Abstract
We herein report a simple and concise route for the synthesis of highly substituted imidazole derivatives via copper-mediated oxidative C–H functionalization in good to high yields. The advantage of the reaction lies in its mild reaction conditions and readily available starting materials.
Keywords: C–H functionalization, Imidazole, Oxidative dehydrogenation
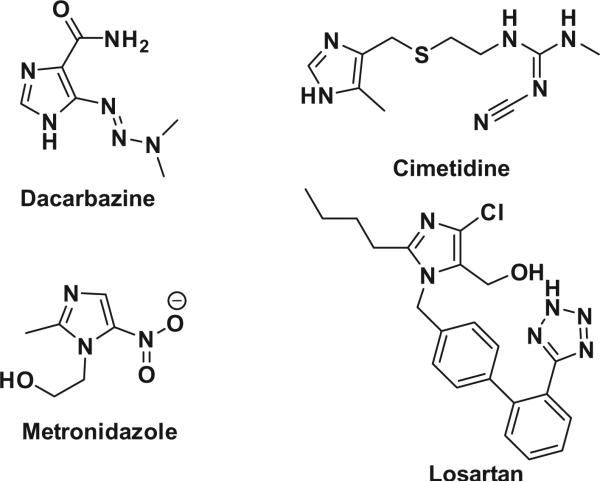
Small heterocyclic molecules can play a critical role in the regulation of macromolecule targets in living systems. Such functions make them a more powerful tool for understanding the various biological pathways for basic research and as pharmacological agents for curing diseases. As a representative of this class of compounds, the imidazole scaffold is emerging as a valuable pharmacophore for biomedical research.1 The imidazole scaffold is present in many natural products and is a bioactive substance in human metabolism.2 There are many clinical drugs being used in the different therapeutic areas based on the imidazole structure, such as anti-cancer (dacarbazine), antihistaminic (cimetidine), anti-parasitic (metronidazole), and antihypertensive (losartan) (Fig. 1).
Figure 1.
Examples of significant imidazole containing pharmaceuticals.
Due to their importance, it has become an attractive target for the synthetic and medicinal chemist. There are many synthetic methodologies that have been developed for assembling and decorating the imidazole ring with diverse functional groups. One of the approaches is using the transition metal catalyzed direct C–H/N–H functionalization3 and other approaches include using various catalytic systems like I2,4 MgSO4,5 FeCl3.6H2O, 6 BF3-SiO2,7 DABCO,8 zeolite-supported reagents,9 bioglycerol-based carbon catalyst,10 HgCl211 and CuI.12 Many reported methods involve expensive starting material or catalytic systems. Therefore, it is still of great importance to explore novel and efficient synthetic approaches for highly substituted imidazoles.
In recent years, transition metal-catalyzed cross-coupling reactions of various reactive functional groups have attracted great interest.13 More specifically, Li et al. have reported cross-dehydrogenative coupling (CDC) reaction in the presence of copper and oxidants.14 Similarly, Wang et al. have prepared 2-phenylquinazolines and oxazoles by using benzylamine as one of the starting materials.15,16 Herein, we report the concise route for the synthesis of highly substituted imidazole via copper-mediated oxidative C–H functionalization from benzylamine and β-enamino esters.
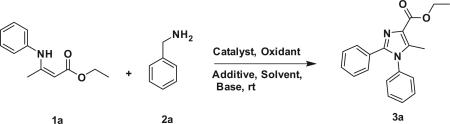
We initiated an investigation of the reaction using β-enamino esters (1a) and benzylamine (2a) as starting point in the presence of a copper source, iodine, and t-BuOOH (TBHP) in hexane at room temperature. The desired product was isolated in 42% yield by the use of CuI as catalyst and acetonitrile as solvent. Then we optimized the reaction condition with different reaction parameters, including catalysts and solvents. Initially, we tried different solvents like DMF, THF, and DMA at room temperature with Cu(OAc)2·H2O and CuI as catalysts. To our delight, the yield was increased by 70% when changing the solvent to DMA in the presence of Cu(OAc)2·H2O catalyst (Table 1, entry-5). Further, we checked the reaction with NaHCO3 as base with the improved reaction solvent (DMA), and to our surprise, the yield was increased by 76% (Table 1, entry-6). With the optimized conditions in hand, we synthesized a series of highly substituted imidazoles with various substitutions with good to high yields.
Table 1.
Optimization of the reaction conditions
| |||||
|---|---|---|---|---|---|
| Entry | Catalyst | Oxidant | Additive | Solvent | Yieldb (%) |
| 1 | CuI | TBHP | I2 | CH3CN | 42 |
| 2 | CuI | TBHP | I2 | DMF | 62 |
| 3 | Cu(OAc)2·H2O | TBHP | I2 | THF | 56 |
| 4 | Cu(OAc)2·H2O | TBHP | I2 | DMF | 68 |
| 5 | Cu(OAc)2·H2O | TBHP | I2 | DMA | 70 |
| 6a | Cu(OAc)2·H2O | TBHP | I2 | DMA | 76 |
Reaction conditions: (Z)-ethyl 3-(phenylamino) but-2-enoate (1a) (1 equiv), benzylamine (2a) (2 equiv), catalyst (20 mmol %), oxidant (2 equiv), additive (1 equiv). Reaction was performed for 12 h.
In the presence of NaHCO3 (1 equiv).
Isolated yields.
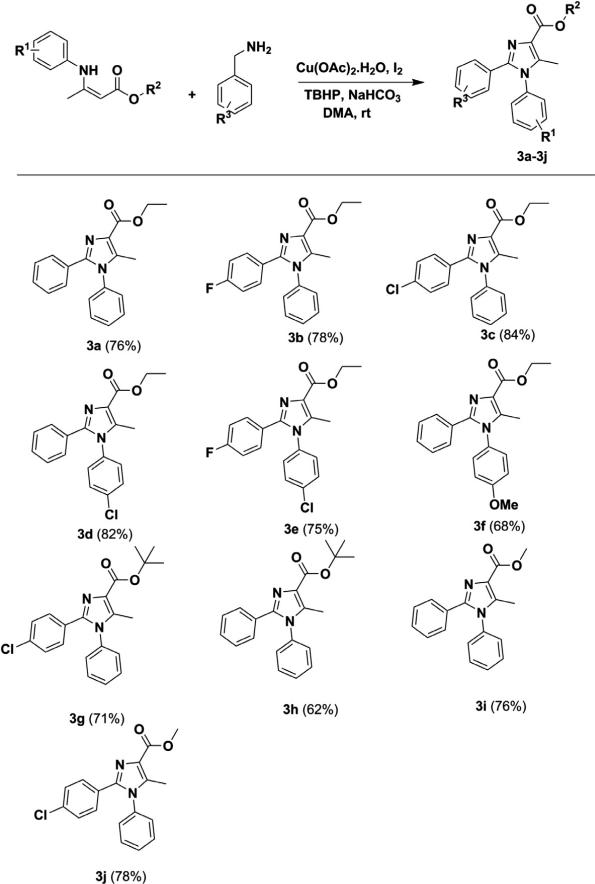
In order to check the functional group tolerance and scope of the substrate, we synthesized highly substituted imidazoles using this protocol by various benzylamine and β-enamino esters. The reaction with the electron withdrawing group (–Cl) present in the aromatic ring of benzylamine (3c, 3g) resulted in an excellent yield compared to the unsubstituted aromatic ring (3a, 3h), but it gave a similar yield when β-enamino methyl ester (3i, 3j) was taken as the starting material. Furthermore, the introduction of electron releasing substituent (3f) at β-enamino ester decreased the yield moderately. The functionality of β-enamino esters was checked by introducing various esters and amines to β-enamino esters. Compounds with ethyl (3a–3f) and methyl (3i, 3j) esters gave good yields compared to t-butyl esters (3g, 3h). Further, aryl amine provided the desired product in good to excellent yields, but the aliphatic amine and unsubstituted amine did not yield the desired product (Fig. 2). This is due to the electron withdrawing property of aryl amine not present in aliphatic and unsubstituted amine. Thus, aryl amine is more nucleophilic and thus more likely to undergo cyclization than aliphatic and unsubstituted amine (Scheme 1).
Figure 2.
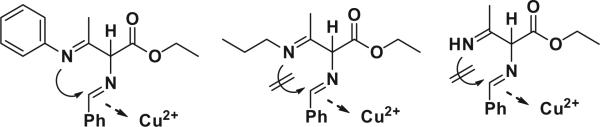
The amine functionality of β-enamino esters.
Scheme 1.
Copper-mediated oxidative functionalization of β-enamino esters with different benzylamine derivatives. Reaction conditions: β-enamino esters (1a) (1 equiv), benzylamine derivatives (2a) (2 equiv), NaHCO3 (1 equiv), Cu(OAc)2 H2O (20 mmol %), TBHP (2 equiv), I2 (1 equiv). Reaction was performed for 12 h.
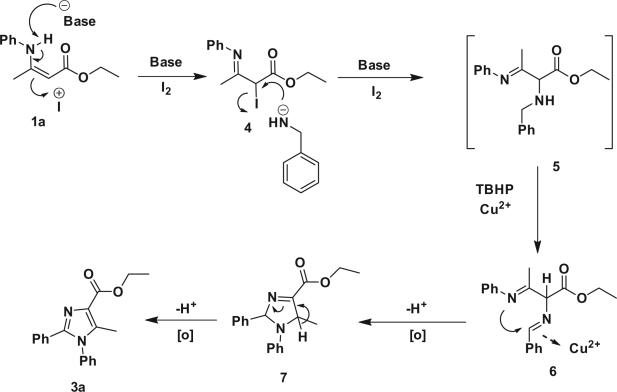
On the basis of the mechanism discussed in the literature,14 plausible reaction mechanism is shown in Scheme 2. Initially, base is involved in the hydrogen abstraction from 1a to give carbanion, which generates 4 via enolization process in the presence of I+· The nucleophilic substitution of benzylamine to intermediate 4 provides 5. Oxidative dehydrogenation and coordination of Cu2+ ion give 6, which undergoes intramolecular cyclization and oxidation of 7, give the desired product 3a.
Scheme 2.
In conclusion, we have developed a concise route for the synthesis of highly substituted imidazoles via copper-mediated oxidative C–H functionalization from readily available starting materials. The notable advantages of the method lie in its mild reaction conditions and low cost, less toxicity, and environmentally benign metal catalyst. This approach could be helpful for the generation of small heterocyclic libraries for the high throughput screening.
Supplementary Material
Acknowledgments
This project was supported by the National Institute of Health research Grant R01HL104516 and R01HL120659 to D.K.A. We thank Dr. James Fletcher (Department of chemistry, Creighton University) for helping in recording Mass and NMR spectra.
Footnotes
Supplementary data
Supplementary data (general experimental procedures, mass and NMR spectral data for representative compounds) associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.tetlet.2014.01.136.
References and notes
- 1.Sullivan JD, Giles RL, Looper RE. Curr. Bioact. Compd. 2009;5:39. [Google Scholar]
- 2.Aleksandrova EV, Kravchenko AN, Kochergin PM. Chem. Heterocycl. Compd. 2011;47:261. [Google Scholar]
- 3.a Sezen B, Sames D. J. Am. Chem. Soc. 2003;125:10580. doi: 10.1021/ja036157j. [DOI] [PubMed] [Google Scholar]; b Lee SH, Yoshida K, Matsushita H, Clapham B, Koch G, Zimmermann J, Janda KD. J. Org. Chem. 2004;69:8829. doi: 10.1021/jo048353u. [DOI] [PubMed] [Google Scholar]
- 4.Kidwai M, Mothsra P. Tetrahedron Lett. 2006;47:5029. [Google Scholar]
- 5.Hu B, Ai N, Wang Z, Xu X, Li X. ARKIVOC. 2012:222. [Google Scholar]
- 6.Heravi MM, Derikv F, Haghighi M. Monatsh. Chem. 2008;139:31. [Google Scholar]
- 7.Sadeghi B, Mirjalili BBF, Hashemi MM. Tetrahedron Lett. 2008;49:2575. [Google Scholar]
- 8.Murthy SN, Madhav B, Nageswar YVD. Tetrahedron Lett. 2010;51:5252. [Google Scholar]
- 9.Sivakumar K, Kathirvel A, Lalitha A. Tetrahedron Lett. 2010;51:3018. [Google Scholar]
- 10.Ramesh K, Narayana Murthy S, Karnakar K, Nageswar YVD, Vijayalakhshmi Kukuma, Prabhavathi Devi Bethala L. A., Prasad RBN. Tetrahedron Lett. 2012;53:1126. [Google Scholar]
- 11.Kaila JC, Baraiya AB, Pandya AN, Jalani HB, Vasu KK, Sudarsanam V. Tetrahedron Lett. 2009;50:3955. [Google Scholar]
- 12.Tang Dong, Wu P, Liu X, Chen YX, Guo SB, Chen WL, Li JG, Chen BH. J. Org. Chem. 2013;78:2746. doi: 10.1021/jo302555z. [DOI] [PubMed] [Google Scholar]
- 13.Li CJ. Acc. Chem. Res. 2009;42:335. doi: 10.1021/ar800164n. [DOI] [PubMed] [Google Scholar]
- 14.Li Z, Li CJ. J. Am. Chem. Soc. 2005;127:3672. doi: 10.1021/ja050058j. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Zhu D, Yu C, Wan C, Wang Z. Org. Lett. 2010;12:2841. doi: 10.1021/ol100954x. [DOI] [PubMed] [Google Scholar]
- 16.Wan C, Zhang J, Wang S, Fan J, Wang Z. Org. Lett. 2010;12:2338. doi: 10.1021/ol100688c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.