Significance
Receptor-interacting protein-1 (RIP1) kinase and caspase-8 are important players in activation of apoptotic pathways. Here we show that RIP1, caspase-8, and RIP3 contribute to infection-induced macrophage cell death and also are required for activation of transcription factor NF-κB and caspase-1 upon infection with the bacterial pathogen Yersinia pestis, the causative agent of plague. Mice lacking caspase-8 and RIP3 are also very susceptible to bacterial infection. This suggests that RIP1, caspase-8, and RIP3 are key molecules with multiple roles in innate immunity during bacterial challenge.
Abstract
A number of pathogens cause host cell death upon infection, and Yersinia pestis, infamous for its role in large pandemics such as the “Black Death” in medieval Europe, induces considerable cytotoxicity. The rapid killing of macrophages induced by Y. pestis, dependent upon type III secretion system effector Yersinia outer protein J (YopJ), is minimally affected by the absence of caspase-1, caspase-11, Fas ligand, and TNF. Caspase-8 is known to mediate apoptotic death in response to infection with several viruses and to regulate programmed necrosis (necroptosis), but its role in bacterially induced cell death is poorly understood. Here we provide genetic evidence for a receptor-interacting protein (RIP) kinase–caspase-8-dependent macrophage apoptotic death pathway after infection with Y. pestis, influenced by Toll-like receptor 4-TIR-domain-containing adapter-inducing interferon-β (TLR4-TRIF). Interestingly, macrophages lacking either RIP1, or caspase-8 and RIP3, also had reduced infection-induced production of IL-1β, IL-18, TNF, and IL-6; impaired activation of the transcription factor NF-κB; and greatly compromised caspase-1 processing. Cleavage of the proform of caspase-1 is associated with triggering inflammasome activity, which leads to the maturation of IL-1β and IL-18, cytokines important to host responses against Y. pestis and many other infectious agents. Our results identify a RIP1–caspase-8/RIP3-dependent caspase-1 activation pathway after Y. pestis challenge. Mice defective in caspase-8 and RIP3 were also highly susceptible to infection and displayed reduced proinflammatory cytokines and myeloid cell death. We propose that caspase-8 and the RIP kinases are key regulators of macrophage cell death, NF-κB and inflammasome activation, and host resistance after Y. pestis infection.
The causative agent of plague, Yersinia pestis is well known to cause significant cell death upon infection (1–3). Like the activation of inflammatory pathways to produce cytokines, triggering cell death pathways is a common response of the mammalian immune system to infection. Death of immune cells can eliminate the replication niche of pathogens found within those cells, thus inhibiting the proliferation of the pathogens and exposing them to bactericidal mechanisms (4). Conversely, elimination of key immune cells can diminish the ability of those cells to respond to infection. Multiple host and microbial factors control cell death pathways (5). Caspase-8–dependent apoptosis, receptor interacting protein-1 (RIP1)- and RIP3-dependent necroptosis, and caspase-1/caspase-11–dependent pyroptosis constitute major modes of regulated cell death during infection (5, 6). Several viruses seem to induce caspase-8–dependent apoptosis (7). Caspase-8 has also been suggested to have additional functions, such as inhibiting necroptosis (7–9) and modulation of NF-κB activation in T and B cells (10). Signaling to the transcription factor NF-κB controls the transcription of cytokines such as IL-6, TNF, pro-IL-1β, and pro-IL-18, and stimulates cell survival. Y. pestis can induce cell death in macrophages and dendritic cells via the type III secretion system (T3SS) effector Yersinia outer protein J (YopJ; YopP in Yersinia enterocolitica), although it is unclear whether this is entirely by apoptosis (11, 12). All human-pathogenic Yersiniae (Y. pestis, Yersinia pseudotuberculosis, and Y. enterocolitica) harbor cytotoxic properties toward host cells, and YopJ production is associated with cell death in vivo and in vitro (13–16). YopJ-mediated inhibition of NF-κB by acetylation of Inhibitor of κB Kinase β (IKKβ), MAP kinase kinases, and TAK1 may modulate macrophage death via effects on inflammatory and prosurvival signals (2, 17–21). Inflammasome activation, culminating in the activation and processing of caspase-1, leads to the production of IL-18 and IL-1β, key inflammatory cytokines and antibacterial defenses, but can also be associated with caspase-1–dependent pyroptotic cell death (22). YopJ also participates in inflammasome activation (16, 23), leading to a host immune response. Thus, this single bacterial effector may induce both protective and harmful effects for the host. In the present study we investigated the mechanisms for Y. pestis-induced cell death, NF-κB activation, and triggering of inflammasome activation.
Results and Discussion
Yersinia Induces Cell Death via RIP1, Caspase-8, and RIP3.
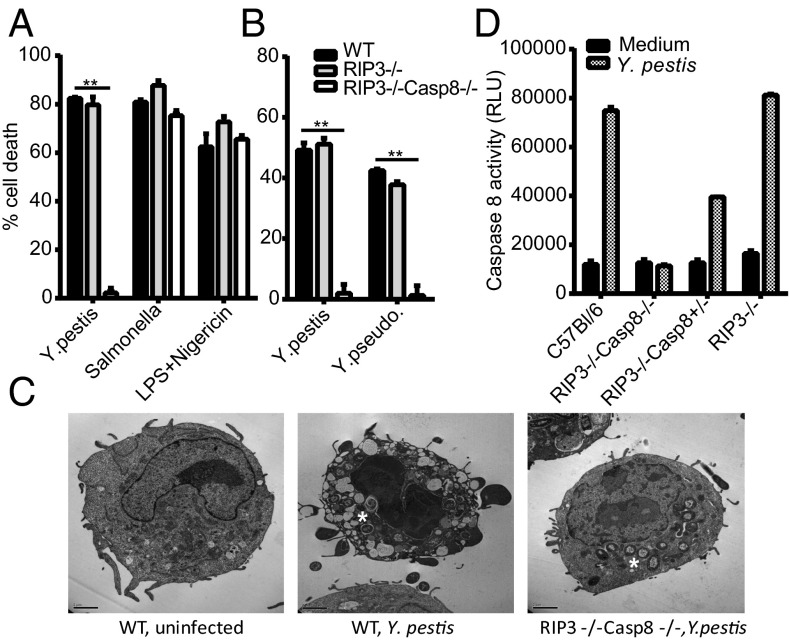
Viable Y. pestis KIM5 can induce rapid cell death via YopJ (Fig. S1A). Rapid death in bone marrow-derived macrophages (BMDMs) is induced in a YopJ-dependent manner by Y. pestis or Y. pseudotuberculosis temperature-shifted from 26 °C to 37 °C (Fig. S1 A and B), a condition that mimics the temperature change associated with infection via a fleabite. In addition to arming the T3SS, the temperature shift ensures the initial presence of some TLR4-stimulatory LPS (Fig. S1C) (24). Although caspase-1 is activated by Y. pestis (1, 25), macrophage death was independent of caspase-1/caspase-11, suggesting nonpyroptotic cytotoxicity (Fig. S2 A and B). Death was unaffected by Fas ligand (FasL) or TNF, indicating that those death receptor-mediated mechanisms are not involved; and independent of inflammasome-related NOD-like receptors (NLRs), the RNA-dependent protein kinase (PKR), the inflammasome adaptor apoptosis-associated speck-like protein containing a CARD (ASC), IL-1β, and IL-18 (Fig. S2 C–G) (1). Caspase-8 is a key enzyme in cell death induced by some viruses (26). Caspase-8 deficiency results in embryonic lethality, but mice deficient in both caspase-8 and RIP3 [RIP3−/− caspase-8−/− mice, double knockout (dKO)] are rescued. These data indicate a vital role for caspase-8 in suppressing necroptosis by targeting a component of the RIP3 pathway (8, 9). Macrophages from RIP3−/−caspase-8−/− mice were remarkably resistant to cell death induced by Y. pestis and Y. pseudotuberculosis, but not by Salmonella, which induces pyroptotic death (4), or with the NLRP3 inflammasome-specific trigger nigericin (Fig. 1 A and B). YopJ-induced death is likely not necroptosis because RIP3-deficient cells are not protected (Fig. 1 A and B). Electron microscopy revealed that macrophages infected with Y. pestis displayed features consistent with apoptotic death, such as membrane blebbing and nuclear condensation and fragmentation. These effects were absent in visibly infected dKO cells (Fig. 1C). Moreover, infection of macrophages with Y. pestis led to DNA fragmentation patterns typically associated with apoptosis, and this was blocked by zVAD pan-caspase inhibition (Fig. S3A). Taken together, our data strongly suggest that Yersinia induces rapid macrophage death by apoptosis via caspase-8.
Fig. 1.
Caspase-8–RIP3-deficient macrophages are protected against Y. pestis induced cytotoxicity. (A and B) Caspase-8−/− RIP3−/− (dKO), but not RIP3 KO BMDM, are protected from Yersinia-induced cytotoxicity measured by LDH release assay or (C) electron microscopy. (Scale bars, 2 µm.) Asterisks in C indicate bacteria. (D) Caspase-8 activity induced by Y. pestis infection (MOI 40, 2 h) in WT, RIP3−/− and dKO BMDMs. BMDM were infected with 10–40 MOI of Yersiniae or 1.5 MOI of Salmonella typhimurium for 4 h (A and B) or 2 h (C and D), and gentamycin was added after 1 h. Figures are representative for three to eight experiments performed. Bars indicate mean plus SD. **P < 0.01 (two-tailed t test).
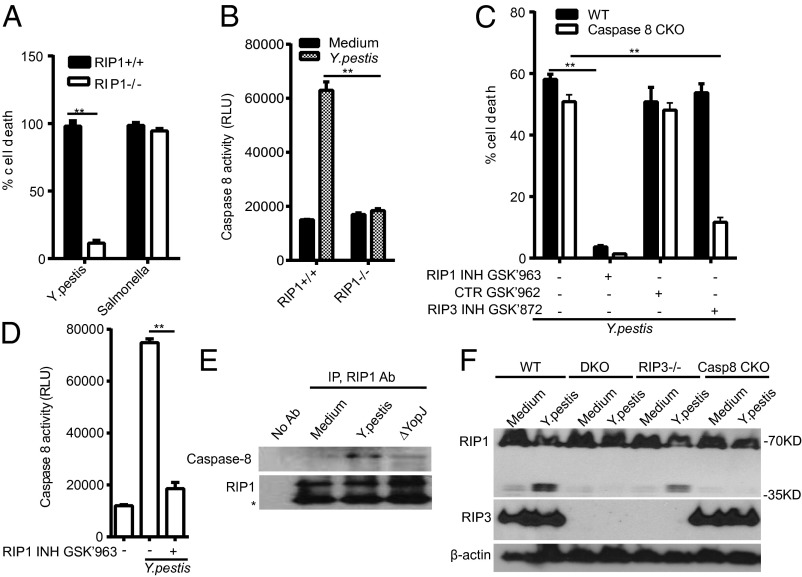
RIP3-mediated necroptotic death requires RIP1 (27–29), a serine/threonine kinase that can also contribute to NF-κB signaling (30) and apoptosis. RIP1−/− mice die shortly after birth (31), but fetal liver macrophages from RIP1−/− mice, in contrast to RIP3−/− macrophages, displayed a rescue from death induced by Y. pestis (Fig. 2A) and DNA laddering (Fig. S3C), suggesting that RIP1 activity contributes to apoptotic cell death upon infection, likely mediated by the induction of caspase-8 enzymatic activity and cleavage of procaspase-8 that precedes cell death (Figs. 1D, and 2 A, B, E, and F and Fig. S3 D and E). RIP1−/− macrophages were also protected from necroptotic cell death induced by heat-killed KIM5 plus zVAD or LPS plus zVAD (Fig. S3F). Potent and specific inhibitors of RIP3 (32) or RIP1 [GlaxoSmithKline (GSK): P.A.H., J.B., P.J.G.] kinase activity have recently been identified. The RIP1 inhibitor GSK’963, but not inactive enantiomer GSK’962, blocks Y. pestis-induced cell death (Fig. 2C and Fig. S3B) and caspase-8 activity (Fig. 2D). In addition to the genetic and pharmacological interactions between RIP1 and caspase-8, we found that RIP1 biochemically interacted with caspase-8 after Y. pestis challenge (Fig. 2E). Cell death, cleavage of procaspase-8, and enzymatic activity were partially reduced in the absence of TLR4 and TRIF, but not MyD88 (Fig. S4). Reduced death was also seen for bacteria grown at 37 °C and Y. pestis-EcLpxL, which constitutively generates a hexa-acylated LPS (Fig. S5 A and B). TLR4 signaling seems to enhance early caspase-8–mediated effects by Y. pestis YopJ, similar to those proposed for Y. enterocolitica YopP (33–35). Cell death induced by Y. pestis grown at 37 °C was inhibited by the presence of CaF1 capsule protein (Fig. S5 C and D), suggesting that the capsule prevented close contact between bacteria and host cells needed for T3SS effects.
Fig. 2.
RIP1 inhibition or deficiency protect macrophages from Y. pestis-induced cell death. (A) RIP1-deficient fetal liver macrophages are resistant to Y. pestis-induced killing (MOI 40, 4 h), detected by LDH release. (B and D) RIP1, but not RIP3, mediates caspase-8 enzymatic activity after infection of BMDM (D) or fetal liver macrophages (B) with Y. pestis for 2 h. (C) Caspase-8 conditional KO macrophages are protected from Y. pestis-induced death in the presence of RIP1 (GSK’963) or RIP3 (GSK’872) kinase inhibitors, but not by inactive compound GSK’962. (D) RIP1 kinase inhibitor GSK’963 inhibits caspase-8 enzyme activity after infection. (E) RIP1 forms a complex with caspase-8 upon infection (1 h), measured by co-IP. (F) RIP1 is cleaved after Y. pestis infection in a caspase-8 dependent fashion. Figures are representative for three to eight experiments performed. Bars indicate mean plus SD. **P < 0.01 (two-tailed t test).
The targeted deletion of caspase-8 in myeloid cells [conditional KO (cKO) caspase-8fl/fl LysM cre+/+ generated by D.M.S.; Fig. S6 A and B] had little effect on Y. pestis-induced macrophage death (Fig. 2C). Although the generation of other mice with defects in caspase-8 in macrophages has been reported (36), our caspase-8 cKO BMDM appeared healthy in culture and did not display increased cell death in the presence or absence of infection (Fig. 2C). Blockade of RIP3 kinase activity with GSK’872 strongly reduced macrophage death in the absence of caspase-8, suggesting that deletion of caspase-8, or caspase inhibition by zVAD (Fig. 2C and Fig. S6C), may promote necroptosis by RIP3, presumably influenced by reduced Y. pestis-induced cleavage of RIP1 in the absence of caspase-8 (Fig. 2F).
Cleavage and activation of the downstream apoptotic executioner caspase-3 was also dependent upon YopJ and caspase-8–RIP3 (Fig. S6D). The caspase-8–RIP3 pathway also influenced death induced by Y. enterocolitica but not by Salmonella or Pseudomonas, which also harbor a T3SS (Fig. S6E). Thus, all human-pathogenic Yersiniae, but not all bacteria containing a T3SS, trigger cell death via the same pathway. Our results provide an explanation for how Yersinia induces macrophage cell death via caspase-8 and RIP kinases. In this model, caspase-8–dependent apoptosis represents the default, whereas caspase-8 absence may lead to RIP3-dependent necroptosis. RIP1 has a key upstream role for both modes of death, perhaps influenced by its ability to direct apoptosis under conditions of cIAP1 depletion (37) as seen with Y. pestis (Fig. S6F).
Effects on NF-κB Activity.
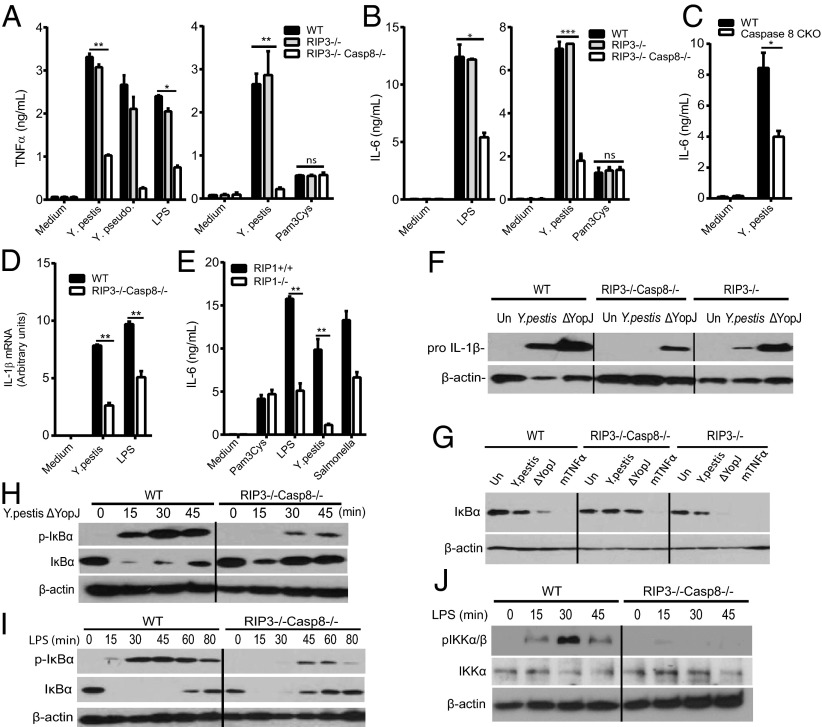
Caspase-8 has also been suggested to regulate NF-κB activity (10, 38, 39). We found a reduction in TNF and IL-6 release, and pro-IL-1β expression, all controlled by NF-κB, in RIP1 KO, caspase-8 cKO, and RIP3−/−caspase-8−/−, but not in RIP3−/− macrophages upon infection or LPS treatment (Fig. 3 A–F). However, cytokine production by the TLR2 ligand Pam3Cys (Fig. 3 A and B) or Sendai virus (Fig. S7A) was largely preserved. The defect in cytokine release could be explained by a decreased NF-κB activation, as suggested by reduced IκBα degradation, IκBα phosphorylation, IKKα/β phosphorylation, and p65 nuclear translocation, particularly at later time points during Y. pestis or LPS challenge (Fig. 3 G–J and Fig. S7 B and D). Reduced signaling could also be observed in RIP1−/− macrophages (Fig. 3E and Fig. S7E). How caspase-8 controls NF-κB activation is unclear and may not involve the enzymatic activity of caspase-8 (39) (Fig. S7C); However, TRIF-mediated pathways may be targeted because MyD88-dependent TLR2 signaling is not affected.
Fig. 3.
Caspase-8 and RIP1 contribute to cytokine release and NF-κB activation. (A–C) WT or mutant BMDM were infected with Y. pestis, Y. pseudotuberculosis (MOI 10), or Salmonella (Sal, MOI 1.5) or treated with LPS (100 ng/mL) or Pam3Cys (500 ng/mL) for 6 h, and cytokine release was measured by ELISA. (D) BMDMs were infected with Y. pestis for 4 h, mRNA was isolated, and quantitative PCR for pro-IL-1β was performed. (E) WT or RIP1−/− fetal liver macrophages were stimulated with LPS (50 ng/mL), Pam3Cys (500 ng/mL), Y. pestis (MOI 10), or Salmonella (MOI 1.5) for 6 h. IL-6 release was measured by ELISA. (F) BMDMs were infected for 6 h and cell lysates probed for pro-IL-1β. (G–J) BMDMs were infected or treated with LPS, mouse TNF-α (10 min), and cell lysates were probed by immunoblot for the indicated proteins (IκBα, phospho-IκBα, phospho-IKKα/β, or β-actin). Figures are representative of two to five experiments performed. Bars indicate means plus SD. **P < 0.01, *P < 0.05 (two-tailed t test).
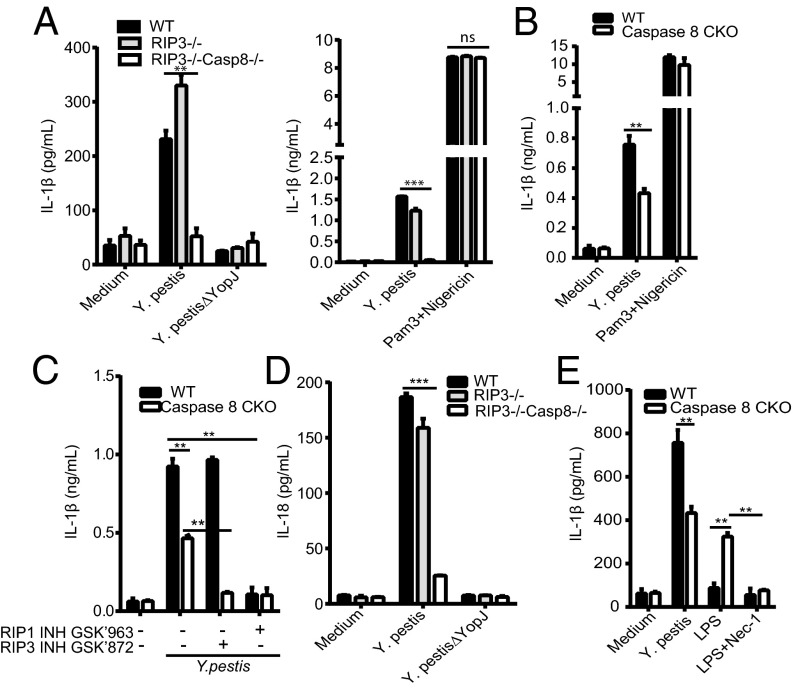
Subsequent experiments indicated that YopJ-dependent Y. pestis-induced IL-1β or IL-18 release was reduced in the absence of caspase-8 and showed further reduction by the absence or blockade of RIP3 or RIP1 kinase activity (Fig. 4 A–E). Cytokine release after stimulation with Pam3Cys and nigericin was unaffected (Fig. 4 A and B), implying that NLRP3 activation was not decreased. Although the absence of caspase-8 alone in macrophages decreased IL-1β release induced by infection, it increased IL-1β induced by LPS alone (Fig. 4E), as suggested for dendritic cells (40). Thus, more complex stimulations, as observed during infection, yield a different result than a purified ligand, possibly reflecting combined effects induced by both LPS and the Yersinia T3SS in the context of live bacteria.
Fig. 4.
Y. pestis-induced release of IL-1β and IL-18 is severely reduced in caspase-8/RIP3-deficient macrophages. (A–E) BMDMs were infected with Y. pestis or Y. pestis ΔYopJ for 6 h as indicated in Fig. 1, or stimulated with nigericin (10 µg/mL) for 1 h after priming with Pam3Cys (4 h, 500 ng/mL). IL-1β and IL-18 were analyzed by ELISA. (C) Some BMDMs were treated with RIP1 inhibitor GSK’963 (1 µM) or RIP3 inhibitor GSK’872 (10 µM) for 1 h before infection. (E) BMDMs were challenged with Y. pestis (MOI 10) for 6 h or LPS (50 ng/mL) for 10 h with or without Nec-1 pretreatment (20 µM). Figures are representative of three to five experiments. Bars indicate means plus SD. **P < 0.01, *P < 0.05 (two-tailed t test in A, B, and D, and two-way ANOVA with Tukey’s posttest in C and E).
RIP1, Caspase-8, and RIP3 Mediate Inflammasome Activation.
Our previous data (Fig. 3) could partially explain reduced IL-1β release. However, caspase-8 or RIP1 inhibition minimized infection-induced caspase-1 cleavage (Fig. S7 F and G), indicating direct effects on inflammasome action. RIP3 has been involved in inflammasome activation under certain conditions with cIAP inhibiton (41). Infection-induced caspase-1 processing, only partially dependent upon NLRP12 (25), was not affected in RIP3−/− cells but was reduced in TLR4 or TRIF KO (Fig. S8 A and B) and caspase-8 cKO cells, and severely reduced in RIP1−/− or RIP3−/−caspase-8−/− cells after Y. pestis infection (Fig. 5 A, B, and D). IL-1β processing was also affected (Fig. 5 C and D). Caspase-1 cleavage induced by Salmonella and Pam3Cys plus nigericin was not affected (Fig. 5 A and B), indicating that NLRC4- and NLRP3-mediated caspase-1 cleavage is not inherently reduced in RIP1−/− or dKO cells. Caspase-8 has been proposed to control IL-1β maturation and release in response to FasL stimulation or fungal and bacterial challenge (42, 43), perhaps by directly cleaving pro-IL-1β (44), and we cannot exclude this possibility. However, we propose that caspase-8 directs caspase-1 processing and activation, in a RIP3-enhanced manner, after Y. pestis challenge (Fig. 5 B and D), but caspase-1 does not control caspase-8 activation (Fig. S8C). Caspase-8 may be a critical component, but deletion or inhibition of RIP3 may block an alternative pathway in the absence of caspase-8, redundancy between caspase-8 and RIP3 may occur, or both molecules may be needed for stabilization of a signaling complex. The mechanism we describe seems independent of FasL, TNF, or type I IFN (Fig. S8 D–F) and may have some common features with responses induced by ER stress, certain chemotherapeutic drugs, or Citrobacter (45–47). The effect on caspase-1 cleavage may be mediated by the inflammasome adaptor ASC (Fig. 5E), because ASC can associate with caspase-8 after Francisella or Salmonella infection (38, 48), although the role of ASC may differ depending upon conditions and source of YopJ (16, 23, 25).
Fig. 5.
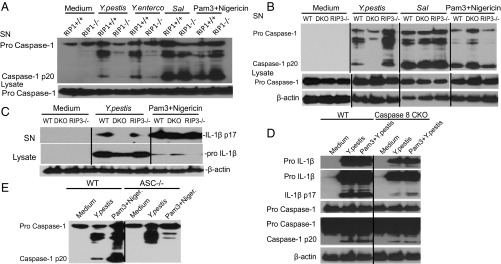
RIP kinases and caspase-8 control caspase-1 cleavage induced by Y. pestis. (A–E) BMDM (WT, RIP3−/−, RIP3−/−caspase-8−/− dKO, caspase-8 cKO) or fetal liver macrophages (RIP1+/+, RIP1−/−) were infected with Y. pestis, Y. enterocolitica, or Salmonella (Sal) for 6 h or primed with Pam3Cys followed by nigericin for 1 h, and supernatants (SN) or lysates were analyzed for caspase-1 or IL-1β processing by immunoblots. Figures are representative of three to five experiments.
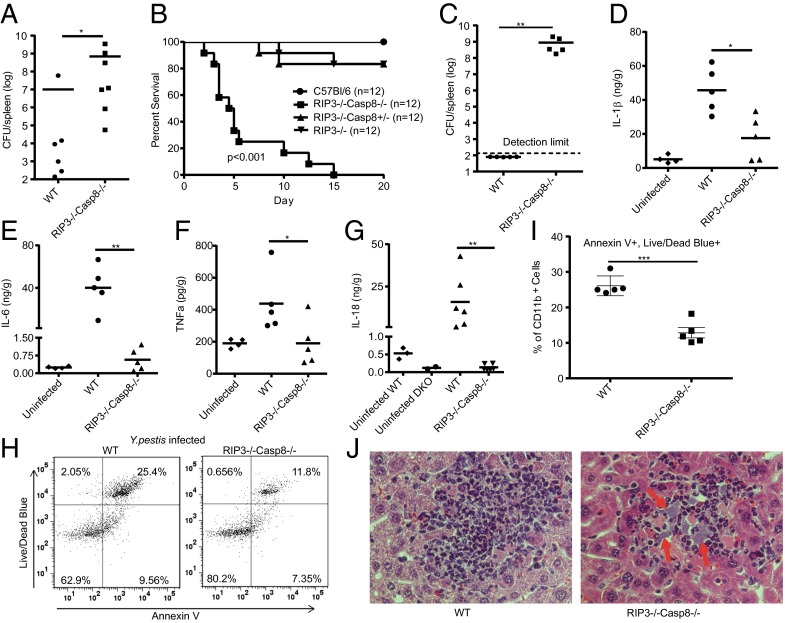
Role of Caspase-8 and RIP3 for in Vivo Resistance to Bacterial Infection.
The in vivo relevance of our findings was emphasized by the fact that RIP3−/−caspase-8−/− mice were more susceptible to s.c. infection with virulent Y. pestis KIM1001 (Fig. 6A). Because LD50 is very low for KIM1001 we used the attenuated strain KIM1001-EcLpxL, which constitutively generates a TLR4-activating hexa-acylated LPS (24), for survival analysis. dKO, or lethally irradiated WT mice with bone marrow transplant (BMT) from dKO, succumbed to s.c. infection with Y. pestis-EcLpxL (Fig. 6B and Fig. S9A). Resistance to Y. pestis-LpxL is heavily influenced by IL-18 and IL-1 (25). Moribund mice had large numbers of bacteria in their spleens compared with WT controls, suggesting that death occurred from uncontrolled systemic bacterial replication (Fig. 6C). This correlated with depressed IL-18, IL-1β, TNF, and IL-6 cytokine levels and reduced myeloid cell death (cells positive for live/dead stain and annexin V) in spleens (Fig. 6 D–I and Fig. S9 B–G) after i.v. infection. Reduced ability to suppress bacterial growth was also suggested by the presence of visible bacteria-containing pockets in inflammatory foci in the livers (Fig. 6J) of dKO BMT mice upon i.v. infection. Because irradiated mice that received RIP3−/−caspase-8−/− BMT behaved similarly as dKO animals, we propose that protection toward infection is mediated by cells originating from the bone marrow, expressing caspase-8 and RIP3. Some questions still remain with respect to certain details of how caspase-8 and RIP3 are involved in caspase-1 processing, although it is possible that ASC has a central role. Our results provide a basis for increased understanding of how bacterial pathogens, via their T3SS, can interact with several aspects of host innate immunity via RIP kinases and caspase-8. The data also show how apoptosis, generally viewed as a “silent” cell death, can be accompanied by strong inflammatory reactions, via pathways with several common players. The host may have developed these pathways as an effective means of alerting cells to the infection. We propose that caspase-8 and RIP kinases are central regulators of cell death and innate immune responses to Y. pestis, and we establish a role for these components in antibacterial innate immune responses. Therapies that modulate the activity of these pathways may be useful in the treatment of bacterial infections.
Fig. 6.
Caspase-8 with RIP3 is critical for in vivo resistance to bacterial infection. RIP3−/−caspase-8−/− dKO or WT mice were infected s.c. with virulent Y. pestis KIM1001 (300 cfu) for 68 h and spleens analyzed for bacterial growth (A). Lethally irradiated mice, subjected to bone marrow transplantation (BMT) from the indicated genotypes (B and C), were infected s.c. with 500 cfu of Y. pestis KIM1001-EcLpxL and monitored for survival (B), P < 0.001 dKO vs. WT (log−rank test). Spleens from moribund dKO BMT mice and controls were analyzed for bacterial contents (C). (D–J) Mice from BMT as above (D–F, H–J) or regular dKO (G) were infected i.v. with KIM1001-EcLpxL (500 cfu) for 42 h. Spleens were homogenized and analyzed for cytokines by ELISA (as cytokine/g tissue) (D–G). (H and I) CD11b-positive myeloid cells in spleens were analyzed for cell death with live/dead blue and annexin V stain. (J) Liver sections were stained with hematoxylin and eosin and subjected to microscopy (400×). Foci containing inflammatory cells (mostly neutrophils) are shown, with visible pockets containing bacteria indicated by arrows. Shown is a representative experiment out of two to three performed. *P < 0.05, **P < 0.01 (Mann-Whitney U test).
Methods
Mice.
RIP3 KO (49) and caspase-8−/− RIP3−/− (dKO) (9) have been reported. Caspase-8fl/fl LysM cre+/+ cKO mice were generated by D.M.S. C57BL/6 mice were bred in house or from Jackson Laboratories. BMT was performed on lethally (900 rads) irradiated mice. Mice were infected s.c. or i.v. with 500 cfu of KIM1001-pEcLpxL and monitored for survival. Tissue for analysis was harvested at 42 h after infection, or at 68 h after s.c. infection with KIM1001 (300 cfu).
Bacterial Strains and Growth Conditions.
Y. pestis KIM5 or KIM5ΔYopJ (24) (25) were grown in tryptose-beef extract broth with 2.5 mM CaCl2 overnight with shaking at 26 °C. The next day the bacteria was diluted 1:8 in fresh media, cultured for 1 h at 26 °C, and shifted to 37 °C for 2 h or grown continuously at 37 °C when indicated. Y. pseudotuberculosis IP2666, Y. enterocolitica 8081, and Salmonella enterica serovar Typhimurium strain SL1344 were as reported (25) and grown at 37 °C. KIM5-EcLpxL and KIM1001-EcLpxL were as previously published (24).
Cell Stimulations.
BMDMs were prepared by maturing bone marrow cells for 6–7 d in the presence of L929 supernatant containing M-CSF. Some experiments were performed with BMDM immortalized with J2 retrovirus (42), or J2 immortalized RIP1+/+ and RIP1−/− fetal liver macrophages (31). Cells were plated overnight and infected with bacteria at multiplicities of infection (MOIs) of 10 or 40, or stimulated with LPS from Y. pestis 26 °C (24) or Escherichia coli, or Pam3Cys (Invivogen). Gentamycin was added 1–2 h after infection. Cell death was estimated at 4 h by measuring lactate dehydrogenase (LDH) release (Promega). In some experiments, cells were pretreated with 1 µM GSK’963 or GSK’962, or 3 µM GSK’872 [RIP1 and RIP3 inhibitors (32) and GSK: P.A.H., J.B., P.J.G.], 20 µM Nec-1 (Enzo), 20 µM zIETD, zYVAD, or zVAD (Promega) for 1 h before infection. Cytokines and caspase-1 cleavage were measured as previously indicated (25). Caspase-8 activity (Promega) was measured after 2 h.
Supplementary Material
Acknowledgments
We thank Kelly Army, Gail Germain, and Anna Cerny for help with mice; Shubhendu Ghosh for assistance with the manuscript; TeChen Tzeng for help with microscopy; Vishva Dixit (Genentech, Inc.) for providing RIP3 KO; Douglas R. Green and Christopher Dillon for sending caspase-8 RIP3 dKO mice; Joan Mecsas and Mary O’Riordan for providing Y. pseudotuberculosis, Y. enterocolitica, and Salmonella; D.M.S. (dmitryshay@emory.edu) for sharing cells from previously unpublished casp8 cKO mice; and GSK: P.A.H., J.B., P.J.G. (peter.j.gough@gsk.com), for providing RIP3 inhibitors and previously unpublished RIP1 inhibitors. The work was supported by National Institutes of Health (NIH) Grants AI07538 and AI057588-American Recovery and Reinvestment Act (to E.L.), AI060025 (to N.S.), AI64349 and AI083713 (to K.A.F.), and AI095213 (to G.I.V. and N.S.), the Norwegian Cancer Society, and the Research Council of Norway. The study also used core services supported by University of Massachusetts Diabetes and Endocrinology Research Center Grant DK32520 and the University of Massachusetts Core Electron Microscopy Facility (supported by NIH/National Center for Research Resources Award S10RR027897).
Footnotes
Conflict of interest statement: E.L. and J.D.G. have a patent application on the use of modified bacteria as used in vaccines. P.A.H., J.B., and P.J.G. are employees and shareholders of GlaxoSmithKline.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1403477111/-/DCSupplemental.
References
- 1.Lilo S, Zheng Y, Bliska JB. Caspase-1 activation in macrophages infected with Yersinia pestis KIM requires the type III secretion system effector YopJ. Infect Immun. 2008;76(9):3911–3923. doi: 10.1128/IAI.01695-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Philip NH, Brodsky IE. Cell death programs in Yersinia immunity and pathogenesis. Front Cell Infect Microbiol. 2012;2:149. doi: 10.3389/fcimb.2012.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lukaszewski RA, et al. Pathogenesis of Yersinia pestis infection in BALB/c mice: Effects on host macrophages and neutrophils. Infect Immun. 2005;73(11):7142–7150. doi: 10.1128/IAI.73.11.7142-7150.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miao EA, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11(12):1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamkanfi M, Dixit VM. Manipulation of host cell death pathways during microbial infections. Cell Host Microbe. 2010;8(1):44–54. doi: 10.1016/j.chom.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Vanlangenakker N, Vanden Berghe T, Vandenabeele P. Many stimuli pull the necrotic trigger, an overview. Cell Death Differ. 2012;19(1):75–86. doi: 10.1038/cdd.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mocarski ES, Upton JW, Kaiser WJ. Viral infection and the evolution of caspase 8-regulated apoptotic and necrotic death pathways. Nat Rev Immunol. 2012;12(2):79–88. doi: 10.1038/nri3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oberst A, et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471(7338):363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaiser WJ, et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471(7338):368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su H, et al. Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science. 2005;307(5714):1465–1468. doi: 10.1126/science.1104765. [DOI] [PubMed] [Google Scholar]
- 11.Gröbner S, et al. Catalytically active Yersinia outer protein P induces cleavage of RIP and caspase-8 at the level of the DISC independently of death receptors in dendritic cells. Apoptosis. 2007;12(10):1813–1825. doi: 10.1007/s10495-007-0100-x. [DOI] [PubMed] [Google Scholar]
- 12.Zheng Y, Lilo S, Mena P, Bliska JB. YopJ-induced caspase-1 activation in Yersinia-infected macrophages: Independent of apoptosis, linked to necrosis, dispensable for innate host defense. PLoS ONE. 2012;7(4):e36019. doi: 10.1371/journal.pone.0036019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monack DM, Mecsas J, Bouley D, Falkow S. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J Exp Med. 1998;188(11):2127–2137. doi: 10.1084/jem.188.11.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Bliska JB. YopJ-promoted cytotoxicity and systemic colonization are associated with high levels of murine interleukin-18, gamma interferon, and neutrophils in a live vaccine model of Yersinia pseudotuberculosis infection. Infect Immun. 2010;78(5):2329–2341. doi: 10.1128/IAI.00094-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brodsky IE, Medzhitov R. Reduced secretion of YopJ by Yersinia limits in vivo cell death but enhances bacterial virulence. PLoS Pathog. 2008;4(5):e1000067. doi: 10.1371/journal.ppat.1000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brodsky IE, et al. A Yersinia effector protein promotes virulence by preventing inflammasome recognition of the type III secretion system. Cell Host Microbe. 2010;7(5):376–387. doi: 10.1016/j.chom.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukherjee S, et al. Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science. 2006;312(5777):1211–1214. doi: 10.1126/science.1126867. [DOI] [PubMed] [Google Scholar]
- 18.Mittal R, Peak-Chew SY, McMahon HT. Acetylation of MEK2 and I kappa B kinase (IKK) activation loop residues by YopJ inhibits signaling. Proc Natl Acad Sci USA. 2006;103(49):18574–18579. doi: 10.1073/pnas.0608995103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paquette N, et al. Serine/threonine acetylation of TGFβ-activated kinase (TAK1) by Yersinia pestis YopJ inhibits innate immune signaling. Proc Natl Acad Sci USA. 2012;109(31):12710–12715. doi: 10.1073/pnas.1008203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meinzer U, et al. Yersinia pseudotuberculosis effector YopJ subverts the Nod2/RICK/TAK1 pathway and activates caspase-1 to induce intestinal barrier dysfunction. Cell Host Microbe. 2012;11(4):337–351. doi: 10.1016/j.chom.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Greten FR, et al. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell. 2007;130(5):918–931. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vladimer GI, Marty-Roix R, Ghosh S, Weng D, Lien E. Inflammasomes and host defenses against bacterial infections. Curr Opin Microbiol. 2013;16(1):23–31. doi: 10.1016/j.mib.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng Y, et al. A Yersinia effector with enhanced inhibitory activity on the NF-κB pathway activates the NLRP3/ASC/caspase-1 inflammasome in macrophages. PLoS Pathog. 2011;7(4):e1002026. doi: 10.1371/journal.ppat.1002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montminy SW, et al. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat Immunol. 2006;7(10):1066–1073. doi: 10.1038/ni1386. [DOI] [PubMed] [Google Scholar]
- 25.Vladimer GI, et al. The NLRP12 inflammasome recognizes Yersinia pestis. Immunity. 2012;37(1):96–107. doi: 10.1016/j.immuni.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oberst A, Green DR. It cuts both ways: Reconciling the dual roles of caspase 8 in cell death and survival. Nat Rev Mol Cell Biol. 2011;12(11):757–763. doi: 10.1038/nrm3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho YS, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137(6):1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang DW, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325(5938):332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 29.Moquin D, Chan FK. The molecular regulation of programmed necrotic cell injury. Trends Biochem Sci. 2010;35(8):434–441. doi: 10.1016/j.tibs.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cusson-Hermance N, Khurana S, Lee TH, Fitzgerald KA, Kelliher MA. Rip1 mediates the Trif-dependent toll-like receptor 3- and 4-induced NF-kappaB activation but does not contribute to interferon regulatory factor 3 activation. J Biol Chem. 2005;280(44):36560–36566. doi: 10.1074/jbc.M506831200. [DOI] [PubMed] [Google Scholar]
- 31.Kelliher MA, et al. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8(3):297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 32.Kaiser WJ, et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288(43):31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gröbner S, et al. Absence of Toll-like receptor 4 signaling results in delayed Yersinia enterocolitica YopP-induced cell death of dendritic cells. Infect Immun. 2007;75(1):512–517. doi: 10.1128/IAI.00756-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Bliska JB. Role of Toll-like receptor signaling in the apoptotic response of macrophages to Yersinia infection. Infect Immun. 2003;71(3):1513–1519. doi: 10.1128/IAI.71.3.1513-1519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haase R, et al. A dominant role of Toll-like receptor 4 in the signaling of apoptosis in bacteria-faced macrophages. J Immunol. 2003;171(8):4294–4303. doi: 10.4049/jimmunol.171.8.4294. [DOI] [PubMed] [Google Scholar]
- 36.Kang TB, et al. Caspase-8 serves both apoptotic and nonapoptotic roles. J Immunol. 2004;173(5):2976–2984. doi: 10.4049/jimmunol.173.5.2976. [DOI] [PubMed] [Google Scholar]
- 37.Feoktistova M, et al. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell. 2011;43(3):449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Man SM, et al. Salmonella infection induces recruitment of Caspase-8 to the inflammasome to modulate IL-1β production. J Immunol. 2013;191(10):5239–5246. doi: 10.4049/jimmunol.1301581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang TB, et al. Mutation of a self-processing site in caspase-8 compromises its apoptotic but not its nonapoptotic functions in bacterial artificial chromosome-transgenic mice. J Immunol. 2008;181(4):2522–2532. doi: 10.4049/jimmunol.181.4.2522. [DOI] [PubMed] [Google Scholar]
- 40.Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity. 2013;38(1):27–40. doi: 10.1016/j.immuni.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 41.Vince JE, et al. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity. 2012;36(2):215–227. doi: 10.1016/j.immuni.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 42.Bossaller L, et al. Cutting edge: FAS (CD95) mediates noncanonical IL-1β and IL-18 maturation via caspase-8 in an RIP3-independent manner. J Immunol. 2012;189(12):5508–5512. doi: 10.4049/jimmunol.1202121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gringhuis SI, et al. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1β via a noncanonical caspase-8 inflammasome. Nat Immunol. 2012;13(3):246–254. doi: 10.1038/ni.2222. [DOI] [PubMed] [Google Scholar]
- 44.Maelfait J, et al. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1beta maturation by caspase-8. J Exp Med. 2008;205(9):1967–1973. doi: 10.1084/jem.20071632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Antonopoulos C, El Sanadi C, Kaiser WJ, Mocarski ES, Dubyak GR. Proapoptotic chemotherapeutic drugs induce noncanonical processing and release of IL-1β via caspase-8 in dendritic cells. J Immunol. 2013;191(9):4789–4803. doi: 10.4049/jimmunol.1300645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gurung P, et al. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol. 2014;192(4):1835–1846. doi: 10.4049/jimmunol.1302839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shenderov K, et al. Cutting edge: Endoplasmic reticulum stress licenses macrophages to produce mature IL-1β in response to TLR4 stimulation through a caspase-8- and TRIF-dependent pathway. J Immunol. 2014;192(5):2029–2033. doi: 10.4049/jimmunol.1302549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pierini R, et al. AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages. Cell Death Differ. 2012;19(10):1709–1721. doi: 10.1038/cdd.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Newton K, Sun X, Dixit VM. Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol Cell Biol. 2004;24(4):1464–1469. doi: 10.1128/MCB.24.4.1464-1469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.