Significance
Apoptosis refers to the ability of a cell to undergo programmed cell death under normal physiological conditions or in response to stress signals. During infection, influenza A viruses have the capacity to induce early apoptosis of immune cells, thereby preventing them from performing their antiviral function. In this study, we identified that a host innate immune sensor [NLRX1 (nucleotide-binding oligomerization domain-like receptor X1)] has the capacity to bind a small death-inducing protein from influenza A virus [PB1-F2 (polymerase basic protein 1-frame 2)] and defend immune cells against virus-driven apoptosis. This phenomenon allows the immune cells to survive longer and effectively restrict viral replication, protecting the host against the detrimental consequences of influenza.
Keywords: innate immunity, Nod-like receptor
Abstract
To subvert host immunity, influenza A virus (IAV) induces early apoptosis in innate immune cells by disrupting mitochondria membrane potential via its polymerase basic protein 1-frame 2 (PB1-F2) accessory protein. Whether immune cells have mechanisms to counteract PB1-F2–mediated apoptosis is currently unknown. Herein, we define that the host mitochondrial protein nucleotide-binding oligomerization domain-like receptor (NLR)X1 binds to viral protein PB1-F2, preventing IAV-induced macrophage apoptosis and promoting both macrophage survival and type I IFN signaling. We initially observed that Nlrx1-deficient mice infected with IAV exhibited increased pulmonary viral replication, as well as enhanced inflammatory-associated pulmonary dysfunction and morbidity. Analysis of the lungs of IAV-infected mice revealed markedly enhanced leukocyte recruitment but impaired production of type I IFN in Nlrx1−/− mice. Impaired type I IFN production and enhanced viral replication was recapitulated in Nlrx1−/− macrophages and was associated with increased mitochondrial mediated apoptosis. Through gain- and loss-of-function strategies for protein interaction, we identified that NLRX1 directly bound PB1-F2 in the mitochondria of macrophages. Using a recombinant virus lacking PB1-F2, we confirmed that deletion of PB1-F2 abrogated NLRX1-dependent macrophage type I IFN production and apoptosis. Thus, our results demonstrate that NLRX1 acts as a mitochondrial sentinel protecting macrophages from PB1-F2–induced apoptosis and preserving their antiviral function. We further propose that NLRX1 is critical for macrophage immunity against IAV infection by sensing the extent of viral replication and maintaining a protective balance between antiviral immunity and excessive inflammation within the lungs.
Influenza A virus (IAV) infection may progress to fatal viral pneumonia if the virus spreads from the upper airways to the alveolar space in the lower respiratory tract. In fact, some highly pathogenic strains of IAV such as those of the highly pathogenic H5N1 subtype preferentially infect the human lower respiratory tract resulting in severe pneumonia and high mortality rate (1). During infection, alveolar macrophages (Mφ) are among the first immune cells to encounter IAV and are indispensable for the early detection and clearance of the virus. Following recognition of the virus via pattern-recognition receptors (PRRs), Mφ produce a wide range of cytokines/chemokines, including IFN-α and -β, collectively referred to as type I IFN (2, 3). There are three major types of PRRs involved in IAV recognition by Mφ: the Toll-like receptors (TLRs), the NOD-like receptors (NLRs), and the RIG-like helicase receptors (RLRs) (4). NLRX1 is a recently identified PRR belonging to the NLR family that has an N-terminal effector domain containing a mitochondrion localization signal (5). Although NLRX1 was initially thought to localize to the mitochondrial outer membrane (6), a recent study suggests that NLRX1 is found in the mitochondrial matrix and interacts with a matrix protein of respiratory chain complex III (UQCRC2) (7); these results have been validated by others (8, 9). Importantly, ligands for NLRX1 are as yet unknown.
Early innate antiviral immunity mainly relies on the production of type I IFN (10–14). Secreted type I IFNs function in an autocrine and paracrine fashion are critical for the early control of viral replication by activating the transcription of intrinsic antiviral factors. Upon virus sensing by host cells, two members of the IFN regulatory factor (IRF) family, IRF3 and IRF7, activate type I IFN gene transcription and initiate the first wave of IFN secretion. Subsequent binding of type I IFNs to their cognate receptor leads to the activation of the JAK/STAT pathway, generating a positive-feedback loop that prolongs activation of IFN-stimulated genes, mediates a second wave of IFN secretion, and leads to the production of antiviral proteins such as ISG56/IFIT1 and STAT2 (15, 16). The success of IAV depends on its capacity to either subvert or subdue the host immune response for a productive replication (13, 17). As such, it encodes several virulence factors that inhibit type I IFN signaling and/or promote viral-induced early apoptosis of immune cells (13, 18).
IAV polymerase basic protein 1-frame 2 (PB1-F2) is a 75- to 90-aa-long accessory protein encoded by an ORF overlapping the PB1 ORF on segment 2 of the viral genome (19, 20). PB1-F2 has been shown to modulate the induction of type I IFN (21–23); however, its main function is to induce apoptosis in host immune cells by targeting mitochondria via the basic amphipathic helix in its C-terminal region, which results in alteration of their morphology, as well as dissipation of the mitochondrial inner membrane potential (ΔΨm) (19, 24–27). Considering their early and central role in anti-influenza immunity, it is not surprising that macrophages (Mφ) are the main target of PB1-F2–mediated apoptosis (19, 28).
Here, we report that Nlrx1−/− mice infected with IAV exhibited increased pulmonary viral titer and enhanced recruitment of inflammatory cells to the lungs, leading to increased airway hyperreactivity (AHR) and morbidity. Increased viral titer in Nlrx1−/− mice was associated with impaired type I IFN production in the lungs, a finding that was recapitulated in vitro in IAV-infected Nlrx1−/− Mφ. The inability of IAV-infected Nlrx1-deficient Mφ to produce type I IFN was not attributable to an intrinsic impairment in type I IFN signaling but rather to increased mitochondrial mediated apoptosis. We further demonstrate that the effect of NLRX1 on the fate of Mφ was attributable to its ability to directly interact with viral protein PB1-F2 and preventing mitochondrial damage. Overall, this study reveals a central role for NLRX1 in promoting Mφ survival and enhancing Mφ type I IFN-dependent antiviral immunity against IAV infection.
Results
Nlrx1 Deficiency Leads to Increased Pulmonary Viral Titer and Inflammation and Reduced Pulmonary Function During IAV Infection.
To evaluate the ability of Nlrx1-deficient mice to control IAV infection, we initially performed infections with a lethal dose (LD50) of IAV (150 pfu). Similar to a previous study (29), Nlrx1-deficient mice revealed significant morbidity as shown by increased weight loss (Fig. 1A) but not mortality (Fig. 1B). Interestingly, Nlrx1−/− mice had significantly higher viral loads in the lungs at days 3 and 6 postinfection (pi) compared with WT mice (Fig. 1C). To study the full kinetic of infection, we next challenged the mice with a sublethal dose of IAV (50 pfu). Similar to the kinetics of human IAV infection (30), sublethal challenge of mice led to productive viral replication in the lungs peaking at day 3 pi and then progressively reduced leading to complete eradication by 12 d pi (Fig. 1D). Although both WT and Nlrx1−/− mice were able to clear the infection by day 12 pi, Nlrx1-deficient mice had significantly higher viral particles in the lungs at the peak of viral replication (day 3 pi; Fig. 1 D and E).
Fig. 1.
NLRX1 restricts early viral replication and prevents excessive inflammation and morbidity during IAV infection. (A–I) WT and Nlrx1−/− mice were infected with a lethal dose (∼150 pfu) (A–C and I, Left) or a sublethal dose (∼50 pfu) (D–H and I, Right) of IAV. (A and B) Weight loss (n = 10 mice per group) (A) and mortality (n = 17 mice per group) (B) were evaluated in lethally infected mice. (C) Lungs from mice infected with ∼150 pfu of IAV were harvested at specific time points and homogenized to assess viral titers. (D) Mice were infected with ∼50 pfu of IAV and lungs were collected at specific time points to assess the kinetic of viral titers. (E) Total RNA was extracted from IAV infected lungs and viral NS1 mRNA was quantified by qPCR. (F) Micrographs of H&E-stained lung sections at days 3 and 12 following infection. (G and H) Total number of leukocytes (CD45.2+) and the frequency of alveolar Mφ (AM), interstitial Mφ (IM), Gr1+ monocytes (Mono), Gr1− mono, neutrophils (Neutro), NK cells (NK), T cells, and B cells were determined in the lungs (G) and the BAL fluid (H). Pie charts of the frequencies of the different leukocyte populations are depicted. See also Figs. S1 and S2. (I) Using flexiVent, the total lung resistance upon methacholine challenge in uninfected and IAV-infected mice was measured at day 6 (∼150 pfu) and day 12 (∼50 pfu) pi. Data are presented as fold increases in pulmonary resistance relative to baseline resistance measured before methacholine challenge. Data are depicted as means ± SEM and are representative of three independent experiments. *P < 0.05; ***P < 0.001; ****P < 0.0001 between mouse genotypes or as indicated (except in A and B, n = 3–6 mice per group per time point).
The significant increase in viral load in Nlrx1−/− mice was also associated with markedly enhanced inflammation because histological analysis of the lungs revealed a noticeably higher level of leukocyte infiltration, as well as increased airway plugging and alveolar wall thickening, in Nlrx1−/− mice compared with WT mice at both days 3 and 12 pi (Fig. 1F and Fig. S1A). To quantify this histological observation, we next performed flow cytometric analysis of the lungs and bronchoalveolar lavage (BAL) fluid of WT and Nlrx1−/− mice (Fig. 1 G and H and Figs. S1B and S2). At day 3 pi, the numbers of CD45.2+ leukocytes was significantly increased in the lungs and BAL fluid of Nlrx1−/− mice compared with WT (Fig. 1 G and H). Analysis of the frequency of different leukocyte populations in the lung of IAV-infected WT and Nlrx1−/− mice showed no significant difference in cellular recruitment. Indeed, the frequencies of alveolar Mφ (AM), interstitial Mφ (IM), “inflammatory” monocytes (Gr1+ Mono), “patrolling” monocytes (Gr1− Mono) neutrophils, eosinophils, natural killer (NK) cells, T cells, and B cells were not significantly different between WT and Nlrx1−/− mice (Fig. 1 G and H). Considering that high levels of viral replication coupled with early robust host immune responses are major determinants of the severity of pneumonia (31), the increased lung inflammation in IAV-infected Nlrx1−/− mice was associated with increased AHR at both days 6 and 12 pi (Fig. 1I). Collectively, these results suggest that NLRX1 plays a key role in restricting the early phase of viral replication thus preventing excessive inflammation and morbidity mediated by IAV infection.
Nlrx1-Deficient Mice Have an Impaired Type I IFN Response to IAV Infection.
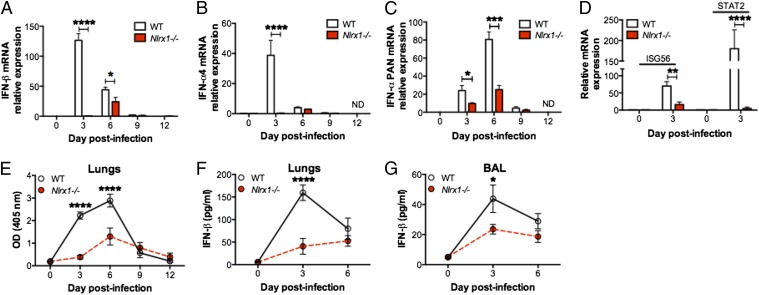
Upon infection, IAV actively suppresses type I IFN production to replicate (17). The ability of the host to counteract this suppression by inducing a strong and rapid type I IFN response is therefore critical to limit the extent of early viral replication. The observation that Nlrx1−/− mice infected with a sublethal dose of IAV had more viral particles in the lungs at day 3 pi, but not at a later time, was an indication of a defect in innate antiviral immunity. To determine whether mice deficient in Nlrx1 have an altered type I IFN response following IAV infection, we initially characterized IFN-β and IFN-α production in the lungs and BAL fluid of infected mice. Consistent with the kinetic of type I IFN production following viral infection (32), maximum IFN-β and IFN-4α mRNA levels were reached first at day 3 pi (Fig. 2 A and B). This initial induction of type I IFN then leads to the second wave of antiviral response by production of mainly other IFN-α subtypes (32). As shown in Fig. 2C, the maximum expression of IFN-α PAN (consists of all 11 murine subtypes) mRNA was at day 6 pi in WT mice. Strikingly, at the peak of viral replication (day 3 pi), both IFN-β and IFN-4α mRNA transcripts were virtually absent from the lungs of Nlrx1−/− mice (Fig. 2 A and B) and were still significantly lower than in IAV-infected WT mice by day 6 pi. This delayed and impaired type I IFN mRNA response in Nlrx1−/− mice was associated with a significant decrease in mRNA transcripts of IFN-stimulated genes (ISGs) ISG56 and STAT2 at day 3 pi (Fig. 2D). Furthermore, the decreased levels of type I IFN mRNA in IAV-infected Nlrx1−/− mice correlated with reduced type I IFN-α and -β protein secretion in both the lungs and BAL fluid (Fig. 2 E–G). Thus, consistent with the increased viral replication during the early phase of IAV infection, deficiency in Nlrx1 leads to a delayed and severely impaired type I IFN response in the lungs.
Fig. 2.
NLRX1 positively regulates in vivo type I IFN response to IAV. (A–F) WT and Nlrx1−/− mice were infected with a sublethal dose (∼50 pfu) of IAV. (A–D) Total RNA was extracted from IAV-infected lungs, and antiviral gene mRNA expression for IFN-β (A), IFN-4α (B), and IFN-α PAN (C), as well as ISG56 and STAT2 (D), was evaluated by qPCR. (E) Type I IFN protein secretion was measured from lung homogenates using B16-Blue IFN-α/β reporter cells. (F–G) IFN-β protein secretion was measured from lung homogenates (F) and BAL fluid (G) by ELISA. Data are depicted as means ± SEM and are representative of three independent experiments. *P < 0.05; **P < 0.01; ****P < 0.0001 between mouse genotypes or as indicated (n = 4–5 mice per group per time point).
Nlrx1-Deficient Mφ Have an Impaired Type I IFN Response and Are More Apoptotic after IAV Infection.
Mφ are essential for innate antiviral immunity by being the main source of type I IFN in the lungs during IAV infection (2, 33). Because IAV-infected Nlrx1−/− mice had similar frequency of AM but decreased type I IFN production in both the lungs and BAL fluid compared with IAV-infected WT mice, we next determined whether Nlrx1−/− Mφ were impaired in their ability to produce type I IFN. Consistent with the significant decrease in type I IFN mRNA in the lungs of Nlrx1−/− mice, Nlrx1−/− bone marrow-derived Mφ produced significantly lower type I IFN mRNA transcripts upon infection with different doses and at different time points following IAV infection (Fig. 3 A and B). Secreted protein levels of type I IFN (both IFN-β and -α; Fig. 3C) and IFN-β (Fig. 3D) were also significantly reduced in Nlrx1−/− Mφ after IAV infection. In line with their impaired type I IFN response, Nlrx1−/− Mφ were more permissive to viral replication than WT Mφ (Fig. 3 E and F). Previous studies suggest that NLRX1 regulates type I IFN via the RIG-I/MAVS signaling pathway during viral infection (6, 29). To test this possibility, we quantified the type I IFN response in WT and Nlrx1−/− Mφ stimulated with the RIG-I ligands 5′pppRNA or intracellular poly(I:C). Similar to other studies (8, 29, 34), the levels of IFN-β mRNA (Fig. 3G) and type I IFN secretion (Fig. 3H) were similar between WT and Nlrx1−/− Mφ, indicating that NLRX1 does not directly affect type I IFN signaling. To further confirm this finding, we next measured the level of phosphorylation of the IRF3 transcription factor, which is directly involved in the activation of type I IFN transcription downstream of RIG-I (4). Following IAV infection or 5′pppRNA treatment, WT and Nlrx1−/− Mφ showed similar levels of IRF3 phosphorylation (Fig. S3A). We next assessed whether the viability of IAV-infected Mφ was affected by NLRX1. Staining with a viability dye revealed that the frequency of dead cells was significantly higher in Nlrx1−/− Mφ compared with WT Mφ at different time points following infection (Fig. 3I). This was attributable to a significant increase in apoptosis in Nlrx1−/− Mφ but not necrosis (Fig. 3 J–L and Fig. S3B). The JC-1 fluorescent dye can be used to measure mitochondrial permeability transition events, an early indication of cellular apoptosis. In healthy cells, the cationic JC-1 dye localizes inside the negatively charged mitochondria where it aggregates. When the mitochondrial membrane potential (ΔΨm) is reduced in apoptotic cells, the JC-1 reagent diffuses throughout the cell in a monomeric form, which fluoresces green. The frequency of green fluorescent Nlrx1−/− Mφ with disrupted ΔΨm was significantly increased compared with WT Mφ at 16 and 24 h pi (Fig. 3M). To further characterize apoptosis in IAV-infected versus bystander Mφ, IAV-infected WT and Nlrx1−/− Mφ were intracellularly stained with antibodies against IAV-nuclear protein (NP) and activated caspase 3. Similar to increased viral titer (Fig. 3 E and F), the frequency of infected (NP+) Nlrx1−/− Mφ was significantly elevated compared with WT Mφ (Fig. 3 N and O). The frequency of apoptosis in IAV-infected Nlrx1−/− Mφ (NP+Active Casp3+) was also significantly higher than in IAV-infected WT Mφ (Fig. 3 N and P), whereas there was no significant change in apoptosis between noninfected WT and Nlrx1−/− Mφ compared with unstimulated cells (NP−Active Casp3+, Fig. 3 N and Q). Together, these results were indicative of an early onset and increased magnitude of apoptosis in IAV-infected Nlrx1-deficient Mφ. To confirm that Nlrx1−/− Mφ were also more apoptotic in vivo during IAV infection, we infected WT and Nlrx1−/− mice with IAV and analyzed Mφ death modality in the lungs. Apoptosis frequencies of both alveolar and interstitial Mφ were significantly enhanced in Nlrx1−/− mice compared with WT, whereas there was no difference in necrosis (Fig. S4). In addition, the frequency of apoptosis in IAV infected alveolar Mφ was significantly higher in the BAL fluid of infected Nlrx1−/− mice compared with WT and the magnitude of differences was substantially increased using the higher dose of IAV inoculum (Fig. 3R). Together, these results show that Nlrx1-deficient Mφ have an impaired type I IFN response, enhanced mitochondrial damage, and undergo more apoptosis following IAV infection.
Fig. 3.
NLRX1 enhances type I IFN production, restricts viral replication and inhibits apoptosis in IAV-infected Mφ. (A–Q) WT and Nlrx1−/− BMDMs were infected or not with different doses of IAV (MOI of 1, 5, or 10) for indicated times. (A and B) Total RNA was extracted and antiviral gene mRNA expression for IFN-β (A) and IFN-α4 (B) was evaluated by qPCR. (C) Type I IFN protein secretion was measured from supernatants using B16-Blue IFN-α/β reporter cells. (D) IFN-β protein secretion was measured from supernatants by ELISA. (E and F) Viral replication was measured by extracting total RNA and quantifying viral gene M1 and NS1 mRNA expression by qPCR (E) or by standard plaque assay using supernatants from infected Mφ (F). (G and H) Uninfected WT and Nlrx1−/− BMDMs were transfected with 5′pppRNA or poly(I:C) for the indicated time. (G) Total RNA was extracted and IFN-β mRNA level was assessed by qPCR. (H) Type I IFN protein secretion was measured from supernatants as in C. (I) The frequency of dead Mφ was evaluated by flow cytometry after staining with the LIVE/DEAD viability dye. (J and K) The frequency of Mφ undergoing apoptosis (AnnexinV+7AAD−) (J) and necrosis (AnnexinV−7AAD+) (K) was determined by flow cytometry. (L) Fold increase in Mφ apoptosis relative to uninfected cells was measured by cell death ELISA. (M) The frequency of Mφ with disrupted mitochondrial membrane potential (ΔΨm) upon infection with IAV was measured by flow cytometry using the JC-1 dye. Data are plotted as percentages of green fluorescent J-monomers (ΔΨm disrupted) in IAV-infected Mφ. (N–Q) WT and Nlrx1−/− BMDMs were infected with 5 or 10 MOI of IAV. Micrographs are representative of the data depicted in O–Q, and numbers indicate the frequency of Mφ within quadrants. Frequency of NP+ Mφ (O), NP+Active Caspase-3+ Mφ undergoing apoptosis (P), and NP−Active Caspase-3+ Mφ undergoing apoptosis (Q) was evaluated at specified time points. (R) WT and Nlrx1−/− mice were infected with 150 or 1,000 pfu of IAV for 12 h, and the frequency of NP+Active Caspase-3+ alveolar Mφ undergoing apoptosis was evaluated from the BAL fluid. In A–M, data are depicted as means ± SEM from triplicate wells of at least two independent experiments. In N–Q, data are depicted as means ± SEM of one representative experiment out of three. Except O–R: *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 between mouse genotypes or as indicated. In O and P: *P < 0.05; **P < 0.01; ****P < 0.0001 compared with unstimulated cells of the same genotype or as indicated.
NLRX1 Binds IAV Virus Proapoptotic PB1-F2 Protein.
NLRX1 is a mitochondrial protein (7) and IAV-infected Nlrx1−/− Mφ were more prone to mitochondrial damage and apoptosis. In addition, the only known IAV protein that targets Mφ mitochondria to induce apoptosis is PB1-F2 (19, 26). Therefore, we next addressed the possibility that NLRX1 could prevent Mφ mitochondrial damage and apoptosis by binding to PB1-F2. To do so, we first ectopically expressed NLRX1 and HA-tagged PB1-F2–HA in HEK293T cells. By using a coimmunoprecipitation approach, we successfully pulled down NLRX1 by immunoprecipitating HA-tagged PB1-F2 from transiently transfected cells, suggesting that these two proteins physically interact (Fig. 4A). Similar results were obtained when by immunoprecipitating NLRX1; HA-PB1-F2 protein was detected in the precipitated complex. Furthermore, confocal microscopy analysis supported the colocalization of endogenous NLRX1 and PB1-F2 (Fig. 4B). To verify whether NLRX1 and myc-PB1-F2 interact during IAV infection, HEK293T cells were infected with the parental IAV virus or with recombinant IAV virus with disrupted PB1-F2 expression (IAV ΔPB1-F2) and pull-down assays of endogenous PB1-F2 or endogenous NLRX1 were performed. Immunoprecipitation detected a prominent endogenous complex between viral PB1-F2 and NLRX1 protein (Fig. 4C). In addition, the NLRX1/PB1-F2 complex could only be detected in cells infected with IAV but not IAV ΔPB1-F2 (Fig. 4C). This interaction was specific, considering PB1-F2 did not coimmunoprecipitate with other proteins localize at various mitochondrial locations, including Tom 20 (mitochondrial outer membrane), apoptosis-inducing factor (AIF) (mitochondrial intermembrane space), and COX IV (mitochondrial inner membrane) (Fig. 4D). To further validate these findings, we performed coimmunoprecipitation experiment using cell-free GST pull-down assay. We demonstrated that endogenous NLRX1 binds to recombinant GST–PB1-F2, confirming the direct nature of the interaction between two proteins (Fig. 4E). To extend these findings, we infected A549 human airway epithelial cells with either WT or mutated virus. Again, immunoprecipitation of PB1-F2 led to the detection of endogenous NLRX1 by immunoblot only in cells infected with IAV but not IAV ΔPB1-F2 (Fig. 4F). Consistent with these data, colocalization of endogenous PB1-F2 and NLRX1 in mitochondria was further supported using confocal microscopy (Fig. 4G). To determine whether the interaction between NLRX1 and PB1-F2 could be detected inside the mitochondria of Mφ, we collected mitochondrial lysates from WT and Nlrx1−/− Mφ infected with IAV. The coimmunoprecipitation experiment with anti-NLRX1 and anti–PB1-F2 polyclonal serum confirmed the interaction between NLRX1 and PB1-F2, which only occurred in isolated mitochondria of WT but not Nlrx1−/− Mφ (Fig. 4H). Thus, NLRX1 binds PB1-F2 and this interaction occurs endogenously in mitochondria.
Fig. 4.
NLRX1 interacts with PB1-F2. (A) NLRX1 expressing construct was cotransfected with either empty vector or HA-tagged PB1-F2 in HEK293T cells; 48 h later, cells were lysed and immunoprecipitated proteins were blotted for either HA-PB1-F2 or NLRX1 as indicated. The two lower gels show expression of PB1-F2 and NLRX1 in whole cell extracts (WCEs). (B) Confocal microscopy analysis demonstrating colocalization of NLRX1 and PB1-F2. A549 cells were transiently transfected with myc-tagged PB1-F2 expressing vector or empty vector; 24 h later, cells were fixed, and yellow regions are the areas of NLRX1 and PB1-F2 colocalization. Nuclei were stained using DAPI (blue). (C) HEK293T cells were infected with 5 MOI of IAV or IAV ΔPB1-F2. Cell lysates were collected 24 h pi to perform immunoprecipitation using either isotype-antibody control, anti-NLRX1 antibodies, or anti-PB1-F2 polyclonal serum. Samples were analyzed by Western blot. WCEs (Input) from infected cells served as a control to detect NLRX1 and viral PB1-F2 protein. Actin was used as a loading control. (D) PB1-F2 specifically interacts with NLRX1 but not other mitochondrial proteins. HEK293T cells were infected either with parental IAV or IAV ΔPB1-F2 strain, and pull-down assay was performed. PB1-F2 interacted with NLRX1 but not other mitochondrial proteins, Tom20, AIF, and Cox IV (Left). WCEs (Input) from infected cells served as a control to detect NLRX1 and other mitochondrial proteins (Right). (E) Direct interaction between PB1-F2 and NLRX1 proteins. Recombinant GST–PB1-F2 protein was incubated with the mitochondrial extract and immunoprecipitated complex was analyzed by immunoblot. Coomassie gel served as a control to demonstrate the presence of GST–PB1-F2 (37 kDa) and GST control (26 kDa) proteins. (F) A549 lung epithelial cells were infected with 1 MOI of IAV or IAV ΔPB1-F2. Endogenous interaction in infected cells was evaluated by coimmunoprecipitation and immunoblot with anti NLRX1. The two bottom gels show expression of NLRX1 and PB1-F2 in whole cell extracts. Actin served as a loading control in this experiment. (G) Confocal microscopy analysis demonstrating colocalization of endogenous NLRX1 and PB1-F2. A549 cells were infected (MOI of 5) with IAV (Top and Middle) or with IAV Δ PB1-F2 (Lower); 24 h after infection, cells were stained with the mitochondrial tracker CMXRos (red), fixed, and then stained for NLRX1 and PB1-F2. IAV-infected cells costained with NLRX1-specific rabbit polyclonal antibody (green), PB1-F2–specific mouse polyclonal serum (blue), and mitochondria (red). White regions are areas of NLRX1 and PB1-F2 colocalization on the mitochondria. (H) WT and Nlrx1−/− deficient BMDMs were infected with 5 MOI of IAV; 16 h later, cells were collected and mitochondria lysates were prepared. Coimmunoprecipitation assay was performed using isotype control, anti-NLRX1 antibodies and anti–PB1-F2 polyclonal serum. WCEs (Input) and mitochondrial fractions were analyzed via Western blot to visualize NLRX1, viral PB1-F2, and mitochondrial VDAC1 proteins. Data are representative of two to three independent experiments.
NLRX1 Enhances Type I IFN Production in IAV-Infected Mφ by Protecting Them from PB1-F2–Mediated Apoptosis.
Because NLRX1 interacted with PB1-F2 in Mφ mitochondria, we next hypothesized that NLRX1-dependent suppression of apoptosis in IAV-infected Mφ would be lost upon infection with IAV ΔPB1-F2. Indeed, the frequency of cell death (Fig. 5A), mitochondrial ΔΨm (Fig. 5B), apoptosis (Fig. 5C and Fig. S5A), or necrosis (Fig. 5D and Fig. S5A) did not differ between WT and Nlrx1−/− Mφ following infection with IAV ΔPB1-F2. Additionally, there was no difference in type I IFN at either transcriptional or translational levels between WT and Nlrx1-deficient Mφ infected with IAV ΔPB1-F2 (Fig. 5 E–G). Consistent with the similar levels of type I IFN production, the levels of viral replication were similar between WT and Nlrx1-deficient Mφ (Fig. 5H) and both pulmonary type I IFN production and viral replication did not differ between WT and Nlrx1−/− mice at day 3 following IAV ΔPB1-F2 infection (Fig. S5 B–D). To evaluate the effect of PB1-F2 in the modulation of type I IFN production and cell death, we next infected WT Mφ with either IAV or IAV ΔPB1-F2. Similar to another study showing the anti-type I IFN function of PB1-F2 (23), we found that the expression levels of IFN-β were significantly higher in IAV ΔPB1-F2–infected Mφ than in IAV-infected Mφ (Fig. 5I). We also confirmed the well-established proapoptotic function of PB1-F2 (19, 27, 34) because Mφ apoptosis but not necrosis was significantly reduced in the absence of PB1-F2 (Fig. 5 K and L). To directly test whether the reduced antiviral activity of Nlrx1−/− Mφ translated to an impaired control of IAV infection in vivo, alveolar Mφ derived from WT and Nlrx1−/− mice were infected with IAV or IAV ΔPB1-F2 in vitro and adoptively transferred intratracheally to Rag-1−/− mice (lacking T and B cells) (Fig. 5M). Although the number of viruses in WT and Nlrx1−/− Mφ was similar before transfer (Fig. 5N), the mice that received infected Nlrx1−/− Mφ showed increased pulmonary viral titer (Fig. 5O) and decreased IFN-β secretion (Fig. 5P) compared with the mice receiving infected WT Mφ. Most importantly, the absence of NLRX1 in alveolar Mφ infected with IAV ΔPB1-F2 had no effect on type I IFN production and pulmonary viral titer following adoptive transfer (Fig. 5 N–P). Collectively, these data suggest that NLRX1 plays a critical role in preventing mitochondrial damage and apoptosis during IAV infection by binding to viral PB1-F2 protein, thereby allowing Mφ to produce more type I IFN and restrict viral replication.
Fig. 5.
NLRX1-dependent type I IFN production and apoptosis inhibition is lost in Mφ infected with IAV ΔPB1-F2. WT and Nlrx1−/− BMDMs were infected or not with different doses of IAV or IAV ΔPB1-F2 (MOI of 1, 5, or 10) for the indicated times. (A) The frequency of dead Mφ was evaluated by flow cytometry after staining with the LIVE/DEAD viability dye. (B) The frequency of Mφ with disrupted mitochondrial membrane potential (ΔΨm) was measured by flow cytometry using the JC-1 dye. Data are plotted as the percentages of green fluorescent J-monomers (ΔΨm disrupted) in IAV-infected Mφ. (C and D) The frequency of Mφ undergoing apoptosis (AnnexinV+7AAD−) (C) and necrosis (AnnexinV−7AAD+) (D) was determined by flow cytometry. (E) Total RNA was extracted and antiviral gene mRNA expression for IFN-β was evaluated by qPCR. (F) Type I IFN protein secretion was measured from supernatants using B16-Blue IFN-α/β reporter cells. (G) IFN-β protein secretion was measured from supernatants by ELISA. (H) Viral replication was measured by standard plaque assay from Mφ supernatants. (I–L) WT and Nlrx1−/− BMDMs were infected or not with different doses of parental IAV and recombinant IAV ΔPB1-F2 for indicated times. Total RNA was extracted, and antiviral gene mRNA expression for IFN-β was evaluated by qPCR (I). The frequency of dead Mφ was evaluated by flow cytometry after staining with the LIVE/DEAD viability dye (J). (K and L) The frequency of Mφ undergoing apoptosis (AnnexinV+7AAD−) (K) and necrosis (AnnexinV−7AAD+) (L) was determined by flow cytometry. (M–P) Alveolar Mφ from naïve WT and Nlrx1−/− mice were isolated and infected with IAV or IAV ΔPB1-F2 in vitro and then adoptively transferred (intratracheally) to Rag-1−/− mice (n = 5). (N) Viral load in infected alveolar Mφ from WT and Nlrx1−/− mice before adoptive transfer in Rag-1−/− mice. Viral loads (O) and IFN-β concentration (P) in the lungs of Rag-1−/− mice 3 d following adoptive transfer with IAV-infected WT or Nlrx1−/− Mφ. Data are depicted as means ± SEM from triplicate wells of at least two independent experiments.
Discussion
The innate immune response is critical for the detection of IAV viruses and subsequent activation of adaptive immunity. To counteract host defense mechanisms, IAV have evolved strategies to replicate efficiently in the airways and transmit to other hosts. Virions initially replicate in airway epithelial cells and are then released from the apical side of these cells toward the airspace where they encounter alveolar Mφ (35). Upon detection of IAV viral particles via PRRs, pulmonary Mφ convert into highly active cells and become the major source of inflammatory cytokines/chemokines including type I IFNs, which are essential to restrict early viral replication. These signals also orchestrate the recruitment of other leukocytes to the lungs to mount a potent antiviral immune response. Although IAV inhibits early apoptosis in epithelial cells of the respiratory tract to preserve a niche for replication, it preferentially induces early apoptosis in Mφ via PB1-F2 (19). Hence, by subverting Mφ function, IAV delays the antiviral immune response and replicates more efficiently. We demonstrate here for the first time, to our knowledge, that a PRR could effectively interact with a viral proapoptotic protein in Mφ mitochondria, thereby preventing early apoptosis and leading to increased type I IFN production by these cells. Although in the current study we have demonstrated that the interaction between host Mφ NLRX1 and viral PB1-F2 is critical for the control of IAV replication both in vitro and in vivo, further study is necessary to evaluate the consequences of interaction between PB1-F2 and NLRX1 in other immune cells or structural cells especially lung epithelial cells. Thus, our results collectively suggest that NLRX1 plays a unique role in Mφ by maintaining mitochondrial fitness and preventing viral-induced cell death to maximize type I IFN production following IAV infection (Fig. 6).
Fig. 6.
NLRX1-mediated recognition of IAV PB1-F2 accessory protein protects Mφ mitochondria and enhances innate antiviral immunity. After early replication in airway epithelial cells, IAV virions are released in the airways, where they encounter and infect alveolar Mφ. IAV virus proapoptotic PB1-F2 accessory protein enters the mitochondria and induces apoptosis of infected Mφ, thereby suppressing early antiviral immunity, including type I IFN production. This immune evasion strategy is counteracted by the host mitochondrial PRR NLRX1, which binds to PB1-F2 and prevents apoptosis of infected Mφ. Enhanced survival of alveolar Mφ increases their antiviral functions and capacity to restrict viral replication, thus maintaining a protective balance between antiviral immunity and excessive inflammation.
There are conflicting reports with regards to the role of NLRX1 in immunity to viral infection. Early studies have suggested that NLRX1 inhibits RIG-I/MAVS-dependent signaling and type I IFN production (6, 29). In contrast with these studies but in line with others (8, 36), we found that NLRX1 does not inhibit RIG-I/MAVS signaling because Nlrx1−/− Mφ stimulated with RIG-I ligands 5′pppRNA and intracellular poly(I:C) were not impaired in their ability to produce type I IFN. In further support of this conclusion, we did not find appreciable differences in IRF3 phosphorylation between IAV-infected WT and Nlrx1−/− Mφ at early time points (e.g., before significant induction of apoptosis in Nlrx1−/− cells). Following careful characterization of WT and Nlrx1−/− mice during IAV infection, our data suggest that the major role of NLRX1 is in the regulation mitochondrial fitness and maintenance of cell viability by interacting with IAV PB1-F2 protein rather than inhibiting RIG-I/MAVS-dependent type I IFN signaling. Thus, our study may potentially explain the discrepancy in the literature considering that in the absence of PB1-F2, the function of NLRX1 is dispensable in immunity to IAV infection. In Nlrx1-deficient mice infected with IAV, despite increased pulmonary viral load, pathology and morbidity, there was no effect on survival which is consistent with the previous study by Allen et al. (29). The absence of correlation between morbidity and mortality may be attributable to adaptive immunity, because IAV-infected Nlrx1-deficient Mφ undergo more apoptosis, which may enhance T cell-mediated immunity via antigen cross-presentation (37). In addition, the early excessive host inflammatory responses in IAV-infected Nlrx1-deficient mice that contributed to the respiratory tissue damage and dysfunction may be explained by two major factors mediated by IAV virus PB1-F2 protein: (i) the increased early viral propagation attributable to significant increase in apoptosis of the pulmonary Mφ, which led to the significant reduction in type I IFN-mediated antiviral immunity; and (ii) the increased levels of PB1-F2 expression, which intensifies the NF-κB and NLRP3 signaling pathways (38, 39). Thus, NLRX1 strongly attenuates the early function of PB1-F2 and influences the early host response to IAV infection. More in vivo studies will be needed to assess the function of NLRX1 in regulating inflammation and adaptive immunity to IAV infection.
Mitochondria are essential platforms conveying both antiviral and cell death signals (5). Thus, mitochondria must have evolved regulatory mechanisms to maintain health and promote antiviral signaling. PB1-F2 is the only known IAV protein that targets mitochondria to induce apoptosis preferentially in monocytes/Mφ (19). A study by Zamarin et al. (27) has proposed a biphasic model of PB1-F2–induced apoptosis, which consists of an early phase of infection when PB1-F2 localizes to the mitochondria without inducing apoptosis and late phase of infection when PB1-F2 activates apoptosis. We envision that NLRX1, by binding to PB1-F2, potentially disarms the ability of PB1-F2 to induce apoptosis during the early phase of infection when the level of PB1-F2 is relatively low and perhaps matches the number of NLRX1 (Fig. 6). However, when this balance is shifted toward PB1-F2 during the late phase of infection, exceeding the number of available NLRX1 (as mimicked by Nlrx1 deficiency), PB1-F2 induces mitochondria-dependent apoptosis. Although the exact mechanism of how NLRX1 prevents PB1-F2–dependent apoptosis remains to be elucidated, we speculate that binding of NLRX1 to PB1-F2 may reduce the availability of PB1-F2 for binding to the components of the permeability transition pore complex (PTPC) and promoting apoptosis. Whether PB1-F2 is simply neutralized or further targeted for proteasomal degradation is currently under investigation. Furthermore, because PB1-F2 targets mitochondria via the basic amphipathic helix in its C-terminal region, the pronounced apoptotic effects of PB1-F2 specifically in cells of immune origin as opposed to all cell types is surprising. However, we predict that the levels of NLRX1 in different cell types may have effects on the ability of PB1-F2 to induce apoptosis. Certainly, further experiments are needed to determine the link between PB1-F2, PTPT, and NLRX1.
To fully understand the function of NLRX1, it will be essential to study the interaction between NLRX1 and PB1-F2 with other strains of IAV. Recently, the analysis of 2,566 available PB1-F2 sequences belonging to the H3N2 subtype revealed that many H3N2 strains harbor PB1-F2 proteins that vary in length because of N-terminal or C-terminal truncations (40). Additionally, the full length of PB1-F2 may have either proinflammatory or noninflammatory properties, which is influenced by its amino acid sequence. The proinflammatory PB1-F2 protein has been directly linked to the pathogenicity of IAV H3N2 virus (e.g., influenza A/Hong Kong/1/68), whereas the noninflammatory PB1-F2 has been associated with the reduced pathogenicity of IAV infection during seasonal H3N2 influenza (A/Wuhan/359/95) (41). It was also demonstrated that a single mutation in PB1-F2 of the highly pathogenic strains of IAV H5N1 (HK/97) and 1918 H1N1 significantly contributed to their pathogenicity and lethality (42). Thus, we envision that further characterization of the PB1-F2 protein identifying its domain responsible for the interaction with NLRX1 will significantly facilitate future studies with other strains of IAV and may explain some of differences in PB1-F2 function between these strains.
In conclusion, the results presented here demonstrate that NLRX1 does not regulate type I IFN signaling per se during IAV infection but rather regulates mitochondrial-dependent cell death program by binding to proapoptotic IAV PB1-F2 protein. NLRX1 thus acts as a sentinel monitoring the levels of viral replication by detecting PB1-F2. When viral replication overreaches the threshold of NLRX1 in mitochondria, apoptosis signaling is activated. Additional studies are required to understand whether these findings can be generalized to other pathogens having the ability to target host mitochondria or unique to Influenza virus.
Methods
Mice.
Six- to eight-week-old C57BL/6 mice were obtained from The Jackson Laboratory. Nlrx1-deficient mice (provided by S. Girardin, University of Toronto) were bred at McGill University. Infected mice were monitored daily for morbidity and mortality up to 21 d postinfection. Experiments were carried out in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of McGill University.
Isolation and Culture of Primary Cells and Cell Lines.
Murine bone marrow derived macrophages (BMDMs) were prepared from aseptically dissected and flushed tibias and femurs of 7- to 9-wk-old mice. Bone marrow cells were differentiated into BMDMs for 7 d in RPMI-1640 supplemented with 30% (vol/vol) L929 cell-conditioned [American Type Culture Collection (ATCC)] medium, 10% (vol/vol) FBS, 2 mM l-glutamine, 1 mM sodium pyruvate, 1% essential and nonessential amino acids, and 100 U/mL penicillin/streptomycin. Viral infection was performed in fresh complete medium without L929 cell-conditioned medium. Madin–Darby Canine Kidney (MDCK), 293T, A549, and MLE12 cells were obtained from the ATCC and were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% (vol/vol) FBS, 10 mM Hepes, and 100 U/mL of penicillin/streptomycin or in Hams DMEM: F12 supplemented with 5% (vol/vol) FBS, 10 mM Hepes, 1× insulin-transferin-selenium, and penicillin/streptomycin. Unless indicated, all cells were plated at the density of 0.5–1 × 106 cells per well in six-well plate. All media and supplements were from GIBCO.
Viruses and Infections.
All in vivo and in vitro infections were performed using influenza A/Puerto Rico/8/34 (H1N1) virus (IAV) or engineered influenza A/Puerto Rico/8/34 virus lacking expression of PB1-F2 (34) (IAV ΔPB1-F2) (both obtained from J.A.M.). For in vivo infection, WT and Nlrx1−/− mice were challenged intranasally (in 25 μL PBS) with IAV at a sublethal dose of 50 pfu or a lethal dose of 150 pfu unless indicated otherwise. BMDMs and epithelial cells were seeded in 6- or 12-well tissue culture plates the day before infection unless indicated otherwise, and all in vitro infections were performed with 1, 5, or 10 multiplicities of infection (MOI) of virus. On the day of infection, the BMDM culture medium was replaced with 1% FBS supplemented medium deprived of L929 cells supernatant. Infection with IAV was performed for 1 h, after which cell monolayer was washed once with PBS, medium was replaced with 10% FBS-containing RPMI medium, and cells were cultured for the indicated time. Viruses were propagated and isolated from MDCK cells and titrated using standard plaque assay in MDCK cells (43).
RNA Isolation and Real-Time Quantitative PCR.
RNA from BMDMs, lung tissue, and epithelial cells (5 × 105) was extracted using TRIzol Reagent (Invitrogen) according to the manufacturer’s instructions; 500 ng of RNA was reverse-transcribed using the iScriptcDNA Synthesis Kit (Bio-Rad), and measurements of gene expression were carried out using iQ SYBR Green SuPermix (Bio-Rad) according to the manufacturer’s instructions. Raw threshold cycle (Ct) values were obtained from Bio-Rad CXT96 quantitive (q)PCR instrument. Results were analyzed using the 2-ΔCt formula normalizing target gene expression to mGAPDH. qPCR primer pairs are listed in Table S1.
Histology and Analysis of Pulmonary Function.
For histopathologic examination, lungs were fixed by inflation and immersion in buffered formalin and subjected to H&E staining. Airway hyperreactivity in response to methacholine was evaluated using the flexiVent apparatus and flexiVent 5.1 software as previously described (44).
Flow Cytometry.
Single-cell suspension was obtained from perfused lungs, which were incubated at 37 °C for 2 h in a mixture of collagenase (300 U per organ) (Sigma) and DNase I (80 U/mL) (Sigma). BAL was conducted by cannulating the trachea with a 22-gauge catheter. Lungs were then washed by instilling 3 × 800 μL of PBS. The total lavage fluid recovered was ∼2 mL. Lung cells and BAL fluid were first incubated with anti-CD16/32 (BD Biosciences) in 0.5% BSA/PBS to block nonspecific Ab interaction with Fc receptors. Then, cells were surface-stained with different combinations of FITC-conjugated anti-CD45.2, V500-conjugated anti-MHC II (I-A/I-E), PE-conjugated anti–Siglec-F, APC-Cy7–conjugated anti-CD11c, PerCP-conjugated anti-CD115, APC-conjugated anti-F4/80, PE-Cy7-conjugated anti-Gr1, PE-Cy7–conjugated anti-CD19, PE-conjugated anti-CD3, and APC-conjugated anti-NK1.1 (all from BD Biosciences). Intracellular staining for IAV NP and active Caspase-3 was performed using Cytofix/Cytoperm Fixation/Permeabilization Solution Kit (BD Biosciences) with FITC-conjugated anti-IAV NP (Abcam), and PE-conjugated anti-Active Caspase-3 (BD Biosciences). Flow cytometry was performed using BD LSR II (BD Biosciences) with FACSDiva Software Version 6.1.2 (BD Biosciences), and analysis was performed using FlowJo Software Version 10.0.6 (Tree Star).
Mφ Stimulation and Cytokine Quantification.
Transfection of BMDMs was performed using Lipofectamine reagent (Invitrogen) according to the manufacturer’s instructions. Lipofectamine was used to transfect cells with 5′pppdsRNA (InvivoGen) at a concentration of 0.1 μg/mL. BMDMs were also transfected with the dsRNA synthetic analog poly(I:C) [poly(I:C) LyoVec; InvivoGen] at a concentration 0.5 μg/mL according to the manufacturer’s instructions. Cells were harvested at different time points posttransfection, and cDNA was prepared to measure mRNA expression level of indicated genes (see Table S1 for list). The level of IFN-β in the supernatant of BMDMs, lung homogenate, and BAL fluid was measured by ELISA (PBL InterferonSource) following the manufacturer’s instructions. Similarly, type I IFN (both IFN-α and IFN-β) secretion was assessed using B16-Blue IFNα/β reporter cells (InvivoGen) according to the specifications of the manufacturer.
Cell Death Analysis.
For in vitro experiments, apoptosis measurements of BMDMs were performed using a cell death detection ELISAPLUS photometric enzyme immunoassay (Roche Applied Science) according to the manufacturer’s instruction. The fluorescent dye JC-1 (MitoPT JC-1 kit; ImmunoChemistry) was used to monitor the extent of mitochondrial depolarization following influenza virus infection relative to the control cells (mock-infected) and carbonylcyanide m-chlorophenylhydrazone (CCCP)-treated cells (used as a positive control). Loss of ∆Ψm was assessed by measuring JC-1 monomers level by flow cytometry. LIVE/DEAD Fixable Violet Dead Cell staining was implemented to determine cell viability following viral infection (Molecular Probes). Necrosis and apoptosis level in vitro and in vivo was assessed using the PE-AnnexinV and 7-amino-actinomycin D (7AAD) Apoptosis Detection Kit I (BD Biosciences) according to the manufacturer’s instructions and analyzed by flow cytometry.
Subcellular Fractionation, Immunoprecipitation, and Western Blot Analysis.
To isolate mitochondrial fractions from cell preparations, the mitochondrial isolation kit (MACS reagent; Miltenyi Biotec) for tissue and cultured cells was used according to the manufacturer’s instructions. For coimmunoprecipitation experiments, mitochondrial extract (50–100 μg) or total cell lysates from transfected 293T cells or IAV-infected 293T and A549 cells suspended in lysis buffer [1% Triton X-100, 150 mM NaCl, 20 mM Hepes (pH 7.5), 10% (vol/vol) glycerol, 1 mM EDTA, protease inhibitors] were incubated with antibody at 4 °C for 2 h. Protein A- and G-agarose beads (Sigma) were then added, followed by incubation at 4 °C overnight. The beads were washed three times with lysis buffer and boiled in 2× SDS loading buffer for 5 min. Immunoprecipitated proteins were then analyzed by Western blotting. For GST pull-down experiments, beads-bound GST–PB1-F2 was blocked with H-buffer [20 mM Hepes (pH 7.7), 75 mM KCl, 0.1 mM EDTA, 25 mM MgCl2, 0.05% Nonidet P-40, 1 mM DTT, 1 mg/mL BSA]. Blocked beads were incubated with mitochondrial lysate for 16 h at 4 °C. Pelleted beads were then washed in H-buffer, and bound proteins were analyzed by immunoblots. pGEX6-PB1-F2 plasmid was a generous gift from W. Wixler (Munster University, Germany). Recombinant GST–PB1-F2 protein was produced and purified according to the GST-tagged protein purification manual (GE Healthcare Life Sciences). Rabbit anti- NLRX1 (cross-reactivity against human NLRX1) (1:1,000) (no. 8583), rabbit anti-IRF3 (1:1,000), rabbit anti-phIRF3 (1:500), rabbit anti-AIF (1:1,000), mouse anti-COX IV (1:1,000), and rabbit anti-VDAC1 (1:1,000) antibodies were purchased from Cell Signaling Technology. Rabbit anti-NLRX1 (cross-reactivity against mouse NLRX1) (1:1,000) (H142) and rabbit anti-Tom20 (1:1,000) were purchased from Santa Cruz Biotechnology. Rabbit anti-HA (1:1,000) (H6908) and mouse anti-Actin (1:10,000) were purchased from Sigma. Anti-IAV PB1-F2 polyclonal serum (1:5) was kindly provided by W. Wixler. Finally, HRP-labeled anti-secondary Ab was used at a dilution 1:10,000 for detection of bound primary Ab by ECL (Amersham). For phospho-IRF3 protein detection, stimulated cells were lysed in ice-cold buffer A [50 mM Tris⋅Cl (pH 7.5), 1 mM EGTA, 1 mM EDTA, 1% (vol/vol) Triton X-100, 1 mM sodium orthovanadate, 5 mM sodium pyrophosphate, 0.27 M sucrose, complete miniprotease inhibitor mixture, and 2 mM DTT]; 20 μg of lysates was submitted to SDS/PAGE, transferred to nitrocellulose, blocked with 5% (vol/vol) BSA-TBS for 1 h at room temperature, and immunoblotted overnight at 4 °C with specific antibodies diluted in 2% (vol/vol) Tris-buffered saline with Tween 20 (TBST) [pIRF3 (Ser396) Cell Signaling no. 4947 (1:1,000); GAPDH Millipore no. MAB374 (1:4,000)]. The signal was detected using goat anti-mouse DyLight 680 and goat anti-rabbit DyLight 800 diluted 1:15,000 in 1% BSA-TBST and quantified using Licor Odyssey imaging system.
Confocal Microscopy.
A549 cells were cultured on glass coverslips coated with Collagen I (Sigma). In some experiments, cells were transfected with myc-tagged PB1-F2–expressing vector or empty vector. In other conditions, A549 cells were infected with PR8 strain of Influenza virus (MOI of 5) or PR8 ΔPB1-F2. Cells were processed for indirect immunofluorescence at 24 h posttransfection or postinfection by fixing in 4% (vol/vol) paraformaldehyde for 20 min. Cell membranes were permeabilized by incubating with PBS buffer containing 0.2% Triton X-100 for 5 min. Samples were blocked in 1% BSA for 30 min before incubation with primary and secondary antibodies. All washes used PBS. Mitochondria were stained using mitochondrial stain CMXRos, according to the manufacturer’s protocol (Molecular Probes, Invitrogen). NLRX1 protein was detected with rabbit polyclonal antibody anti-NLRX1 (Cell Signaling Technology) at a 1:50 dilution. Myc tag was detected by using mouse mAb anti-myc (Sigma) at a 1:750 dilution. Viral PB1-F2 protein in infected cells was detected with mouse polyclonal anti–PB1-F2 serum at a dilution 1:5. Secondary antibodies were Alexa Fluor 488-conjugated goat anti-rabbit (Invitrogen), Alexa Fluor 555-conjugated goat anti-mouse (Invitrogen), and Alexa Fluor 350-conjugated donkey anti-mouse (Invitrogen). Images of nuclei were visualized by DAPI staining (1:2,000; Molecular Probes). Coverslips were mounted (ProLong Gold Anti Fade; Invitrogen) onto microscope slides, and stained cells were viewed under Leica TCS SP2 laser-scanning confocal microscope.
Adoptive Transfer Model of Infection.
Alveolar Mφ were collected from the BAL fluid of naïve WT and Nlrx1−/− mice and suspended Mφ were infected for 2 h in vitro with 5 MOI of IAV or IAV ΔPB1-F2. Free viruses were then removed by three washes with PBS, each followed by centrifugation for 10 min at 200 × g and 4 °C. Cells were resuspended in PBS at a density of 5 × 105 cells per 40 μL and then transferred by the intratracheal route into naïve Rag-1−/− mice (45, 46).
Statistical Analysis.
Statistical analysis was performed using GraphPad Prism Version 6.0 (GraphPad Software). Data were compared using a two-tailed Student t test or two-way ANOVA with Sidak’s multiple comparison test and are expressed as means ± SEM. The log-rank test was used to compare mouse survival rates. Differences were considered significant if P < 0.05.
Supplementary Material
Acknowledgments
We thank Dr. W. Wixler for providing anti-influenza virus A PB1-F2 polyclonal serum. This work was supported by Canadian Institute of Health Research (CIHR) Operating Grant MOP-106488 (to M.D.), and M.D. holds a CIHR New Investigator Award. F.C. is supported by F. Banting and C. Best Canada graduate scholarships from CIHR.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1322118111/-/DCSupplemental.
References
- 1.Fukuyama S, Kawaoka Y. The pathogenesis of influenza virus infections: The contributions of virus and host factors. Curr Opin Immunol. 2011;23(4):481–486. doi: 10.1016/j.coi.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim HM, et al. Alveolar macrophages are indispensable for controlling influenza viruses in lungs of pigs. J Virol. 2008;82(9):4265–4274. doi: 10.1128/JVI.02602-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J, et al. Innate immune response of human alveolar macrophages during influenza A infection. PLoS ONE. 2012;7(3):e29879. doi: 10.1371/journal.pone.0029879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeuchi O, Akira S. Innate immunity to virus infection. Immunol Rev. 2009;227(1):75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnoult D, Soares F, Tattoli I, Girardin SE. Mitochondria in innate immunity. EMBO Rep. 2011;12(9):901–910. doi: 10.1038/embor.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore CB, et al. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature. 2008;451(7178):573–577. doi: 10.1038/nature06501. [DOI] [PubMed] [Google Scholar]
- 7.Arnoult D, et al. An N-terminal addressing sequence targets NLRX1 to the mitochondrial matrix. J Cell Sci. 2009;122(Pt 17):3161–3168. doi: 10.1242/jcs.051193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rebsamen M, et al. NLRX1/NOD5 deficiency does not affect MAVS signalling. Cell Death Differ. 2011;18(8):1387. doi: 10.1038/cdd.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sasaki O, et al. A structural perspective of the MAVS-regulatory mechanism on the mitochondrial outer membrane using bioluminescence resonance energy transfer. Biochim Biophys Acta. 2013;1833(5):1017–1027. doi: 10.1016/j.bbamcr.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Arimori Y, et al. Type I interferon limits influenza virus-induced acute lung injury by regulation of excessive inflammation in mice. Antiviral Res. 2013;99(3):230–237. doi: 10.1016/j.antiviral.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Malmgaard L. Induction and regulation of IFNs during viral infections. J Interferon Cytokine Res. 2004;24(8):439–454. doi: 10.1089/1079990041689665. [DOI] [PubMed] [Google Scholar]
- 12.Mattei F, Schiavoni G, Tough DF. Regulation of immune cell homeostasis by type I interferons. Cytokine Growth Factor Rev. 2010;21(4):227–236. doi: 10.1016/j.cytogfr.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 13.van de Sandt CE, Kreijtz JH, Rimmelzwaan GF. Evasion of influenza A viruses from innate and adaptive immune responses. Viruses. 2012;4(9):1438–1476. doi: 10.3390/v4091438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan N, Chen ZJ. Intrinsic antiviral immunity. Nat Immunol. 2012;13(3):214–222. doi: 10.1038/ni.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, et al. ISG56 is a negative-feedback regulator of virus-triggered signaling and cellular antiviral response. Proc Natl Acad Sci USA. 2009;106(19):7945–7950. doi: 10.1073/pnas.0900818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hambleton S, et al. STAT2 deficiency and susceptibility to viral illness in humans. Proc Natl Acad Sci USA. 2013;110(8):3053–3058. doi: 10.1073/pnas.1220098110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moltedo B, et al. Cutting edge: Stealth influenza virus replication precedes the initiation of adaptive immunity. J Immunol. 2009;183(6):3569–3573. doi: 10.4049/jimmunol.0900091. [DOI] [PubMed] [Google Scholar]
- 18.Lowy RJ. Influenza virus induction of apoptosis by intrinsic and extrinsic mechanisms. Int Rev Immunol. 2003;22(5-6):425–449. doi: 10.1080/08830180305216. [DOI] [PubMed] [Google Scholar]
- 19.Chen W, et al. A novel influenza A virus mitochondrial protein that induces cell death. Nat Med. 2001;7(12):1306–1312. doi: 10.1038/nm1201-1306. [DOI] [PubMed] [Google Scholar]
- 20.Wise HM, et al. A complicated message: Identification of a novel PB1-related protein translated from influenza A virus segment 2 mRNA. J Virol. 2009;83(16):8021–8031. doi: 10.1128/JVI.00826-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Goffic R, et al. Influenza A virus protein PB1-F2 exacerbates IFN-beta expression of human respiratory epithelial cells. J Immunol. 2010;185(8):4812–4823. doi: 10.4049/jimmunol.0903952. [DOI] [PubMed] [Google Scholar]
- 22.Varga ZT, et al. The influenza virus protein PB1-F2 inhibits the induction of type I interferon at the level of the MAVS adaptor protein. PLoS Pathog. 2011;7(6):e1002067. doi: 10.1371/journal.ppat.1002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varga ZT, Grant A, Manicassamy B, Palese P. Influenza virus protein PB1-F2 inhibits the induction of type I interferon by binding to MAVS and decreasing mitochondrial membrane potential. J Virol. 2012;86(16):8359–8366. doi: 10.1128/JVI.01122-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibbs JS, Malide D, Hornung F, Bennink JR, Yewdell JW. The influenza A virus PB1-F2 protein targets the inner mitochondrial membrane via a predicted basic amphipathic helix that disrupts mitochondrial function. J Virol. 2003;77(13):7214–7224. doi: 10.1128/JVI.77.13.7214-7224.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chanturiya AN, et al. PB1-F2, an influenza A virus-encoded proapoptotic mitochondrial protein, creates variably sized pores in planar lipid membranes. J Virol. 2004;78(12):6304–6312. doi: 10.1128/JVI.78.12.6304-6312.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada H, Chounan R, Higashi Y, Kurihara N, Kido H. Mitochondrial targeting sequence of the influenza A virus PB1-F2 protein and its function in mitochondria. FEBS Lett. 2004;578(3):331–336. doi: 10.1016/j.febslet.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 27.Zamarin D, García-Sastre A, Xiao X, Wang R, Palese P. Influenza virus PB1-F2 protein induces cell death through mitochondrial ANT3 and VDAC1. PLoS Pathog. 2005;1(1):e4. doi: 10.1371/journal.ppat.0010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coleman JR. The PB1-F2 protein of Influenza A virus: Increasing pathogenicity by disrupting alveolar macrophages. Virol J. 2007;4:9. doi: 10.1186/1743-422X-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen IC, et al. NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I-MAVS and TRAF6-NF-κB signaling pathways. Immunity. 2011;34(6):854–865. doi: 10.1016/j.immuni.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrat F, et al. Time lines of infection and disease in human influenza: A review of volunteer challenge studies. Am J Epidemiol. 2008;167(7):775–785. doi: 10.1093/aje/kwm375. [DOI] [PubMed] [Google Scholar]
- 31.Kash JC, et al. Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature. 2006;443(7111):578–581. doi: 10.1038/nature05181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trinchieri G. Type I interferon: Friend or foe? J Exp Med. 2010;207(10):2053–2063. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Julkunen I, et al. Molecular pathogenesis of influenza A virus infection and virus-induced regulation of cytokine gene expression. Cytokine Growth Factor Rev. 2001;12(2-3):171–180. doi: 10.1016/s1359-6101(00)00026-5. [DOI] [PubMed] [Google Scholar]
- 34.McAuley JL, et al. Expression of the 1918 influenza A virus PB1-F2 enhances the pathogenesis of viral and secondary bacterial pneumonia. Cell Host Microbe. 2007;2(4):240–249. doi: 10.1016/j.chom.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mora R, Rodriguez-Boulan E, Palese P, García-Sastre A. Apical budding of a recombinant influenza A virus expressing a hemagglutinin protein with a basolateral localization signal. J Virol. 2002;76(7):3544–3553. doi: 10.1128/JVI.76.7.3544-3553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soares F, et al. NLRX1 does not inhibit MAVS-dependent antiviral signalling. Innate Immun. 2013;19(4):438–448. doi: 10.1177/1753425912467383. [DOI] [PubMed] [Google Scholar]
- 37.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392(6671):86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 38.Le Goffic R, et al. Transcriptomic analysis of host immune and cell death responses associated with the influenza A virus PB1-F2 protein. PLoS Pathog. 2011;7(8):e1002202. doi: 10.1371/journal.ppat.1002202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McAuley JL, et al. Activation of the NLRP3 inflammasome by IAV virulence protein PB1-F2 contributes to severe pathophysiology and disease. PLoS Pathog. 2013;9(5):e1003392. doi: 10.1371/journal.ppat.1003392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chakrabarti AK, Pasricha G. An insight into the PB1F2 protein and its multifunctional role in enhancing the pathogenicity of the influenza A viruses. Virology. 2013;440(2):97–104. doi: 10.1016/j.virol.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 41.Alymova IV, et al. Immunopathogenic and antibacterial effects of H3N2 influenza A virus PB1-F2 map to amino acid residues 62, 75, 79, and 82. J Virol. 2011;85(23):12324–12333. doi: 10.1128/JVI.05872-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conenello GM, Zamarin D, Perrone LA, Tumpey T, Palese P. A single mutation in the PB1-F2 of H5N1 (HK/97) and 1918 influenza A viruses contributes to increased virulence. PLoS Pathog. 2007;3(10):1414–1421. doi: 10.1371/journal.ppat.0030141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaush CR, Smith TF. Replication and plaque assay of influenza virus in an established line of canine kidney cells. Appl Microbiol. 1968;16(4):588–594. doi: 10.1128/am.16.4.588-594.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coulombe F, Fiola S, Akira S, Cormier Y, Gosselin J. Muramyl dipeptide induces NOD2-dependent Ly6C(high) monocyte recruitment to the lungs and protects against influenza virus infection. PLoS ONE. 2012;7(5):e36734. doi: 10.1371/journal.pone.0036734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Divangahi M, et al. Mycobacterium tuberculosis evades macrophage defenses by inhibiting plasma membrane repair. Nat Immunol. 2009;10(8):899–906. doi: 10.1038/ni.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Divangahi M, Desjardins D, Nunes-Alves C, Remold HG, Behar SM. Eicosanoid pathways regulate adaptive immunity to Mycobacterium tuberculosis. Nat Immunol. 2010;11(8):751–758. doi: 10.1038/ni.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.