may be responsible for the down-regulation of the R1α protein that was observed by immunoblotting and immunohistochemistry. These data are similar to what we have published before in thyroid, adrenal and other cancers where PRKAR1A mutations were not found [4–6]. What is, however, different in the present study was that yet another of the PKA R subunits, R2β, was also down-regulated at the protein level in endometrial cancer tissues.
Down-regulation of these widely expressed molecules that are largely responsible for inhibition of PKA activation in all tissues was most likely responsible for the increase in PKA activity that we detected in endometrial cancers. Interestingly, this increase in PKA activity may not be cAMP-dependent: following exposure to cAMP, both normal and cancer tissues responded with an increase in PKA activity as expected. However, the stimulating effect of cAMP was less profound in tumor tissues compared to normal endometrium; following cAMP withdrawal, free PKA activity was again found to be higher in cancers. Overall cancers demonstrated lower cAMP-binding than normal tissue. These data suggest that increased PKA activity in endometrial cancer may be at least in part cAMP-independent due to the down-regulation of the R subunits R1α and R2β.
This is a different mechanism of PKA’s involvement in cancer than what has been previously described in PRKAR1A-inactiviating mutations leading to R1α haploinsufficiency and increased total PKA activity [1, 14–17]: increased PKA activity in these R1α defects is cAMP-dependent and associated with increased expression of other R subunits, including R2α and R2β.
In conclusion, although no mutations have been found in the PRKAR1A coding sequence, the significant aberrations, which were detected in the function and expression of the PKA subunits between the malignant and the normal tissues, underline the implication of the cAMP pathway in human endometrial tumor genesis.
Acknowledgements
We thank all patients and their referring physicians for their participation in this study. This work was supported, in part, by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), intramural National Institutes of Health (NIH) project Z01-HD-000642-04 to Dr. C. A. Stratakis.
Footnotes
Conflict of interest statement: All authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported
Authors Contributions: AT participated in the design of the study, samples collection and preparation and carried out the manuscript, EB carried out molecular assays and statistical analysis for this study. MN, AH, SB and CL carried out the molecular genetic studies, immunoassays and PKA binding assays. TP participated in the design of the study, and performed surgical operations indicated. CD participated in data collection, design of the study and revised criticaly the manuscript. AR confered the surgical background for the samples of this research. CS conceived of the study, and participated in its design and coordination and helped to draft the manuscript. AA participated in the design and coordination of the study and helped in data collection. All authors read and approved the final manuscript.
References
- 1.Robinson-White AJ, et al. PRKAR1A inactivation leads to increased proliferation and decreased apoptosis in human B lymphocytes. Cancer Res. 2006;66(21):10603–10612. doi: 10.1158/0008-5472.CAN-06-2200. [DOI] [PubMed] [Google Scholar]
- 2.Meoli E, et al. Protein kinase A effects of an expressed PRKAR1A mutation associated with aggressive tumors. Cancer Res. 2008;68(9):3133–3141. doi: 10.1158/0008-5472.CAN-08-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirschner LS, et al. Genetic heterogeneity and spectrum of mutations of the PRKAR1A gene in patients with the carney complex. Hum Mol Genet. 2000;9(20):3037–3046. doi: 10.1093/hmg/9.20.3037. [DOI] [PubMed] [Google Scholar]
- 4.McDaid HM, et al. Increased expression of the RIalpha subunit of the cAMP-dependent protein kinase A is associated with advanced stage ovarian cancer. Br J Cancer. 1999;79(5–6):933–939. doi: 10.1038/sj.bjc.6690149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertherat J, et al. Molecular and functional analysis of PRKAR1A and its locus (17q22-24) in sporadic adrenocortical tumors 17q losses, somatic mutations, and protein kinase A expression and activity. Cancer Res. 2003;63(17):5308–5319. [PubMed] [Google Scholar]
- 6.Sandrini F, et al. Regulatory subunit type I-alpha of protein kinase A (PRKAR1A): a tumor-suppressor gene for sporadic thyroid cancer. Genes Chromosomes Cancer. 2002;35(2):182–192. doi: 10.1002/gcc.10112. [DOI] [PubMed] [Google Scholar]
- 7.Dabizzi S, et al. Luteinizing hormone increases human endometrial cancer cells invasiveness through activation of protein kinase A. Cancer Res. 2003;63(14):4281–4286. [PubMed] [Google Scholar]
- 8.Bulun SE, Simpson ER. Aromatase expression in women's cancers. Adv Exp Med Biol. 2008;630:112–132. doi: 10.1007/978-0-387-78818-0_8. [DOI] [PubMed] [Google Scholar]
- 9.Stratakis CA, Kirschner LS, Carney JA. Carney complex: diagnosis and management of the complex of spotty skin pigmentation, myxomas, endocrine overactivity, and schwannomas. Am J Med Genet. 1998;80(2):183–185. doi: 10.1002/(sici)1096-8628(19981102)80:2<183::aid-ajmg19>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 10.Nesterova MV, SL, Vasiliev VY, Severin ES. A cyclic adenosine 3',5'-monophosphate-dependent histone kinase from pig brain. Purification and some properties of the enzyme. Biochim Biophys Acta. 1975;19(377):271–281. doi: 10.1016/0005-2744(75)90309-5. [DOI] [PubMed] [Google Scholar]
- 11.Gilman AG. A protein binding assay for adenosine 3' 5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murk W, et al. Extracellularly Signal-Regulated Kinase Activity in the Human Endometrium: Possible Roles in the Pathogenesis of Endometriosis. J Clin Endocrinol Metab. 2008;93(9):3532–3540. doi: 10.1210/jc.2007-2051. [DOI] [PubMed] [Google Scholar]
- 13.Tierney EP, et al. Activation of the protein kinase A pathway in human endometrial stromal cells reveals sequential categorical gene regulation. Physiol Genomics. 2003;16(1):47–66. doi: 10.1152/physiolgenomics.00066.2003. [DOI] [PubMed] [Google Scholar]
- 14.Greene EL, et al. In vitro functional studies of naturally occurring pathogenic PRKAR1A mutations that are not subject to nonsense mRNA decay. Hum Mutat. 2008;29(5):633–639. doi: 10.1002/humu.20688. [DOI] [PubMed] [Google Scholar]
- 15.Kirschner LS, et al. A mouse model for the Carney complex tumor syndrome develops neoplasia in cyclic AMP-responsive tissues. Cancer Res. 2005;65(11):4506–4514. doi: 10.1158/0008-5472.CAN-05-0580. [DOI] [PubMed] [Google Scholar]
- 16.Robinson-White A, et al. PRKAR1A Mutations and protein kinase A interactions with other signaling pathways in the adrenal cortex. J Clin Endocrinol Metab. 2006;91(6):2380–2388. doi: 10.1210/jc.2006-0188. [DOI] [PubMed] [Google Scholar]
- 17.Griffin KJ, et al. A transgenic mouse bearing an antisense construct of regulatory subunit type 1A of protein kinase A develops endocrine and other tumours: comparison with Carney complex and other PRKAR1A induced lesions. Journal of Medical Genetics. 2004;41(12):923–931. doi: 10.1136/jmg.2004.028043. [DOI] [PMC free article] [PubMed] [Google Scholar]