Abstract
Background
Elevated blood alcohol content is a risk factor for injury. Associations of blood alcohol content with acute respiratory distress syndrome have not been conclusively established. We evaluated the association of a BAC >0 mg/dL with the intermediate outcomes, Injury Severity Score and Glasgow Coma Score, and their association with acute respiratory distress syndrome development.
Methods
Observational retrospective cohort study of 26,305 primary trauma admissions to a statewide referral trauma center from July 11, 2003 to October 31, 2011. Logistic regression was performed to assess the relationship between Admission blood alcohol content, Injury Severity Score, Glasgow Coma Score, and acute respiratory distress syndrome development within five days of admission.
Results
The case-rate for acute respiratory distress syndrome was 5.5% (1447). Blood alcohol content >0 mg/dL was associated with acute respiratory distress syndrome development in adjusted analysis (Odds Ratio 1.50; 95% Confidence Interval 1.33–1.71, p<0.001). High Injury Severity Score (≥16) had a stronger association with acute respiratory distress syndrome development (Odds Ratio 17.99; 95% Confidence Interval 15.51–20.86); as did low Glasgow Coma Score (≤8) (Odds Ratio 8.77; 95% Confidence Interval 7.64–10.07, p<0.001). Patients with low Glasgow Coma Score and high Injury Severity Score had the most frequent acute respiratory distress syndrome (33.6%) and the highest case fatality rate without acute respiratory distress syndrome (24.7%).
Conclusions
Elevated blood alcohol content is associated with acute respiratory distress syndrome development. In the analysis of alcohol exposure, Injury Severity Score and Glasgow Coma Score occur after alcohol ingestion, making them intermediate outcomes. Injury Severity Score and Glasgow Coma Score were strong predictors of acute respiratory distress syndrome and may be useful to identify at-risk patients. Elevated blood alcohol content may increase the frequency of the acute respiratory distress syndrome through influence on injury severity or independent molecular mechanisms which can be discriminated only in experimental models
Keywords: Acute Respiratory Distress Syndrome, Trauma Severity Indices, Ethanol, Alcohol Drinking
BACKGROUND
Chronic alcohol use carries a two to three fold risk for acute respiratory distress syndrome (ARDS) development1,2. Subsequent studies have shown mechanisms in the lung with altered epithelial cell permeability, decreased alveolar liquid clearance, and decreased glutathione production and redox potential3. Investigations into the relationship of acute alcohol exposure and ARDS are not available. One observational study investigating the effects of both elevated BAC and chronic alcohol use on outcomes in trauma patients found an increased risk of respiratory failure and mortality among chronic alcohol use patients and not patients with BAC ≥100 mg/dL4, but those investigators did not assess ARDS according to an international consensus definition or ascertain BAC systematically.
Alcohol intoxication is a risk factor for injury,5 and elevated BAC is present in up to 50% of trauma patients4,5. Studies assessing the acute alcohol-injury severity relationship have focused on the behavioral effects of an elevated BAC such as speeding, seat belt use or mechanism, severity, and location of injury6. The deleterious biological effects of an elevated BAC with injury have been shown in murine models7. Observational studies have shown an increased susceptibility to pneumonia and infections from an elevated BAC8,9, but the association with ARDS was not described.
Studies examining acute alcohol exposure and outcomes in trauma patients have used trauma severity indices as confounders when, in fact, they occur after alcohol exposure and are intermediate outcomes. Severe trauma is a strong predictor of mortality10 and may activate the systemic inflammatory response syndrome (SIRS)11; leading to multiple organ dysfunctions (MODS) including ARDS12,13. ARDS occurs in 4–8% of trauma patients; percentages as high as 33% have been reported in patients admitted with injury severity scores (ISS) ≥ 1614–16.
We hypothesized that elevated BAC is associated with ARDS development. In a cohort of patients from a statewide referral trauma center with routine BAC testing, we evaluated the relationships of BAC with incident ARDS within five days of injury. ISS and Glasgow Coma Score (GCS) were assessed as intermediate outcomes and in relationship to the risk for ARDS and mortality.
METHODS
Environment
We analyzed data from a cohort of 26,305 patients admitted to the R. Adams Cowley Shock Trauma Center (STC) at the University of Maryland Medical Center between July 11, 2003 and October 31, 2011. The STC) is a free-standing, urban, Level I trauma center which admits over 5,000 primary trauma patients annually from a catchment of urban, suburban, and rural communities of over 6 million people. The STC has been the central healthcare resource for critically injured adults in the State of Maryland for more than three decades and has maintained a trauma registry since the mid-1980s17.
Patient Selection
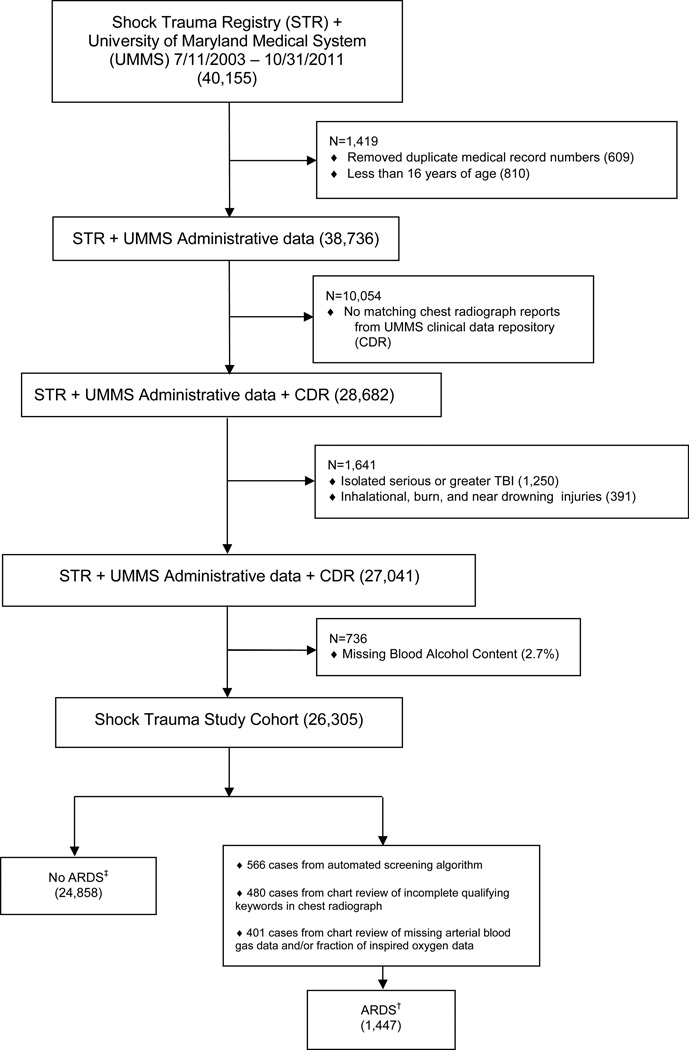
We assessed 40,155 patient records (Figure 1) which did not include patients with length of stay less than 15 minutes17 (323, 0.8%), in order to avoid patients who arrived dead or died immediately after arrival to the trauma center. This proportion is similar to that in the National Trauma Data Bank (1.8%)18. Patients younger than 16 years of age, inhalational injuries, burn injuries, near drowning, and isolated traumatic brain injury (TBI) defined by an Abbreviated Injury Score (AIS) ≥ 3 for head/neck but ≤ 1 for each of the other body regions were excluded16. Patients 16–21 years old were included, as 910/4149 (22%) had BAC >0 mg/dL.
Figure 1.
CONSORT Diagram
† 1441 cases of ARDS in patients with Injury Severity Scores
‡ 24,131 cases without ARDS in patients with Injury Severity Scores
Acute Respiratory Distress Syndrome Definition
A validated surveillance algorithm19,20 incorporating the Berlin definition21 was adapted to identify patients with ARDS. In study of ICU patients, the surveillance algorithm had a sensitivity of 97.6% and specificity of 97.6%20. Qualifying chest radiograph reports were linked within 24 hours to qualifying PaO2/FiO2 ratios in mechanically ventilated patients. Patients receiving mechanical ventilation were identified using administrative data. In a random sample of 461 (1.7%) patients, overall accuracy was 93% for the ventilator administrative data, consistent with prior use of administrative data from our institution22. Over 92% of the cohort had chest radiographs performed during initial evaluation in the trauma resuscitation unit however reports were not routinely recorded in the registry. An electronic query process used the STC registry medical record numbers (MRN) to retrieve chest radiograph reports from the University of Maryland Clinical Data Repository, succeeding in 28,682/38,736 (74.0%) patients. Patients who did not have radiographs linkable by MRN were excluded; errors in record numbers have been documented to cause inconsistencies with similar frequency between trauma registries and administrative databases23. The removal of patients with unmatched radiograph reports did not affect the characteristics of the cohort when compared to all patients in the registry.
After screening, there were 8,179 chest radiograph reports in 3,890 patients with keyword results that were inconclusive for ARDS criteria. Chart review of those radiograph reports identified an additional 480 (12.3%) patients with ARDS. Patients who were mechanically ventilated and had qualifying chest radiographs but were missing registry data for PaO2 or FiO2 (925) had chart reviews performed to identify an additional 401 (43.4%) cases of ARDS; a validated measure of the pulse oximetry SpO2/ FiO2 ratio was used in 25 of those patients because of missing PaO2 data24.
Analytic Approach
Our primary outcome was ARDS development. We identified ARDS occurring within five days of trauma16. BAC exposure was evaluated in our primary analysis as a dichotomous variable (0 versus >0 mg/dL) to account for variations in transport time and resuscitation. In secondary analyses, BAC was evaluated as a continuous variable and in four ordered categories. BAC values were recorded in 26,305/27,041 (97.3%) of patients. Of the 736 patients who had missing BAC values, 549 (74.6%) were discharged home, 157 (21.3%) were discharged to acute and chronic care facilities, and 30 (4.1%) died; 42 (5.7%) developed ARDS in the first five days. In a random sample chart review of 74 (10.1%) missing BAC patients, 95.9% had true missing values.
ISS and GCS were intermediate outcomes that were evaluated dichotomously (ISS <16 versus ISS ≥ 16 and GCS >8 versus ≤8)16,25. In the final analysis cohort, 733 (2.8%) were missing ISS scores. Of the patients missing ISS scores, 647 (88.3%) were discharged home, 81 (11.1%) were discharged to acute and chronic care facilities, and 5 (0.7%) died; < 1% developed ARDS in the first five days. In the data analysis for ISS and GCS, only patients with ISS and GCS scores were evaluated (n=25,572).
Baseline characteristics were presented as medians with interquartile ranges and compared using Wilcoxon-Mann-Whitney or Kruskal Wallis non-parametric tests with continuous variables. A comparison of two or more proportions was performed using a Chi-Square test or Fisher Exact Test. Logistic regression was used to assess the risk for ARDS development. Variable selection for the multivariable logistic regression was made a priori and based on review of prior literature and biologic plausibility26,27. Logistic regression model performance was measured with likelihood ratio tests. All the models showed adequate performance with the likelihood ratio having a p<0.0001. The comorbidities were recorded in the registry from attending physician documentation. Test for linear and quadratic trend was performed for ordered BAC categories. The Pearson product-moment correlation coefficient was used to test the correlation between ISS and GCS.
Analysis was performed using SAS Version 9.1.3 (SAS Institute, Cary, NC). The institutional review board of the University of Maryland Baltimore approved this study with waiver of consent and Health Insurance Portability and Accountability Act authorization.
RESULTS
Demographics and characteristics by BAC are shown in Table 1. Estimated time from injury to trauma center presentation was 55.7 (±41.7) minutes. Among the 7360 (28.0%) patients with a BAC >0 mg/dL, levels greater than 100 mg/dL accounted for 5328 (20.3%) of the cohort. The large majority of trauma patients suffered blunt injury, but penetrating injuries were more common in patients with BAC >0 mg/dL. Mechanical ventilation was used in 5537 (21.0%) of the cohort. The five day case-fatality rate was 509 (1.9%).
Table 1.
Patient Characteristics by Alcohol Exposure (BAC in mg/dL)
| Dichotomous BAC | Ordered BAC | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Total (26,305) |
0 (18,945) |
> 0 (7,360) |
P-value | 1–100 (2,032) |
101–230 (3,273) |
>230 (2,055) |
P-value |
| Age (years) | 36.0 (23 to 51) | 38.0 (23 to 53) | 33.0 (23 to 46) | <0.001 | 31.0 (23 to 45) | 30.0 (23 to 44) | 36.0 (26 to 48) | <0.001 |
| Sex (Male) | 18394 (69.9) | 12463 (65.8) | 5931 (80.6) | <0.001 | 1599 (78.7) | 2631 (80.4) | 1701 (82.8) | <0.001 |
| Race (White) | 16212 (61.6) | 11966 (63.2) | 4246 (57.7) | <0.001 | 1024 (50.4) | 1973 (60.3) | 1249 (60.8) | <0.001 |
| Chronic Alcohol Use | 2122 (8.1) | 364 (1.9) | 1758 (23.9) | <0.001 | 240 (11.8) | 828 (25.3) | 690 (33.6) | <0.001 |
| Tobacco Use | 8330 (31.7) | 5147 (27.2) | 3183 (43.2) | <0.001 | 809 (39.8) | 1466 (44.8) | 908 (44.2) | <0.001 |
| DM | 1624 (6.2) | 1390 (7.3) | 234 (3.2) | <0.001 | 74 (3.6) | 92 (2.8) | 68 (3.3) | <0.001 |
| Immunosuppressive Medication | 50 (0.2) | 26 (0.1) | 24 (0.3) | 0.002 | 9 (0.4) | 12 (0.4) | 3 (0.2) | 0.002 |
| CHF or MI <12 mo | 233 (0.9) | 201 (1.1) | 32 (0.4) | <0.001 | 9 (0.4) | 17 (0.5) | 6 (0.3) | <0.001 |
| Injury Type | ||||||||
| Blunt | 22832 (86.8) | 16750 (88.4) | 6082 (82.6) | 1539 (75.7) | 2719 (83.1) | 1824 (88.8) | ||
| Penetrating | 3164 (12.0) | 1933 (10.2) | 1231 (16.7) | 481 (23.7) | 530 (16.2) | 220 (10.7) | ||
| Crush | 199 (0.8) | 178 (0.9) | 21 (0.3) | <0.001 | 5 (0.2) | 11 (0.3) | 5 (0.2) | <0.001 |
| Hanging | 39 (0.2) | 32 (0.2) | 7 (0.1) | 3 (0.2) | 4 (0.1) | 0 (0.0) | ||
| Ingestion | 9 (0.0) | 3 (0.0) | 6 (0.1) | 2 (0.1) | 3 (0.1) | 1 (0.1) | ||
| Other | 62 (0.2) | 49 (0.3) | 13 (0.2) | 2 (0.1) | 6 (0.2) | 5 (0.2) | ||
| ISS ≥ 16 | 6660 (26.0) (n=25,572) | 4613 (25.2) | 2047 (28.3) | <0.001 | 575 (30.0) | 960 (29.7) | 512 (25.3) | <0.001 |
| Admission GCS ≤8 | 1501 (5.9) (n=25,572) | 835 (4.5) | 666 (9.2) | <0.001 | 151 (7.6) | 287 (8.9) | 228 (11.3) | <0.001 |
| Mechanical Ventilation | 5537 (21.0) | 3430 (18.1) | 2107 (28.6) | <0.001 | 522 (25.7) | 954 (29.2) | 631 (30.7) | <0.001 |
| ARDS | 1447 (5.5) | 1010 (5.3) | 437 (5.9) | 0.05 | 121 (6.0) | 204 (6.2) | 112 (5.5) | 0.09 |
CHF = Congestive Heart Failure; MI = Myocardial Infarction; DM = Diabetes Mellitus; GCS = Glasgow Coma Score; ISS = Injury Severity Score; ARDS = Acute Respiratory Distress Syndrome
Continuous values are presented as medians with interquartile range, and categorical values are presented as numbers with percentages.
The frequency of comorbidities was low (Table 1). Fewer than 1% had cirrhosis, chronic renal failure, or heart failure. Diabetes was documented in 1624 (6.2%) of patients and hypertension in 4209 (16.0%). Characteristics associated with BAC >0 mg/dL included lower age, male gender, non-white race, chronic alcohol use, tobacco use, no previous diagnosis of diabetes, and prior use of immunosuppressive medication.
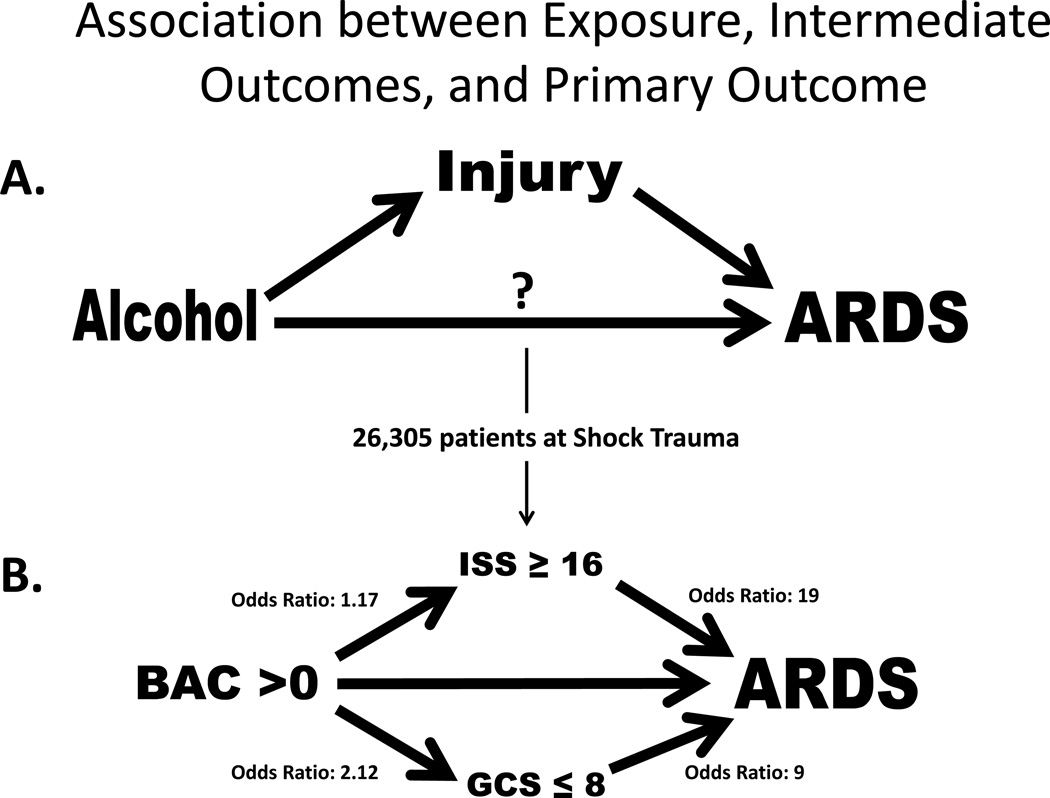
The case-rate for ARDS development in the first five days was 5.5%. The unadjusted odds ratio (OR) for ARDS development in patients with a BAC >0 mg/dL was 1.12; 95% CI 1.00–1.26, p= 0.05 (Table 2). In adjusted analysis, the strength of association of BAC >0 mg/dL with ARDS development increased to an OR 1.50; 95% CI 1.33–1.71, p<0.001. Higher category of BAC was associated with a greater risk for ARDS development (p<0.001). The result for continuous BAC was similar (SDC 1). In unadjusted analysis of the intermediate outcomes ISS and GCS, BAC >0 mg/dL was associated with increased risk for high ISS (≥16) (OR 1.17; 95% CI 1.10–1.25, p<0.001) and for low GCS (≤8) (OR 2.12; 95% CI 1.91–2.36, p<0.001) (Figure 2). In adjusted analysis, BAC >0 mg/dL was associated with an OR 1.17 (95% CI 1.09–1.25, p<0.001) for high ISS and OR 2.52 (95% CI 2.25–2.83, p<0.001) for low GCS.
Table 2.
Univariate and Multivariable Logistic Model for ARDS Development by BAC, GCS, and ISS
| UNIVARIATE | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood Alcohol Content (BAC) | Glasgow Coma Score (GCS) | Injury Severity Score (ISS) | |||||||||
| (26,305) | ARDS (OR and 95% CI) |
p-value | (25,572) | ARDS (OR and 95% CI) |
p-value | (25,572) | ARDS (OR and 95% CI) |
p-value | |||
| 0 mg/dL | 1.00 (ref group) | >8 | 1.00 (ref group) | <16 | 1.00 (ref group) | ||||||
| >0 mg/dL | 1.12 (1.00–1.26) | 0.05 | ≤8 | 9.08 (7.98–10.32) | <0.001 | ≥16 | 19.07 (16.46–22.08) | <0.001 | |||
| MULTIVARIABLE | |||||||||||
| Blood Alcohol Content (BAC) | Glasgow Coma Score (GCS) | Injury Severity Score (ISS) | |||||||||
| (26,305) |
ARDS (OR and 95% CI) |
p-value | (25,572) |
ARDS (OR and 95% CI) |
p-value | (25,572) |
ARDS (OR and 95% CI) |
p-value | |||
| Age | 1.02 (1.02–1.03) | <0.001 | Age | 1.02 (1.02–1.03) | <0.001 | Age | 1.02 (1.01–1.02) | <0.001 | |||
| Sex = Male | 1.95 (1.71–2.22) | <0.001 | Sex = Male | 1.86 (1.62–2.13) | <0.001 | Sex = Male | 1.68 (1.46–1.94) | <0.001 | |||
| Race = Non-White | 0.73 (0.65–0.83) | <0.001 | Race = Non-White | 0.76 (0.67–0.85) | <0.001 | Race = Non-White | 0.85 (0.75–0.96) | 0.02 | |||
| Chronic Alcohol Use (ref = No) | 0.46 (0.35–0.60) | <0.001 | Chronic Alcohol Use (ref = No) | 0.48 (0.36–0.63) | <0.001 | Chronic Alcohol Use (ref = No) | 0.37 (0.28–0.49) | <0.001 | |||
| Diabetes Mellitus (ref = No) | 0.87 (0.71–1.08) | 0.21 | Diabetes Mellitus (ref = No) | 0.98 (0.79–1.22) | 0.83 | Diabetes Mellitus (ref = No) | 1.06 (0.85–1.34) | 0.59 | |||
| Immunosupp Medication (ref = No) | 5.15 (2.50–10.6) | <0.001 | Immunosupp Medication (ref = No) | 4.75 (2.20–10.25) | <0.001 | Immunosupp Medication (ref = No) | 5.07 (2.19–11.71) | <0.0001 | |||
| Tobacco use (ref = No) | 0.44 (0.39–0.51) | <0.001 | Tobacco use (ref = No) | 0.61 (0.53–0.71) | <0.001 | Tobacco use (ref = No) | 0.58 (0.50–0.67) | <0.001 | |||
| BAC >0mg/dL | 1.17 (1.02–1.34) | 0.02 | BAC>0mg/dL | 1.39 (1.21–1.59) | <0.001 | ||||||
| BAC>0 mg/dL | 1.50 (1.33–1.71) | <0.001 | GCS ≤8 | 8.77 (7.64–10.07) | <0.001 | ISS ≥16 | 17.99 (15.51–20.86) | <0.001 | |||
Univariate panel: Unadjusted logistic regression for ARDS development by: BAC in column 1, GCS in column 2, and ISS in column 3
Multivariable panel: Adjusted logistic regression for ARDS development by: BAC in column 1, GCS in column 2, and ISS in column 3
ARDS = acute respiratory distress syndrome; ISS = Injury Severity Score; GCS = Glasgow Coma Score
Figure 2.
A. Primary exposure of alcohol is temporally associated and occurs prior to injury which is an intermediate outcome prior to ARDS development. Alcohol may have a direct relationship with ARDS development also. B. Unadjusted analyses from Shock Trauma Registry showing association between the primary exposure blood alcohol content with the intermediate outcomes ISS and GCS. The question of direct association of BAC with ARDS development cannot be answered without the ability to control the risk factors independently of each other.
The case-rate for ARDS development in patients with high ISS was 18.3% (1221) and low GCS was 28.5% (428). In unadjusted analysis, high ISS as well as low GCS each had a strong positive association for ARDS development. The inclusion of BAC >0 mg/dL in the adjusted analysis of ARDS development’s association with low GCS or high ISS made little change in the odds ratios (Table 2).
Patients with high ISS and thorax injury score ≥3 (serious chest injury) were more likely to develop ARDS than those without serious chest injury (73.1% vs. 27.0%, p<0.001) (SDC 2). Head/neck injury score ≥4 (severe) was determinative of high ISS by definition but was not determinative of low GCS although severe head/neck injury and low GCS are strongly associated (OR 28.3, 95% CI 25.1–31.9; p<0.001). Among patients with high ISS, the interaction between GCS and head/neck injury was not statistically significant (p=0.09) for ARDS development. Among patients with high ISS, severe head/neck injury made a small but statistically significant difference for ARDS development in the high GCS stratum (14.3% vs. 18.1%, p=0.002) but not for ARDS development in the low GCS stratum (33.4% vs. 33.6%, p=0.95) (SDC 3).
ISS and GCS were correlated (Pearson’s coefficient 0.48, p<0.001). Patients with high GCS and low ISS accounted for 72.9% of the cohort, and had the lowest case rates for ARDS development (0.1%) and the lowest case fatality rates without ARDS (1.0%). Patients with low GCS and high ISS had the highest rates for ARDS development (33.6%) and the highest case fatality rates without ARDS (24.7%). Low GCS increased the occurrence of ARDS or case-fatality little except in combination with high ISS (Table 3). Sensitivity for ARDS development or case-fatality from high ISS were 86.9% (95% CI 85.3%–88.4%) and specificity 78.7% (95% CI 78.2%–79.2%).
Table 3.
Patient Outcomes during First 5 Days of Hospitalization for Dichotomous BAC by GCS/ISS Strata (GCS Low = ≤8, ISS High = ≥16; n=25,572)
| BAC | |||
|---|---|---|---|
| 0 mg/dL | >0 mg/dL | Total | |
| GCS_Low/ISS_High | |||
| ARDS | 252 (35.5) | 154 (30.8) | 406 (33.6) |
| Dead without ARDS | 195 (27.5) | 104 (20.8) | 299 (24.7) |
| Alive without ARDS | 263 (37.0) | 242 (48.4) | 505 (41.7) |
| 710 (58.7) | 500 (41.3) | 1210 (4.7) | |
| GCS_High/ISS_High | |||
| ARDS | 586 (15.0) | 229 (14.8) | 815 (15.0) |
| Dead without ARDS | 69 (1.8) | 14 (0.9) | 83 (1.5) |
| Alive without ARDS | 3248 (83.2) | 1304 (84.3) | 4552 (83.5) |
| 3903 (71.6) | 1547 (28.4) | 5450 (21.3) | |
| GCS_Low/ISS_Low | |||
| ARDS | 13 (10.4) | 9 (5.4) | 22 (7.6) |
| Dead without ARDS | 6 (4.8) | 0 (0.0) | 6 (2.0) |
| Alive without ARDS | 106 (84.8) | 157 (94.6) | 263 (90.4) |
| 125 (43.0) | 166 (57.0) | 291 (1.1) | |
| GCS_High/ISS_Low | |||
| ARDS | 155 (1.1) | 43 (0.9) | 198 (1.0) |
| Dead without ARDS | 14 (0.1) | 1 (0.0) | 15 (0.1) |
| Alive without ARDS | 13427 (98.8) | 4981 (99.1) | 18408 (98.9) |
| 13596 (73.0) | 5025 (27.0) | 18621 (72.9) | |
| TOTAL | |||
| ARDS | 1006 (5.5) | 435 (6.0) | 1441 (5.6) |
| Dead without ARDS | 284 (1.5) | 119 (1.6) | 403 (1.6) |
| Alive without ARDS | 17044 (93.0) | 6684 (92.4) | 23728 (92.8) |
| 18334 (71.7) | 7238 (28.3) | 25572 | |
BAC = blood alcohol content; ARDS = acute respiratory distress syndrome; ISS = Injury Severity Score; GCS = Glasgow Coma Score
DISCUSSION
BAC >0 mg/dL was present in 28% of the patients; most of those had a BAC >100 mg/dL. We found BAC >0 mg/dL was associated with an increased risk for ARDS development; this risk was dose related. ISS and GCS result from the injury event and occur after alcohol ingestion, making them intermediate outcomes rather than baseline characteristics. BAC >0 mg/dL was associated with high ISS and low GCS. ISS and GCS were stronger predictors of ARDS development than BAC.
Controlled experimental and epidemiological studies have shown alcohol exposure can increase the severity of injury4,6. Epidemiologic studies that adjusted for injury severity in their analyses of outcomes from acute alcohol exposure may have obscured the association of mortality and other outcomes with BAC28–30 by overadjustment31. We showed an increased risk for ARDS development from BAC >0 mg/dL after adjusting for characteristics that were present prior to alcohol ingestion. Experimental studies with investigator control of injury severity are needed to better define the roles of acute alcohol exposure and trauma in the etiology of ARDS (Figure 2).
ISS is a useful and commonly-applied measure of trauma severity. Previously, trauma severity has been identified as a risk factor for ARDS, with higher ISS placing patients at greater risk18. In studying the association of BAC >0 mg/dL with clinical outcomes in trauma patients, it is important to recognize that injury temporally occurs after alcohol ingestion but previous epidemiologic studies adjusted for injury severity in their analyses, which may account for differing results in mortality risk,28,30. In our analysis, BAC >0 mg/dL increased the risk for ISS ≥16, but it had little effect on the development of ARDS in patients with high ISS. We found a high risk for ARDS development in patients with high ISS regardless of alcohol exposure. We also found serious chest trauma contributed to high ISS scores and was present in nearly two-thirds of the ARDS cases in our cohort. Serious chest trauma makes a strong contribution to ARDS development and can be used to predict ARDS development32. Pulmonary contusions of at least 20% of the chest have been associated with serious chest trauma, placing patients at-risk for ARDS12.
Similar to high ISS, BAC >0 mg/dL had little effect on the role of low GCS in ARDS development. Traumatic brain injury is one source of neurogenic ARDS not associated with other injury27. Although GCS and head/neck injury are the two most widely used clinical markers to assess traumatic brain injury, they are poorly correlated33. We found severe head/neck injury was strongly associated with a depressed sensorium (low GCS) but not all patients with a low GCS that developed ARDS had severe head/neck injury. Our findings are inconclusive as to whether severe head/neck injury made a difference in ARDS development in combination with depressed sensorium (low GCS). Among patients with clear sensorium (high GCS,) severe head/neck injury appears to add some risk for ARDS development. A depressed sensorium (low GCS) on admission may entail some risk for ARDS that does not derive from high ISS or severe head/neck injury.
The greatest risk for ARDS development occurs in patients with high ISS and low GCS. Patients admitted with severe injury (high ISS) and a depressed sensorium (low GCS) are more likely to experience ARDS or die than not, regardless of BAC level. Patients with a low ISS and a clear sensorium (high GCS)--who comprise the majority-- are unlikely to die or experience ARDS in the first five days of their hospital course.
It is difficult to parse alcohol’s direct pathophysiologic effects in the injured patient. Murine models have demonstrated that alcohol can impair pathogen clearance and alter lung inflammation after toxin exposure, via effects on neutrophil and alveolar macrophage recruitment and function34,35. These murine studies focused on isolated components of host defense during the immediate post injury period35. In a human study of trauma patients, alcohol-intoxicated trauma patients (excluding chronic alcoholics) had attenuation in immune response in the first several days after injury and an augmented immune response more than nine days after injury36.
One mechanism for ARDS development associated with depressed sensorium or alcohol exposure may be pulmonary aspiration syndromes37, which we did not capture in our data. We did exclude conditions that may lead to aspiration and ARDS development such as inhalational injuries, burns, and drownings38,39. We also excluded patients with isolated and serious TBI so we may address the effects of BAC on ARDS risk without entirely separate TBI effects.
Some factors for ARDS development produced different results in our cohort than previously reported. Prior studies showed chronic alcohol use was a positive risk factor for ARDS development in mixed ICU and medical ICU patients1,2. The frequency of chronic alcohol use in our cohort was lower than others observed3 and it was protective for ARDS development. Interaction of BAC >0 mg/dL and chronic alcohol use was present and was protective for ARDS development but we could not discern the reason in our data. Decreased risk for ARDS development from tobacco use was also shown whereas in another trauma cohort the risk was increased27. Chronic alcohol use is best measured with a validated questionnaire and tobacco use with plasma cotinine levels2,27. Our trauma registry utilized attending physician notes; explanations for differing results require more rigorous methods. Given the deleterious biological effects of chronic alcohol use3, a next step would be to better quantify the relationships among chronic alcohol use and acute alcohol exposure on trauma patient outcomes.
Though a single center, R. Adams Cowley Shock Trauma Center is a regional trauma referral center for the State of Maryland. The volume and characteristics of the center’s patients is comparable to patients treated in multiple trauma centers throughout the United States40. The ARDS case-rate in our statewide referral trauma center is similar to previously reported regional and national rates14,15.
Nearly all our patients had BAC recorded and those with missing BAC had a similar case-rate for ARDS development to the rest of the patients suggesting missing BAC values were not selected for occurrence of ARDS. We chose a dichotomous exposure with BAC as our primary analysis to reduce the influence of variations in transit time from injury scene to the trauma resuscitation unit. Medevac systems customarily provide fluid resuscitation en route that may alter admission BAC levels too. Time from injury to admission may have allowed clearance of low levels of BAC resulting in some alcohol exposed patients being counted as BAC negative and led us to underestimate the association with ARDS.
Patients missing ISS scores probably had low scores; nearly all those patients were without ARDS, discharged alive and would not have changed our results. Our cohort’s large size, wide distribution of age (including many younger individuals), and low rate of comorbidities offer a clearer picture of the relationship between elevated BAC and outcomes than other investigators may have been able to capture. We believe these features mitigate limitations, and we would apply our conclusions widely.
CONCLUSION
Elevated BAC is associated with ARDS development. BAC >0 mg/dL is associated also with severe injury (high ISS) and depressed sensorium (low GCS). Patients admitted with high ISS and low GCS are more likely than not to experience ARDS or die. Elevated BAC may increase the frequency of ARDS through influence on injury severity or independent molecular mechanisms which can be discriminated only in experimental models. High ISS and low GCS scores may be useful to identify patients at high risk for ARDS development.
Supplementary Material
ACKNOWLEDGMENTS
All sources of support: This research was supported in part by National Institute of Health grants R01 AA018707 (Gordon Smith) and K12RR023250 (Giora Netzer) and F32AA022553-01 (Majid Afshar).
Betsy Kramer, Project Manager for Clinical Information Systems contributed to the manuscript by providing the dataset from the Shock Trauma Registry. Prerna Mahod and Mark Daly in Medical Informatics contributed in data collection by performing the automated query for keywords in the chest radiograph reports from the Clinical Data Repository at University of Maryland. Marty Reynolds at University of Maryland provided ventilator administrative data.
Footnotes
Meetings for abstract of paper: American Thoracic Society Scientific Session, 2013
Conflict of Interest and Source of Funding: No conflicts of interest or other funding sources to disclose amongst the authors. Majid Afshar and Giora Netzer had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
AUTHOR’S CONTRIBUTIONS
Study concept and design: Giora Netzer, Michael Terrin, Gordon Smith, and Majid Afshar
Acquisition of Data: Giora Netzer, Gordon Smith, Majid Afshar, Jean Jeudy, Matthew Barrett, Sahar Mansoor
Analysis and Interpretation of Data: Majid Afshar, Gordon Smith, Michael Terrin, Matthew Lissauer, and Giora Netzer
Drafting of the manuscript: Majid Afshar, Gordon Smith, Michael Terrin, Matthew Lissauer, and Giora Netzer
Contributor Information
Majid Afshar, Email: mafshar@medicine.umaryland.edu.
Gordon S. Smith, Email: gssmith@som.umaryland.edu.
Michael L. Terrin, Email: mterrin@epi.umaryland.edu.
Matthew Barrett, Email: mbarrett@umm.edu.
Matthew E. Lissauer, Email: Mlissauer@umm.edu.
Sahar Mansoor, Email: smansoor.sahar@gmail.com.
Jean Jeudy, Email: jjeudy@umm.edu, jjeudymd@gmail.com.
Giora Netzer, Email: gnetzer@medicine.umaryland.edu.
REFERENCES
- 1.Moss M, Bucher B, Moore FA, Moore EE, Parsons PE. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA. 1996;275(1):50–54. [PubMed] [Google Scholar]
- 2.Moss M, Parsons PE, Steinberg KP, Hudson LD, Guidot DM, Burnham EL, Eaton S, Cotsonis GA. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med. 2003;31(3):869–877. doi: 10.1097/01.CCM.0000055389.64497.11. [DOI] [PubMed] [Google Scholar]
- 3.Moss M, Burnham EL. Alcohol abuse in the critically ill patient. Lancet. 2006;368(9554):2231–2242. doi: 10.1016/S0140-6736(06)69490-7. [DOI] [PubMed] [Google Scholar]
- 4.Jurkovich GJ, Rivara FP, Gurney JG, Fligner C, Ries R, Mueller BA, Copass M. The effect of acute alcohol intoxication and chronic alcohol abuse on outcome from trauma. JAMA. 1993;270(1):51–56. [PubMed] [Google Scholar]
- 5.Waller PF, Stewart JR, Hansen AR, Stutts JC, Popkin CL, Rodgman EA. The potentiating effects of alcohol on driver injury. JAMA. 1986;256(11):1461–1466. [PubMed] [Google Scholar]
- 6.Li G, Keyl PM, Smith GS, Baker SP. Alcohol and injury severity: reappraisal of the continuing controversy. J Trauma. 1997;42(3):562–569. doi: 10.1097/00005373-199703000-00032. [DOI] [PubMed] [Google Scholar]
- 7.Greiffenstein P, Molina PE. Alcohol-Induced Alterations on Host Defense After Traumatic Injury. J Trauma. 2008;64(1):230–240. doi: 10.1097/TA.0b013e318158a4ad. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz M, Ewig S, Torres A, Arancibia F, Marco F, Mensa J, Sanchez M, Martinez JA. Severe community-acquired pneumonia. Risk factors and follow-up epidemiology. Am J Respir Crit Care Med. 1999;160(3):923–929. doi: 10.1164/ajrccm.160.3.9901107. [DOI] [PubMed] [Google Scholar]
- 9.Gentilello LM, Cobean RA, Walker AP, Moore EE, Wertz MJ, Dellinger EP. Acute ethanol intoxication increases the risk of infection following penetrating abdominal trauma. J Trauma. 1993;34(5):669–674. doi: 10.1097/00005373-199305000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Kuhls DA, Malone DL, McCarter RJ, Napolitano LM. Predictors of mortality in adult trauma patients: the physiologic trauma score is equivalent to the Trauma and Injury Severity Score. J Am Coll Surg. 2002;194(6):695–704. doi: 10.1016/s1072-7515(02)01211-5. [DOI] [PubMed] [Google Scholar]
- 11.Hoch RC, Rodriguez R, Manning T, Bishop M, Mead P, Shoemaker WC, Abraham E. Effects of accidental trauma on cytokine and endotoxin production. Crit Care Med. 1993;21(6):839–845. doi: 10.1097/00003246-199306000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Miller PR, Croce MA, Bee TK, Qaisi WG, Smith CP, Collins GL, Fabian TC. ARDS after pulmonary contusion: accurate measurement of contusion volume identifies high-risk patients. J Trauma. 2001;51(2):223–228. doi: 10.1097/00005373-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Salim A, Martin M, Constantinou C, Sangthong B, Brown C, Kasotakis G, Demetriades D, Belzberg H. Acute respiratory distress syndrome in the trauma intensive care unit: Morbid but not mortal. Arch Surg. 2006;141(7):655–658. doi: 10.1001/archsurg.141.7.655. [DOI] [PubMed] [Google Scholar]
- 14.Miller PR, Croce MA, Kilgo PD, Scott J, Fabian TC. Acute respiratory distress syndrome in blunt trauma: identification of independent risk factors. Am Surg. 2002;68(10):845–850. [PubMed] [Google Scholar]
- 15.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 16.Shah CV, Localio AR, Lanken PN, Kahn JM, Bellamy S, Gallop R, Finkel B, Gracias VH, Fuchs BD, Christie JD. The impact of development of acute lung injury on hospital mortality in critically ill trauma patients. Crit Care Med. 2008;36(8):2309–2315. doi: 10.1097/CCM.0b013e318180dc74. [DOI] [PubMed] [Google Scholar]
- 17.Dutton RP, Stansbury LG, Leone S, Kramer E, Hess JR, Scalea TM. Trauma Mortality in Mature Trauma Systems: Are We Doing Better? An Analysis of Trauma Mortality Patterns, 1997–2008. J Trauma. 2010;69(3):620–626. doi: 10.1097/TA.0b013e3181bbfe2a. [DOI] [PubMed] [Google Scholar]
- 18.Haut ER, Kalish BT, Cotton BA, Efron DT, Haider AH, Stevens KA, Kieninger AN, Cornwell EE, Chang DC. Prehospital intravenous fluid administration is associated with higher mortality in trauma patients: a National Trauma Data Bank analysis. Ann Surg. 2011;253(2):371–377. doi: 10.1097/SLA.0b013e318207c24f. [DOI] [PubMed] [Google Scholar]
- 19.Azzam HC, Khalsa SS, Urbani R, Shah CV, Christie JD, Lanken PN, Fuchs BD. Validation Study of an Automated Electronic Acute Lung Injury Screening Tool. J Am Med Inform Assoc. 2009;16(4):503–508. doi: 10.1197/jamia.M3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koenig HC, Finkel BB, Khalsa SS, Lanken PN, Prasad M, Urbani R, Fuchs BD. Performance of an automated electronic acute lung injury screening system in intensive care unit patients. Crit Care Med. 2011;39(1):98–104. doi: 10.1097/CCM.0b013e3181feb4a0. [DOI] [PubMed] [Google Scholar]
- 21.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 22.Parker AM, Liu X, Harris AD, Harris AD, Shanholtz CB, Smith RL, Hess DR, Reynolds M, Netzer G. Respiratory therapy organizational changes are associated with increased respiratory care utilization. Respir Care. 2013;58(3):438–449. doi: 10.4187/respcare.01562. [DOI] [PubMed] [Google Scholar]
- 23.Wynn A, Wise M, Wright MJ, Rafaat A, Wang YZ, Steeb G, McSwain N, Beuchter KJ, Hunt JP. Accuracy of administrative and trauma registry databases. J Trauma. 2001;51:464–468. doi: 10.1097/00005373-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Rice TW, Wheeler AP, Bernard GR, Hayden DL, Schoenfeld DA, Ware LB. Comparison of the SpO2/FIO2 Ratio and the PaO2/FIO2 Ratio in Patients With Acute Lung Injury or ARDS. Chest. 2007;132(2):410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 25.Mascia L. Acute Lung Injury in Patients with Severe Brain Injury: A Double Hit Model. Neurocritical Care. 2009;11(3):417–426. doi: 10.1007/s12028-009-9242-8. [DOI] [PubMed] [Google Scholar]
- 26.Gajic O, Dabbagh O, Park PK, Adesanya A, Chang SY, Hou P, Anderson H, Hoth JJ, Mikkelsen ME, Gentile NT, Gong MN, Talmor D, Bajwa E, Watkins TR, Festic E, Yilmaz M, Iscimen R, Kaufman DA, Esper AM, Sadikot R, Douglas I, Sevransky J. Early Identification of Patients at Risk of Acute Lung Injury: Evaluation of Lung Injury Prediction Score in a Multicenter Cohort Study. Am J Respir Crit Care Med. 2011;183(4):462–470. doi: 10.1164/rccm.201004-0549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calfee CS, Matthay MA, Eisner MD, Benowitz N, Call M, Pittet JF, Cohen MJ. Active and Passive Cigarette Smoking and Acute Lung Injury after Severe Blunt Trauma. Am J Respir Crit Care Med. 2011;183(12):1660–1665. doi: 10.1164/rccm.201011-1802OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fabbri A, Marchesini G, Morselli-Labate AM, Rossi F, Cicognani A, Dente M, Iervese T, Ruggeri S, Mengozzi U, Vandelli A. Blood alcohol concentration and management of road trauma patients in the emergency department. J Trauma. 2001;50:521–528. doi: 10.1097/00005373-200103000-00018. [DOI] [PubMed] [Google Scholar]
- 29.Friedman LS. Dose–response relationship between in-hospital mortality and alcohol following acute injury. Alcohol. 2012;46:769–775. doi: 10.1016/j.alcohol.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Madan AK, Yu K, Beech DJ. Alcohol and drug use in victims of life-threatening trauma. The Journal of Trauma Injury, Infection, and Critical Care. 1999;47:568–571. doi: 10.1097/00005373-199909000-00026. [DOI] [PubMed] [Google Scholar]
- 31.VanderWeele TJ. On the relative nature of overadjustment and unnecessary adjustment. Epidemiology. 2009;20(4):496–499. doi: 10.1097/EDE.0b013e3181a82f12. [DOI] [PubMed] [Google Scholar]
- 32.Haider AH, Crompton JG, Oyetunji T, Risucci D, DiRusso S, Basdag H, Villegas CV, Syed ZU, Haut ER, Efron DT. Mechanism of injury predicts case fatality and functional outcomes in pediatric trauma patients: the case for its use in trauma outcomes studies. J Pediatr Surg. 2011;46(8):1557–1563. doi: 10.1016/j.jpedsurg.2011.04.055. [DOI] [PubMed] [Google Scholar]
- 33.Foreman BP, Caesar RR, Parks J, Madden C, Gentilello LM, Shafi S, Carlile MC, Harper CR, Diaz-Arrastia RR. Usefulness of the abbreviated injury score and the injury severity score in comparison to the Glasgow Coma Scale in predicting outcome after traumatic brain injury . J Trauma. 2007;62(4):946–950. doi: 10.1097/01.ta.0000229796.14717.3a. [DOI] [PubMed] [Google Scholar]
- 34.Zhang P, Nelson S, Summer WR, Spitzer JA. Acute ethanol intoxication suppresses the pulmonary inflammatory response in rats challenged with intrapulmonary endotoxin. Alcohol Clin Exp Res. 1997;21(5):773–778. [PubMed] [Google Scholar]
- 35.Shukla SD, Pruett SB, Szabo G, Arteel GE. Binge Ethanol and Liver: New Molecular Developments. Alcohol Clin Exp Res. 2013;37(4):550–557. doi: 10.1111/acer.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szabo G, Mandrekar P, Verma B, Isaac A, Catalano D. Acute ethanol consumption synergizes with trauma to increase monocyte tumor necrosis factor alpha production late postinjury. J Clin Immunol. 1994;14(6):340–352. doi: 10.1007/BF01546318. [DOI] [PubMed] [Google Scholar]
- 37.Adnet F, Baud F. Relation between glasgow coma scale and aspiration pneumonia. Lancet. 1996;348:123–124. doi: 10.1016/s0140-6736(05)64630-2. [DOI] [PubMed] [Google Scholar]
- 38.Gregorakos L, Markou N, Psalida V, Kanakaki M, Alexopoulou A, Sotiriou E, Damianos A, Myrianthefs P. Near-drowning: Clinical course of lung injury in adults. Lung. 2009;187:93–97. doi: 10.1007/s00408-008-9132-4. [DOI] [PubMed] [Google Scholar]
- 39.Dancey DR, Hayes J, Gomez M, Schouten D, Fish J, Peters W, Slutsky AS, Stewart TE. Ards in patients with thermal injury. Intensive Care Med. 1999;25:1231–1236. doi: 10.1007/pl00003763. [DOI] [PubMed] [Google Scholar]
- 40.Champion HR, Copes WS, Sacco WJ, Lawnick MM, Keast SL, Bain LW, Jr, Flanagan ME, Frey CF. The Major Trauma Outcome Study: establishing national norms for trauma care. J Trauma. 1990;30(11):1356–1365. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.