Abstract
PURPOSE
Over 80% of US patients initiate HD with a tunneled dialysis catheter (TDC). Published data on TDC outcomes are based on a case-mix of prevalent and incident TDCs. We analyzed factors affecting patency and complications of first TDCs ever placed in a large cohort of incident HD patients.
MATERIALS AND METHODS
We retrospectively queried a prospective, computerized vascular access database to identify 472 patients receiving a first ever TDC. Multiple variable survival analysis was used to identify clinical parameters affecting TDC patency (from placement to non-elective removal) and infection (from placement to first episode of catheter-related bacteremia).
RESULTS
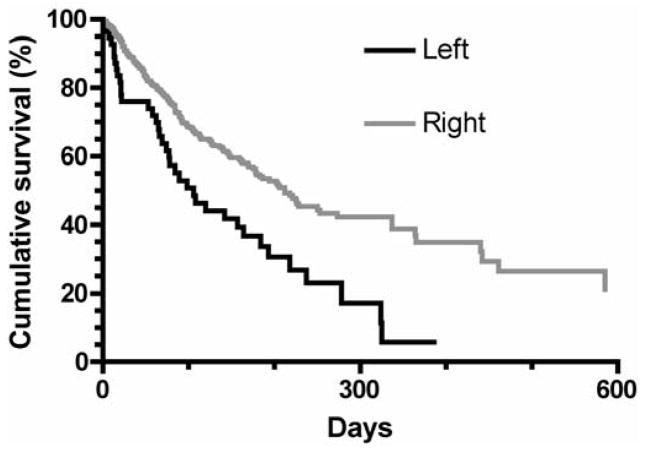
The median patency of all TDCs was 202 days. Left-sided placement of TDCs was the only variable associated with inferior TDC patency (hazard ratio 1.98; 95% CI, 1.39–2.81, p<0.0001). The 6-month TDC patency was 37% for left interval jugular (LIJ) vein catheters vs 54% for right internal jugular (RIJ) vein catheters. The one-year patency was 6% for LIJ catheters vs 35% for RIJ catheters. Catheter patency was not associated with patient age, sex, race, hypertension, diabetes, coronary artery disease, peripheral vascular disease, cerebrovascular disease, or heart failure. The median time to the first episode of catheter-related bacteremia was 163 days. None of the clinical variables was associated with TDC infection.
CONCLUSIONS
TDCs are plagued by high rates of infection. Right IJ vein TDC should be used preferentially to maximize catheter patency.
Introduction
Tunneled dialysis catheters (TDC) are the least desirable type of vascular access for long-term hemodialysis, owing to the high rate of thrombosis, infection, and central vein stenosis, as well as their association with high patient mortality (1–4). Whereas nationwide efforts to improve vascular access outcomes have reduced the prevalent TDC use in the U.S. to 24%, the vast majority (83%) of patients with end-stage renal disease (ESRD) in the U.S. initiate HD with a TDC (5, 6). The number of patients in the U.S. receiving TDCs is approximated to be 110,000 annually (6).
Most published studies reporting on the outcomes of TDC have lumped together prevalent and incident HD patients. These populations may differ in terms of the patient comorbidities (7, 8) and vascular anatomy (9), factors that may affect TDC outcomes. For example, TDC may produce central vein stenosis and fibrin sheaths, thereby contributing to shortened patency of subsequent TDC. Likewise, patients developing catheter-related bacteremia (CRB) may be at higher risk of CRB with their subsequent TDC. For this reason, it would be valuable to assess the outcomes and complications in incident HD patients receiving their first TDCs ever. The present study evaluated a large cohort of patients at a large medical center fulfilling this definition.
Methods
Study population
There are approximately 500 hemodialysis patients followed at the study medical center. They receive their treatments at 5 in-center hemodialysis units. Most of the patients’ vascular access procedures and hospitalizations take place at one hospital, allowing two dedicated access coordinators to maintain a prospective computerized access database of all vascular access procedures, complications and outcomes.
TDC management
Four interventional radiologists and one interventional nephrologist placed the TDCs under sterile conditions in a radiology suite, using real-time ultrasound for venous cannulation and fluoroscopic guidance to ensure optimal positioning of the catheter tip. Catheters were placed preferentially in the right internal jugular vein (IJV), unless the right IJV was occupied by another tunneled catheter (e.g., Mediport) or was thrombosed. Catheters were inserted following a technique previously described (10). Interventionalists used 14 French Ash-Split and Titan (Medcomp, Harleysville, PA), 14.5 French Decathlon Gold (Spire Biomedical, Bedford, MA), and 15 French Hemosplit (Bard Access Systems, Salt Lake City, Utah) and 15.5 French Duraflow (AngioDynamics, Latham, NY) catheters according to an operator’s preference. To position the distal catheter tip at the junction of right atrium and superior vena cava estimated to be 5–6 cm below the right tracheobronchial angle on posterior-anterior projection, the cuff-to-tip catheter length of 19 cm was used for right internal jugular vein (IJV) and 23 cm for left IJV. TDC maintenance included instillation of heparin at concentration of 1000 units/mL into both lumens at the end of each HD session.
Catheter dysfunction was defined as the inability to maintain dialysis blood flow rate of at least 250 mL/min (11). In patients with catheter dysfunction, both TDC lumens were instilled with recombinant tissue plasminogen activator (tPA) (Genentech, San Francisco, CA) at 2 mg per port for 30–60 minutes. Dialysis was reattempted the same day after the tPA treatment and if catheter dysfunction persisted, the TDC was exchanged over a guidewire. Original TDC remained in place for as long as tPA treatment (even if recurring) was effective. Catheter-related bacteremia (CRB) was defined as the presence of systemic signs and symptoms of infection (fever or chills) in combination with positive blood cultures (12). Other potential sources of infection were ruled out on the basis of clinical presentation and appropriate testing (e.g., chest radiograph or soft tissue ultrasound)(13). CRBs were treated with a 3-week course of appropriate intravenous antibiotics, in conjunction with an antibiotic lock instilled into both catheter lumens at the end of each HD session (14). TDCs were exchanged over a guidewire if the patient’s symptoms persisted 48 hours after initiation of antibiotics, or if the patient had positive surveillance blood cultures one week after completing the antibiotic course. The database did not include complete information about exit site or tunnel infections.
Data analysis
The prospective, computerized vascular access database was queried to identify 705 patients with newly diagnosed ESRD who received their first TDC for initiation of dialysis during the six-year period from January 1, 2004 to December 31, 2009. There were 63 patients excluded because of fistula or graft surgery prior to initiation of dialysis. An additional 170 patients were excluded because of referral to an outside dialysis center shortly after initiation of HD, such that no follow-up data were available. The remaining 472 patients constituted the study population for the current analysis. Information on demographic and comorbidities was obtained from the hospital electronic medical record database containing clinic notes documenting the above data. Information on procedures and complications (including microbiology data) was extracted from the prospective vascular access database. The local Institutional Review Board approved the analysis of the existing medical information for research purposes.
Most of the studied patients were black, reflecting the racial distribution of our dialysis patient population. Men comprised 50% of the group. Mean patient age was 54 ± 15 years. There was high prevalence of hypertension, diabetes and vascular disease among the patients. Thirty-four patients (7.2%) had a history of dialysis access surgery preceding the TDC placement. The side of the TDC was not recorded for 4 patients. Of the remaining 468 patients, 57 (or 12%) received a left IJ TDC.
Because previous studies have suggested inferior patency of TDC placed in the left internal jugular vein, the characteristics of patients receiving left vs right internal jugular vein TDC was compared (Table 1). The two groups were comparable in terms of age, sex, race, and frequency of hypertension, diabetes, coronary artery disease, peripheral vascular disease, cerebrovascular disease, and congestive heart failure.
Table 1.
Baseline demographic and clinical characteristics of the study patients.
| Parameter | Left TDC | Right TDC | p-value |
|---|---|---|---|
| Total patients | 57 | 411 | |
| Age | 53 ± 13 | 54 ± 16 | 0.89 |
| Black race | 44 (77%) | 310 (75%) | 0.77 |
| Female sex | 30 (53%) | 206 (50%) | 0.42 |
| Hypertension | 49 (86%) | 348 (85%) | 0.80 |
| Diabetes | 29 (51%) | 210 (51%) | 0.98 |
| Coronary artery disease | 13 (23%) | 92 (22%) | 0.94 |
| Peripheral vascular disease | 9 (16%) | 54 (13%) | 0.58 |
| Cerebrovascular disease | 11 (19%) | 54 (13%) | 0.21 |
| Congestive heart failure | 13 (23%) | 80 (19%) | 0.55 |
TDC, tunneled dialysis catheter
Coronary artery disease, defined as history of myocardial infarction, unstable angina, percutaneous coronary intervention, or coronary artery bypass surgery.
Peripheral vascular disease, defined as claudication, peripheral artery angioplasty or bypass surgery, or lower limb amputation.
Cerebrovascular disease, defined as history of transient ischemic attack or cerebrovascular disease documented by imaging studies.
Congestive heart failure, defined as a low systolic ejection fraction by echocardiography or history of symptomatic pulmonary edema that resolved after ultrafiltration by hemodialysis.
Catheter patency was defined as the ability to use it for hemodialysis with adequate blood flows (> 250 ml/min), and was calculated from the time of TDC placement to its exchange or non-elective removal. Catheter failure was defined as a non-elective removal of TDC because of an infection or dysfunction. Rare cases of accidental TDC loss or TDC cuff exposure necessitating catheter replacement were classified as having “catheter dysfunction”. Time to first infection was calculated from TDC placement to the first documented CRB, even if TDC exchange was not required. Patient follow-up was censored for death, transfer to another facility or elective TDC removal due to use of a fistula or graft for HD.
Statistical analysis
SAS Enterprise Guide 4.3 software was used to perform the statistical analyses. Multiple variable survival analysis was applied to evaluate variables affecting catheter survival, using a selection criteria with a p-value = 0.1 and a “kick out” value of 0.05. Kaplan-Meier methodology was used to generate survival curves for catheter survival and time to first infection. Differences between outcomes of patient subsets were compared using log-rank tests. Hazard ratios (HRs) and their confidence intervals (CIs) were calculated. P < 0.05 was considered statistically significant.
Results
The overall median TDC patency for both groups was 202 days with 69% of the original catheters still in place at 3 months, 53% at 6 months and 34% at 12 months. The TDC was removed non-electively due to catheter failure in 206 (or 43.6%) of the patients. Overall, TDC failure was due to catheter dysfunction in 55% of cases, and catheter-related bacteremia in 45%. The proportion of TDC failure due to dysfunction was 70% in the first 3 months after TDC placement, 30% for months 4 to 6, and 28% for months 7 to 12. Of the 266 patients without catheter failure, 186 (70%) had their TDC removed electively due to use of a permanent access, 40 (15%) died, 17 (6%) were lost to follow-up, 16 (6%) transferred to a non-UAB dialysis unit, 4 (1.5%) had kidney transplantation, and 3 (1%) regained renal function and discontinued dialysis.
On univariate survival analysis, catheter patency was not associated with patient age, sex, race, diabetes, hypertension, coronary artery disease, peripheral vascular disease, cerebrovascular disease, or congestive heart failure (Table 2). TDC patency was significantly shorter for catheters placed in the left internal jugular vein, as compared to those placed in the right internal jugular vein (HR = 1.93, 95% CI, 1.50–3.66; P = 0.0002 (Figure 1). The 6-month TDC patency was 37% for LIJ catheters vs 54% for RIJ catheters. The one-year patency was 6% for LIJ catheters vs 35% for RIJ catheters. On multiple variable survival analysis, using step-wise selection criteria, the only variable associated with catheter failure was the catheter side (HR = 1.98, 95% CI, 1.39–2.81; p< 0.0001).
Table 2.
Association of TDC failure with clinical parameters
| Variable | HR | 95% CI | p-value |
|---|---|---|---|
| Age ≥65 vs <65 yr | 1.01 | (0.73, 1.40) | 0.93 |
| Sex, female vs male | 1.13 | (0.86, 1.49) | 0.38 |
| Race, black vs white | 0.87 | (0.63,1.20) | 0.40 |
| Diabetes | 0.96 | (0.73, 1.26) | 0.76 |
| Hypertension | 0.81 | (0.54, 1.23) | 0.33 |
| Coronary artery disease | 0.82 | (0.58, 1.15) | 0.24 |
| Peripheral vascular disease | 0.90 | (0.60, 1.34) | 0.60 |
| Cerebrovascular disease | 1.17 | (0.80,1.70) | 0.43 |
| Congestive heart failure | 0.86 | (0.60, 1.22) | 0.40 |
| Catheter side, left vs right | 1.93 | (1.50–3.66) | 0.0002 |
TDC, Tunneled dialysis catheter; HR, hazard ratio; CI, confidence interval
Figure 1.
Patency of tunneled dialysis catheters placed in right vs. left internal jugular vein. Catheter patency was decreased for left IJ vs right IJ catheters (p<0.0001).
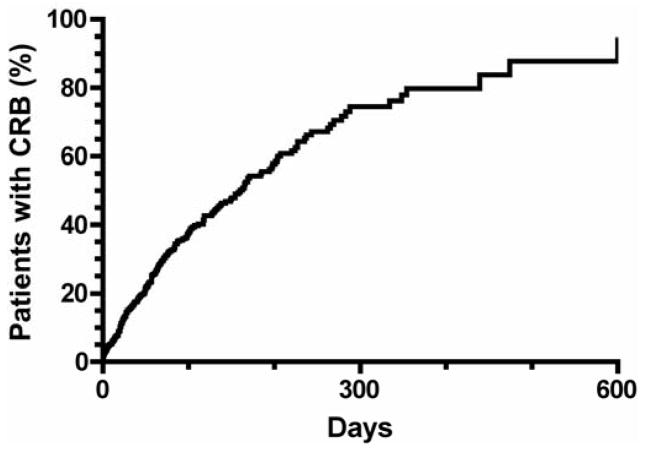
The median time to catheter-related bacteremia was 163 days, with 35% of patients infected at 3 months, 54% at 6 months and 79% at 12 months (Figure 2). Time to catheter infection was not significantly associated with patient age, sex, race, diabetes, hypertension, coronary artery disease, peripheral vascular disease, cerebrovascular disease, congestive heart failure or catheter side (Table 3).
Figure 2.
Time to first catheter-related bacteremia in patients with tunneled dialysis catheters.
Table 3.
Association of catheter-related bacteremia with clinical parameters
| Variable | HR | 95% CI | p-value |
|---|---|---|---|
| Age ≥65 vs <65 yr | 0.91 | (0.65, 1.28) | 0.59 |
| Sex, female vs male | 0.95 | (0.72, 1.26) | 0.73 |
| Race, black vs white | 1.31 | (0.91, 1.90) | 0.15 |
| Diabetes | 1.10 | (0.81, 1.41) | 0.64 |
| Hypertension | 0.91 | (0.58, 1.40) | 0.66 |
| Coronary artery disease | 0.94 | (0.67, 1.31) | 0.71 |
| Peripheral vascular disease | 1.02 | (0.68, 1.52) | 0.94 |
| Cerebrovascular disease | 1.17 | (0.80, 1.72) | 0.44 |
| Congestive heart failure | 0.98 | (0.69, 1.39) | 0.91 |
| Catheter side, left vs right | 1.38 | (0.92, 2.10) | 0.12 |
HR, Hazard ratio; CI, confidence interval
Discussion
Incident ESRD patients receiving TDCs may differ from prevalent ESRD patients. In one recent report, incident ESRD patients were older and had a higher comorbidity index (7). At least two comorbidities – diabetes and hypertension – have been associated with early catheter failure (15, 16). Incident ESRD patients also have greater proportion (25–30%) of TDCs placed in the left IJV (17, 18). However, the reported catheter outcomes for incident ESRD patients in the current study are similar to those reported previously for prevalent hemodialysis patients (Table 4). More than 10 years ago, Ewing et al reported 6 month TDC survival at 47.5%, albeit with a lower 3-month infection rate of 18.6% (10). A study by Rocklin et al from 2001 reported a 50% 3-month TDC patency with median of 80 days to the first CRB (19). We suspect that any clinical advantage of being a prevalent ESRD patient is counterbalanced by gradual emergence of vascular anatomical pathology, such as central venous stenosis or fibrin sheath formation, that may predispose to catheter failure (15, 20, 21).
Table 4.
Summary of previously published studies documenting tunneled dialysis catheter outcomes.
| References | N of patients | TDC survival (days) | Non- Elective TDC Removal Rate | Proportion of non-elective TDC removal due to CRB | Major Findings |
|---|---|---|---|---|---|
| Rocklin et al (17) | 86 | 90, median | 87% | 40% | Same TDC patency with right and left IJV placement |
| Little et al (13) | 336 | 312, median | 66% | 20% | Worse TDC patency is associated with diabetes and number of previous TDCs |
| Ewing et al (10) | 83 | 190, mean | 65% | 49% | TDC thrombosis rate is 21%, documented radiographically |
| Lee et al (14) | 182 | 87, mean | 52% | 33% | Higher rate of TDC complications is associated with hypertension |
| Develter et al (19) | 157 | 310, median | 51% | 50% | Longer TDC patency is associated with right IJV placement |
| Alomari et al (16) | 207 | 66, median | 70% | 48% | Fibrin sheath rate is 76%, documented radiographically |
| Jain et al (15) | 175 | 175, median | - | - | Longer TDC patency is associated with right IJV placement |
| Wilkin et al (24) | 143 | 288, mean | - | 35% | Right IJV thrombosis in 26% |
| Current Study | 472 | 202, median | 44% | 45% | Longer TDC patency is associated with right IJV placement |
All the studies were retrospective in design.
TDC, tunneled dialysis catheter; CRB, catheter-related bacteremia; IJV, internal jugular vein
We confirm the previously reported association of left-sided TDCs with inferior TDC patency (17, 22). There are three potential explanations for this observation. First, the left-sided TDC travels through a more tortuous path than does the right-sided TDC, thereby increasing the likelihood of tip migration out of the right atrium into the superior vena cava (23). Second, patients whose initial TDC is inserted via the left IJV may already have a pre-existing abnormal vasculature, as a consequence of co-existent or previous right IJV non-dialysis catheters. In fact, central vein thrombosis has been reported in 26% of patients with a history of central vein dialysis catheters (24). The prior vascular injury may itself affect the patency of the first TDC. Finally, it is likely that those patients receiving a left IJV catheter were sicker, and possibly more pro-thrombotic, a factor that may predispose to TDC failure.
CRB is the Damocles’ sword for patients relying on TDC as their access. Nearly half of non-elective TDC removals in the current study were necessitated by an infection, in close agreement with previous reports (Table 4). Despite novel strategies to prevent CRB, this complication remains a common event among catheter-dependent hemodialysis patients, and often results in hospitalizations or metastatic infection (15). At our dialysis clinics, suspected catheter infections are treated aggressively with broad-spectrum systemic antibiotics, in conjunction with an antibiotic-heparin locking solution instilled into the lumens after the dialysis session. The antibiotic locks are utilized in an attempt to salvage the existing TDC. The TDC is removed only in patients not responding to this approach, or those with blood-stream infections caused by Staphylococcus aureus, Pseudomonas aeruginosa, or fungal species, as recommended by Infectious Diseases Society of America (12). Notwithstanding the efforts to salvage the catheter with an antibiotic lock, 57% of study catheters were eventually removed due to infection after the first CRB episode, and only 12% survived after two CRB episodes. These findings are in keeping with other published series, in which infection accounted for a substantial proportion of non-elective TDC removals (Table 4).
The strengths of the current study included the large number of patients included, the restriction of analysis to the first TDC for each patient, and the prospective data collection regarding TDC outcomes and complications. Our study also has some limitations. First, this study reports the outcomes obtained at a single medical center, which may not generalize to other medical centers. However, our results are remarkably similar to those reported elsewhere, despite vastly different patients’ characteristics (Table 4). Second, it was impossible to ascertain the history of non-dialysis catheter use that might explain why a subset of patients required their first ever TDC to be placed via the left internal jugular vein. Finally, we did not obtain imaging studies to identify specific causes of catheter dysfunction (e.g., intraluminal thrombus vs. tip migration), which might provide some insight into the observed difference in right and left IJV catheter outcomes.
In conclusion, the current study describes the natural history of first ever TDCs placed in ESRD patients at HD initiation. In this patient population, CRB continues to be a significant detriment to catheter survival. Right IJV placement should always be pursued first to minimize catheter dysfunction and maximize catheter patency.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Xue JL, Dahl D, Ebben JP, Collins AJ. The association of initial hemodialysis access type with mortality outcomes in elderly Medicare ESRD patients. Am J Kidney Dis. 2003;42:1013–9. doi: 10.1016/j.ajkd.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Mokrzycki MH, Lok CE. Traditional and non-traditional strategies to optimize catheter function: go with more flow. Kidney Int. 2010;78:1218–31. doi: 10.1038/ki.2010.332. [DOI] [PubMed] [Google Scholar]
- 3.Allon M. Dialysis catheter-related bacteremia: treatment and prophylaxis. Am J Kidney Dis. 2004;44:779–91. [PubMed] [Google Scholar]
- 4.Lok CE, Mokrzycki MH. Prevention and management of catheter-related infection in hemodialysis patients. Kidney Int. 2011;79:587–98. doi: 10.1038/ki.2010.471. [DOI] [PubMed] [Google Scholar]
- 5.Wish JB. Vascular access for dialysis in the United States: progress, hurdles, controversies, and the future. Semin Dial. 2010;23:614–8. doi: 10.1111/j.1525-139X.2010.00797.x. [DOI] [PubMed] [Google Scholar]
- 6.Foley RN, Chen SC, Collins AJ. Hemodialysis access at initiation in the United States, 2005 to 2007: still “catheter first”. Hemodial Int. 2009;13:533–42. doi: 10.1111/j.1542-4758.2009.00396.x. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Huang Z, Gilbertson DT, Foley RN, Collins AJ. An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int. 2010;77:141–51. doi: 10.1038/ki.2009.413. [DOI] [PubMed] [Google Scholar]
- 8.Bradbury BD, Fissell RB, Albert JM, et al. Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Clin J Am Soc Nephrol. 2007;2:89–99. doi: 10.2215/CJN.01170905. [DOI] [PubMed] [Google Scholar]
- 9.Bozof R, Kats M, Barker J, Allon M. Time to symptomatic vascular stenosis at different locations in patients with arteriovenous grafts. Semin Dial. 2008;21:285–8. doi: 10.1111/j.1525-139X.2008.00436.x. [DOI] [PubMed] [Google Scholar]
- 10.Ewing F, Patel D, Petherick A, Winney R, McBride K. Radiological placement of the AshSplit haemodialysis catheter: a prospective analysis of outcome and complications. Nephrol Dial Transplant. 2002;17:614–9. doi: 10.1093/ndt/17.4.614. [DOI] [PubMed] [Google Scholar]
- 11.National Kidney Foundation. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Vascular Access. Am J Kidney Dis. 2006;48:S176–S322.2006. doi: 10.1053/j.ajkd.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 12.Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Solaiman Y, Estrada E, Allon M. The spectrum of infections in catheter-dependent hemodialysis patients. Clin J Am Soc Nephrol. 2011;6:2247–52. doi: 10.2215/CJN.03900411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poole CV, Carlton D, Bimbo L, Allon M. Treatment of catheter-related bacteraemia with an antibiotic lock protocol: effect of bacterial pathogen. Nephrol Dial Transplant. 2004;19:1237–44. doi: 10.1093/ndt/gfh041. [DOI] [PubMed] [Google Scholar]
- 15.Little MA, O’Riordan A, Lucey B, et al. A prospective study of complications associated with cuffed, tunnelled haemodialysis catheters. Nephrol Dial Transplant. 2001;16:2194–200. doi: 10.1093/ndt/16.11.2194. [DOI] [PubMed] [Google Scholar]
- 16.Lee O, Raque JD, Lee LJ, Wivell W, Block CA, Bettmann MA. Retrospective assessment of risk factors to predict tunneled hemodialysis catheter outcome. J Vasc Interv Radiol. 2004;15:457–61. doi: 10.1097/01.rvi.0000124942.24134.62. [DOI] [PubMed] [Google Scholar]
- 17.Jain G, Allon M, Saddekni S, Barker JF, Maya ID. Does heparin coating improve patency or reduce infection of tunneled dialysis catheters? Clin J Am Soc Nephrol. 2009;4:1787–90. doi: 10.2215/CJN.03920609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alomari AI, Falk A. The natural history of tunneled hemodialysis catheters removed or exchanged: a single-institution experience. J Vasc Interv Radiol. 2007;18:227–35. doi: 10.1016/j.jvir.2006.12.719. [DOI] [PubMed] [Google Scholar]
- 19.Rocklin MA, Dwight CA, Callen LJ, Bispham BZ, Spiegel DM. Comparison of cuffed tunneled hemodialysis catheter survival. Am J Kidney Dis. 2001;37:557–63. [PubMed] [Google Scholar]
- 20.Timsit JF, Farkas JC, Boyer JM, et al. Central vein catheter-related thrombosis in intensive care patients: incidence, risks factors, and relationship with catheter-related sepsis. Chest. 1998;114:207–13. doi: 10.1378/chest.114.1.207. [DOI] [PubMed] [Google Scholar]
- 21.MacRae JM, Ahmed A, Johnson N, Levin A, Kiaii M. Central vein stenosis: a common problem in patients on hemodialysis. ASAIO J. 2005;51:77–81. doi: 10.1097/01.mat.0000151921.95165.1e. [DOI] [PubMed] [Google Scholar]
- 22.Develter W, De Cubber A, Van Biesen W, Vanholder R, Lameire N. Survival and complications of indwelling venous catheters for permanent use in hemodialysis patients. Artif Organs. 2005;29:399–405. doi: 10.1111/j.1525-1594.2005.29067.x. [DOI] [PubMed] [Google Scholar]
- 23.Haygood TM, Malhotra K, Ng C, Chasen B, McEnery KW, Chasen M. Migration of central lines from the superior vena cava to the azygous vein. Clin Radiol. 2012;67:49–54. doi: 10.1016/j.crad.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Wilkin TD, Kraus MA, Lane KA, Trerotola SO. Internal jugular vein thrombosis associated with hemodialysis catheters. Radiology. 2003;228:697–700. doi: 10.1148/radiol.2283020681. [DOI] [PubMed] [Google Scholar]