Significance
Although scientists have identified surprising cognitive flexibility in animals and potentially unique features of human psychology, we know less about the selective forces that favor cognitive evolution, or the proximate biological mechanisms underlying this process. We tested 36 species in two problem-solving tasks measuring self-control and evaluated the leading hypotheses regarding how and why cognition evolves. Across species, differences in absolute (not relative) brain volume best predicted performance on these tasks. Within primates, dietary breadth also predicted cognitive performance, whereas social group size did not. These results suggest that increases in absolute brain size provided the biological foundation for evolutionary increases in self-control, and implicate species differences in feeding ecology as a potential selective pressure favoring these skills.
Keywords: psychology, behavior, comparative methods, inhibitory control, executive function
Abstract
Cognition presents evolutionary research with one of its greatest challenges. Cognitive evolution has been explained at the proximate level by shifts in absolute and relative brain volume and at the ultimate level by differences in social and dietary complexity. However, no study has integrated the experimental and phylogenetic approach at the scale required to rigorously test these explanations. Instead, previous research has largely relied on various measures of brain size as proxies for cognitive abilities. We experimentally evaluated these major evolutionary explanations by quantitatively comparing the cognitive performance of 567 individuals representing 36 species on two problem-solving tasks measuring self-control. Phylogenetic analysis revealed that absolute brain volume best predicted performance across species and accounted for considerably more variance than brain volume controlling for body mass. This result corroborates recent advances in evolutionary neurobiology and illustrates the cognitive consequences of cortical reorganization through increases in brain volume. Within primates, dietary breadth but not social group size was a strong predictor of species differences in self-control. Our results implicate robust evolutionary relationships between dietary breadth, absolute brain volume, and self-control. These findings provide a significant first step toward quantifying the primate cognitive phenome and explaining the process of cognitive evolution.
Since Darwin, understanding the evolution of cognition has been widely regarded as one of the greatest challenges for evolutionary research (1). Although researchers have identified surprising cognitive flexibility in a range of species (2–40) and potentially derived features of human psychology (41–61), we know much less about the major forces shaping cognitive evolution (62–71). With the notable exception of Bitterman’s landmark studies conducted several decades ago (63, 72–74), most research comparing cognition across species has been limited to small taxonomic samples (70, 75). With limited comparable experimental data on how cognition varies across species, previous research has largely relied on proxies for cognition (e.g., brain size) or metaanalyses when testing hypotheses about cognitive evolution (76–92). The lack of cognitive data collected with similar methods across large samples of species precludes meaningful species comparisons that can reveal the major forces shaping cognitive evolution across species, including humans (48, 70, 89, 93–98).
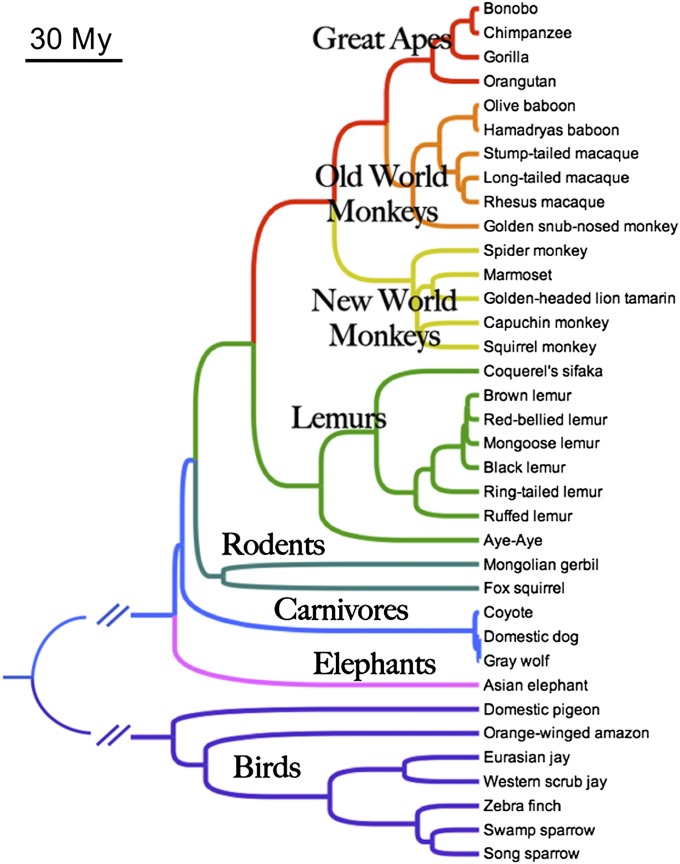
To address these challenges we measured cognitive skills for self-control in 36 species of mammals and birds (Fig. 1 and Tables S1–S4) tested using the same experimental procedures, and evaluated the leading hypotheses for the neuroanatomical underpinnings and ecological drivers of variance in animal cognition. At the proximate level, both absolute (77, 99–107) and relative brain size (108–112) have been proposed as mechanisms supporting cognitive evolution. Evolutionary increases in brain size (both absolute and relative) and cortical reorganization are hallmarks of the human lineage and are believed to index commensurate changes in cognitive abilities (52, 105, 113–115). Further, given the high metabolic costs of brain tissue (116–121) and remarkable variance in brain size across species (108, 122), it is expected that the energetic costs of large brains are offset by the advantages of improved cognition. The cortical reorganization hypothesis suggests that selection for absolutely larger brains—and concomitant cortical reorganization—was the predominant mechanism supporting cognitive evolution (77, 91, 100–106, 120). In contrast, the encephalization hypothesis argues that an increase in brain volume relative to body size was of primary importance (108, 110, 111, 123). Both of these hypotheses have received support through analyses aggregating data from published studies of primate cognition and reports of “intelligent” behavior in nature—both of which correlate with measures of brain size (76, 77, 84, 92, 110, 124).
Fig. 1.
A phylogeny of the species included in this study. Branch lengths are proportional to time except where long branches have been truncated by parallel diagonal lines (split between mammals and birds ∼292 Mya).
With respect to selective pressures, both social and dietary complexities have been proposed as ultimate causes of cognitive evolution. The social intelligence hypothesis proposes that increased social complexity (frequently indexed by social group size) was the major selective pressure in primate cognitive evolution (6, 44, 48, 50, 87, 115, 120, 125–141). This hypothesis is supported by studies showing a positive correlation between a species’ typical group size and the neocortex ratio (80, 81, 85–87, 129, 142–145), cognitive differences between closely related species with different group sizes (130, 137, 146, 147), and evidence for cognitive convergence between highly social species (26, 31, 148–150). The foraging hypothesis posits that dietary complexity, indexed by field reports of dietary breadth and reliance on fruit (a spatiotemporally distributed resource), was the primary driver of primate cognitive evolution (151–154). This hypothesis is supported by studies linking diet quality and brain size in primates (79, 81, 86, 142, 155), and experimental studies documenting species differences in cognition that relate to feeding ecology (94, 156–166).
Although each of these hypotheses has received empirical support, a comparison of the relative contributions of the different proximate and ultimate explanations requires (i) a cognitive dataset covering a large number of species tested using comparable experimental procedures; (ii) cognitive tasks that allow valid measurement across a range of species with differing morphology, perception, and temperament; (iii) a representative sample within each species to obtain accurate estimates of species-typical cognition; (iv) phylogenetic comparative methods appropriate for testing evolutionary hypotheses; and (v) unprecedented collaboration to collect these data from populations of animals around the world (70).
Here, we present, to our knowledge, the first large-scale collaborative dataset and comparative analysis of this kind, focusing on the evolution of self-control. We chose to measure self-control—the ability to inhibit a prepotent but ultimately counterproductive behavior—because it is a crucial and well-studied component of executive function and is involved in diverse decision-making processes (167–169). For example, animals require self-control when avoiding feeding or mating in view of a higher-ranking individual, sharing food with kin, or searching for food in a new area rather than a previously rewarding foraging site. In humans, self-control has been linked to health, economic, social, and academic achievement, and is known to be heritable (170–172). In song sparrows, a study using one of the tasks reported here found a correlation between self-control and song repertoire size, a predictor of fitness in this species (173). In primates, performance on a series of nonsocial self-control control tasks was related to variability in social systems (174), illustrating the potential link between these skills and socioecology. Thus, tasks that quantify self-control are ideal for comparison across taxa given its robust behavioral correlates, heritable basis, and potential impact on reproductive success.
In this study we tested subjects on two previously implemented self-control tasks. In the A-not-B task (27 species, n = 344), subjects were first familiarized with finding food in one location (container A) for three consecutive trials. In the test trial, subjects initially saw the food hidden in the same location (container A), but then moved to a new location (container B) before they were allowed to search (Movie S1). In the cylinder task (32 species, n = 439), subjects were first familiarized with finding a piece of food hidden inside an opaque cylinder. In the following 10 test trials, a transparent cylinder was substituted for the opaque cylinder. To successfully retrieve the food, subjects needed to inhibit the impulse to reach for the food directly (bumping into the cylinder) in favor of the detour response they had used during the familiarization phase (Movie S2).
Thus, the test trials in both tasks required subjects to inhibit a prepotent motor response (searching in the previously rewarded location or reaching directly for the visible food), but the nature of the correct response varied between tasks. Specifically, in the A-not-B task subjects were required to inhibit the response that was previously successful (searching in location A) whereas in the cylinder task subjects were required to perform the same response as in familiarization trials (detour response), but in the context of novel task demands (visible food directly in front of the subject).
Results
Across species and accounting for phylogeny, performance on the two tasks was strongly correlated (r = 0.53, n = 23, P < 0.01). Thus, species that participated in both cognitive tasks were assigned a composite score averaging performance across tasks (Table S5). Because the two tasks assessed complementary but not identical abilities, the composite score serves as a broader index of self-control across tasks. Phylogenetic analyses revealed that scores were more similar among closely related species, with the maximum likelihood estimate of λ, a measure of phylogenetic signal, significantly greater than zero in most cases (Table 1). For both tasks, scores from multiple populations of the same species (collected by different researchers at different sites) were highly correlated (cylinder task: r = 0.95, n = 5, P = 0.01; A-not-B task: r = 0.87, n = 6, P = 0.03; SI Text and Table S6). To control for the nonindependence of species level data, we used phylogenetic generalized least squares (PGLS) to test the association between performance on the cognitive tasks and the explanatory variables associated with each hypothesis. Our neuroanatomical predictors included measures of absolute brain volume [endocranial volume (ECV)], residual brain volume [residuals from a phylogenetic regression of ECV predicted by body mass (ECV residuals)], and Jerrison’s (108) encephalization quotient (EQ) (Methods).
Table 1.
Phylogenetic signal in the cognitive data
| Log likelihood |
|||||
| Data source | Dependent measure | λ, ML* | λ = ML | λ = 0 | P† |
| All species | Cylinder score | 0.83 | −2.14 | −4.13 | 0.05 |
| A-not-B score | 0.72 | −12.60 | −14.90 | 0.03 | |
| Composite score | 0.76 | −2.00 | −3.47 | 0.09 | |
| Primates | Cylinder score | 0.95 | −0.62 | −3.63 | 0.01 |
| A-not-B score | 0.48 | −6.05 | −7.54 | 0.08 | |
| Composite score | 0.86 | −0.98 | −3.32 | 0.03 | |
The maximum likelihood estimate for λ, a statistical measure of phylogenetic signal (201).
P values are based on a likelihood ratio test comparing the model with the maximum likelihood estimate of λ to a model where λ is fixed at 0 (the null alternative representing no phylogenetic signal).
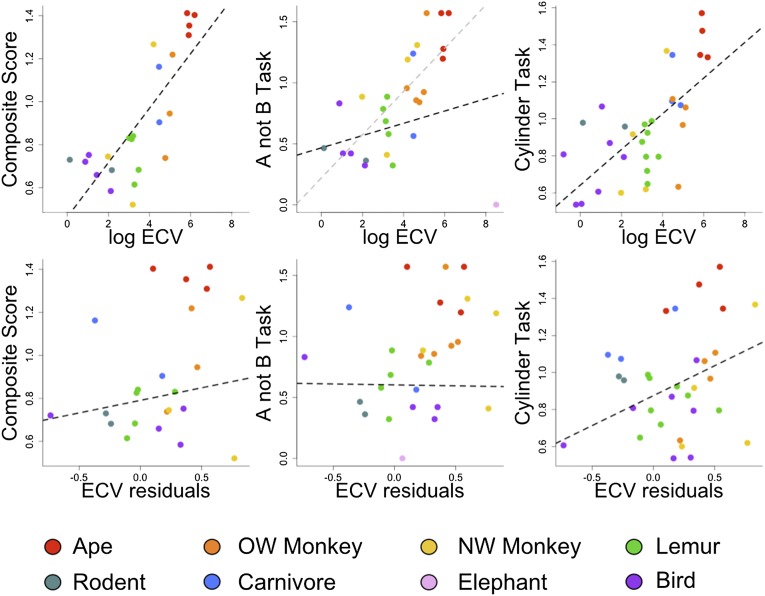
Across species, absolute brain volume (measured as ECV) was a robust predictor of performance (Fig. 2 and Table 2), supporting the predictions of the cortical reorganization hypothesis. ECV covaried positively with performance on the cylinder task and the composite score and explained substantial variance in performance (r2 = 0.43–0.60; Table 2). This association was much weaker for the A-not-B task, reflecting that the largest-brained species (Asian elephant) had the lowest score on this measure (Fig. 2 and Table 2). The same analysis excluding the elephant yielded a strong and significant positive association between ECV and scores on the A-not-B task (Fig. 2 and Table 2). Across the entire sample, residual brain volume was far less predictive than absolute brain volume: it explained only 3% of variance in composite scores, and was a significant predictor of performance in only one of the tasks (Table 2, SI Text, and Fig. 2). EQ was positively related to composite scores across species (β = 0.28, t21 = 3.23, P < 0.01, λ = 0, r2 = 0.33) but again explained far less variance than absolute brain volume.
Fig. 2.
Cognitive scores as a function of log endocranial volume (ECV) and residual brain volume (ECV residuals). In both tasks and in the composite measure, ECV was a significant predictor of self-control. Relative brain volume universally explained less variance. Plots show statistically transformed data (see Methods for details). The gray dashed line shows an alternate model excluding the elephant from analysis. NW, New World; OW, Old World.
Table 2.
The relationship between brain volume, socioecology, observational measures of cognition, and performance on the cognitive tasks
| Data source | Explanatory variable | Dependent measure | t | df | P | r2 | λ |
| All species | Absolute brain volume | Cylinder | 4.79 | 30 | <0.01 | 0.43 | 0.00 |
| Absolute brain volume | A-not-B | 1.03 | 25 | 0.16 | 0.04 | 0.69 | |
| Absolute brain volume | A-not-B (no elephant) | 5.44 | 24 | <0.01 | 0.55 | 0.00 | |
| Absolute brain volume | Composite | 5.67 | 21 | <0.01 | 0.60 | 0.00 | |
| Residual brain volume | Cylinder | 2.31 | 30 | 0.01 | 0.15 | 0.98 | |
| Residual brain volume | A-not-B | 0.05 | 25 | 0.96 | <0.01 | 0.72 | |
| Residual brain volume | A-not-B (no elephant) | 0.33 | 24 | 0.37 | <0.01 | 0.58 | |
| Residual brain volume | Composite | 0.78 | 21 | 0.22 | 0.03 | 0.67 | |
| Nonprimates | Absolute brain volume | Cylinder | 3.30 | 10 | <0.01 | 0.52 | 0.00 |
| Absolute brain volume | A-not-B | −0.59 | 7 | 0.71 | 0.05 | 0.00 | |
| Absolute brain volume | Composite | 2.54 | 6 | 0.02 | 0.52 | 0.00 | |
| Residual brain volume | Cylinder | 1.12 | 10 | 0.14 | 0.11 | 0.69 | |
| Residual brain volume | A-not-B | −1.83 | 7 | 0.95 | 0.32 | 0.00 | |
| Residual brain volume | Composite | −0.58 | 6 | 0.71 | 0.05 | 0.25 | |
| Primates | Absolute brain volume | Cylinder | 5.01 | 18 | <0.01 | 0.58 | 0.00 |
| Absolute brain volume | A-not-B | 4.39 | 16 | <0.01 | 0.55 | 0.00 | |
| Absolute brain volume | Composite | 5.27 | 13 | <0.01 | 0.68 | 0.00 | |
| Residual brain volume | Cylinder | 2.26 | 18 | 0.02 | 0.22 | 0.93 | |
| Residual brain volume | A-not-B | 2.64 | 16 | 0.01 | 0.30 | 0.00 | |
| Residual brain volume | Composite | 1.69 | 13 | 0.06 | 0.18 | 0.60 | |
| Primates | Population group size | Composite | −0.75 | 13 | 0.77 | 0.04 | 0.83 |
| Foraging group size | Composite | −0.33 | 13 | 0.63 | 0.01 | 0.82 | |
| Percent fruit in diet | Composite | 0.11 | 13 | 0.46 | <0.01 | 0.85 | |
| Dietary breadth | Composite | 4.99 | 12 | <0.01 | 0.68 | 0.69 | |
| Social learning | Composite | 2.63 | 9 | 0.03 | 0.44 | 0.00 | |
| Innovation | Composite | 1.99 | 9 | 0.08 | 0.31 | 0.00 | |
| Extractive foraging | Composite | 3.10 | 9 | 0.01 | 0.52 | 0.00 | |
| Tool use | Composite | 3.12 | 9 | 0.01 | 0.52 | 0.00 | |
| Tactical deception | Composite | 4.06 | 9 | <0.01 | 0.65 | 0.00 | |
| gs | Composite | 3.61 | 9 | <0.01 | 0.59 | 0.00 | |
| PCA 1 | Composite | 3.61 | 9 | <0.01 | 0.59 | 0.00 |
The sign of the t statistic indicates the direction of the relationship between variables. Data regarding social learning, innovation, extractive foraging, tool use, tactical deception (all of which covary), and primate gs scores were adjusted for research effort and obtained from Reader et al. (92) and Byrne and Corp (124). PCA 1 is equivalent to the gs score calculated by Reader et al. (92) restricted to species in this dataset. We used the arcsine square-root transformed mean proportion of correct responses for each species as the dependent measure in all analyses, as this best met the statistical assumptions of our tests. Socioecological data were log transformed (group size) or arcsine square root transformed (proportion fruit in diet) for analysis.
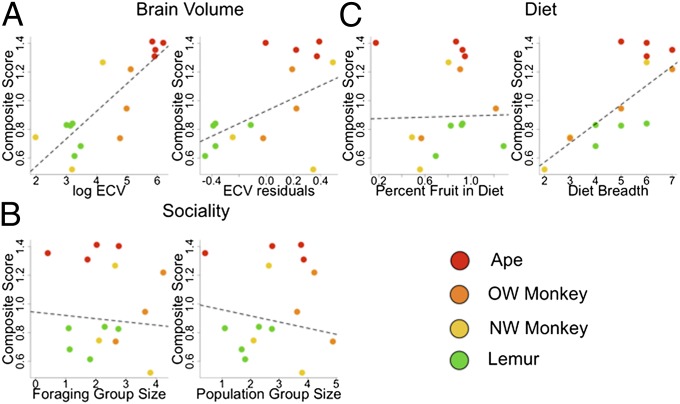
We conducted the same analyses using only primates (23 species, 309 subjects), the best-represented taxonomic group in our dataset. Within primates, absolute brain volume was the best predictor of performance across tasks and explained substantial variation across species (r2 = 0.55–0.68; Fig. 3 and Table 2). In contrast to the analysis across all species, residual brain volume was predictive of performance on both tasks within primates, although it explained much less variance than absolute brain volume (r2 = 0.18–0.30; Fig. 3 and Table 2). Within primates the analysis using EQ as a predictor of composite scores was similar to that using ECV residuals (β = 0.24, t13 = 1.65, P = 0.06, λ = 0.66, r2 = 0.17).
Fig. 3.
Cognitive scores for primates as a function of (A) absolute and residual endocranial volume (ECV), (B) foraging and population social group size, and (C) frugivory and dietary breadth. Absolute ECV, residual ECV, and dietary breadth covaried positively with measures of self-control. Plots show statistically transformed data (see Methods and Table 2 for details).
We also restricted the analyses to only the nonprimate species in our sample (13 species, 258 subjects). Within the nonprimate species, ECV was again the best predictor of self-control, and was significantly and positively associated with composite scores and scores on the cylinder task, but not the A-not-B task (Table 2). Removing the Asian elephant from the analysis of the A-not-B task did not change this result (β = 0.09, t6 = 1.37, P = 0.11, λ = 0, r2 = 0.24). Residual brain volume was not a significant predictor of any of these measures (Table 2), and EQ was unrelated to composite scores (β = −0.01, t6 = −0.08, P = 0.53, λ = 0.28, r2 < 0.01).
We used the experimentally derived measures of self-control to investigate the two leading ecological hypotheses that have been proposed as catalysts of primate cognitive evolution. We focused on primates because these species are best represented in our dataset, and the ecological data have been systematically compiled and related to neuroanatomical proxies for cognition in these species. As a measure of social complexity, we tested the hypothesis that social group size, which covaries with the neocortex ratio in anthropoid primates (129), would predict performance in the self-control tasks. To explore multiple variants of this hypothesis, we investigated both species-typical population group size and foraging group size as predictor variables. Neither measure of group size was associated with task performance (Fig. 3, Table 2, and Table S7), echoing findings using observational data on behavioral flexibility (92). We tested the foraging hypotheses by examining whether the degree of frugivory (percent fruit in diet) or dietary breadth (number of dietary categories reported to have been consumed by each species) (92) predicts performance. The percent of fruit in a species’ diet was not a significant predictor of any of the cognitive measures (Fig. 3, Table 2, and Table S7). However, dietary breadth covaried strongly with our measures of self-control (Fig. 3, Table 2, and Table S7). Supplemental analyses involving home range size, day journey length, the defensibility index, and substrate use revealed no significant associations (SI Text and Fig. S1).
To provide an integrated test of variance explained by absolute brain volume and dietary breadth, we fit a multiple regression including both terms as predictors of primates’ composite cognitive scores. This model explained 82% of variance in performance between species with significant and positive coefficients for both absolute ECV and dietary breadth, controlling for the effects of one another (ECV: t11 = 3.30, P < 0.01; dietary breadth: t11 = 3.02, P < 0.01; λ = 0.00, r2 = 0.82). Thus, while correlated with one another (t = 3.04, P < 0.01, λ = 0, r2 = 0.32), both brain volume and dietary complexity account for unique components of variance in primate cognition, together explaining the majority of interspecific variation on these tasks. In this model the independent effect for dietary breadth (r2 = 0.45) was comparable to that for ECV (r2 = 0.49).
We also assessed the extent to which our experimental data corroborate species-specific reports of intelligent behavior in nature (92). Controlling for observational research effort, our experimental measures covaried positively with reports of innovation, extractive foraging, tool use, social learning, and tactical deception in primates (Table 2, Table S7, and SI Text). Our experimental measure also covaried with a “general intelligence” factor, gs (92), derived from these observational measures (Table 2, Table S7, Fig. S2, and SI Text).
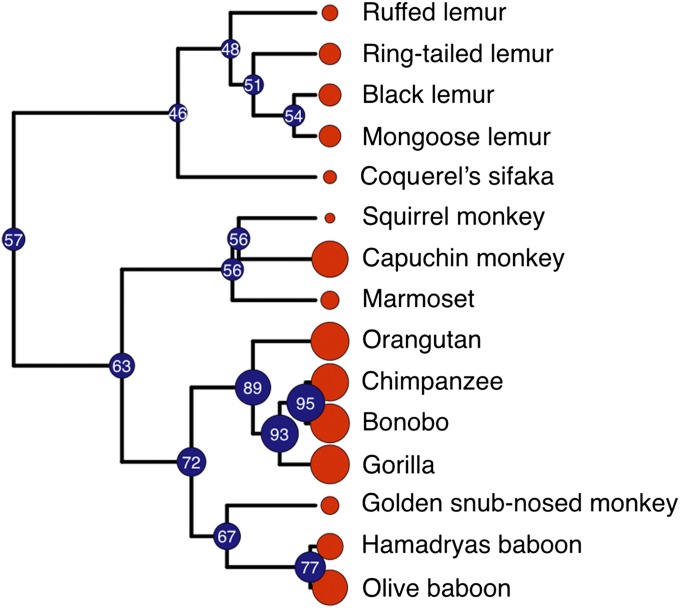
Lastly, we used data from the extant species in our dataset to reconstruct estimated ancestral states in the primate phylogeny. Maximum likelihood reconstruction of ancestral states implies gradual cognitive evolution in the lineage leading to apes, with a convergence between apes and capuchin monkeys (Fig. 4 and SI Text). Thus, in addition to statistical inferences about ancestral species, this model reveals branches in the phylogeny associated with rapid evolutionary change, convergence and divergence, and the historical contexts in which these events occurred.
Fig. 4.
Ancestral state reconstruction of cognitive skills for self-control. We generated the maximum likelihood estimates for ancestral states along the primate phylogeny using data from the composite measure (average score across tasks for species that participated in both tasks). The red circles along the tips of the phylogeny are proportional to the extant species’ composite scores (larger circles represent higher scores). The blue circles at the internal nodes of the phylogeny represent the estimated ancestral states for the composite score, with the estimated value indicated within circles at each node.
Discussion
Our phylogenetic comparison of three dozen species supports the hypothesis that the major proximate mechanism underlying the evolution of self-control is increases in absolute brain volume. Our findings also implicate dietary breadth as an important ecological correlate, and potential selective pressure for the evolution of these skills. In contrast, residual brain volume was only weakly related, and social group size was unrelated, to variance in self-control. The weaker relationship with residual brain volume and lack of relationship with social group size is particularly surprising given the common use of relative brain volume as a proxy for cognition and historical emphasis on increases in social group size as a likely driver of primate cognitive evolution (85).
Why might absolutely larger brains confer greater cognitive advantages than relatively larger brains? One possibility is that as brains get absolutely larger, the total number of neurons increases, and brains tend to become more modularized, perhaps facilitating the evolution of new cognitive networks (91, 101, 102). Indeed, recent data suggest that human brains are notable mainly for their absolute volume, and otherwise conform to the (re)organizational expectations for a primate brain of their volume (99, 100, 104–107, 175). Due to limited comparative data on more detailed aspects of neuroanatomy (e.g., neuron counts, regional volumes, functional connectivity) our analyses were restricted to measures derived from whole brain volumes. However, an important question for future research will be whether finer measures of the neuroanatomical substrates involved in regulating self-control (e.g., prefrontal cortex) explain additional variation in cognition across species. For example, the best performing species in our sample were predominantly anthropoid primates, species that have evolved unique prefrontal areas that are thought to provide a cognitive advantage in foraging decisions that rely on executive function (176–178). Nonetheless, other species without these neuroanatomical specializations also performed well, raising the possibility that the cognitive skills required for success in these tasks may be subserved by diverse but functionally similar neural mechanisms across species (e.g., ref. 179). Thus, although evolutionary increases in brain volume create the potential for new functional areas or cognitive networks, more detailed data from the fields of comparative and behavioral neuroscience will be essential for understanding the biological basis of species differences in cognition (e.g., refs. 180–183).
Within primates we also discovered that dietary breadth is strongly related to levels of self-control. One plausible ultimate explanation is that individuals with the most cognitive flexibility may be most likely to explore and exploit new dietary resources or methods of food acquisition, which would be especially important in times of scarcity. If these behaviors conferred fitness benefits, selection for these traits in particular lineages may have been an important factor in the evolution of species differences in self-control. A second possibility is that dietary breadth represents an ecological constraint on brain evolution, rather than a selective pressure per se (116, 155, 184, 185). Accordingly, species with broad diets may be most capable of meeting the metabolic demands of growing and maintaining larger brains, with brain enlargement favored through a range of ecological selective pressures (86). Nonetheless, after accounting for shared variance between dietary breadth and brain volume, dietary breadth was still strongly associated with performance on self-control tasks. Thus, it is likely that dietary breadth acts both as a selective pressure and a metabolic facilitator of cognitive evolution. Given that foraging strategies have also been linked to species differences in cognition in nonprimate taxa (94, 156–159, 161, 162, 166), it remains an important question whether dietary breadth will have similar explanatory power in other orders of animals.
The data reported here likely represent relatively accurate estimates of species-typical cognition because we collected data from large samples within each species (mean n = 15.3 ± 2.0 subjects per species, range = 6–66), scores from multiple populations of the same species were highly correlated, and performance was not associated with previous experience in cognitive tasks (SI Text). Thus, although populations may vary to some extent (e.g., due to differences in rearing history or experimental experience), these differences are small relative to the interspecific variation we observed. The relationship between our experimental measures of self-control and observational measures of behavioral flexibility also suggest that our measures have high ecological validity, and underscore the complementary roles of observational and experimental approaches for the study of comparative cognition.
Our tasks could be flexibly applied with a range of species because all species we tested exhibited the perceptual, motivational, and motoric requirements for participation. Thus, despite the fact that these species may vary in their reliance on vision, visual acuity, or motivation for food rewards, all species met the same pretest criteria, assuring similar proficiency with basic task demands before being tested. Nonetheless, in any comparative cognitive test it is possible that features of individual tasks are more appropriate for some species than others. One mechanism to overcome this challenge is through the approach implemented here, in which (i) multiple tasks designed to measure the same underlying construct are used, (ii) the correlation between tasks is assessed across species, and (iii) a composite score averaging performance across tasks is used as the primary dependent measure. In cases where data are limited to a single measure from a species, the results must be interpreted extremely cautiously (e.g., performance of the Asian elephant on the A-not-B task).
The relationship between self-control and absolute brain volume is unlikely to be a nonadaptive byproduct of selection for increases in body size for several reasons. First, a comparison of models using only body mass or ECV as the predictor of composite scores yielded stronger support for the ECV model both in an analysis across all species [change in the Akaike information criterion (∆AICc) = 0.77], and within primates (∆AICc = 3.12). However, it is only within primates that the change in AICc between the body mass and ECV models exceeded the two-unit convention for meaningful difference (186). Second, the number of neurons in primate brains scales isometrically with brain size, indicating selection for constant neural density and neuron size, a scaling relationship that contrasts with other orders of animals (100). Thus, the relationship between absolute brain volume and self-control may be most pronounced in the primate species in our sample, and may not generalize to all other large-brained animals (e.g., whales, elephants), or taxa whose brains are organized differently than primates (e.g., birds). Nonetheless, even when removing primate species from the analysis, absolute brain volume remained the strongest predictor of species differences in self-control. Third, ancestral state reconstructions indicate that both absolute and relative brain volume have increased over time in primates, whereas body mass has not (187). Lastly, although not as predictive as absolute brain volume, residual brain volume was a significant predictor of self-control in several of our analyses. Thus, multiple lines of evidence implicate selection for brain volume (and organization) independent of selection for body size, and our data illustrate the cognitive consequences of these evolutionary trends.
With the exception of dietary breadth we found no significant relationships between several socioecological variables and measures of self-control. These findings are especially surprising given that both the percentage of fruit in the diet and social group size correlate positively with neocortex ratio in anthropoid primates (86, 142). Our findings suggest that the effect of social and ecological complexity may be limited to influencing more specialized, and potentially domain-specific forms of cognition (188–196). For example, among lemurs, sensitivity to cues of visual attention used to outcompete others for food covaries positively with social group size, whereas a nonsocial measure of self-control does not (146). Therefore, our ability to evaluate the predicted relationships between socioecology and cognition will depend on measures designed to assess skills in specific cognitive domains (e.g., visual perspective-taking or spatial memory). In addition, more nuanced measures of social and ecological complexity (e.g., coalitions or social networks) may be necessary to detect these relationships (197).
Overall, our results present a critical step toward understanding the cognitive implications of evolutionary shifts in brain volume and dietary complexity. They also underscore the need for future cognitive studies investigating how ecological factors drive cognitive evolution in different psychological domains. These experimental measures will be particularly important given that even the most predictive neuroanatomical measures failed to account for more than 30% of cognitive variance across species in this study. With a growing comparative database on the cognitive skills of animals, we will gain significant insights into the nature of intelligence itself, and the extent to which changes in specific cognitive abilities have evolved together, or mosaically, across species. This increased knowledge of cognitive variation among living species will also set the stage for stronger reconstructions of cognitive evolutionary history. These approaches will be especially important given that cognition leaves so few traces in the fossil record. In the era of comparative genomics and neurobiology, this research provides a critical first step toward mapping the primate cognitive phenome and unraveling the evolutionary processes that gave rise to the human mind.
Methods
In the A-not-B task, subjects were required to resist searching for food in a previous hiding place when the food reward was visibly moved to a novel location. Subjects watched as food was hidden in one of three containers positioned at the exterior of a three-container array and were required to correctly locate the food in this container on three consecutive familiarization trials before advancing to the test. In the test trial, subjects initially saw the food hidden in the same container (container A), but then watched as the food was moved to another container at the other end of the array (container B; Movie S1). Subjects were then allowed to search for the hidden food, and the accuracy of the first search location was recorded. This procedure differs slightly from the original task used by Piaget (198) in which test trials involved the immediate hiding of the reward in location B, without first hiding the reward in location A. Our method followed the procedure of Amici et al. (174), and similarly we conducted one test trial per subject. For the A-not-B task, our dependent measure was the percentage of individuals that responded correctly on the test trial within each species.
In the cylinder task, subjects were first familiarized with finding a piece of food hidden inside an opaque cylinder. Subjects were required to successfully find the food by detouring to the side of the cylinder on four of five consecutive trials before advancing to the test. In the following 10 test trials, a transparent cylinder was substituted for the opaque cylinder. To successfully retrieve the food, subjects needed to inhibit the impulse to reach for the food directly (bumping into the cylinder) in favor of the detour response they had used during the familiarization (Movie S2). Although subjects may have initially failed to perceive the transparent barrier on the first test trial, they had ample opportunity to adjust their behavior through visual, auditory, and tactile feedback across the 10 test trials. For the cylinder task our dependent measure was the percentage of test trials that a subject performed the correct detour response, which was averaged across individuals within species to obtain species means.
In both tasks, all species were required to meet the same pretest criteria, demonstrating a basic understanding of the task, and allowing meaningful comparison of test data across species. Although the number of trials required to meet these criteria varied between species, we found no significant relationship between the number of pretest trials and test performance on either task (A-not-B: t25 = −1.83, λ = 0.52, P = 0.08; cylinder task: t30 = −1.14, λ = 0.69, P = 0.26). For analyses involving brain volume, log ECV was used as the measure of absolute brain volume and we extracted residuals from a PGLS model of log ECV predicted by log body mass as our primary measure of relative brain volume (ECV residuals; SI Text). As an additional measure of relative brain size we incorporated Jerrison’s (108) EQ, calculated as EQ = brain mass/0.12 × body mass0.67. Although EQ and a residuals approach both measure deviation from an expected brain-to-body scaling relationship, they differ in that EQ measures deviation from a previously estimated allometric exponent using a larger dataset of species, whereas ECV residuals are derived from the actual scaling relationship within our sample, while accounting for phylogeny.
To control for the nonindependence of species level data, we used PGLS to test the association between performance on the cognitive tasks and the explanatory variables associated with each hypothesis. We predicted that brain volume, group size, and measures of dietary complexity would covary positively with cognitive performance. Thus, each of these predictions was evaluated using directional tests following the conventions (δ = 0.01, γ = 0.04) recommended by Rice and Gaines (199), which allocates proportionally more of the null distribution in the predicted direction, while retaining statistical power to detect unexpected patterns in the opposite direction. We incorporated the parameter λ in the PGLS models to estimate phylogenetic signal and regression parameters simultaneously, using a maximum likelihood procedure (200, 201). This research was approved by the Duke University Institutional Animal Care and Use Committee (protocol numbers A303-11-12, A199-11-08, and A055-11-03).
Supplementary Material
Acknowledgments
We thank Natalie Cooper and Sunil Suchindran for statistical advice; Jeff Stevens and two anonymous reviewers for comments on drafts of this manuscript; and Ikuma Adachi, Nathan Emery, Daniel Haun, Marc Hauser, Ludwig Huber, Al Kamil, Chris Krupenye, Luke Matthews, Collin McCabe, Alexandra Rosati, Kara Schroepfer, Jeff Stevens, Tara Stoinski, Michael Tomasello, and Victoria Wobber for their helpful discussion during the workshops from which this research emerged. F. Aureli and F. Amici thank Iber Rodriguez Castillo, Roberto Pacheco Mendez, Fernando Victoria Arceo, Liesbeth Sterck, Barbara Tiddi, and all the animal keepers at the facilities where the data were collected for support and cooperation. K.E.S. and A.P. thank Steve Nichols and the staff at The Parrot Zoo. This work was supported by the National Evolutionary Synthesis Center (NESCent) through support of a working group led by C.L.N. and B.H. NESCent is supported by the National Science Foundation (NSF) EF-0905606. For training in phylogenetic comparative methods, we thank the AnthroTree Workshop (supported by NSF BCS-0923791). Y.S. thanks the National Natural Science Foundation of China (Project 31170995) and National Basic Research Program (973 Program: 2010CB833904). E.E.B. thanks the Duke Vertical Integration Program and the Duke Undergraduate Research Support Office. J.M.P. was supported by a Newton International Fellowship from the Royal Society and the British Academy. L.R.S. thanks the James S. McDonnell Foundation for Award 220020242. L.J.N.B. and M.L.P. acknowledge the National Institutes of Mental Health (R01-MH096875 and R01-MH089484), a Duke Institute for Brain Sciences Incubator Award (to M.L.P.), and a Duke Center for Interdisciplinary Decision Sciences Fellowship (to L.J.N.B.). E.V. and E.A. thank the Programma Nazionale per la Ricerca–Consiglio Nazionale delle Ricerche (CNR) Aging Program 2012–2014 for financial support, Roma Capitale–Museo Civico di Zoologia and Fondazione Bioparco for hosting the Istituto di Scienze e Tecnologie della Cognizione–CNR Unit of Cognitive Primatology and Primate Centre, and Massimiliano Bianchi and Simone Catarinacci for assistance with capuchin monkeys. K.F. thanks the Japan Society for the Promotion of Science (JSPS) for Grant-in-Aid for Scientific Research 20220004. F. Aureli thanks the Stages in the Evolution and Development of Sign Use project (Contract 012-984 NESTPathfinder) and the Integrating Cooperation Research Across Europe project (Contract 043318), both funded by the European Community’s Sixth Framework Programme (FP6/2002–2006). F. Amici was supported by Humboldt Research Fellowship for Postdoctoral Researchers (Humboldt ID 1138999). L.F.J. and M.M.D. acknowledge NSF Electrical, Communications, and Cyber Systems Grant 1028319 (to L.F.J.) and an NSF Graduate Fellowship (to M.M.D.). C.H. thanks Grant-in-Aid for JSPS Fellows (10J04395). A.T. thanks Research Fellowships of the JSPS for Young Scientists (21264). F.R. and Z.V. acknowledge Austrian Science Fund (FWF) Project P21244-B17, the European Research Council (ERC) under the European Union’s Seventh Framework Programme (FP/2007–2013)/ERC Grant Agreement 311870 (to F.R.), Vienna Science and Technology Fund Project CS11-026 (to Z.V.), and many private sponsors, including Royal Canin for financial support and the Game Park Ernstbrunn for hosting the Wolf Science Center. S.M.R. thanks the Natural Sciences and Engineering Research Council (Canada). J.K.Y. thanks the US Department of Agriculture–Wildlife Services–National Wildlife Research Center. J.F.C. thanks the James S. McDonnell Foundation and Alfred P. Sloan Foundation. E.L.M. and B.H. thank the Duke Lemur Center and acknowledge National Institutes of Health Grant 5 R03 HD070649-02 and NSF Grants DGE-1106401, NSF-BCS-27552, and NSF-BCS-25172. This is Publication 1265 of the Duke Lemur Center.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323533111/-/DCSupplemental.
References
- 1.Darwin C. 1871. The Descent of Man, and Selection in Relation to Sex. (D. Appleton and Company, New York)
- 2.Beran MJ, Beran MM. Chimpanzees remember the results of one-by-one addition of food items to sets over extended time periods. Psychol Sci. 2004;15(2):94–99. doi: 10.1111/j.0963-7214.2004.01502004.x. [DOI] [PubMed] [Google Scholar]
- 3.Bourjade M, Thierry B, Call J, Dufour V. Are monkeys able to plan for future exchange? Anim Cogn. 2012;15(5):783–795. doi: 10.1007/s10071-012-0502-1. [DOI] [PubMed] [Google Scholar]
- 4.Bugnyar T, Heinrich B. Ravens, Corvus corax, differentiate between knowledgeable and ignorant competitors. Proc Biol Sci. 2005;272(1573):1641–1646. doi: 10.1098/rspb.2005.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Call J, Tomasello M. Does the chimpanzee have a theory of mind? 30 years later. Trends Cogn Sci. 2008;12(5):187–192. doi: 10.1016/j.tics.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Cheney DL, Seyfarth RM. How Monkeys See the World: Inside the Mind of Another Species. Chicago: Univ of Chicago Press; 1990. [Google Scholar]
- 7.Cheney DL, Seyfarth RM. Baboon Metaphysics: The Evolution of a Social Mind. Chicago: Univ of Chicago Press; 2007. [Google Scholar]
- 8.Chittka L, Niven J. Are bigger brains better? Curr Biol. 2009;19(21):R995–R1008. doi: 10.1016/j.cub.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 9.de Waal FB. Putting the altruism back into altruism: The evolution of empathy. Annu Rev Psychol. 2008;59:279–300. doi: 10.1146/annurev.psych.59.103006.093625. [DOI] [PubMed] [Google Scholar]
- 10. Fragaszy DM, Perry S (2003) The Biology of Traditions: Models and Evidence (Cambridge Univ Press, Cambridge, UK)
- 11.Gácsi M, Miklósi A, Varga O, Topál J, Csányi V. Are readers of our face readers of our minds? Dogs (Canis familiaris) show situation-dependent recognition of human’s attention. Anim Cogn. 2004;7(3):144–153. doi: 10.1007/s10071-003-0205-8. [DOI] [PubMed] [Google Scholar]
- 12.Galef BG, Laland KN. Social learning in animals: Empirical studies and theoretical models. Bioscience. 2005;55(6):489–499. [Google Scholar]
- 13.Leavens DA, Hopkins WD. Intentional communication by chimpanzees: A cross-sectional study of the use of referential gestures. Dev Psychol. 1998;34(5):813–822. doi: 10.1037//0012-1649.34.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liedtke J, Werdenich D, Gajdon GK, Huber L, Wanker R. Big brains are not enough: Performance of three parrot species in the trap-tube paradigm. Anim Cogn. 2011;14(1):143–149. doi: 10.1007/s10071-010-0347-4. [DOI] [PubMed] [Google Scholar]
- 15.Maestripieri D. Primate Psychology. Cambridge, MA: Harvard Univ Press; 2003. [Google Scholar]
- 16.Matsuzawa T. Primate Origins of Human Cognition and Behavior. Tokyo: Springer; 2001. [Google Scholar]
- 17.Miklósi Á. Dog Behaviour, Evolution, and Cognition. Oxford: Oxford Univ Press; 2008. [Google Scholar]
- 18.Range F, Horn L, Bugnyar T, Gajdon GK, Huber L. Social attention in keas, dogs, and human children. Anim Cogn. 2009;12(1):181–192. doi: 10.1007/s10071-008-0181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmitt V, Pankau B, Fischer J. Old world monkeys compare to apes in the primate cognition test battery. PLoS ONE. 2012;7(4):e32024. doi: 10.1371/journal.pone.0032024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Udell MA, Dorey NR, Wynne CDL. What did domestication do to dogs? A new account of dogs’ sensitivity to human actions. Biol Rev Camb Philos Soc. 2010;85(2):327–345. doi: 10.1111/j.1469-185X.2009.00104.x. [DOI] [PubMed] [Google Scholar]
- 21.Wasserman E, Kiedinger R, Bhatt R. Conceptual behavior in pigeons: Categories, subcategories, and pseudocategories. J Exp Psychol Anim Behav Process. 1988;14(3):235. [Google Scholar]
- 22.Webb B. Cognition in insects. Philos Trans R Soc Lond B Biol Sci. 2012;367(1603):2715–2722. doi: 10.1098/rstb.2012.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weir AA, Chappell J, Kacelnik A. Shaping of hooks in New Caledonian crows. Science. 2002;297(5583):981. doi: 10.1126/science.1073433. [DOI] [PubMed] [Google Scholar]
- 24.Wright AA, Santiago HC, Sands SF, Kendrick DF, Cook RG. Memory processing of serial lists by pigeons, monkeys, and people. Science. 1985;229(4710):287–289. doi: 10.1126/science.9304205. [DOI] [PubMed] [Google Scholar]
- 25.Arnold K, Zuberbühler K. Language evolution: Semantic combinations in primate calls. Nature. 2006;441(7091):303. doi: 10.1038/441303a. [DOI] [PubMed] [Google Scholar]
- 26.Benson-Amram S, Holekamp KE. Innovative problem solving by wild spotted hyenas. Proc Biol Sci. 2012;279(1744):4087–4095. doi: 10.1098/rspb.2012.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cook RG, Brown MF, Riley DA. Flexible memory processing by rats: Use of prospective and retrospective information in the radial maze. J Exp Psychol Anim Behav Process. 1985;11(3):453–469. [PubMed] [Google Scholar]
- 28.Suddendorf T, Corballis MC. Behavioural evidence for mental time travel in nonhuman animals. Behav Brain Res. 2010;215(2):292–298. doi: 10.1016/j.bbr.2009.11.044. [DOI] [PubMed] [Google Scholar]
- 29.MacLean EL, Hare B. Bonobos and chimpanzees infer the target of another’s attention. Anim Behav. 2012;83(2):345–353. [Google Scholar]
- 30.Melis AP, Hare B, Tomasello M. Chimpanzees recruit the best collaborators. Science. 2006;311(5765):1297–1300. doi: 10.1126/science.1123007. [DOI] [PubMed] [Google Scholar]
- 31.Emery NJ, Clayton NS. The mentality of crows: Convergent evolution of intelligence in corvids and apes. Science. 2004;306(5703):1903–1907. doi: 10.1126/science.1098410. [DOI] [PubMed] [Google Scholar]
- 32.Pepperberg IM. The Alex Studies: Cognitive and Communicative Abilities of Grey Parrots. Cambridge, MA: Harvard Univ Press; 2009. [Google Scholar]
- 33.Bekoff M, Allen C, Burghardt GM, editors. The Cognitive Animal: Empirical and Theoretical Perspectives on Animal Cognition. Cambridge, MA: MIT Press; 2002. [Google Scholar]
- 34.Brannon EM, Terrace HS. Ordering of the numerosities 1 to 9 by monkeys. Science. 1998;282(5389):746–749. doi: 10.1126/science.282.5389.746. [DOI] [PubMed] [Google Scholar]
- 35.Hampton RR, Zivin A, Murray EA. Rhesus monkeys (Macaca mulatta) discriminate between knowing and not knowing and collect information as needed before acting. Anim Cogn. 2004;7(4):239–246. doi: 10.1007/s10071-004-0215-1. [DOI] [PubMed] [Google Scholar]
- 36.Kaminski J, Call J, Fischer J. Word learning in a domestic dog: Evidence for “fast mapping”. Science. 2004;304(5677):1682–1683. doi: 10.1126/science.1097859. [DOI] [PubMed] [Google Scholar]
- 37.Mendes N, Hanus D, Call J. Raising the level: Orangutans use water as a tool. Biol Lett. 2007;3(5):453–455. doi: 10.1098/rsbl.2007.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horner V, Whiten A. Causal knowledge and imitation/emulation switching in chimpanzees (Pan troglodytes) and children (Homo sapiens) Anim Cogn. 2005;8(3):164–181. doi: 10.1007/s10071-004-0239-6. [DOI] [PubMed] [Google Scholar]
- 39.Inoue S, Matsuzawa T. Working memory of numerals in chimpanzees. Curr Biol. 2007;17(23):R1004–R1005. doi: 10.1016/j.cub.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 40.Kaminski J, Call J, Tomasello M. Chimpanzees know what others know, but not what they believe. Cognition. 2008;109(2):224–234. doi: 10.1016/j.cognition.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 41.Hare B. From hominoid to hominid mind: What changed and why? Annu Rev Anthropol. 2011;40:293–309. [Google Scholar]
- 42.Penn DC, Holyoak KJ, Povinelli DJ. Darwin’s mistake: Explaining the discontinuity between human and nonhuman minds. Behav Brain Sci. 2008;31(2):109–130. doi: 10.1017/S0140525X08003543. discussion 130–178. [DOI] [PubMed] [Google Scholar]
- 43.Tomasello M. The Cultural Origins of Human Cognition. Cambridge, MA: Harvard Univ Press; 1999. [Google Scholar]
- 44.Whiten A, Erdal D. The human socio-cognitive niche and its evolutionary origins. Philos Trans R Soc Lond B Biol Sci. 2012;367(1599):2119–2129. doi: 10.1098/rstb.2012.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shettleworth SJ. Modularity, comparative cognition and human uniqueness. Philos Trans R Soc Lond B Biol Sci. 2012;367(1603):2794–2802. doi: 10.1098/rstb.2012.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boyd R, Richerson PJ. Culture and the evolution of human cooperation. Philos Trans R Soc Lond B Biol Sci. 2009;364(1533):3281–3288. doi: 10.1098/rstb.2009.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Csibra G, Gergely G. Natural pedagogy. Trends Cogn Sci. 2009;13(4):148–153. doi: 10.1016/j.tics.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 48.Fitch WT, Huber L, Bugnyar T. Social cognition and the evolution of language: Constructing cognitive phylogenies. Neuron. 2010;65(6):795–814. doi: 10.1016/j.neuron.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haun DB, Rapold CJ, Call J, Janzen G, Levinson SC. Cognitive cladistics and cultural override in Hominid spatial cognition. Proc Natl Acad Sci USA. 2006;103(46):17568–17573. doi: 10.1073/pnas.0607999103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herrmann E, Call J, Hernàndez-Lloreda MV, Hare B, Tomasello M. Humans have evolved specialized skills of social cognition: The cultural intelligence hypothesis. Science. 2007;317(5843):1360–1366. doi: 10.1126/science.1146282. [DOI] [PubMed] [Google Scholar]
- 51.Hill K, Barton M, Hurtado AM. The emergence of human uniqueness: Characters underlying behavioral modernity. Evol Anthropol. 2009;18(5):187–200. [Google Scholar]
- 52.Hill K, Kaplan H, Lancaster J, Hurtado A. A theory of human life history evolution: Diet, intelligence, and longevity. Evol Anthropol. 2000;9(4):156–185. [Google Scholar]
- 53.Kagan J. The uniquely human in human nature. Daedalus. 2004;133(4):77–88. [Google Scholar]
- 54.Melis AP, Semmann D. How is human cooperation different? Philos Trans R Soc Lond B Biol Sci. 2010;365(1553):2663–2674. doi: 10.1098/rstb.2010.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meltzoff AN. Origins of theory of mind, cognition and communication. J Commun Disord. 1999;32(4):251–269. doi: 10.1016/s0021-9924(99)00009-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moll H, Tomasello M. Cooperation and human cognition: The vygotskian intelligence hypothesis. Philos Trans R Soc B-Biol Sci. 2007;362(1480):639–648. doi: 10.1098/rstb.2006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Penn DC, Povinelli DJ. On the lack of evidence that non-human animals possess anything remotely resembling a ‘theory of mind’. Philos Trans R Soc Lond B Biol Sci. 2007;362(1480):731–744. doi: 10.1098/rstb.2006.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Penn DC, Povinelli DJ. Causal cognition in human and nonhuman animals: A comparative, critical review. Annu Rev Psychol. 2007;58:97–118. doi: 10.1146/annurev.psych.58.110405.085555. [DOI] [PubMed] [Google Scholar]
- 59.Dean LG, Kendal RL, Schapiro SJ, Thierry B, Laland KN. Identification of the social and cognitive processes underlying human cumulative culture. Science. 2012;335(6072):1114–1118. doi: 10.1126/science.1213969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jensen K, Call J, Tomasello M. Chimpanzees are rational maximizers in an ultimatum game. Science. 2007;318(5847):107–109. doi: 10.1126/science.1145850. [DOI] [PubMed] [Google Scholar]
- 61.Fehr E, Fischbacher U. Third-party punishment and social norms. Evol Hum Behav. 2004;25(2):63–87. [Google Scholar]
- 62.Beach FA. The snark was a boojum. Am Psychol. 1950;5(4):115–124. [Google Scholar]
- 63.Bitterman ME. Phyletic differences in learning. Am Psychol. 1965;20(6):396–410. doi: 10.1037/h0022328. [DOI] [PubMed] [Google Scholar]
- 64.Griffin DR. Prospects for a cognitive ethology. Behav Brain Sci. 1978;1(4):527–538. [Google Scholar]
- 65.Hodos W, Campbell CB. Scala naturae-why there is no theory in comparative psychology. Psychol Rev. 1969;76(4):337–350. [Google Scholar]
- 66.Macphail EM. The comparative psychology of intelligence. Behav Brain Sci. 1987;10(4):645–656. [Google Scholar]
- 67.Platt ML, Spelke ES. What can developmental and comparative cognitive neuroscience tell us about the adult human brain? Curr Opin Neurobiol. 2009;19(1):1–5. doi: 10.1016/j.conb.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shettleworth SJ. Where is the comparison in comparative cognition?: Alternative research programs. Psychol Sci. 1993;4(3):179–184. [Google Scholar]
- 69.Shettleworth SJ. The evolution of comparative cognition: Is the snark still a boojum? Behav Processes. 2009;80(3):210–217. doi: 10.1016/j.beproc.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 70.MacLean EL, et al. How does cognition evolve? Phylogenetic comparative psychology. Anim Cogn. 2012;15(2):223–238. doi: 10.1007/s10071-011-0448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haun DB, Jordan FM, Vallortigara G, Clayton NS. Origins of spatial, temporal and numerical cognition: Insights from comparative psychology. Trends Cogn Sci. 2010;14(12):552–560. doi: 10.1016/j.tics.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 72.Bitterman ME. Evolution of intelligence. Sci Am. 1965;212(1):92–100. doi: 10.1038/scientificamerican0165-92. [DOI] [PubMed] [Google Scholar]
- 73.Bitterman ME. The comparative analysis of learning. Science. 1975;188(4189):699–709. doi: 10.1126/science.188.4189.699. [DOI] [PubMed] [Google Scholar]
- 74.Bitterman ME. Toward a comparative psychology of learning. Am Psychol. 1960;15(11):704–712. [Google Scholar]
- 75.Reader SM. Evolution of cognition. In: Losos JB, editor. Oxford Bibliographies in Evolutionary Biology. New York: Oxford Univ Press; 2014. [Google Scholar]
- 76.Shultz S, Dunbar RIM. Species differences in executive function correlate with hippocampus volume and neocortex ratio across nonhuman primates. J Comp Psychol. 2010;124(3):252–260. doi: 10.1037/a0018894. [DOI] [PubMed] [Google Scholar]
- 77.Deaner RO, Isler K, Burkart J, van Schaik C. Overall brain size, and not encephalization quotient, best predicts cognitive ability across non-human primates. Brain Behav Evol. 2007;70(2):115–124. doi: 10.1159/000102973. [DOI] [PubMed] [Google Scholar]
- 78.Roth G, Dicke U. Evolution of the brain and intelligence. Trends Cogn Sci. 2005;9(5):250–257. doi: 10.1016/j.tics.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 79.Barton RA. Visual specialization and brain evolution in primates. Proc Biol Sci. 1998;265(1409):1933–1937. doi: 10.1098/rspb.1998.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barton RA. The evolutionary ecology of the primate brain. In: Lee PC, editor. Comparative Primate Socioecology. Cambridge, UK: Cambridge Univ Press; 1999. pp. 167–203. [Google Scholar]
- 81.Barton RA. Primate brain evolution: Integrating comparative, neurophysiological, and ethological data. Evol Anthropol. 2006;15(6):224–236. [Google Scholar]
- 82.Beauchamp G, Fernandez-Juricic E. Is there a relationship between forebrain size and group size in birds? Evol Ecol Res. 2004;6(6):833–842. [Google Scholar]
- 83.Deaner R, van Schaik C, Johnson V. Do some taxa have better domain-general cognition than others? A meta-analysis of nonhuman primate studies. Evol Psychol. 2006;4:149–196. [Google Scholar]
- 84.Deaner RO, Nunn CL, van Schaik CP. Comparative tests of primate cognition: Different scaling methods produce different results. Brain Behav Evol. 2000;55(1):44–52. doi: 10.1159/000006641. [DOI] [PubMed] [Google Scholar]
- 85.Dunbar RI. The social brain hypothesis. Evol Anthropol. 1998;6(5):178–190. [Google Scholar]
- 86.Dunbar RI, Shultz S. Understanding primate brain evolution. Philos Trans R Soc Lond B Biol Sci. 2007;362(1480):649–658. doi: 10.1098/rstb.2006.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dunbar RIM. The social brain: Mind, language, and society in evolutionary perspective. Annu Rev Anthropol. 2003;32:163–181. [Google Scholar]
- 88.Gibson KR. Evolution of human intelligence: The roles of brain size and mental construction. Brain Behav Evol. 2002;59(1-2):10–20. doi: 10.1159/000063730. [DOI] [PubMed] [Google Scholar]
- 89.Healy SD, Rowe C. A critique of comparative studies of brain size. Proc Biol Sci. 2007;274(1609):453–464. doi: 10.1098/rspb.2006.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shultz S, Dunbar R. Encephalization is not a universal macroevolutionary phenomenon in mammals but is associated with sociality. Proc Natl Acad Sci USA. 2010;107(50):21582–21586. doi: 10.1073/pnas.1005246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smaers JB, Soligo C. Brain reorganization, not relative brain size, primarily characterizes anthropoid brain evolution. Proc Biol Sci. 2013;280(1759):20130269. doi: 10.1098/rspb.2013.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reader SM, Hager Y, Laland KN. The evolution of primate general and cultural intelligence. Philos Trans R Soc B-Biol Sci. 2011;366(1567):1017–1027. doi: 10.1098/rstb.2010.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nunn CL. The Comparative Method in Evolutionary Anthropology and Biology. Chicago: Univ of Chicago Press; 2011. [Google Scholar]
- 94.Balda RP, Kamil AC, Bednekoff PA. Predicting cognitive capacities from natural histories: Examples from four corvid species. Current Ornithology. 1996;13:33–66. [Google Scholar]
- 95.Czeschlik T. Animal cognition–the phylogeny and ontogeny of cognitive abilities. Anim Cogn. 1998;1(1):1–2. [Google Scholar]
- 96.Garland T, Adolph SC. Why not to do 2-species comparative-studies - limitations on inferring adaptation. Physiol Zool. 1994;67(4):797–828. [Google Scholar]
- 97.Gómez JC. Species comparative studies and cognitive development. Trends Cogn Sci. 2005;9(3):118–125. doi: 10.1016/j.tics.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 98.Papini MR. Pattern and process in the evolution of learning. Psychol Rev. 2002;109(1):186–201. doi: 10.1037/0033-295x.109.1.186. [DOI] [PubMed] [Google Scholar]
- 99.Sherwood CC, et al. Evolution of increased glia-neuron ratios in the human frontal cortex. Proc Natl Acad Sci USA. 2006;103(37):13606–13611. doi: 10.1073/pnas.0605843103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Herculano-Houzel S, Collins CE, Wong P, Kaas JH. Cellular scaling rules for primate brains. Proc Natl Acad Sci USA. 2007;104(9):3562–3567. doi: 10.1073/pnas.0611396104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Finlay BL, Darlington RB. Linked regularities in the development and evolution of mammalian brains. Science. 1995;268(5217):1578–1584. doi: 10.1126/science.7777856. [DOI] [PubMed] [Google Scholar]
- 102.Kaas JH. Why is brain size so important: Design problems and solutions as neocortex gets biggeror smaller. Brain Mind. 2000;1(1):7–23. [Google Scholar]
- 103.Holloway RL., Jr Cranial capacity and neuron number: A critique and proposal. Am J Phys Anthropol. 1966;25(3):305–314. doi: 10.1002/ajpa.1330250310. [DOI] [PubMed] [Google Scholar]
- 104.Herculano-Houzel S. Brains matter, bodies maybe not: The case for examining neuron numbers irrespective of body size. Ann N Y Acad Sci. 2011;1225(1):191–199. doi: 10.1111/j.1749-6632.2011.05976.x. [DOI] [PubMed] [Google Scholar]
- 105.Herculano-Houzel S. The remarkable, yet not extraordinary, human brain as a scaled-up primate brain and its associated cost. Proc Natl Acad Sci USA. 2012;109(Suppl 1):10661–10668. doi: 10.1073/pnas.1201895109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Herculano-Houzel S. The human brain in numbers: A linearly scaled-up primate brain. Front Hum Neurosci. 2009;3:31. doi: 10.3389/neuro.09.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Azevedo FAC, et al. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009;513(5):532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- 108.Jerison HJ. Evolution of the Brain and Intelligence. New York: Academic; 1973. [Google Scholar]
- 109.Kappelman J. The evolution of body mass and relative brain size in fossil hominids. J Hum Evol. 1996;30(3):243–276. [Google Scholar]
- 110.Lefebvre L, Reader SM, Sol D. Brains, innovations and evolution in birds and primates. Brain Behav Evol. 2004;63(4):233–246. doi: 10.1159/000076784. [DOI] [PubMed] [Google Scholar]
- 111.Sol D, Bacher S, Reader SM, Lefebvre L. Brain size predicts the success of mammal species introduced into novel environments. Am Nat. 2008;172(Suppl 1):S63–S71. doi: 10.1086/588304. [DOI] [PubMed] [Google Scholar]
- 112.Sol D, Duncan RP, Blackburn TM, Cassey P, Lefebvre L. Big brains, enhanced cognition, and response of birds to novel environments. Proc Natl Acad Sci USA. 2005;102(15):5460–5465. doi: 10.1073/pnas.0408145102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rightmire GP. Brain size and encephalization in early to Mid-Pleistocene Homo. Am J Phys Anthropol. 2004;124(2):109–123. doi: 10.1002/ajpa.10346. [DOI] [PubMed] [Google Scholar]
- 114.Lovejoy CO. The origin of man. Science. 1981;211(4480):341–350. doi: 10.1126/science.211.4480.341. [DOI] [PubMed] [Google Scholar]
- 115.Seyfarth RM, Cheney DL. What are big brains for? Proc Natl Acad Sci USA. 2002;99(7):4141–4142. doi: 10.1073/pnas.082105099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aiello LC, Wheeler P. The expensive-tissue hypothesis: The brain and the digestive system in human and primate evolution. Curr Anthropol. 1995;36(2):199–221. [Google Scholar]
- 117.Isler K, van Schaik CP. Metabolic costs of brain size evolution. Biol Lett. 2006;2(4):557–560. doi: 10.1098/rsbl.2006.0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Barrickman NL, Bastian ML, Isler K, van Schaik CP. Life history costs and benefits of encephalization: A comparative test using data from long-term studies of primates in the wild. J Hum Evol. 2008;54(5):568–590. doi: 10.1016/j.jhevol.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 119.Bennett PM, Harvey PH. Brain size, development and metabolism in birds and mammals. J Zool. 1985;207(4):491–509. [Google Scholar]
- 120.Charvet CJ, Finlay BL. Embracing covariation in brain evolution: Large brains, extended development, and flexible primate social systems. Prog Brain Res. 2012;195:71–87. doi: 10.1016/B978-0-444-53860-4.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Isler K, van Schaik CP. The Expensive Brain: A framework for explaining evolutionary changes in brain size. J Hum Evol. 2009;57(4):392–400. doi: 10.1016/j.jhevol.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 122.Martin RD. Relative brain size and basal metabolic rate in terrestrial vertebrates. Nature. 1981;293(5827):57–60. doi: 10.1038/293057a0. [DOI] [PubMed] [Google Scholar]
- 123.Jerison HJ. Animal intelligence as encephalization. Philos Trans R Soc Lond B Biol Sci. 1985;308(1135):21–35. doi: 10.1098/rstb.1985.0007. [DOI] [PubMed] [Google Scholar]
- 124.Byrne RW, Corp N. Neocortex size predicts deception rate in primates. Proc Biol Sci. 2004;271(1549):1693–1699. doi: 10.1098/rspb.2004.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Byrne RW, Whiten AW. Machiavellian Intelligence: Social Expertise and the Evolution of Intellect in Monkeys, Apes, and Humans. Oxford: Clarendon Press; 1988. [Google Scholar]
- 126.Jolly A. Lemur social behavior and primate intelligence. Science. 1966;153(3735):501–506. doi: 10.1126/science.153.3735.501. [DOI] [PubMed] [Google Scholar]
- 127.Humphrey NK. The social function of intellect. In: Bateson P, Hinde R, editors. Growing Points in Ethology. Cambridge, UK: Cambridge Univ Press; 1976. [Google Scholar]
- 128.de Waal FBM, Tyack PL. Animal Social Complexity: Intelligence, Culture, and Individualized Societies. Cambridge, MA: Harvard Univ Press; 2003. [Google Scholar]
- 129.Dunbar RI, Shultz S. Evolution in the social brain. Science. 2007;317(5843):1344–1347. doi: 10.1126/science.1145463. [DOI] [PubMed] [Google Scholar]
- 130.Bond AB, Kamil AC, Balda RP. Social complexity and transitive inference in corvids. Anim Behav. 2003;65(3):479–487. [Google Scholar]
- 131.Byrne RW, Bates LA. Sociality, evolution and cognition. Curr Biol. 2007;17(16):R714–R723. doi: 10.1016/j.cub.2007.05.069. [DOI] [PubMed] [Google Scholar]
- 132.Cunningham E, Janson C. A socioecological perspective on primate cognition, past and present. Anim Cogn. 2007;10(3):273–281. doi: 10.1007/s10071-007-0078-3. [DOI] [PubMed] [Google Scholar]
- 133.Dunbar RIM. Grooming, Gossip and the Evolution of Language. London: Faber and Faber; 1996. [Google Scholar]
- 134.Emery N. The evolution of social cognition. In: Easton A, Emery N, editors. The Cognitive Neuroscience of Social Behaviour. London: Routledge; 2005. pp. 115–156. [Google Scholar]
- 135.Holekamp KE. Questioning the social intelligence hypothesis. Trends Cogn Sci. 2007;11(2):65–69. doi: 10.1016/j.tics.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 136.Kummer H, Daston L, Gigerenzer G, Silk J. The social intelligence hypothesis. In: Weingart P, Mitchell SD, Richerson PJ, Maasen S, editors. Human by Nature: Between Biology and the Social Sciences. Mahwah, NJ: Lawrence Erlbaum Associates; 1997. pp. 157–179. [Google Scholar]
- 137.Maclean EL, Merritt DJ, Brannon EM. Social organization predicts transitive reasoning in prosimian primates. Anim Behav. 2008;76(2):479–486. doi: 10.1016/j.anbehav.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Paz-Y-Miño C G, Bond AB, Kamil AC, Balda RP. Pinyon jays use transitive inference to predict social dominance. Nature. 2004;430(7001):778–781. doi: 10.1038/nature02723. [DOI] [PubMed] [Google Scholar]
- 139.Reader SM, Laland KN. Social intelligence, innovation, and enhanced brain size in primates. Proc Natl Acad Sci USA. 2002;99(7):4436–4441. doi: 10.1073/pnas.062041299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Barrett L, Henzi P, Rendall D. Social brains, simple minds: Does social complexity really require cognitive complexity? Philos Trans R Soc Lond B Biol Sci. 2007;362(1480):561–575. doi: 10.1098/rstb.2006.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Seyfarth RM, Cheney DL, Bergman TJ. Primate social cognition and the origins of language. Trends Cogn Sci. 2005;9(6):264–266. doi: 10.1016/j.tics.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 142.Barton RA. Neocortex size and behavioural ecology in primates. Proc Biol Sci. 1996;263(1367):173–177. doi: 10.1098/rspb.1996.0028. [DOI] [PubMed] [Google Scholar]
- 143.Kudo H, Dunbar RIM. Neocortex size and social network size in primates. Anim Behav. 2001;62(4):711–722. [Google Scholar]
- 144.Pérez-Barbería FJ, Shultz S, Dunbar RIM. Evidence for coevolution of sociality and relative brain size in three orders of mammals. Evolution. 2007;61(12):2811–2821. doi: 10.1111/j.1558-5646.2007.00229.x. [DOI] [PubMed] [Google Scholar]
- 145.Shultz S, Dunbar RIM. The evolution of the social brain: Anthropoid primates contrast with other vertebrates. Proc Biol Sci. 2007;274(1624):2429–2436. doi: 10.1098/rspb.2007.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Maclean EL, et al. Group size predicts social but not nonsocial cognition in lemurs. PLoS ONE. 2013;8(6):e66359. doi: 10.1371/journal.pone.0066359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sandel AA, MacLean E, Hare B. Evidence from four lemur species that ringtailed lemur social cognition converges with that of haplorhine primates. Anim Behav. 2011;81(5):925–931. [Google Scholar]
- 148.Bugnyar T, Stöwe M, Heinrich B. Ravens, Corvus corax, follow gaze direction of humans around obstacles. Proc Biol Sci. 2004;271(1546):1331–1336. doi: 10.1098/rspb.2004.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Plotnik JM, de Waal FB, Reiss D. Self-recognition in an Asian elephant. Proc Natl Acad Sci USA. 2006;103(45):17053–17057. doi: 10.1073/pnas.0608062103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Holekamp KE, Sakai ST, Lundrigan BL. Social intelligence in the spotted hyena (Crocuta crocuta) Philos Trans R Soc Lond B Biol Sci. 2007;362(1480):523–538. doi: 10.1098/rstb.2006.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Milton K. Distribution patterns of tropical plant foods as an evolutionary stimulus to primate mental development. Am Anthropol. 1981;83(3):534–548. [Google Scholar]
- 152.Clutton-Brock TH, Harvey PH. Primates, brains and ecology. J Zool. 1980;190(MAR):309–323. [Google Scholar]
- 153.Barton RA. Embodied cognitive evolution and the cerebellum. Philos Trans R Soc Lond B Biol Sci. 2012;367(1599):2097–2107. doi: 10.1098/rstb.2012.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zuberbühler K, Janmaat K. Foraging cognition in non-human primates. In: Platt ML, Ghazanfar AA, editors. Primate Neuroethology. New York: Oxford University Press; 2010. pp. 64–83. [Google Scholar]
- 155.Fish JL, Lockwood CA. Dietary constraints on encephalization in primates. Am J Phys Anthropol. 2003;120(2):171–181. doi: 10.1002/ajpa.10136. [DOI] [PubMed] [Google Scholar]
- 156.Shettleworth SJ. Cognition, Evolution, and Behavior. 2nd Ed. Oxford: Oxford Univ Press; 2010. [Google Scholar]
- 157.Balda RP, Kamil AC. A comparative study of cache recovery by 3 corvid species. Anim Behav. 1989;38:486–495. [Google Scholar]
- 158.Barkley CL, Jacobs LF. Sex and species differences in spatial memory in food-storing kangaroo rats. Anim Behav. 2007;73(2):321–329. [Google Scholar]
- 159.Bednekoff PA, Balda RP, Kamil AC, Hile AG. Long-term spatial memory in four seed-caching corvid species. Anim Behav. 1997;53(2):335–341. [Google Scholar]
- 160.Heilbronner SR, Rosati AG, Stevens JR, Hare B, Hauser MD. A fruit in the hand or two in the bush? Divergent risk preferences in chimpanzees and bonobos. Biol Lett. 2008;4(3):246–249. doi: 10.1098/rsbl.2008.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Krebs JR. Food-storing birds: Adaptive specialization in brain and behaviour? Philos Trans R Soc Lond B Biol Sci. 1990;329(1253):153–160. doi: 10.1098/rstb.1990.0160. [DOI] [PubMed] [Google Scholar]
- 162.Krebs JR, Sherry DF, Healy SD, Perry VH, Vaccarino AL. Hippocampal specialization of food-storing birds. Proc Natl Acad Sci USA. 1989;86(4):1388–1392. doi: 10.1073/pnas.86.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Platt ML, Brannon EM, Briese TL, French JA. Differences in feeding ecology predict differences in performance between golden lion tamarins (leontopithecus rosalia) and wied’s marmosets (callithrix kuhli) on spatial and visual memory tasks. Anim Learn Behav. 1996;24(4):384–393. [Google Scholar]
- 164.Rosati AG, Stevens JR, Hare B, Hauser MD. The evolutionary origins of human patience: Temporal preferences in chimpanzees, bonobos, and human adults. Curr Biol. 2007;17(19):1663–1668. doi: 10.1016/j.cub.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 165.Rosati AG, Stevens JR, Hauser MD. The effect of handling time on temporal discounting in two new world primates. Anim Behav. 2006;71(6):1379–1387. [Google Scholar]
- 166.Shettleworth SJ. Spatial memory in food-storing birds. Philos Trans R Soc Lond B Biol Sci. 1990;329(1253):143–151. doi: 10.1098/rstb.1990.0160. [DOI] [PubMed] [Google Scholar]
- 167.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324(5927):646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 168.Hauser MD. Perseveration, inhibition and the prefrontal cortex: A new look. Curr Opin Neurobiol. 1999;9(2):214–222. doi: 10.1016/s0959-4388(99)80030-0. [DOI] [PubMed] [Google Scholar]
- 169.Tangney JP, Baumeister RF, Boone AL. High self-control predicts good adjustment, less pathology, better grades, and interpersonal success. J Pers. 2004;72(2):271–324. doi: 10.1111/j.0022-3506.2004.00263.x. [DOI] [PubMed] [Google Scholar]
- 170.Moffitt TE, et al. A gradient of childhood self-control predicts health, wealth, and public safety. Proc Natl Acad Sci USA. 2011;108(7):2693–2698. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mischel W, Shoda Y, Rodriguez MI. Delay of gratification in children. Science. 1989;244(4907):933–938. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- 172.Bezdjian S, Baker LA, Tuvblad C. Genetic and environmental influences on impulsivity: A meta-analysis of twin, family and adoption studies. Clin Psychol Rev. 2011;31(7):1209–1223. doi: 10.1016/j.cpr.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Boogert NJ, Anderson RC, Peters S, Searcy WA, Nowicki S. Song repertoire size in male song sparrows correlates with detour reaching, but not with other cognitive measures. Anim Behav. 2011;81(6):1209–1216. [Google Scholar]
- 174.Amici F, Aureli F, Call J. Fission-fusion dynamics, behavioral flexibility, and inhibitory control in primates. Curr Biol. 2008;18(18):1415–1419. doi: 10.1016/j.cub.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 175.Smaers JB, et al. Primate prefrontal cortex evolution: Human brains are the extreme of a lateralized ape trend. Brain Behav Evol. 2011;77(2):67–78. doi: 10.1159/000323671. [DOI] [PubMed] [Google Scholar]
- 176.Genovesio A, Wise SP, Passingham RE. Prefrontal-parietal function: From foraging to foresight. Trends Cogn Sci. 2014;18(2):72–81. doi: 10.1016/j.tics.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 177.Preuss TM. Do rats have prefrontal cortex? The rose-woolsey-akert program reconsidered. J Cogn Neurosci. 1995;7(1):1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- 178.Passingham RE, Wise SP. The Neurobiology of the Prefrontal Cortex: Anatomy, Evolution, and the Origin of Insight. Oxford: Oxford Univ Press; 2012. [Google Scholar]
- 179.de Kort SR, Clayton NS. An evolutionary perspective on caching by corvids. Proc Biol Sci. 2006;273(1585):417–423. doi: 10.1098/rspb.2005.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rilling JK, Glasser MF, Jbabdi S, Andersson J, Preuss TM. Continuity, divergence, and the evolution of brain language pathways. Front Evol Neurosci. 2011;3:11. doi: 10.3389/fnevo.2011.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mantini D, Corbetta M, Romani GL, Orban GA, Vanduffel W. Evolutionarily novel functional networks in the human brain? J Neurosci. 2013;33(8):3259–3275. doi: 10.1523/JNEUROSCI.4392-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rilling JK, et al. The evolution of the arcuate fasciculus revealed with comparative DTI. Nat Neurosci. 2008;11(4):426–428. doi: 10.1038/nn2072. [DOI] [PubMed] [Google Scholar]
- 183.Mars RB, Sallet J, Neubert F-X, Rushworth MF. Connectivity profiles reveal the relationship between brain areas for social cognition in human and monkey temporoparietal cortex. Proc Natl Acad Sci USA. 2013;110(26):10806–10811. doi: 10.1073/pnas.1302956110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.MacLean EL, Barrickman NL, Johnson EM, Wall CE. Sociality, ecology, and relative brain size in lemurs. J Hum Evol. 2009;56(5):471–478. doi: 10.1016/j.jhevol.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 185.Allen KL, Kay RF. Dietary quality and encephalization in platyrrhine primates. Proc Biol Sci. 2012;279(1729):715–721. doi: 10.1098/rspb.2011.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Burnham KP, Anderson DR. Model Selection and Multi-Model Inference: A Practical Information-Theoretic Approach. New York: Springer; 2002. [Google Scholar]
- 187.Montgomery SH, Capellini I, Barton RA, Mundy NI. Reconstructing the ups and downs of primate brain evolution: Implications for adaptive hypotheses and Homo floresiensis. BMC Biol. 2010;8(1):9. doi: 10.1186/1741-7007-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gigerenzer G. The modularity of social intelligence. In: Whiten A, Byrne RA, editors. Machiavellian Intelligence II: Extensions and Evaluation. Cambridge, UK: Cambridge Univ Press; 1997. pp. 264–288. [Google Scholar]
- 189.Geary DC, Huffman KJ. Brain and cognitive evolution: Forms of modularity and functions of mind. Psychol Bull. 2002;128(5):667–698. doi: 10.1037/0033-2909.128.5.667. [DOI] [PubMed] [Google Scholar]
- 190.Cosmides L. The logic of social exchange: Has natural selection shaped how humans reason? Studies with the Wason selection task. Cognition. 1989;31(3):187–276. doi: 10.1016/0010-0277(89)90023-1. [DOI] [PubMed] [Google Scholar]
- 191. Fodor JA (1983) The Modularity of Mind: An Essay on Faculty Psychology (MIT Press, Cambridge, MA)
- 192.Hirschfeld LA, Gelman SA. Mapping the Mind: Domain Specificity in Cognition and Culture. New York: Cambridge Univ Press; 1994. [Google Scholar]
- 193.Cosmides L, Tooby J. Mapping the Mind: Domain Specificity in Cognition and Culture. New York: Cambridge Univ Press; 1994. Origins of domain specificity: The evolution of functional organization; pp. 85–116. [Google Scholar]
- 194.Leslie AM. Mapping the Mind: Domain Specificity in Cognition and Culture. New York: Cambridge Univ Press; 1994. Tomm, toby, and agency: Core architecture and domain specificity; pp. 119–148. [Google Scholar]
- 195.Gelman R, Williams EM. 1998. Enabling constraints for cognitive development and learning: Domain specificity and epigenesis. Handbook of Child Psychology, Cognition, Perception, and Language, ed Damon W (John Wiley & Sons Inc., Hoboken, NJ), Vol 2, pp 575–630.
- 196.Amici F, Barney B, Johnson VE, Call J, Aureli F. A modular mind? A test using individual data from seven primate species. PLoS ONE. 2012;7(12):e51918. doi: 10.1371/journal.pone.0051918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Silk J, Cheney D, Seyfarth R. A practical guide to the study of social relationships. Evol Anthropol (Melb) 2013;22(5):213–225. doi: 10.1002/evan.21367. [DOI] [PubMed] [Google Scholar]
- 198.Piaget J. The Construction of Reality in the Child. New York: Basic Books; 1954. [Google Scholar]
- 199.Rice WR, Gaines SD. ‘Heads I win, tails you lose’: Testing directional alternative hypotheses in ecological and evolutionary research. Trends Ecol Evol. 1994;9(6):235–237. doi: 10.1016/0169-5347(94)90258-5. [DOI] [PubMed] [Google Scholar]
- 200.Freckleton RP, Harvey PH, Pagel M. Phylogenetic analysis and comparative data: A test and review of evidence. Am Nat. 2002;160(6):712–726. doi: 10.1086/343873. [DOI] [PubMed] [Google Scholar]
- 201.Pagel M. Inferring the historical patterns of biological evolution. Nature. 1999;401(6756):877–884. doi: 10.1038/44766. [DOI] [PubMed] [Google Scholar]