Abstract
Tuberous sclerosis is an autosomal dominant disorder characterized by involvement of skin, nervous system, kidneys, and lungs. It results from mutations in 1 of 2 genes: TSC1 (encoding hamartin) or TSC2 (encoding tuberin), leading to dysregulation and activation of the mammalian target of rapamycin (mTOR) pathway. Constitutive activation of mTOR signaling has recently been reported in systemic lupus erythematosus (SLE), and inhibition of this pathway may benefit patients with SLE nephritis. We report a case of a young woman with tuberous sclerosis who developed fulminant SLE, with lower extremity edema, massive proteinuria, and class IV lupus glomerulonephritis. She died despite treatment with high-dose steroids, plasmapheresis, and cyclophosphamide. Although there are no prior reports of coexistence of these 2 rare diseases, this case is of considerable interest because of the possibility that activation of mTOR by the TSC mutations may have led to activation of the immune system and the development of unusually severe SLE.
Keywords: tuberous sclerosis, systemic lupus erythematosus, rapamycin, mTOR
The tuberous sclerosis complex (TSC) is a multisystem autosomal dominant disorder resulting from mutations in either of 2 genes: TSC1 (encoding hamartin) or TSC2 (encoding tuberin), leading to dysregulation of the mammalian target of rapamycin (mTOR) pathway. Major and minor criteria have been proposed for diagnosis. Tuberous sclerosis complex often causes disabling neurologic disorders such as epilepsy, mental retardation, and autism. Additional major features of disease include facial angiofibromas, renal angiomyolipomas, and pulmonary lymphangiomyomatosis.
mTOR is a key eukaryotic signaling protein conserved from yeast to humans, which regulates protein synthesis and energy expenditure. Constitutive activation of mTOR is a major pathological consequence of mutation in TSC1 or TSC2.
Mutations in the mTOR pathway such as those seen in tuberous sclerosis might be anticipated to put such patients at increased risk for systemic lupus erythematosus (SLE) because mTOR signaling plays a key role in SLE T cells and may trigger autoimmunity.1 Furthermore, inhibition of mTOR signaling with rapamycin has been reported to be effective in the treatment of human SLE.2
We report a patient who suffered from tuberous sclerosis with development of rapidly fatal SLE. We hypothesize that the onset of lupus in the presence of dysregulation of mTOR may have led to the development of virulent autoimmunity. To our knowledge, this is the first report of these 2 diseases in the same patient. This case highlights the possible importance of mTOR in SLE.
CASE
A 26-year-old woman with tuberous sclerosis and a seizure disorder was admitted with progressive upper- and lower-extremity swelling for 10 days and nausea/vomiting and diarrhea. Before this, she was in apparent good health except for TSC. She denied rash, chest pain, oral ulcers, photosensitivity, alopecia, Raynaud phenomenon, fevers, or bruising. Home medications were levetiracetam 1 g twice a day, topiramate 200 mg twice a day, and loratadine 10 mg daily. She had facial angiofibromata. Upon admission, she had nephrotic-range proteinuria (17 g proteinuria in 24 hours), hematuria, and acute renal failure. Her laboratory results and serologies are summarized in the Table 1. Abdominal computed tomography scan showed renal angiolipomata. Renal biopsy showed (1) diffuse lupus nephritis, class IV, with numerous hyaline thrombi suggestive of cryoglobulins; (2) microscopic foci of spindle cell proliferation consistent with angiomyolipomata (Fig. 1). Serum cryoglobulins were not detected.
Table 1.
Laboratory Results Upon Admission and Their Trend During the Hospitalization
| Laboratory Test | On Admission: August 2011 | September 2011 | October 2011 | November 2011 |
|---|---|---|---|---|
| ANA/dsDNA/Sm/RNP | 1:1280 Homogeneous/+/+/+ | |||
| C3 (70–176 mg/dL) | 17.2 | 65.5 | 62.2 | 105 |
| C4 (16–64 mg/dL) | 10 | 18.4 | 10.1 | 12.7 |
| CBC:Hb (11.5–16.0 g/dL) | Hb 6.4 g/dL | Hb 7.6 g/dL | Hb 7.8 g/dL | Hb 7.9 g/dL |
| WBC (4000–11,000/μL) | 4000/μL | 7200/μL | 5000/μL | 12,100/μL |
| Platelets (140,000–400,000/μL) | 212,000/μL | 95,000/μL | 110,000/μL | 224,000/μL |
| BUN (8–20 mg/dL) | 78 | 43 | 80 | 77 |
| Cr (0.40–1.30 mg/dL) | 4.18 | 2.4 | 2.0 | 0.9 |
| 24-h Urine protein | 17,418 mg | 8676 mg | ||
| Cryoglobulins | Negative | |||
| Hepatitis B/C/HIV | Negative | |||
| Urinalysis (protein: | >300 Protein, large blood, large granular | |||
| negative, blood: negative) | casts, packed red blood cells | |||
| ESR (0–20 mm/h) | 137 | 103 | ||
| CRP (0.08–0.80 mg/dL) | 5.72 |
Normal values of each test are shown in the parentheses.
ANA indicates antinuclear antibody; dsDNA, double-stranded DNA; anti-Sm, anti-Smith; RNP, ribonucleoprotein; CBC, complete blood count; Hb, hemoglobin; WBC, white blood count; BUN, blood urea nitrogen; Cr, creatinine; HIV, human immunodeficiency virus; UA, urinalysis; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
Figure 1.
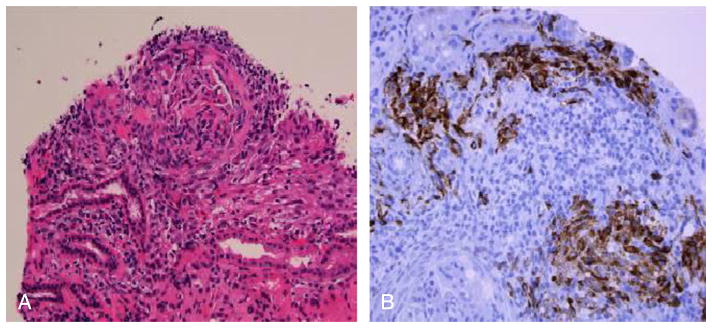
Renal interstitial involvement by angiomyolipoma. A shows low-power view of interstitial spindle cell proliferation accompanied by inflammation. Spindle cells show intense cytoplasmic staining for HMB-45 by immunoperoxidase (B) (original magnification, 100× [A], 400× [B]). Figure A and B Color Figures available online at www.jclinrheum.com
She was treated with pulse methylprednisolone and started on dialysis. Her course was complicated by respiratory failure due to diffuse alveolar hemorrhage (DAH). She received intravenous pulse cyclophosphamide, and renal function improved. She had a prolonged hospital course, during which she developed seizures that were managed with additional medications, gastrointestinal bleeding from gastric ulcers, and multiple episodes of hypoxic respiratory failure attributed to DAH. Her second cyclophosphamide infusion was delayed due to Pseudomonas bacteremia. She also received plasma exchange for DAH with hypoxic episodes. She again developed seizures, and brain magnetic resonance imaging was suggestive of posterior reversible encephalopathy. Her systolic blood pressures ranged from 140 to 170 mm Hg to 90 to 110 mm Hg diastolic during these episodes. She developed Pseudomonas sepsis again that was treated with cefepime. She continued to decline, with worsening DAH, and died 120 days after admission.
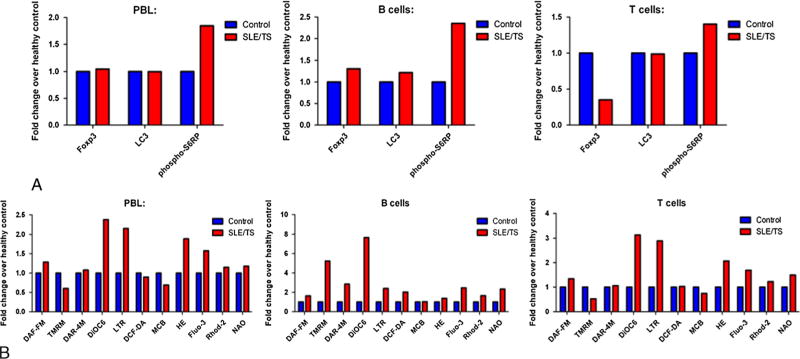
Flow cytometric analysis showed that the patient’s B and T cells displayed increased mTOR activity in monocyte-depleted peripheral blood lymphocytes, CD19+ B cells, and CD3+ T cells relative to a healthy control matched for age and gender (Fig. 2). There was also diminished expression of the Foxp3 transcription factor, possibly reflecting deficiency of regulatory T cells linked to activation of mTOR. The TSC/SLE patient’s lymphocytes also exhibited elevation of the mitochondrial transmembrane potential (mitochondrial hyperpolarization), increased mitochondrial mass, increased hydrogen peroxide, increased cytosolic and mitochondrial calcium, increased nitric oxide and nitrite production, increased lysosomal mass, and reduced intra-cellular glutathione (MCB) (Fig. 2B and 3).
Figure 2.
Increased mTOR activity and underlying metabolic changes in TSC/SLE patient lymphocytes. A, Flow cytometric measurement of mTOR activity via phosphorylation of S6 ribosomal protein (pS6RP) and expression of Foxp3 transcription factor in monocyte-depleted peripheral blood lymphocytes, CD19+ B cells, and CD3+ T cells of TSC/SLE patient and healthy control matched for age and gender. LC3= microtubule associated protein 1 light chain 3. B, Detection of nitric oxide (DAF-FM), mitochondrial transmembrane potential (TMRM, DiOC6) nitrate (DAR-4M), lysosomes (LTR), hydrogen peroxide (DCF-DA), intracellular glutathione (MCB), reactive oxygen intermediates (HE), cytosolic calcium (Flou-3), mitochondrial calcium (Rhod-2), mitochondrial mass (NAO) using flow cytometry. Color Figures available online at www.jclinrheum.com
Figure 3.
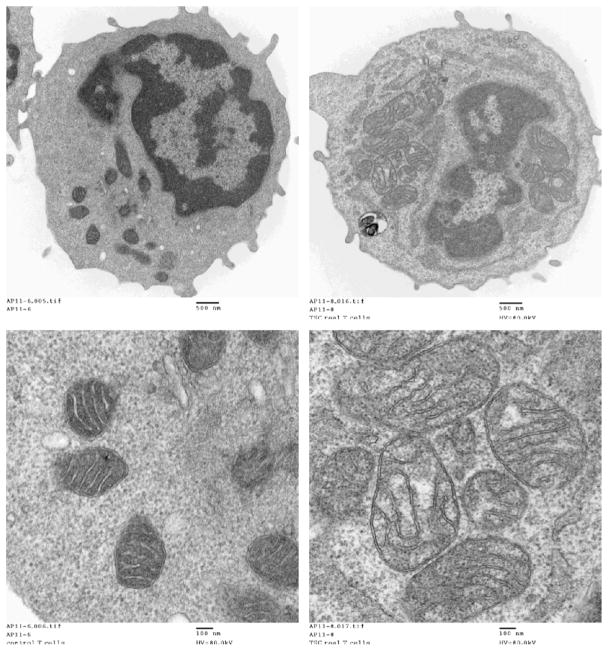
The right panel shows increased size and numbers of mitochondria in T cells isolated from the patient with tuberous sclerosis overlapping with systemic lupus erythematosus (TSC/SLE). T cells were prepared by negative selection of peripheral blood mononuclear cells from a normal age-matched control are shown on the left; 5 × 106 T cells were fixed in 2.5% glutaraldehyde and prepared for electron microscopy. Images shown represent 29,500× magnification of whole cells (top panels) and 108,000× magnification of mitochondria (bottom panels).
DISCUSSION
This patient had a mutation in the TSC complex leading to tuberous sclerosis and subsequently developed severe SLE. The coexistence of these 2 conditions is of interest in light of recent findings concerning the possible role of mTOR in SLE. Extensive biochemical and genetic studies have shown that the TSC complex inhibits the rapamycin-sensitive mTOR signaling pathway, thereby suppressing cell growth. The functional characterization of TSC proteins as intrinsic suppressors of mTOR signaling has suggested a possible therapeutic value of rapamycin for TSC disease. The phosphoinositol-3-kinase/Akt pathway is a major upstream activator of mTOR, and recent studies have also implicated it in the pathogenesis of SLE.5,6 Consistent with this notion, Reddy et al10 found a strong association between mTOR pathway genes and the genes implicated in human lupus by constructing the mTOR pathway interactome. Based on the possible role of mTOR pathway in SLE, use of rapamycin has been explored in SLE. Indeed, in different mouse models of lupus, rapamycin has been shown to be an effective treatment for lupus nephritis.7–9 Warner et al7 showed that rapamycin prevented the rise in anti–double-stranded DNA antibody and urinary albumin levels in lupus-prone MRL/lpr mice and restored T-cell mitogen-stimulated splenocyte proliferation and interleukin 2 production. Results were reproduced in 9 human patients with SLE refractory to other immunosuppressive therapy where rapamycin was beneficial.2
It seems that rapamycin exerts a beneficial role in SLE by different mechanisms. It has been considered that rapamycin acts by inhibiting interleukin 2–mediated signal transduction pathways, thus preventing cell cycle progression from the G1 to the S phase in T cells and several other cell lines.11 Battaglia et al12 have shown in a murine model that rapamycin induces expansion of the naturally occurring CD4+ CD25+ FoxP3+ T regulatory cells, which retain their suppressive functions in vitro and in vivo, supporting the notion that rapamycin might restrict the proliferation and activity of autoreactive T cells by promoting T regulatory cell expansion. Rapamycin also limits proinflammatory interferon α production by plasmacytoid dendritic cells.13 In some studies, it has been shown to promote a TH1-focused immune response, which may limit the T-cell stimulation of autoreactive B cells in SLE. Rapamycin was a consideration in our patient’s case, but she had already been started on cyclophosphamide regimen for lupus nephritis. She did not receive rapamycin.
CONCLUSIONS
To date, there are no reports of the coexistence of SLE and TSC. The independent occurrence of lupus in the presence of the TSC environment, with increased constitutive mTOR signaling, may lead to a particularly aggressive form of SLE, as seen in this case report. It is also possible that the TSC environment may by itself predispose to the development of autoimmunity. It is possible that TSC patients may have undetected autoimmunity due to their mTOR signaling abnormalities. To test this hypothesis, we propose that TSC patient sera should be screened for antinuclear antibodies.
Footnotes
The authors declare no conflict of interest.
References
- 1.Fernandez D, Perl A. mTOR signaling: a central pathway to pathogenesis in systemic lupus erythematosus? Disc Med. 2010;9:173–178. [PMC free article] [PubMed] [Google Scholar]
- 2.Fernandez D, Bonilla E, Mirza N, et al. Rapamycin reduces disease activity and normalizes T cell activation-induced calcium fluxing in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54:2983–2988. doi: 10.1002/art.22085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perl A, Nagy G, Gergely P, Jr, et al. Apoptosis and mitochondrial dysfunction in lymphocytes of patients with systemic lupus erythematosus. In: Perl A, editor. Autoimmunity: Methods and Protocol. 1. Totowa, NJ: Humana; 2004. pp. 87–114. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez DR, Telarico T, Bonilla E, et al. Activation of mTOR controls the loss of TCRzeta in lupus T cells through HRES-1/Rab4-regulated lysosomal degradation. J Immunol. 2009;182:2063–2073. doi: 10.4049/jimmunol.0803600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barber DF, Bartolome A, Hernandez C, et al. PI3Kgamma inhibition blocks glomerulonephritis and extends lifespan in a mouse model of systemic lupus. Nat Med. 2005;11:933–935. doi: 10.1038/nm1291. [DOI] [PubMed] [Google Scholar]
- 6.Stylianou K, Petrakis I, Mavroeidi V, et al. The PI3K/Akt/mTOR pathway is activated in murine lupus nephritis and downregulated by rapamycin. Nephrol Dial Transplant. 2011;26:498–508. doi: 10.1093/ndt/gfq496. [DOI] [PubMed] [Google Scholar]
- 7.Warner LM, Adams LM, Sehgal SN. Rapamycin prolongs survival and arrests pathophysiologic changes in murine systemic lupus erythematosus. Arthritis Rheum. 1994;37:289–297. doi: 10.1002/art.1780370219. [DOI] [PubMed] [Google Scholar]
- 8.Lui SL, Yung S, Tsang R, et al. Rapamycin prevents the development of nephritis in lupus-prone NZB/W F1 mice. Lupus. 2008;17:305–313. doi: 10.1177/0961203307088289. [DOI] [PubMed] [Google Scholar]
- 9.Lui SL, Tsang R, Chan KW, et al. Rapamycin attenuates the severity of established nephritis in lupus-prone NZB/W F1 mice. Nephrol Dial Transplant. 2008;23:2768–2776. doi: 10.1093/ndt/gfn216. [DOI] [PubMed] [Google Scholar]
- 10.Reddy PS, Legault HM, Sypek JP, et al. Mapping similarities in mTOR pathway perturbations in mouse lupus nephritis models and human lupus nephritis. Arthritis Res Ther. 2008;10:R127. doi: 10.1186/ar2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sehgal SN. Rapamune (RAPA, rapamycin, sirolimus): mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin Biochem. 1998;31:335–340. doi: 10.1016/s0009-9120(98)00045-9. [DOI] [PubMed] [Google Scholar]
- 12.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 13.Cao W, Manicassamy S, Tang H, et al. Toll-like receptor- mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin- sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol. 2008;9:1157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]