Significance
The recruitment of host stromal cells, such as macrophages and mesenchymal stem cells (MSCs), to the primary tumor is a critical step toward cancer malignancy. We have identified signals that are exchanged between breast cancer cells (BCCs) and MSCs. This signaling increases the recruitment of both MSCs and macrophages to primary tumors and increases metastasis of BCCs to lymph nodes and lungs. Reduced oxygen levels (hypoxia) in breast cancers are associated with increased risk of metastasis and decreased patient survival. We show that hypoxia stimulates signaling between BCCs and MSCs due to the activity of hypoxia-inducible factors (HIFs). Drugs that block HIF activity prevent signaling and macrophage recruitment, which suggests that they may be useful additions to breast cancer therapy.
Keywords: HIF-1, mammary fat pad, orthotopic implantation, lymph node metastasis, lung metastasis
Abstract
Intratumoral hypoxia induces the recruitment of stromal cells, such as macrophages and mesenchymal stem cells (MSCs), which stimulate invasion and metastasis by breast cancer cells (BCCs). Production of macrophage colony-stimulating factor 1 (CSF1) by BCCs is required for macrophage recruitment, but the mechanisms underlying CSF1 expression have not been delineated. Triple-negative breast cancers have increased expression of genes regulated by hypoxia-inducible factors (HIFs). In this study, we delineate two feed-forward signaling loops between human MDA-MB-231 triple-negative BCCs and human MSCs that drive stromal cell recruitment to primary breast tumors. The first loop, in which BCCs secrete chemokine (C-X-C motif) ligand 16 (CXCL16) that binds to C-X-C chemokine receptor type 6 (CXCR6) on MSCs and MSCs secrete chemokine CXCL10 that binds to receptor CXCR3 on BCCs, drives recruitment of MSCs. The second loop, in which MSCs secrete chemokine (C-C motif) ligand 5 that binds to C-C chemokine receptor type 5 on BCCs and BCCs secrete cytokine CSF1 that binds to the CSF1 receptor on MSCs, drives recruitment of tumor-associated macrophages and myeloid-derived suppressor cells. These two signaling loops operate independent of each other, but both are dependent on the transcriptional activity of HIFs, with hypoxia serving as a pathophysiological signal that synergizes with chemokine signals from MSCs to trigger CSF1 gene transcription in triple-negative BCCs.
Breast cancer metastasis transforms a local disease that is cured by surgical excision into a systemic disease that responds poorly to available therapies and is the major cause of patient mortality (1). Although somatic mutations have been cataloged in hundreds of human breast cancers and many genes that promote or suppress metastasis have been identified, the analysis of genetic alterations cannot reliably distinguish metastatic from nonmetastatic cancers (1–3). Multiple stromal cell types, including mesenchymal stem cells (MSCs) and tumor-associated macrophages (TAMs), are recruited to the tumor microenvironment and promote metastasis (4, 5). In mouse models, MSCs produce chemokines, including chemokine (C-C motif) ligand 5 (CCL5) and chemokine (C-X-C motif) ligand 10 (CXCL10), which bind to their cognate receptors, chemokine receptor type 5 (CCR5) and C-X-C chemokine receptor type 3 (CXCR3), respectively, on breast cancer cells (BCCs) to stimulate invasion and metastasis (6–9). TAMs are abundant in breast cancer and outnumber the BCCs in some cases (10). The density of TAMs in primary breast cancer biopsies is correlated with metastasis and patient mortality (11–13). In mouse models, macrophage colony-stimulating factor 1 (CSF1) and the chemokine CCL2 are secreted by BCCs and bind to their cognate receptors, CSF1 receptor (CSF1R) and CCR2, on TAMs, leading to their recruitment to the tumor microenvironment, where they produce EGF and other secreted proteins that promote invasion and metastasis (14–19).
Intratumoral hypoxia is another major microenvironmental factor that is associated with invasion, metastasis, and patient mortality (20–22). Cancer cells respond to the hypoxic microenvironment through the activity of hypoxia-inducible factors (HIFs), which are heterodimeric transcription factors composed of an O2-regulated HIF-1α or HIF-2α subunit and a constitutively expressed HIF-1β subunit (23). In primary tumor biopsies, elevated HIF-1α or HIF-2α protein levels are associated with an increased risk of metastasis and mortality that is independent of breast cancer grade or stage (24–28). HIF-1α, HIF-2α, or both are required for the transcriptional activation of a battery of hypoxia-inducible genes whose protein products are required for discrete steps in the process of breast cancer invasion and metastasis (29–34). High expression of HIF target genes is commonly observed in triple-negative breast cancers (TNBCs), which lack estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 (HER2) expression and respond poorly to chemotherapy (2).
Both MSCs and TAMs are recruited to the hypoxic breast tumor microenvironment (9, 35), although the underlying mechanisms are not fully understood. In the present study, we hypothesized that the presence of MSCs in the primary breast tumor may facilitate TAM recruitment. Our studies of human MDA-MB-231 TNBC cells in immunodeficient mice revealed that HIFs regulate the hypoxia-induced expression of CXCL16 in BCCs, which was required for MSC recruitment. CCL5→CCR5 signaling between MSCs and BCCs was required for CSF1 expression by BCCs, which was also induced by hypoxia. Expression of CSF1 and CCR5 by BCCs was required for TAM recruitment and BCC metastasis. HIF-dependent recruitment of TAMs was also demonstrated after implantation of mouse 4T1 TNBC cells into the mammary fat pad (MFP) of immunocompetent mice. Taken together, these results delineate molecular mechanisms by which intratumoral hypoxia regulates the recruitment of MSCs and TAMs, and their interaction with TNBCs, to stimulate invasion and metastasis.
Results
CXCL16 Expression by BCCs Stimulates MSC Recruitment.
We previously demonstrated that hypoxia-induced expression of placental growth factor (PGF) by MDA-MB-231 BCCs provides a signal for the recruitment of MSCs to primary breast tumors and stimulates MSCs to express CXCL10, which binds to CXCR3 on BCCs (CXCL10MSC→CXCR3BCC) to stimulate invasion and metastasis (9). We also showed that CXCR3 expression by BCCs was required for CXCL10 expression by MSCs, but the responsible signal from BCCs to MSCs was not identified (9). CXCL16 expression by prostate cancer cells was shown to promote MSC recruitment (36), and CXCL16 expression by BCCs has been reported (37). We cocultured GFP-expressing human MDA-MB-231 BCCs with human MSCs at a 1:1 ratio for 48 h under 20% or 1% O2, and flow cytometry was performed using GFP and CD105 immunofluorescence to sort for BCCs and MSCs, respectively. RNA was isolated from the sorted cells and RT-quantitative real-time PCR (qPCR) revealed that CXCL16 expression by BCCs was induced following coculture and that hypoxia further enhanced expression (Fig. 1A). The expression of CXCR6, a receptor for CXCL16, was induced by hypoxia in BCCs, as previously reported (38), as well as in MSCs, but coculture did not enhance its expression (Fig. 1B).
Fig. 1.
CXCL16 expression by MDA-MB-231 BCCs is induced by hypoxia and is required for MSC recruitment. (A) GFP-expressing BCCs were cocultured with MSCs at 20% or 1% O2 for 48 h and then flow-sorted based on GFP fluorescence for BCCs and CD105 immunofluorescence for MSCs. CXCL16 mRNA levels were determined in flow-sorted BCCs and MSCs from cocultures and in BCCs or MSCs cultured alone. RNA levels were normalized to results for BCCs cultured alone at 20% O2. *P < 0.05 vs. 20%; ##P < 0.001 vs. BCCs alone. (B) BCCs, MSCs, and BCCs + MSCs were incubated at 20% or 1% O2 for 48 h, and CXCR6 mRNA levels were determined and normalized to BCCs at 20%. *P < 0.05 vs. 20%. (C) MDA-MB-231 subclones expressing shRNA against CXCR3 (shCR3-1 and shCR3-3) or an NTC shRNA were cultured alone or with MSCs and exposed to 20% or 1% O2 for 48 h. CXCL16 mRNA levels were normalized to NTC − MSCs at 20%. *P < 0.05; **P < 0.001 vs. 20%; #P < 0.05 vs. NTC; ##P < 0.001 vs. NTC. (D) BCCs + MSCs were treated with CXCL10 NAb or IgG and exposed to 20% or 1% O2. CXCL16 mRNA levels were normalized to IgG at 20%. **P < 0.001 vs. 20%; ##P < 0.001 vs. IgG. (E) MDA-MB-231 subclones expressing shRNA against CXCL16 (shCX16-1 and shCX16-3) or NTC shRNA were cultured alone or with MSCs and exposed to 20% or 1% O2. CXCL10 mRNA levels were normalized to NTC at 20%. *P < 0.05; **P < 0.001 vs. NTC − MSCs; ##P < 0.001 vs. NTC + MSCs. (F) Migration of MSCs in response to CM from BCC subclones cultured at 20% or 1% O2 was determined and normalized to NTC at 20%. *P < 0.01 vs. 20% NTC; #P < 0.01 vs. 1% NTC. (G) BCC subclones were implanted in the MFP, and MSC recruitment to the primary tumor was analyzed by qPCR for Y chromosome sequences and normalized to NTC. *P < 0.01 vs. NTC. (H) Bidirectional signaling between BCCs and MSCs generates a feed-forward loop that stimulates MSC recruitment.
We hypothesized that CXCL10MSC→CXCR3BCC signaling might stimulate CXCL16BCC→CXCR6MSC reciprocal signaling. To test this hypothesis, we analyzed BCC subclones stably transfected with shRNAs that inhibit CXCR3 expression (9). CXCL16 expression in CXCR3-deficient BCCs was not induced by coculture with MSCs or by hypoxia (Fig. 1C). We next added neutralizing antibody (NAb) against CXCL10 or control IgG to cocultures of MSCs and BCCs. CXCL16 mRNA expression was significantly decreased in the presence of CXCL10 NAb (Fig. 1D).
We also hypothesized that CXCL16BCC→CXCR6MSC signaling might stimulate CXCL10MSC→CXCR3BCC reciprocal signaling. To test this hypothesis, we generated BCC subclones that were stably transfected with shRNAs that inhibit CXCL16 expression (Fig. S1). CXCL10 expression in MSCs cocultured with CXCL16-deficient BCCs was significantly decreased and was not induced by hypoxia (Fig. 1E).
To investigate whether CXCL16 secretion from BCCs stimulated the motility of MSCs, we isolated conditioned medium (CM) from CXCL16-deficient BCCs or BCCs expressing a nontargeting control (NTC) shRNA. CM from CXCL16-deficient BCCs induced significantly less MSC migration in a Boyden chamber assay, and the augmented effect of CM from hypoxic control BCCs was not observed with CXCL16-deficient BCCs (Fig. 1F). To determine whether CXCL16 secretion from BCCs promotes recruitment of MSCs to the primary tumor, BCCs were orthotopically implanted in the MFP of female SCID mice. When the tumors reached a volume of 250 mm3, MSCs that were originally derived from a male donor were injected via the tail vein. Primary tumors were harvested 16 h later, and MSC recruitment was determined by qPCR assay of genomic DNA using Y chromosome-specific primers (9). CXCL16 deficiency in BCCs significantly decreased the recruitment of MSCs to the primary tumor (Fig. 1G). Taken together, these results delineate CXCL16BCC→CXCR6MSC and CXCL10MSC→CXCR3BCC reciprocal signaling, which creates a feed-forward loop between BCCs and MSCs that drives MSC recruitment to the primary tumor (Fig. 1H).
CXCL16 Promotes Metastasis of BCCs to Lymph Nodes and Lungs.
Because CXCL16BCC→CXCR6MSC signaling was required for CXCL10MSC→CXCR3BCC signaling, which stimulates invasion and metastasis (9), we hypothesized that CXCL16 expression was required for efficient metastasis. To test this hypothesis, control or CXCL16-deficient BCCs were orthotopically implanted in the MFP of female SCID mice. CXCL16 deficiency had no effect on primary tumor growth (Fig. 2A). However, mice bearing CXCL16-deficient tumors had significantly decreased numbers of circulating tumor cells (Fig. 2B), metastatic cancer cells in the lungs by qPCR (Fig. 2C) and metastatic foci in the lungs by histology (Fig. 2 D and E), and metastatic cancer cells in the ipsilateral axillary lymph node (Fig. 2 F and G). Taken together, these results demonstrate that CXCL16 expression by BCCs plays a significant role in promoting breast cancer metastasis.
Fig. 2.
CXCL16 promotes lung and lymph node metastasis of MDA-MB-231 BCCs. BCC subclones were implanted in the MFP of SCID mice. (A) Primary tumor volumes were determined serially. (B) Circulating tumor cells in peripheral blood were determined by qPCR using primers specific for human 18S rRNA and normalized to NTC. *P < 0.01 vs. NTC. (C) Lung DNA was analyzed by qPCR with primers specific for human HK2 sequences and normalized to NTC. *P < 0.01 vs. NTC. (D) Photomicrographs of H&E-stained lung sections. (Scale bars: 2 mm.) (E) Lung sections were scored for metastatic foci per field. **P < 0.001 vs. NTC. (F) Lymph node sections were stained with an antibody that specifically recognizes human vimentin. (Scale bars: 0.5 mm.) (G) Stained area was quantified by image analysis. **P < 0.001 vs. NTC.
HIF-Dependent BCC–MSC Interactions Promote TAM and Myeloid-Derived Suppressor Cell Recruitment.
To investigate whether interaction of MSCs with BCCs facilitates the recruitment of TAMs to primary breast tumors, we cocultured MSCs with BCCs for 48 h, followed by MFP implantation of 1 × 106 cocultured cells. Controls included mice implanted with 1 × 106 BCCs alone or BCCs mixed with MSCs immediately before implantation (Fig. 3A). When primary tumors reached 450 mm3, they were excised and single-cell suspensions were analyzed by flow cytometry for the presence of TAMs, which express CSF1R and F4/80 on their cell surface, and myeloid-derived suppressor cells (MDSCs; TAM progenitors), which express CD11b and Ly6C. CSF1R+F4/80+ and CD11b+Ly6C+ cells were increased in tumors derived from BCC + MSC coculture compared with tumors derived from BCCs alone or BCC + MSC coinjection (Fig. 3 B and C and Fig. S2 A and B).
Fig. 3.
Macrophage recruitment is dependent on HIF activity. (A) 1 × 106 MDA-MB-231 BCCs alone, 1 × 106 BCCs mixed with 1 × 106 MSCs immediately before injection, or 1 × 106 BCCs cocultured with 1 × 106 MSCs for 48 h were implanted in the MFP of SCID mice. The percentage of CSF1R+F4/80+ (B) and CD11b+Ly6C+ (C) cells in 450-mm3 primary tumors was determined by fluorescence-activated cell sorting (FACS). *P < 0.05; **P < 0.01 vs. BCCs. (D) MDA-MB-231 EV or DKD cells were implanted in the MFP of SCID mice. The percentage of CSF1R+F4/80+ (E) and CD11b+Ly6C+ (F) cells in 450-mm3 primary tumors was determined by FACS. **P < 0.01 vs. EV. (G) Mice bearing 200-mm3 MDA-MB-231 tumors were treated with saline or digoxin for 7 d. The percentage of CSF1R+F4/80+ (H) and CD11b+Ly6C+ (I) cells in primary tumors was determined. **P < 0.01 vs. saline.
Recent studies have shown that exposure of macrophages to levels of hypoxia that are comparable to what is observed in tumors leads to induction of HIF-1α and HIF-2α, which, in turn, activate a broad array of genes with proangiogenic, proinvasive, and prometastatic functions (39, 40). TAM numbers are generally higher in tumors containing high overall levels of hypoxia, as seen in primary human breast carcinomas (41) and various animal tumors (42). To investigate whether HIF activity in BCCs plays a role in TAM recruitment, we used the MDA-MB-231 double-knockdown (DKD) subclone, which is stably transfected with vectors encoding shRNAs that inhibit HIF-1α and HIF-2α, and the empty vector (EV) subclone (9). The BCC subclones were injected into the MFP of female SCID mice, and tumors were harvested for flow cytometric analysis when they reached a volume of 450 mm3 (Fig. 3D). The recruitment of TAMs and MDSCs to tumors derived from DKD cells was significantly decreased compared with EV tumors (Fig. 3 E and F and Fig. S2 C and D).
We next examined the effect of HIFs on macrophage recruitment by treating tumor-bearing mice with digoxin to inhibit HIF activity (30, 31). When tumors reached 200 mm3, mice received daily i.p. injections of either saline or digoxin (2 mg/kg) for 7 d, followed by tumor excision and flow cytometric analysis (Fig. 3G). Recruitment of TAMs and MDSCs was significantly decreased in primary tumors of digoxin-treated mice (Fig. 3 H and I and Fig. S2 E and F). Taken together, these results demonstrate that HIFs play an important role in recruiting TAMs and MDSCs to primary breast tumors.
Hypoxia and Coculture Induce HIF-Dependent CSF1 and CSF1R Expression.
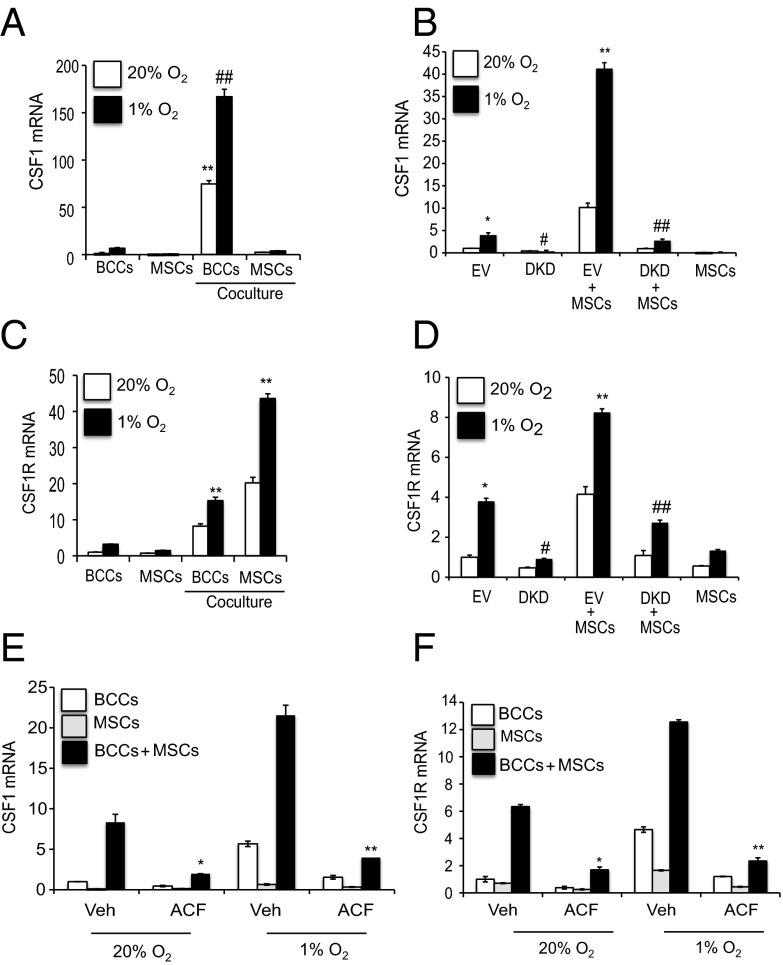
Previous studies demonstrated reciprocal paracrine interactions in which BCCs secrete CSF1 and sense EGF, whereas TAMs sense CSF1 and secrete EGF (16–18), but the trigger for CSF1 expression was not determined. CSF1 binds to its cognate receptor, CSF1R, which is expressed by TAMs; however, CSF1R is also expressed in >50% of breast tumors, which suggests that CSF1 mediates both autocrine and paracrine signaling (14–18). Coculture of GFP-expressing human MDA-MB-231 BCCs with human MSCs and flow sorting of GFP+ BCCs and CD105+ MSCs revealed that CSF1 mRNA expression was induced in BCCs (Fig. 4A), whereas CSF1R mRNA expression was induced in both MSCs and BCCs (Fig. 4C). Expression of both CSF1 and CSF1R was augmented in BCCs subjected to hypoxia (Fig. 4 A and C).
Fig. 4.
HIFs mediate coculture- and hypoxia-induced expression of CSF1 and CSF1R. (A and C) GFP-expressing MDA-MB-231 BCCs were cultured individually or cocultured with MSCs at 20% or 1% O2 for 48 h, and GFP+ BCCs and CD105+ MSCs were then sorted by FACS. CSF1 (A) and CSF1R (C) mRNA levels were determined and normalized to BCCs at 20% O2. **P < 0.001 vs. 20% BCCs; ##P < 0.001 vs. 1% BCCs. (B and D) EV BCCs, DKD BCCs, MSCs, EV + MSCs or DKD + MSCs were cultured at 20% or 1% O2 for 48 h. CSF1 (B) and CSF1R (D) mRNA levels were determined and normalized to EV at 20%. *P < 0.05 vs. 20% EV; **P < 0.001 vs. 1% EV; #P < 0.01 vs. 1% EV; ##P < 0.001 vs. 1% EV + MSCs. (E and F) BCCs, MSCs, or BCCs + MSCs were treated with 1 μM acriflavine (ACF) or DMSO vehicle (Veh) and exposed to 20% or 1% O2 for 48 h. CSF1 (E) and CSF1R (F) mRNA levels were normalized to BCC Veh at 20%. *P < 0.05 vs. Veh; **P < 0.001 vs. Veh.
To investigate the role of HIFs in these phenomena, MSCs were cocultured with the DKD or EV subclone of BCCs. Expression of CSF1 and CSF1R mRNA was significantly decreased in DKD + MSC, compared with EV + MSC, cocultures at both 20% and 1% O2 (Fig. 4 B and D). Levels of secreted CSF1 protein were also significantly decreased in CM isolated from DKD + MSC, compared with EV + MSC, cocultures (Fig. S3A). Pharmacological inhibition of HIF activity using acriflavine, which blocks the dimerization of HIF-1α or HIF-2α with HIF-1β (43), inhibited coculture- and hypoxia-induced CSF1 and CSF1R mRNA expression (Fig. 4 E and F). Taken together, these results indicate that cross-talk between BCCs and MSCs is mediated through HIF-dependent CSF1→CSF1R signaling.
CSF1 Promotes Macrophage Recruitment and Metastasis.
We generated MDA-MB-231 subclones that were stably transfected with vector encoding either of two different shRNAs targeting CSF1. Efficient knockdown of CSF1 mRNA expression and protein secretion was confirmed by RT-qPCR and ELISA, respectively (Fig. S3 B and C). Coculture-induced CSF1 secretion was also abrogated (Fig. S3C). CSF1 deficiency had no effect on primary tumor growth after MFP implantation (Fig. 5A). Mice bearing tumors derived from CSF1-deficient BCCs had significantly decreased numbers of circulating tumor cells (Fig. 5B), metastatic cancer cells (Fig. 5C) and metastatic foci (Fig. 5 D and E) in the lungs, metastatic cancer cells in the ipsilateral axillary lymph node (Fig. 5 F and G), and CSF1R+F4/80+ TAMs (Fig. 5H) and CD11b+Ly6C+ MDSCs (Fig. 5I) recruited to the primary tumor. These data demonstrate that CSF1 expression in BCCs plays a significant role in promoting macrophage recruitment and metastasis to both lymph nodes and lungs.
Fig. 5.
CSF1 promotes metastasis of MDA-MB-231 BCCs. MDA-MB-231 subclones expressing shRNA against CSF1 (shCSF1-1 and shCSF1-5) or NTC shRNA were implanted in the MFP of female SCID mice. (A) Primary tumor volumes were determined serially. (B) Circulating tumor cells were determined by qPCR and normalized to NTC. *P < 0.01 vs. NTC. (C) Metastatic BCCs in the lungs were determined by qPCR and normalized to NTC. **P < 0.001 vs. NTC. (D) Photomicrographs of H&E-stained lung sections. (Scale bars: 0.5 mm.) (E) Lung sections were scored for metastatic foci per field. **P < 0.001 vs. NTC. (F) Lymph node sections were stained with human-specific vimentin. (Scale bars: 0.5 mm.) (G) Stained lymph node (LN) area was quantified by image analysis. **P < 0.001 vs. NTC. (H and I) The percentage of CSF1R+F4/80+ (H) and CD11b+Ly6C+ (I) cells in primary tumors was determined by FACS. *P < 0.01 vs. NTC; **P < 0.001 vs. NTC.
CCL5→CCR5 Signaling Between MSCs and BCCs Is Required for CSF1 Expression.
Previous studies showed that CCL5→CCR5 signaling stimulates breast cancer metastasis (6) and that coculture and hypoxia induce the expression of CCL5 in MSCs and its cognate receptor CCR5 in BCCs (9, 44). However, neither the upstream trigger nor the downstream effector of CCL5→CCR5 signaling was delineated. To investigate whether CCL5→CCR5 signaling regulates CSF1 expression, CCL5 NAb was added to cocultures of MSCs and BCCs. Expression of both CCL5 (Fig. 6A) and CCR5 (Fig. 6B) mRNA was significantly decreased in the presence of CCL5 NAb. Blocking CCL5→CCR5 signaling also significantly decreased CSF1 mRNA levels (Fig. 6C). Next, we generated MDA-MB-231 subclones stably transfected with vector encoding either of two different shRNAs, which inhibited expression of CCR5 mRNA (Fig. S4A) and cell surface protein (Fig. S4B). CCR5 deficiency in BCCs blocked the induction of CSF1 mRNA expression by coculture or hypoxia (Fig. 6D). Remarkably, CSF1 deficiency in BCCs abrogated hypoxia- and coculture-induced CCL5 mRNA expression by MSCs (Fig. 6E). Taken together, these results demonstrate that CSF1 expression by BCCs is regulated by CCL5MSC→CCR5BCC signaling and that CCL5 expression by MSCs is regulated by CSF1BCC→CSF1RMSC signaling, indicating the existence of a second feed-forward loop, which, in this case, promotes the recruitment of TAMs and MDSCs to primary breast tumors (Fig. 6F).
Fig. 6.
CCL5-CCR5 signaling between MSCs and BCCs is required for CSF1 expression. (A–C) MDA-MB-231 BCCs + MSCs were treated with CCL5 NAb or IgG and exposed to 20% or 1% O2 for 48 h. CCL5 (A), CCR5 (B), and CSF1 (C) mRNA levels were normalized to 20% IgG. *P < 0.01 vs. 20% IgG; **P < 0.001 vs. 20% IgG; #P < 0.01 vs. 1% IgG; ##P < 0.001 vs. 1% IgG. (D) MDA-MB-231 subclones expressing NTC shRNA or shRNA against CCR5 (shCCR5-4 and shCCR5-5) were cultured alone or with MSCs and exposed to 20% or 1% O2 for 48 h. CSF1 mRNA levels were normalized to 20% NTC. *P < 0.05 vs. 20% NTC; **P < 0.001 vs. 20% NTC; #P < 0.05 vs. 1% NTC; ##P < 0.001 vs. 1% NTC + MSCs. (E) MDA-MB-231 subclones expressing NTC shRNA or shRNA against CSF1 (shCSF1-1 and shCSF1-5) were cultured alone or with MSCs and exposed to 20% or 1% O2 for 48 h. CCL5 mRNA levels were normalized to 20% NTC. **P < 0.001 vs. 20% NTC + MSCs; ##P < 0.001 vs. 1% NTC + MSCs. (F) Bidirectional signaling between BCCs and MSCs generates a feed-forward loop that stimulates tumor-associated macrophage (TAM) recruitment.
CCR5 Promotes Macrophage Recruitment and Metastasis.
To investigate the role of CCR5 in breast cancer pathogenesis further, CCR5-deficient BCCs were implanted in the MFP of female SCID mice. CCR5 deficiency had no effect on primary tumor growth (Fig. 7A). However, mice bearing CCR5-deficient tumors showed significantly decreased numbers of circulating tumor cells (Fig. 7B), metastatic cancer cells (Fig. 7C) and metastatic foci (Fig. 7 D and E) in the lungs, metastatic cancer cells in lymph nodes (Fig. 7 F and G), and TAMs (Fig. 7H) and MDSCs (Fig. 7I) recruited to the primary tumor. These results indicate that CCR5 expression by BCCs promotes macrophage recruitment and metastasis.
Fig. 7.
CCR5 promotes macrophage recruitment and breast cancer metastasis. MDA-MB-231 subclones were implanted in the MFP of SCID mice. (A) Primary tumor volumes were determined serially. (B) Circulating tumor cells were determined by qPCR and normalized to NTC. *P < 0.05 vs. NTC. (C) Metastatic BCCs in the lungs were determined by qPCR. **P < 0.001 vs. NTC. (D) Photomicrographs of H&E-stained lung sections. (Scale bars: 0.5 mm.) (E) Lung sections were scored for metastatic foci per field. **P < 0.001 vs. NTC. (F and G) Lymph node sections were stained with human-specific vimentin antibody (F), and staining was quantified by image analysis (G). **P < 0.001 vs. NTC. (Scale bars: F, 0.5 mm.) The percentage of CSF1R+F4/80+ (H) and CD11b+Ly6C+ (I) cells in primary tumors was determined by FACS. *P < 0.01 vs. NTC.
CSF1 and CCR5 Are HIF Target Genes.
Analysis of the human CSF1 gene sequence revealed two candidate HIF binding sites located 0.6 kb (site 1) and 2.5 kb (site 2) 5′ to the transcription start site (Fig. 8A). To determine whether HIFs bind to these sites, ChIP assays were performed with MDA-MB-231 BCCs, which demonstrated hypoxia-induced binding of HIF-1α (Fig. 8B), HIF-1β (Fig. 8C), and HIF-2α (Fig. 8D) to site 1 and hypoxia-induced binding of HIF-1α (Fig. 8E) and HIF-1β (Fig. 8F), but not HIF-2α (Fig. 8G), to site 2.
Fig. 8.
CSF1 and CCR5 are HIF target genes. (A) Candidate HIF binding sites were identified in the 5′-flanking region of the human CSF1 gene. (B–G) MDA-MB-231 cells were incubated at 20% or 1% O2 for 16 h, and ChIP assays were performed using IgG or antibody against HIF-1α, HIF1β, or HIF-2α. Primers flanking site 1 and site 2 were used for qPCR, and values were normalized to IgG 20%. *P < 0.05 vs. all other conditions, Student's t test on log-converted values. (H) Candidate HIF binding sites were identified in the 5′-flanking region (site 1) and 3′-untranslated region (site 2) of the human CCR5 gene. (I–N) MDA-231 cells were incubated at 20% or 1% O2 for 16 h, and ChIP assays were performed using IgG or antibodies against HIF-1α, HIF-1β, or HIF-2α. Primers flanking site 1 or site 2 were used for qPCR, and values were normalized to IgG 20%. *P < 0.05 vs. all other conditions; **P < 0.001.
Analysis of the human CCR5 gene sequence revealed candidate HIF binding sites in the 5′-flanking and 3′-untranslated regions, 1,370 bp 5′ (site 1) and 8,065 bp 3′ (site 2) of the transcription start site, respectively (Fig. 8H). ChIP assays revealed hypoxia-induced binding of HIF-2α and HIF-1β, but not HIF-1α, to site 1 (Fig. 8 I–K) and hypoxia-induced binding of HIF-1α and HIF-1β, but not HIF-2α, to site 2 (Fig. 8 L–N). Taken together, the ChIP data demonstrate that the human CSF1 and CCR5 genes are directly regulated by both HIF-1 and HIF-2.
CCL5 and CSF1 Stimulate Macrophage Migration.
To study macrophage migration directly, we isolated bone marrow cells from BALB/c mice and incubated them in the presence of CSF1 to stimulate macrophage (BM-Mϕ) differentiation, with CSF1R+F4/80+ cells representing 80% of the final population (Fig. 9A). CM from BCCs + MSCs cocultured at 20% O2 stimulated BM-Mϕ migration, and the effect was augmented when CM from hypoxic cocultures was used (Fig. 9B). The stimulatory effect of CM from cocultures was abrogated when CCL5 NAb was added to the CM (Fig. 9C) or when CSF1-deficient BCCs were used (Fig. 9D). These results indicate that coculture- and hypoxia-induced CCL5 and CSF1 expression stimulate BM-Mϕ migration.
Fig. 9.
Coculture- and hypoxia-induced CCL5 and CSF1 expression is required for migration of bone marrow-derived macrophages (BM-Mϕ). (A) Bone marrow cells were isolated from the BALB/c mice and differentiated into macrophages (BM-Mϕ) by supplementing the culture media with CSF1. The efficiency of differentiation was determined by FACS analysis of CSF1R and F4/80 surface antigen expression, using antibodies conjugated to phycoerythrin (PE-A) or allophycocyanin (APC-A). (B) BM-Mϕ were seeded in the upper compartment of a Boyden chamber, and the number of cells that migrated through the filter in response to CM from nonhypoxic (20% O2) or hypoxic (1% O2) MDA-MB-231 cells (BCCs), MSCs, or BCCs + MSCs in the lower compartment were counted under light microscopy after staining with crystal violet. (Scale bars: 0.2 mm.) Data were normalized to CM from BCCs at 20% O2. *P < 0.05 vs. 20% BCCs; **P < 0.01 vs. 20% BCCs; #P < 0.01 vs. 20% BCCs + MSCs. (C) BM-Mϕ migration in response to CM isolated from BCCs or BCCs + MSCs (supplemented with IgG or CCL5 NAb) was determined and normalized to CM from BCCs at 20% O2. (Scale bars: 0.2 mm.) *P < 0.05 vs. 20% BCCs; **P < 0.01 vs. 20% BCCs; #P < 0.05 vs. 20% IgG; ##P < 0.001 vs. 1% IgG. (D) BM-Mϕ migration in response to CM isolated from NTC or shCSF1-1 BCCs (alone or cocultured with MSCs) was determined and normalized to CM from NTC cells at 20% O2. (Scale bars: 0.2 mm.) *P < 0.05 vs. 20% NTC − MSCs; **P < 0.01 vs. 20% NTC − MSCs; #P < 0.05 vs. 1% NTC - MSCs; ##P < 0.001 vs. 1% NTC + MSCs.
HIFs Are Required for Macrophage Recruitment and Metastasis in a Syngeneic Mouse Mammary Carcinoma Model.
To investigate the role of HIFs in macrophage recruitment in an immunocompetent model of TNBC, we used 4T1 mouse mammary carcinoma cells, which form primary tumors and metastases similar to human TNBC after implantation into the MFP of syngeneic BALB/c mice (45). We generated 4T1 subclones that were stably transfected with vectors encoding NTC shRNA or shRNA(s) that inhibited the expression of HIF-1α (sh1α), HIF-2α (sh2α), or both (DKD) (Fig. S5A). After orthotopic implantation in the MFP of female BALB/c mice, the growth of primary tumors derived from sh1α or DKD cells was significantly decreased compared with tumors derived from sh2α or NTC cells (Fig. 10 A and B). The numbers of metastatic nodules in lungs (Fig. 10C) and metastatic foci in axillary lymph nodes (Fig. 10D) harvested from mice implanted with sh1α, sh2α, or DKD cells were significantly decreased compared those from mice implanted with NTC cells. Recruitment of TAMs (Fig. 10E and Fig. S5B) and MDSCs (Fig. 10F and Fig. S5C) was significantly decreased in primary tumors derived from sh1α and DKD cells compared with tumors derived from sh2α or NTC cells. Taken together, these data indicate that HIFs are required for primary tumor growth, macrophage recruitment, and metastasis of TNBCs in immunocompetent mice.
Fig. 10.
HIFs are required for macrophage recruitment and metastasis in the immunocompetent 4T1 mouse mammary carcinoma model of TNBC. (A) 4T1 subclones expressing NTC shRNA or shRNA against HIF-1α (sh1α), HIF-2α (sh2α), or both HIF-1α and HIF-2α (DKD) were implanted in the MFP of female syngeneic BALB/c mice. Primary tumor volumes were determined serially. *P < 0.05 vs. NTC or sh2α. (B) Tumor weights were measured at the end of the experiment. **P < 0.01 vs. NTC. (C) Whole-mount inflated lungs were stained with India blue dye. The number of metastatic nodules (arrows) was counted. *P < 0.05 vs. NTC; **P < 0.01 vs. NTC. (D) Lymph node sections were stained with H&E. (Scale bars: 2 mm.) The number of metastatic foci (arrows) was counted. *P < 0.05 vs. NTC; **P < 0.01 vs. NTC. (E and F) The percentage of CSF1R+F4/80+ (E) and CD11b+Ly6C+ (F) cells in primary tumors was determined by FACS. *P < 0.05 vs. NTC; **P < 0.01 vs. NTC.
Breast Cancer Gene Expression Data Suggest Signaling Pathways Are Clinically Relevant.
To determine whether the two bidirectional signaling loops that were delineated in BCC-MSC cocultures are clinically relevant, we analyzed microarray gene expression data obtained from 530 primary human breast cancers, 63 samples of adjacent normal breast tissue, and 6 metastases (2). We demonstrated that CXCR3 expression (and activation) in BCCs was required for the induction of CXCL16 expression in BCCs in response to coculture with MSCs and exposure to hypoxia (Fig. 1), and Pearson’s test revealed that CXCR3 and CXCL16 mRNA levels were highly correlated (P = 10−13) in the clinical specimens. Similarly, we demonstrated that CCR5 expression (and activation) in BCCs was required for the induction of CSF1 expression in BCCs in response to coculture and hypoxia (Fig. 6), and Pearson’s test revealed that CCR5 and CSF1 mRNA levels were highly correlated (P = 10−16) in the clinical specimens.
Discussion
In this study, we have delineated two HIF-regulated feed-forward signaling loops between BCCs and MSCs that drive stromal cell recruitment to primary breast tumors (Fig. 11). The first loop, involving CXCL16BCC→CXCR6MSC and CXCL10MSC→CXCR3BCC signaling arms, with each arm stimulating the activity of the other, drives recruitment of MSCs. The second loop, involving CCL5MSC→CCR5BCC and CSF1BCC→CSF1RMSC signaling arms, with each arm stimulating the activity of the other, drives recruitment of TAMs and MDSCs. These two pathways operate independent of each other, but both are dependent on HIF activity, with hypoxia serving as a physiological signal that synergizes with chemokine signals from MSCs to trigger CSF1 and CCR5 gene transcription in BCCs.
Fig. 11.
Signaling pathways involved in MSC and MDSC/TAM recruitment that promote BCC invasion and metastasis are shown. Intercellular signaling and intracellular signaling are indicated by wide colored arrows and thin black arrows, respectively. The blue symbols represent five HIF-regulated gene products in BCCs. CSF1→CSF1R→EGF→EGF receptor (BCC-TAM) signaling (14–16), CCL5→CCR5 (MSC-BCC) signaling (6), and PGF→VEGFR1→CXCL10→CXCR3 (BCC-MSC) signaling (9) were described previously. Data from the current study have delineated a feed-forward loop involving CXCL10, CXCR3, CXCL16, and CXCR6 that drives recruitment of MSCs and a second feed-forward loop involving CCL5, CCR5, CSF1, and CSF1R that drives recruitment of MDSCs and TAMs.
The recruitment of MSCs promotes cancer progression and metastasis (6–9). In a previous study, we showed that PGF secretion by BCCs facilitates the recruitment of MSCs to breast tumors and that PGF binds to VEGF receptor 1 (VEGFR1) on MSCs to stimulate CXCL10 expression (9). In the present study, we have demonstrated that in addition to PGF, CXCL16 secretion by hypoxic BCCs recruits MSCs to primary breast tumors. Furthermore, whereas PGFBCC→VEGFR1MSC signaling is unidirectional, we show that CXCL16BCC→CXCR6MSC signaling is part of a bidirectional feed-forward loop that activates CXCR3 signaling in BCCs (Fig. 11), which is proinvasive and prometastatic (9). As a result, CXCL16 deficiency markedly impaired lymph node and lung metastasis of BCCs.
Elegant studies have demonstrated that CSF1 expression by BCCs promotes macrophage recruitment and metastasis in mouse models of breast cancer (15–18). Elevated serum levels of CSF1 predict lymph node involvement in women with early- stage breast cancer and decreased overall survival in postmenopausal patients with breast cancer (46). However, the mechanisms by which CSF1 expression is induced in BCCs had not been delineated. Similarly, CCL5MSC→CCR5BCC signaling was linked to invasion and metastasis (6, 47), but the upstream trigger and downstream effector had not been delineated. We have now unified these observations by showing that CCL5MSC→CCR5BCC signaling induces CSF1 expression in BCCs, which serves to recruit CSF1R+ TAMs, as well as feeding back to stimulate CCL5 expression by MSCs (Fig. 6H).
HIF activity in cancer cells plays critical roles in the production of angiogenic growth factors and the mobilization of bone marrow-derived angiogenic cells, which are blocked by treating tumor-bearing mice with HIF inhibitors (43). Treatment of mice bearing primary breast tumors with acriflavine or digoxin potently inhibits metastatic niche formation and blocks lung and lymph node metastasis (30, 31, 48). Hypoxia induces HIF-dependent expression of genes in BCCs that activate cell motility (34), ECM remodeling (32, 33), lymphangiogenesis, and lymph node metastasis (31), as well as extravasation and lung metastasis (30). The present study now identifies recruitment of MSCs, TAMs, and MDSCs as additional components of the metastatic process that are stimulated by intratumoral hypoxia in an HIF-dependent manner. Finally, both human MDA-MB-231 and mouse 4T1 cells are preclinical models for the 15% of human breast cancers, designated as TNBC, that do not express progesterone, estrogen, or HER2 receptors and manifest the basal/claudin-low gene expression pattern. Further studies are required to determine whether these same HIF-driven intercellular signaling mechanisms are also activated by hypoxia in estrogen/progesterone receptor-positive and HER2+ breast cancers.
Targeted therapies are not available for TNBCs, which are treated with cytotoxic chemotherapy to which <20% of patients show a durable response. The basal/claudin-low gene expression pattern is characterized by increased expression of HIF target genes (2). Our data show that two HIF inhibitors, acriflavine and digoxin, blocked signaling and recruitment of TAMs and MDSCs, suggesting that addition of HIF inhibitors to existing therapeutic regimens may improve the clinical outcome in patients with TNBC.
Materials and Methods
Cell Lines and Culture.
Mycoplasma-free and molecularly authenticated human MDA-MB-231 BCCs were maintained in high-glucose (4.5 mg/mL) DMEM with 10% FBS and 1% penicillin/streptomycin. The 4T1 mouse mammary carcinoma cells (American Type Culture Collection) were maintained in RPMI-1640 with 10% FBS and 1% penicillin/streptomycin. Human bone marrow-derived MSCs (49) were obtained from the Tulane Center for Gene Therapy. MSCs were maintained in α-MEM supplemented with 20% FBS and 1% penicillin/streptomycin. Cells were maintained at 37 °C in a 5% CO2 and 95% air incubator (20% O2). Hypoxic cells were maintained at 37 °C in a modular incubator chamber (Billups–Rothenberg) flushed with a gas mixture containing 1% O2, 5% CO2, and 94% N2. For coculture experiments, equal numbers of MSCs and BCCs were seeded in a 1:1 ratio of DMEM/10% FBS and α-MEM/20% FBS.
Transduction with shRNA Vectors.
The pLKO.1-puro lentiviral vectors encoding shRNA targeting human CSF1 (clone ID: NM_000757), human CCR5 (clone ID: NM_000579), mouse HIF-1α (clone ID: NM_010431), and mouse HIF-2α (clone ID: NM_010137) were purchased from Sigma–Aldrich. The recombinant vectors were cotransfected with plasmid pCMV-dR8.91 and plasmid encoding vesicular stomatitis virus G protein into 293T cells using FuGENE 6 (Roche Applied Science). Viral supernatant was collected 48 h posttransfection, filtered (0.45-μm pore size), and added to MDA-MB-231 cells in the presence of 8 μg/mL polybrene (Sigma–Aldrich). Puromycin (0.5 μg/mL) was added to the medium of cells transduced with pLKO.1-puro vectors for selection.
RT-qPCR.
Total RNA was extracted from cells using TRIzol (Invitrogen) and treated with DNase I (Ambion). One microgram of total RNA was used for first-strand DNA synthesis with the iScript cDNA Synthesis system (BioRad). qPCR was performed using human-specific primers and SYBR Green qPCR Master Mix (Fermentas). For each primer pair, the annealing temperature was optimized by gradient PCR. The expression of each target mRNA relative to 18S rRNA was calculated based on the threshold cycle (Ct) as 2−Δ(ΔCt), where ΔCt = Cttarget − Ct18S and Δ(ΔCt) = ΔCttest − ΔCtcontrol (22). Primer sequences are provided in Table S1.
Animal Studies.
Female 5- to 7-wk-old SCID (National Cancer Institute) or BALB/c (Charles River Laboratories) mice were studied according to protocols approved by the Johns Hopkins University Animal Care and Use Committee that were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (50). Digoxin and saline for injection were obtained from the research pharmacy of the Johns Hopkins Hospital. BCCs were harvested by trypsinization, washed twice in PBS, counted, and suspended at 107 cells/mL in a 1:1 solution of PBS and Matrigel (Corning). Mice were anesthetized, and 2 × 106 cells were injected into the MFP. Primary tumors were measured in three dimensions (a, b, and c), and volume (V) was calculated as V = abc × 0.52. Primary tumors and the ipsilateral axillary lymph node were harvested (31). Lungs were perfused with PBS, and one lung was inflated for formalin fixation, paraffin embedding, and staining with H&E; the other lung was used to isolate genomic DNA for qPCR to quantify human HK2 and mouse 18S rRNA gene sequences, as previously described (30).
Circulating Tumor Cell Assay.
Total RNA isolated from 0.5 mL of whole blood was subjected to qPCR using primers specific for human 18S rRNA (31).
MSC Recruitment Assay.
2 × 106 MDA-MB-231 cells were implanted in the MFP of female SCID mice. When the tumor reached a volume of 450 mm3, 5 × 105 MSCs (derived from a male donor) were injected i.v. and the primary tumor was harvested after 16 h. MSCs recruited to the primary tumor were determined by qPCR analysis of SRY copy number (9).
Flow Cytometry.
Tumor tissue was minced and digested with 1 mg/mL type 1 collagenase (Sigma) at 37 °C for 30 min. Digested tissues were filtered through 70-μm cell strainers. Cells were incubated with Fc Block (BD Pharmingen). To identify TAMs, cells were stained with peridinin chlorophyll protein-conjugated CSF1R antibody (BD Biosciences) and allophycocyanin-conjugated F4/80 antibody (Novus Biologicals), and subjected to flow cytometry. To identify MDSCs, cells were stained with allophycocyanin-conjugated CD11b antibody (BD Biosciences) and peridinin chlorophyll protein-conjugated Ly6C antibody (BD Biosciences), and subjected to flow cytometry. Unstained control and single-stained cells were prepared in every experiment for gating. Dead cells were gated out by side-scatter and forward-scatter analysis.
Isolation of Mouse Bone Marrow-Derived Macrophages.
Bone marrow cells were isolated and cultured for 10 d in RPMI-1640 supplemented with 10% FBS, 1% penicillin/streptomycin, and 100 ng/mL CSF1 (R&D Systems) according to standard protocols (51, 52).
Migration Assays.
Cells were seeded onto an uncoated filter in the upper compartment of a 24-well Boyden chamber (8-mm pore size; Costar) and allowed to migrate for 8 h in response to CM in the lower compartment. The cells that migrated to the underside of the filter were stained with crystal violet and counted under bright-field microscopy.
India Ink Staining of Lungs.
Mice were euthanized, and India ink (15%) was injected into the lungs through the trachea. The lungs were fixed in Feketet’s solution (100 mL of 70% alcohol, 10 mL of formalin, and 5 mL of glacial acetic acid) at room temperature. After de-staining, metastatic nodules appear white on a black background (53).
Analysis of Lymph Node Metastasis.
Immunohistochemical analyses were performed on formalin-fixed, paraffin-embedded lymph node sections as previously described (9). Staining was performed using an antibody that specifically recognizes human vimentin (Santa Cruz Biotechnology) and analyzed by ImageJ software (National Institutes of Health). The acquired images in red-green-blue (RGB) color were separated into different color channels by a color deconvolution method (54).
ChIP Assays.
MDA-MB-231 cells were cross-linked with formaldehyde and lysed with SDS lysis buffer. Chromatin was sheared by sonication, and lysates were precleared with salmon sperm DNA/protein A-agarose slurry (Millipore) and incubated with antibody against HIF-1α (Santa Cruz Biotechnology), HIF-1β (Novus Biologicals), or HIF-2α (Novus Biologicals), or with IgG (Santa Cruz Biotechnology or Novus Biologicals) as previously described (9).
Statistical Analysis.
All data are presented as mean ± SEM (n = 3). Differences between groups were analyzed by one-way ANOVA, unless otherwise stated, before normalization of data. Level 3 gene expression data from 596 patients in the Breast Invasive Carcinoma dataset (2) were obtained from The Cancer Genome Atlas Data Portal (http://tcga-data.nci.nih.gov/tcga/tcgaHome2.jsp). GraphPad Prism software was used to calculate Pearson’s correlation coefficients and determine P values for coexpression.
Supplementary Material
Acknowledgments
We thank Karen Padgett of Novus Biologicals, Inc. for providing IgG and antibodies against F4/80, HIF-1β, and HIF-2α. We thank Darwin Prockop for providing human MSCs from the Tulane Center for Gene Therapy, which was supported by Grant P40-RR017447 from the National Institutes of Health. G.L.S. is an American Cancer Society Research Professor and the C. Michael Armstrong Professor at Johns Hopkins University School of Medicine. This work was supported by funds from the American Cancer Society and National Cancer Institute. D.M.G. was supported by a postdoctoral fellowship from the Susan G. Komen Foundation, and N.T. was supported by the Japan Science and Technology Agency.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1406655111/-/DCSupplemental.
References
- 1.Marino N, et al. Breast cancer metastasis: Issues for the personalization of its prevention and treatment. Am J Pathol. 2013;183(4):1084–1095. doi: 10.1016/j.ajpath.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohl CR, Harihar S, Denning WL, Sharma R, Welch DR. Metastasis suppressors in breast cancers: Mechanistic insights and clinical potential. J Mol Med (Berl) 2014;92(1):13–30. doi: 10.1007/s00109-013-1109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9(4):239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanahan D, Coussens LM. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 6.Karnoub AE, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 7.Mi Z, et al. Osteopontin promotes CCL5-mesenchymal stromal cell-mediated breast cancer metastasis. Carcinogenesis. 2011;32(4):477–487. doi: 10.1093/carcin/bgr009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Haibi CP, et al. Critical role for lysyl oxidase in mesenchymal stem cell-driven breast cancer malignancy. Proc Natl Acad Sci USA. 2012;109(43):17460–17465. doi: 10.1073/pnas.1206653109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaturvedi P, et al. Hypoxia-inducible factor-dependent breast cancer-mesenchymal stem cell bidirectional signaling promotes metastasis. J Clin Invest. 2013;123(1):189–205. doi: 10.1172/JCI64993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly PMA, Davison RS, Bliss E, McGee JO. Macrophages in human breast disease: A quantitative immunohistochemical study. Br J Cancer. 1988;57(2):174–177. doi: 10.1038/bjc.1988.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leek RD, Landers RJ, Harris AL, Lewis CE. Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast. Br J Cancer. 1999;79(5-6):991–995. doi: 10.1038/sj.bjc.6690158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsutsui S, et al. Macrophage infiltration and its prognostic implications in breast cancer: The relationship with VEGF expression and microvessel density. Oncol Rep. 2005;14(2):425–431. [PubMed] [Google Scholar]
- 13.Medrek C, Pontén F, Jirström K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306. doi: 10.1186/1471-2407-12-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Sullivan C, Lewis CE, Harris AL, McGee JO. Secretion of epidermal growth factor by macrophages associated with breast carcinoma. Lancet. 1993;342(8864):148–149. doi: 10.1016/0140-6736(93)91348-p. [DOI] [PubMed] [Google Scholar]
- 15.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193(6):727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wyckoff J, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64(19):7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 17.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 18.Goswami S, et al. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;65(12):5278–5283. doi: 10.1158/0008-5472.CAN-04-1853. [DOI] [PubMed] [Google Scholar]
- 19.Qian BZ, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris AL. Hypoxia—A key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2(1):38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 21.Vaupel P, Mayer A, Höckel M. Tumor hypoxia and malignant progression. Methods Enzymol. 2004;381:335–354. doi: 10.1016/S0076-6879(04)81023-1. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan R, Graham CH. Hypoxia-driven selection of the metastatic phenotype. Cancer Metastasis Rev. 2007;26(2):319–331. doi: 10.1007/s10555-007-9062-2. [DOI] [PubMed] [Google Scholar]
- 23.Semenza GL. Hypoxia-inducible factors: Mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33(4):207–214. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schindl M, et al. Austrian Breast and Colorectal Cancer Study Group Overexpression of hypoxia-inducible factor 1α is associated with an unfavorable prognosis in lymph node-positive breast cancer. Clin Cancer Res. 2002;8(6):1831–1837. [PubMed] [Google Scholar]
- 25.Bos R, et al. Levels of hypoxia-inducible factor-1α independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer. 2003;97(6):1573–1581. doi: 10.1002/cncr.11246. [DOI] [PubMed] [Google Scholar]
- 26.Dales JP, et al. Overexpression of hypoxia-inducible factor HIF-1α predicts early relapse in breast cancer: Retrospective study in a series of 745 patients. Int J Cancer. 2005;116(5):734–739. doi: 10.1002/ijc.20984. [DOI] [PubMed] [Google Scholar]
- 27.Generali D, et al. Hypoxia-inducible factor-1α expression predicts a poor response to primary chemoendocrine therapy and disease-free survival in primary human breast cancer. Clin Cancer Res. 2006;12(15):4562–4568. doi: 10.1158/1078-0432.CCR-05-2690. [DOI] [PubMed] [Google Scholar]
- 28.Helczynska K, et al. Hypoxia-inducible factor-2α correlates to distant recurrence and poor outcome in invasive breast cancer. Cancer Res. 2008;68(22):9212–9220. doi: 10.1158/0008-5472.CAN-08-1135. [DOI] [PubMed] [Google Scholar]
- 29.Wong CC, et al. Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proc Natl Acad Sci USA. 2011;108(39):16369–16374. doi: 10.1073/pnas.1113483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang H, et al. HIF-1-dependent expression of angiopoietin-like 4 and L1CAM mediates vascular metastasis of hypoxic breast cancer cells to the lungs. Oncogene. 2012;31(14):1757–1770. doi: 10.1038/onc.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Schito L, et al. Hypoxia-inducible factor 1-dependent expression of platelet-derived growth factor B promotes lymphatic metastasis of hypoxic breast cancer cells. Proc Natl Acad Sci USA. 2012;109(40):E2707–E2716. doi: 10.1073/pnas.1214019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilkes DM, et al. Collagen prolyl hydroxylases are essential for breast cancer metastasis. Cancer Res. 2013;73(11):3285–3296. doi: 10.1158/0008-5472.CAN-12-3963. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Gilkes DM, et al. Procollagen lysyl hydroxylase 2 is essential for hypoxia-induced breast cancer metastasis. Mol Cancer Res. 2013;11(5):456–466. doi: 10.1158/1541-7786.MCR-12-0629. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Gilkes DM, et al. Hypoxia-inducible factors mediate coordinated RhoA-ROCK1 expression and signaling in breast cancer cells. Proc Natl Acad Sci USA. 2014;111(3):E384–E393. doi: 10.1073/pnas.1321510111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104(8):2224–2234. doi: 10.1182/blood-2004-03-1109. [DOI] [PubMed] [Google Scholar]
- 36.Jung Y, et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat Commun. 2013;4:1795. doi: 10.1038/ncomms2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsumura S, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181(5):3099–3107. doi: 10.4049/jimmunol.181.5.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin S, et al. Chemokine C-X-C motif receptor 6 contributes to cell migration during hypoxia. Cancer Lett. 2009;279(1):108–117. doi: 10.1016/j.canlet.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 39.Lewis C, Murdoch C. Macrophage responses to hypoxia: Implications for tumor progression and anti-cancer therapies. Am J Pathol. 2005;167(3):627–635. doi: 10.1016/S0002-9440(10)62038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murdoch C, Lewis CE. Macrophage migration and gene expression in response to tumor hypoxia. Int J Cancer. 2005;117(5):701–708. doi: 10.1002/ijc.21422. [DOI] [PubMed] [Google Scholar]
- 41.Leek RD, Harris AL. Tumor-associated macrophages in breast cancer. J Mammary Gland Biol Neoplasia. 2002;7(2):177–189. doi: 10.1023/a:1020304003704. [DOI] [PubMed] [Google Scholar]
- 42.De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013;23(3):277–286. doi: 10.1016/j.ccr.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 43.Lee K, et al. Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proc Natl Acad Sci USA. 2009;106(42):17910–17915. doi: 10.1073/pnas.0909353106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Lin S, et al. Chemokine C-C motif receptor 5 and C-C motif ligand 5 promote cancer cell migration under hypoxia. Cancer Sci. 2012;103(5):904–912. doi: 10.1111/j.1349-7006.2012.02259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52(6):1399–1405. [PubMed] [Google Scholar]
- 46.Aharinejad S, et al. Elevated CSF1 serum concentration predicts poor overall survival in women with early breast cancer. Endocr Relat Cancer. 2013;20(6):777–783. doi: 10.1530/ERC-13-0198. [DOI] [PubMed] [Google Scholar]
- 47.Velasco-Velázquez M, et al. CCR5 antagonist blocks metastasis of basal breast cancer cells. Cancer Res. 2012;72(15):3839–3850. doi: 10.1158/0008-5472.CAN-11-3917. [DOI] [PubMed] [Google Scholar]
- 48.Wong CC, et al. Inhibitors of hypoxia-inducible factor 1 block breast cancer metastatic niche formation and lung metastasis. J Mol Med (Berl) 2012;90(7):803–815. doi: 10.1007/s00109-011-0855-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci USA. 2000;97(7):3213–3218. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Committee on Care and Use of Laboratory Animals . Guide for the Care and Use of Laboratory Animals. Bethesda: Natl Inst Health; 1985. DHHS Publ. No. (NIH) 85-23. [Google Scholar]
- 51.Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol. 2008;Chapter 14:Unit 14. 1. doi: 10.1002/0471142735.im1401s83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weischenfeldt J, Porse B. Bone Marrow-Derived Macrophages (BMM): Isolation and Applications. CSH Protoc. 2008;2008:pdb prot5080. doi: 10.1101/pdb.prot5080. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, et al. Integrin subunits α5 and α6 regulate cell cycle by modulating the chk1 and Rb/E2F pathways to affect breast cancer metastasis. Mol Cancer. 2011;10:84. doi: 10.1186/1476-4598-10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol. 2001;23(4):291–299. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.