Significance
Hitherto, both the autonomic nervous system and innate immune system were regarded as systems that cannot be voluntarily influenced. The present study demonstrates that, through practicing techniques learned in a short-term training program, the sympathetic nervous system and immune system can indeed be voluntarily influenced. Healthy volunteers practicing the learned techniques exhibited profound increases in the release of epinephrine, which in turn led to increased production of anti-inflammatory mediators and subsequent dampening of the proinflammatory cytokine response elicited by intravenous administration of bacterial endotoxin. This study could have important implications for the treatment of a variety of conditions associated with excessive or persistent inflammation, especially autoimmune diseases in which therapies that antagonize proinflammatory cytokines have shown great benefit.
Keywords: LPS, cathecholamines, cortisol
Abstract
Excessive or persistent proinflammatory cytokine production plays a central role in autoimmune diseases. Acute activation of the sympathetic nervous system attenuates the innate immune response. However, both the autonomic nervous system and innate immune system are regarded as systems that cannot be voluntarily influenced. Herein, we evaluated the effects of a training program on the autonomic nervous system and innate immune response. Healthy volunteers were randomized to either the intervention (n = 12) or control group (n = 12). Subjects in the intervention group were trained for 10 d in meditation (third eye meditation), breathing techniques (i.a., cyclic hyperventilation followed by breath retention), and exposure to cold (i.a., immersions in ice cold water). The control group was not trained. Subsequently, all subjects underwent experimental endotoxemia (i.v. administration of 2 ng/kg Escherichia coli endotoxin). In the intervention group, practicing the learned techniques resulted in intermittent respiratory alkalosis and hypoxia resulting in profoundly increased plasma epinephrine levels. In the intervention group, plasma levels of the anti-inflammatory cytokine IL-10 increased more rapidly after endotoxin administration, correlated strongly with preceding epinephrine levels, and were higher. Levels of proinflammatory mediators TNF-α, IL-6, and IL-8 were lower in the intervention group and correlated negatively with IL-10 levels. Finally, flu-like symptoms were lower in the intervention group. In conclusion, we demonstrate that voluntary activation of the sympathetic nervous system results in epinephrine release and subsequent suppression of the innate immune response in humans in vivo. These results could have important implications for the treatment of conditions associated with excessive or persistent inflammation, such as autoimmune diseases.
The innate immune system is crucial to our survival, but excessive or persistent proinflammatory cytokine production can result in tissue damage and organ injury, such as in autoimmune diseases. Biological therapies that antagonize proinflammatory cytokines or their receptors are very effective and have revolutionized the treatment of autoimmune diseases, such as rheumatoid arthritis and inflammatory bowel disease (1, 2). However, these drugs are expensive and have serious side effects (3, 4). Therefore, innovative therapies aimed at limiting inflammatory cytokine production in a more physiological manner are warranted.
Acute activation of the sympathetic nervous system attenuates inflammation via activation of β2-adrenoreceptors by catecholamines, exemplified by the fact that (nor)epinephrine attenuates lipopolysaccharide (LPS)-induced TNF-α release in vitro (5, 6) and short-term infusion of epinephrine limits production of proinflammatory cytokines in vivo during experimental endotoxemia (i.v. administration of LPS in healthy volunteers) (7). In addition, as part of a stress response, increased levels of catecholamines are often accompanied by elevations of the well-known immunosuppressive hormone cortisol [via activation of the hypothalamic–pituitary–adrenal (HPA) axis] (8, 9).
Next to exogenous (i.e., pharmacological or electrical) modulation of the autonomic nervous system (ANS), endogenous stimulation of ANS activity may also limit the inflammatory response, but the ANS is generally regarded as a system that cannot be voluntarily influenced. However, results from a recently performed case study on a Dutch individual, who holds several world records with regard to withstanding extreme cold, suggest otherwise (10). It was shown that this individual was able to voluntarily activate the sympathetic nervous system through a self-developed method involving meditation, exposure to cold, and breathing techniques. This resulted in increased catecholamine and cortisol release and a remarkably mild innate immune response during experimental endotoxemia compared with more than 100 subjects who previously underwent experimental endotoxemia. In the present study, we investigated the effects of his training program (see Movie S1 for an impression) on sympathetic nervous system parameters and the innate immune response in healthy male volunteers during experimental endotoxemia in a randomized controlled fashion.
Results
Baseline characteristics of subjects that underwent experimental endotoxemia in both groups were similar (Table 1).
Table 1.
Subject demographic characteristics
| Parameter | Trained group, n = 12 | Control group, n = 12 | P value |
| Age, y | 24 (19–27) | 22 (19–27) | 0.43 |
| Height, cm | 181 (172–190) | 185 (179–189) | 0.30 |
| Weight, kg | 75 (58–92) | 78 (65–91) | 0.25 |
| BMI, kg/m2 | 23 (19–26) | 23 (20–27) | 0.98 |
| HR, beats/min | 60 (41–80) | 61 (40–75) | 0.88 |
| MAP, mmHg | 92 (82–113) | 94 (78–105) | 0.89 |
Parameters were measured during screening visit. BMI, body mass index; HR, heart rate; MAP, mean arterial blood pressure. Data are presented as median (range). P values were calculated using Mann–Whitney u test.
Cardiorespiratory Parameters, Temperature, and Symptoms.
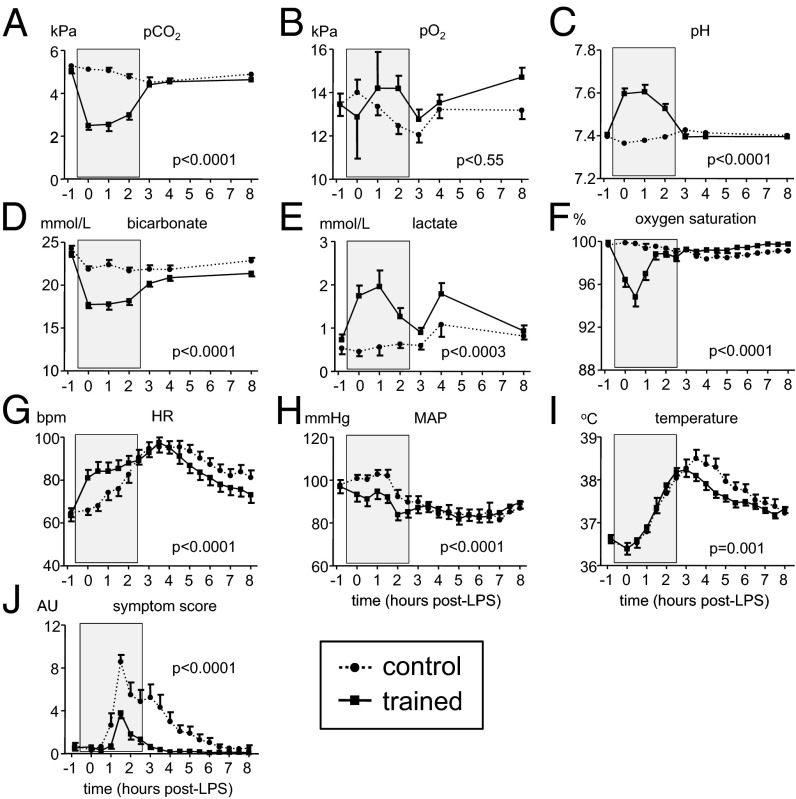
In the control group, arterial blood gas parameters pCO2, pO2, pH, bicarbonate, lactate, and oxygen saturation were normal and did not substantially change during endotoxemia (Fig. 1 A–F). In contrast, in trained individuals, practicing the learned breathing techniques resulted in an immediate and profound decrease of pCO2 and bicarbonate, and an increase in pH (reaching up to 7.75 in individual subjects; Fig. 2 and Movie S2), indicating acute respiratory alkalosis, which normalized quickly after cessation of the breathing techniques. Mean pO2 remained virtually unaltered in trained subjects, whereas lactate levels were significantly elevated, but not to clinically relevant levels. A significant decrease in oxygen saturation was observed in the trained group during practicing of the breathing techniques (Fig. 1F). Minimum oxygen saturation levels in each cycle of hyper/hypoventilation (after cessation of breathing for several minutes) typically dropped to around 50% in trained individuals for a short period (∼10 s; Fig. 2 and Movie S2). Heart rate and mean arterial blood pressure (MAP) showed a pattern typical for endotoxemia in the control group: a gradual decrease in MAP and a compensatory rise in heart rate after LPS administration (Fig. 1 G and H). In the trained group, heart rate increased after commencing the breathing techniques and normalized earlier compared with the control group, whereas MAP decreased during the breathing techniques and thereafter followed the same pattern as in the control group. LPS administration resulted in fever, with a maximum temperature increase in the control group of 1.9 ± 0.2 °C (mean ± SEM), whereas this increase was less pronounced and normalized earlier in the trained group (Fig. 1I). Self-reported symptoms (nausea, headache, shivering, and muscle and back pain on a six-point Likert scale) peaked 1.5 h after LPS administration in both groups, but were attenuated in the trained individuals compared with the control group (reduction of 56% in peak levels; Fig. 1J).
Fig. 1.
Cardiorespiratory parameters, temperature, and symptoms during experimental endotoxemia in control and trained subjects. (A) Carbon dioxide partial pressure (pCO2) in arterial blood. (B) Oxygen partial pressure (pO2) in arterial blood. (C) pH in arterial blood. (D) Bicarbonate (HCO3−) in arterial blood. (E) Lactate in arterial blood. (F) Oxygen saturation measured by pulse oximetry. (G) Heart rate (HR). (H) Mean arterial pressure (MAP). (I) Temperature. (J) Score of self-reported symptoms. Data are expressed as mean ± SEM of 12 subjects per group. Gray box indicates period in which the trained subjects practiced their learned breathing techniques. P values between groups were calculated using repeated measures two-way analysis of variance (ANOVA, interaction term). AU, arbitrary units; bpm, beats per minute.
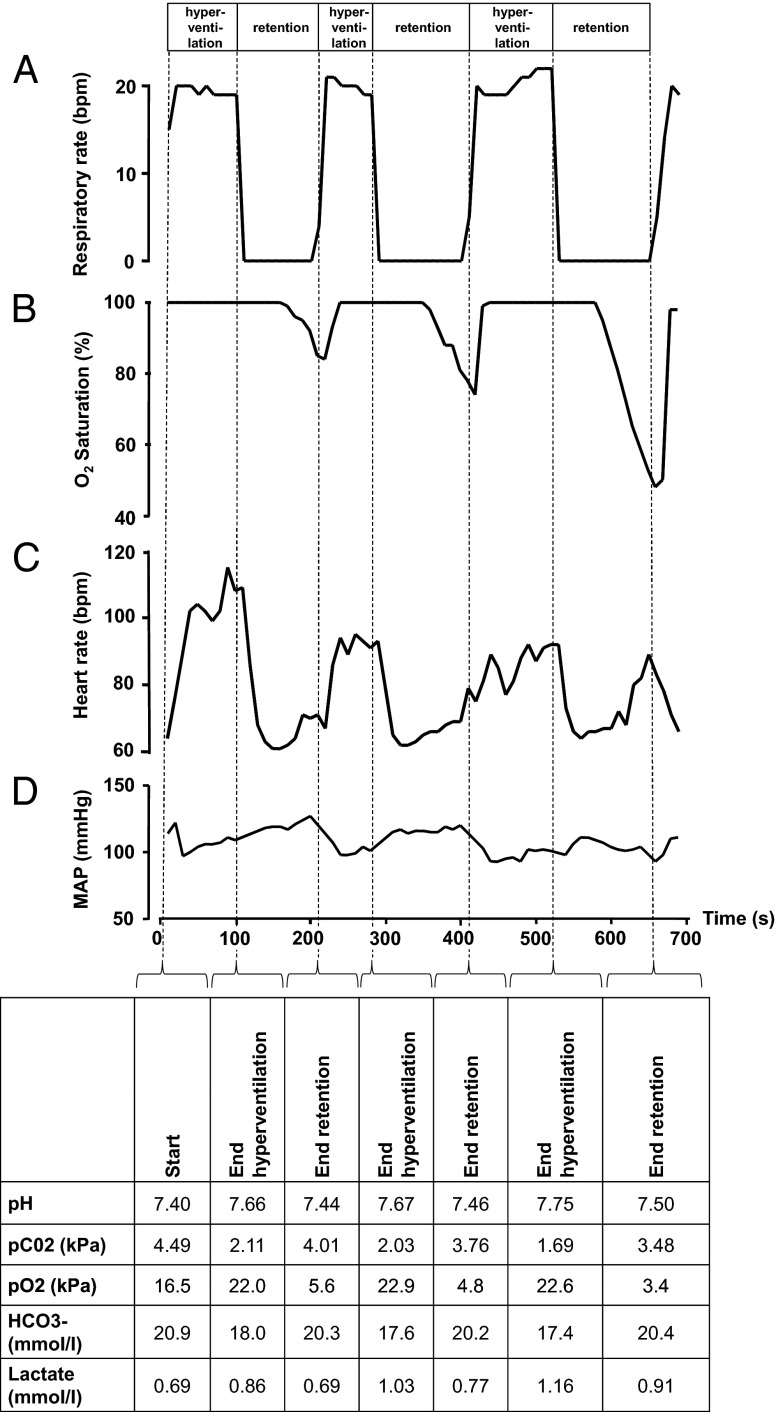
Fig. 2.
Cardiorespiratory and biochemical changes during cyclic hyperventilation and breath retention in a representative subject of the trained group. (A) The respiratory rate alternately increased to around 20 breaths per minute (bpm) for several minutes, and then dropped to zero during voluntary breath retention. These cyclic changes in respiration resulted in profound changes in (B) oxygen saturation, (C) heart rate, and (D) mean arterial pressure. The data depicted were sampled from the monitor every 10 s. At the end of each hyperventilation phase and breath retention phase, an arterial blood sample was drawn for arterial blood gas analysis, of which the results are listed in the table below D. The cycles of hyper/hypoventilation in this particular subject can be viewed in Movie S2.
Catecholamine and Cortisol Levels.
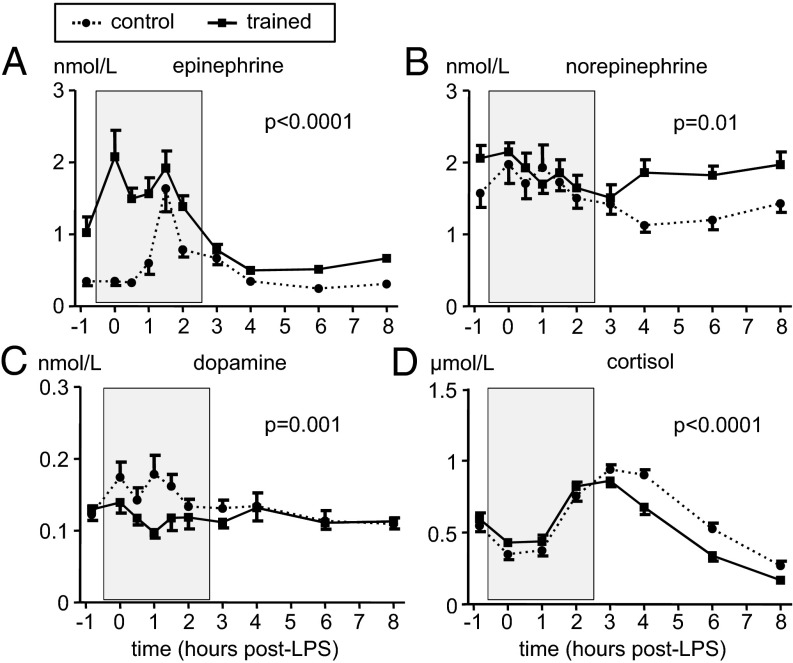
Plasma epinephrine levels (Fig. 3A) increased sharply 1 h after LPS administration and peaked at T = 1.5 h in the control group. In trained subjects, baseline epinephrine levels were significantly higher compared with the control group (mean ± SEM: 1.02 ± 0.22 vs. 0.35 ± 0.06 nmol/L, P = 0.007) (unpaired Student t test). After starting practicing the learned breathing techniques, epinephrine levels further increased in this group and peaked just before administration of LPS (mean ± SEM: 2.08 ± 0.37 nmol/L at T = 0 h, with individual subjects reaching up to 5.3 nmol/L) and remained elevated until cessation of the breathing techniques. In contrast to epinephrine, norepinephrine and dopamine levels remained within the reference range throughout the experiment (Fig. 3 B and C). Norepinephrine levels were similar between groups during the breathing period, although trained subjects displayed higher levels at baseline and after cessation of the breathing techniques. In contrast, dopamine levels were slightly lower in trained individuals during the breathing techniques but were similar between groups before and afterward. There were no differences in serum levels of the stress hormone cortisol between the groups before or during the period in which the trained group practiced their techniques; however, levels normalized more quickly in trained individuals (Fig. 3D).
Fig. 3.
Plasma cathecholamine concentrations and serum cortisol concentrations during experimental endotoxemia in control and trained subjects. (A) Plasma epinephrine. (B) Plasma norepinephrine. (C) Plasma dopamine. (D) Serum cortisol. Data are expressed as mean ± SEM of 12 subjects per group. Gray box indicates period in which the trained subjects practiced their learned breathing techniques. P values between groups were calculated using repeated measures two-way analysis of variance (ANOVA, interaction term).
Leukocyte Counts.
Total leukocyte counts in both groups showed the typical endotoxemia-induced biphasic pattern with an initial leukopenia followed by leukocytosis (Fig. S1A). Leukocyte concentrations were markedly higher in trained individuals. 30 min after start of the breathing techniques (T = 0 h), an increase in lymphocytes was observed in trained individuals, which was not present in the control group (Fig. S1B). Concentrations of neutrophils and monocytes were similar between groups at this early time point, but were distinctly higher in the trained group at later time points (Fig. S1 C and D).
Plasma Cytokines.
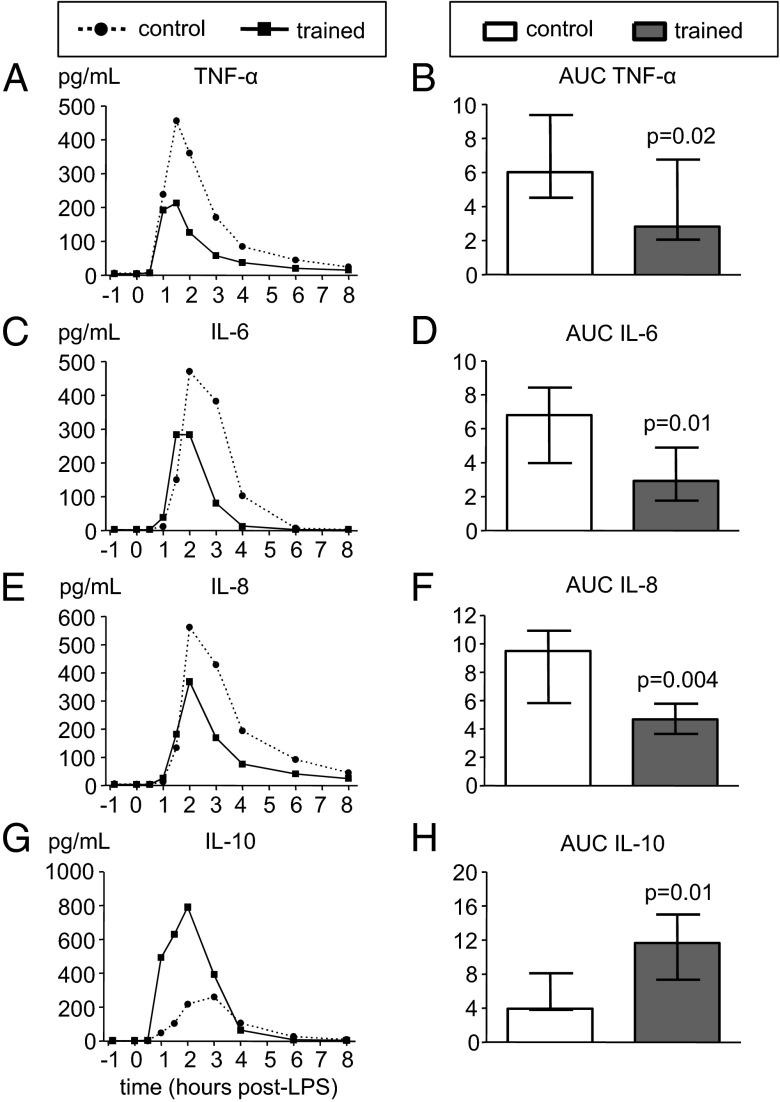
Plasma concentrations of proinflammatory cytokines TNF-α, IL-6, and IL-8, and the anti-inflammatory cytokine IL-10 all markedly increased after LPS administration in both groups (Fig. 4). However, in trained individuals, TNF-α, IL-6, and IL-8 levels were significantly attenuated, whereas the IL-10 response was greatly augmented compared with the control group (TNF-α, IL-6, and IL-8 levels 53%, 57%, and 51% lower; IL-10 levels 194% higher). Furthermore, IL-10 levels in the trained group increased sharply early after LPS administration (at T = 1 h) and peaked 1 h before the peak observed in the control group. In line with previous reports (11), plasma levels of the proinflammatory cytokine IL-1β were barely detectable during human endotoxemia. Concentrations were below the detection limit (3.9 pg/mL) in all but four subjects (two in each group, showing very low concentrations (4–6 pg/mL) at one to three time points with no apparent kinetics over time). Concentrations of the anti-inflammatory cytokine TGF-β showed no kinetics after administration of LPS and were not different between groups (Fig. S2A). We also measured plasma concentrations of leptin, an adipokine that exerts proinflammatory activity. At baseline (T = −1 h), there was a trend toward lower levels of leptin in the trained group compared with the control group (mean ± SEM: 3.36 ± 0.55 vs. 4.99 ± 0.74 ng/mL, P = 0.09, unpaired Student t test), which remained apparent at all subsequent time points (Fig. S2B). Leptin kinetics showed a biphasic pattern with an initial modest decrease followed by a gradual increase in both groups. However, there were no differences between groups over time.
Fig. 4.
Plasma cytokine concentrations during endotoxemia in control and trained subjects. (A, C, E, and G) Median values of pro- (TNF-α, IL-6, and IL-8) and anti-inflammatory (IL-10) cytokines (n = 12 per group). (B, D, F, and H) Median ± interquartile range of area under curve (AUC) of pro- (TNF-α, IL-6, and IL-8) and anti-inflammatory (IL-10) cytokines (n = 12 per group; unit: ×104 pg/mL·h). P values were calculated using Mann–Whitney u tests.
Correlation Analyses.
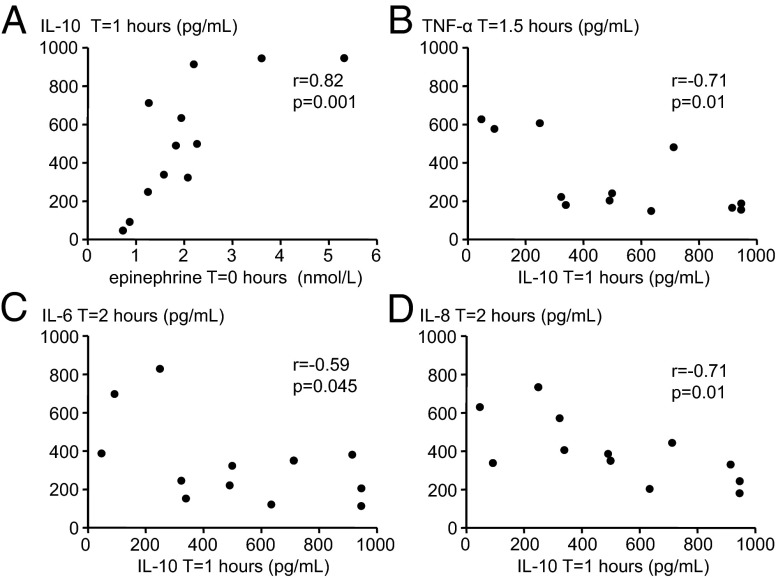
As depicted in Fig. 5A, there was a strong positive correlation (rs = 0.82, P = 0.001) between epinephrine levels in the trained group at T = 0 h (30 min after commencing the breathing techniques) and the early increase in IL-10 levels at T = 1 h, which was not present in the control group (rs = 0.18, P = 0.571). Furthermore, there were significant inverse correlations between levels of the anti-inflammatory cytokine IL-10 at T = 1 h and peak levels of the proinflammatory mediators TNF-α (at T = 1.5 h), IL-6 (at T = 2 h), and IL-8 (at T = 2 h) in the trained group (Fig. 5 B–D). In the control group, no such inverse correlations between IL-10 and proinflammatory cytokines were observed. In fact, we found significant positive correlations between preceding TNF-α and IL-6 levels on the one hand and IL-10 levels at later time points (TNF-αT = 1 vs. IL-10T = 2: rs = 0.59, P = 0.045 and IL-6T = 1.5 vs. IL-10T = 2: rs = 0.60, P = 0.039).
Fig. 5.
Correlations in trained individuals. (A) Correlation between peak plasma levels of epinephrine (at T = 0 h) and plasma levels of the anti-inflammatory cytokine IL-10 at T = 1 h. (B) Correlation between plasma levels of the anti-inflammatory cytokine IL-10 at T = 1 h and peak plasma levels of the proinflammatory cytokine TNF-α (at T = 1.5 h). (C) Correlation between plasma levels of the anti-inflammatory cytokine IL-10 at T = 1 h and peak plasma levels of the proinflammatory cytokine IL-6 (at T = 2 h). (D) Correlation between plasma levels of the anti-inflammatory cytokine IL-10 at T = 1 h and peak plasma levels of the proinflammatory cytokine IL-8 (at T = 2 h). R and P values were calculated using Spearman correlation.
Discussion
Herein, we show that a short-term training program and practicing breathing techniques learned during this training program results in release of epinephrine, induction of early anti-inflammatory IL-10 production, and consequently attenuation of the proinflammatory innate immune response during experimental human endotoxemia. Also, trained individuals experienced fewer endotoxemia-associated flu-like symptoms, and a more swift normalization of fever and cortisol levels, which are likely the result of the attenuated proinflammatory response.
This study demonstrates that the in vivo innate immune response can be voluntarily influenced in a nonpharmacological manner through voluntary activation of the sympathetic nervous system. In accordance with the data of our control group, human endotoxemia in itself has been shown previously to result in increased levels of epinephrine (12). However, in trained individuals epinephrine levels were already profoundly increased 30 min after start of practicing the breathing techniques, before LPS administration. Epinephrine levels in trained individuals were even higher than those reported in a recent study in which acute stress elicited by a bungee jump was found to suppress cytokine production by leukocytes ex vivo stimulated with LPS (13). As norepinephrine, dopamine, and cortisol levels were not increased in the training group, it appears that the techniques predominantly result in stimulation of the sympathetic input to the adrenal medulla, because this is the most abundant source of epinephrine in the body and epinephrine-producing chromaffin cells in the adrenal medulla are much more abundant than those producing norepinephrine (14).
The observed potentiating effects on anti-inflammatory IL-10 production as well as the attenuation of proinflammatory cytokine levels are in agreement with a previously performed study, where epinephrine was i.v. administered before LPS in healthy volunteers and resulted in early and increased IL-10 production (7), and with studies showing that pretreatment with IL-10 results in attenuation of the proinflammatory response in healthy volunteers (15, 16). In the training group, strong inverse correlations between IL-10 levels at an early time point and later-occurring peak levels of the proinflammatory mediators were found, whereas in the control group the opposite was found: positive correlations between preceding levels of proinflammatory mediators with the later-occurring peak levels of IL-10. These findings indicate that the proinflammatory response drives IL-10 production in the control group, whereas the epinephrine-induced early increase in IL-10 production inhibits proinflammation in the trained group. The early increases in lymphocytes and subsequent higher concentrations of circulating neutrophils in the training group compared with the control group can also be attributed to the elevated epinephrine levels found in trained individuals, as catecholamines induce leukocytosis characterized by an initial lymphocytosis followed by an increase of other subpopulations (17). Furthermore, similar changes in leukocyte counts were previously observed during voluntary hyperventilation (18). Our study is limited by the fact that we did not measure specific leukocyte subtypes such as CD3, CD4, and CD8 numbers as well as B cells, dendritic cells, and natural killer (NK) cells, some of which have been shown to be specifically altered by catecholamines and/or stress (19, 20).
It appears that mainly the breathing techniques used by the trained individuals account for the increase in epinephrine and subsequent attenuation of the inflammatory response. A limitation of our study design is that it does not allow the identification of the particular component of the practiced breathing exercises that results in increased epinephrine levels. Furthermore, the effect of the length of the training and the length of propensity for altered responses after training has yet to be determined. However, the effects on epinephrine are likely a consequence of both the hyperventilation phase and hypoxia due to breath retention, as both have been demonstrated to increase epinephrine levels (18, 21–24). The hyperventilation-induced increase in epinephrine was shown to be dependent on decreased levels of bicarbonate, as hyperventilation combined with bicarbonate infusion (resulting in hypocapnia and alkalosis, but normal bicarbonate levels) nullified epinephrine increase (24). In concordance, in the present study, bicarbonate levels were significantly lower in the trained subjects during practicing of the breathing techniques compared with control subjects. The attenuated cytokine response is unlikely to be a direct result from low pCO2 and high pH levels because hypocapnic alkalosis, as opposed to hypercapnic acidosis (25), is not associated with anti-inflammatory effects. Therefore, epinephrine is the most probable intermediate factor (7). Nevertheless, it cannot be ruled out that other elements of the training, apart from practicing the breathing exercises, ultimately affected the LPS-induced innate immune response. For instance, the exposition to extreme cold and subsequent rewarming during the training sessions might have resulted ischemic preconditioning and/or release of danger associated molecular patterns (DAMPs), which could result in a tolerant state toward a subsequent LPS challenge.
It remains to be determined whether the results of this study using an acute model of inflammation in healthy volunteers can be extrapolated to patients with chronic autoimmune diseases. For instance, chronic stress might be harmful in these conditions due to induction of proinflammatory mediators (26), whereas bouts of short-term stress, similar to the effects of the training intervention described in this study, may be beneficial due to immunosuppressive effects (26). Of interest, the in vivo anti-inflammatory potential in humans of biologics currently used in the treatment of rheumatoid arthritis was first established in proof-of-principle human endotoxemia studies (27, 28), illustrating the relevance of the model to investigate novel therapies for this type of disease.
In conclusion, the present proof-of-principle study demonstrates that the sympathetic nervous system and immune system can be voluntarily influenced through practicing techniques that are relatively easy to learn within a short time frame. It therefore could have important implications for the treatment of a variety of conditions associated with excessive or persistent inflammation, especially auto-immune diseases.
Materials and Methods
Subjects.
This parallel randomized controlled study was registered at ClinicalTrials.gov as NCT01835457. After approval by the local ethics committee of the Radboud University Nijmegen Medical Centre (CMO 2012/455), 30 healthy, nonsmoking, Dutch male volunteers were included in the trial. All subjects provided written informed consent and experiments were in accordance with the Declaration of Helsinki, including current revisions, and Good Clinical Practice guidelines. Subjects were screened before the start of the experiment and had a normal physical examination, electrocardiography, and routine laboratory values. Exclusion criteria were: febrile illness during the 2 wk before the endotoxemia experiment, taking any prescription medication, history of spontaneous vagal collapse, practicing or experience with any kind of meditation, or participation in a previous trial where LPS was administered. The subjects were randomly allocated to the trained group (n = 18) or the control group (n = 12) by the opening of a sealed envelope prepared by a research nurse not involved in the study. After having fulfilled the training program, 12 of the 18 trained subjects were randomly assigned to participate in the experimental endotoxemia experiments (further explained in Study Design and Training Procedure below).
Three subjects in the control group that underwent endotoxemia on the same day and received LPS from the same ampoule were excluded from the trial and replaced. Their symptoms, temperature rise, hemodynamic response, and cytokine response were inconsistent with having received an adequate dose of 2 ng/kg LPS. Batchwise determination of cytokine levels revealed exceptionally low levels in all three subjects: Their peak cytokine response (TNF-α and IL-6) was less than half of that of the lowest recorded in a cohort of 112 healthy male subjects that previously underwent experimental endotoxemia (10) and peaked at atypical time points (subject 1, TNF-α = 39 pg/mL at 4 h after LPS administration and IL-6 = 27 pg/mL at 4 h after LPS administration; subject 2, TNF-α = 32 pg/mL at 3 h after LPS administration and IL-6 = 31 pg/mL at 3 h after LPS administration; and subject 3, TNF-α = 9 pg/mL at 2 h after LPS administration and IL-6 = 7 pg/mL at 3 h after LPS administration). Therefore, a endotoxin dose administration error was assumed and the subjects were replaced.
Study Design and Training Procedure.
The study was sequentially conducted in two identical blocks, each consisting of nine subjects in the trained group (of which six finally participated in the endotoxemia experiments, further explained below) and six subjects in the control group. This design was chosen to minimize the bias due to differences in the interval between the end of the training period and the endotoxemia experiments. As the aim of our study was to investigate the effects of the training intervention on the innate immune response in a standardized model of systemic inflammation, we did not assess the effects of the training intervention on immune system parameters in the absence of endotoxemia. A schematic overview of the study design (one block) is depicted in Fig. S3. The trained group was trained by Dutch individual Wim Hof and three trainers who previously received an instructor course by Wim Hof to become a trainer. A medical doctor of the study team (L.T.v.E.) and the principal investigator (M.K.) were present during all training sessions (in Poland and in The Netherlands), and during the experimental endotoxemia experiments. The first 4 d of the training program took place in Poland and were most intensive. The program consisted of three main elements: meditation, exposure to cold, and breathing techniques (see Movie S1 for an impression of the training program).
-
i)
Meditation, so-called “third eye meditation,” a form of meditation including visualizations aimed at total relaxation.
-
ii)
During the training, subjects voluntarily exposed themselves to cold in several ways: standing in the snow barefoot for up to 30 min and lying bare chested in the snow for 20 min; daily dipping/swimming in ice-cold water (0–1 °C) for up to several minutes (including complete submersions); and hiking up a snowy mountain (elevation: 1,590 m) bare chested, wearing nothing but shorts and shoes at temperatures ranging from −5 to −12 °C (wind chill: −12 to −27 °C).
-
iii)
Breathing techniques, consisting of two exercises. In the first exercise subjects were asked to hyperventilate for an average of 30 breaths. Subsequently, the subjects exhaled and held their breath for ∼2–3 min (“retention phase”). The duration of breath retention was entirely at the discretion of the subject himself. Breath retention was followed by a deep inhalation breath, that was held for 10 s. Subsequently a new cycle of hyper/hypoventilation began. The second exercise consisted of deep inhalations and exhalations in which every inhalation and exhalation was followed by breath holding for 10 s, during which the subject tightened all his body muscles. These two breathing exercises were also performed during the endotoxemia experiments. Additional element of the training program consisted of strength exercises (e.g., push-ups and yoga balance techniques).
After returning from Poland, the subjects practiced the techniques they learned daily by themselves at home (2–3 h/d; cold exposure was achieved through taking cold showers) until the endotoxemia experiment day (5–9 d later). In addition, a final group training took place and at the end of this day, six of the nine trained subjects (in each block) were randomly selected for participation in the endotoxemia experiments, using the sealed envelope method. This selection was performed to allow for subject replacement in case of an adverse event or illness in one of the trained subjects selected for the endotoxemia experiments. The selected subjects practiced in a final training session led by Wim Hof on the day before the endotoxemia experiment day. Wim Hof was present to coach the subjects during the endotoxemia experiment days during the 3 h that the subjects in the trained group practiced the learned techniques. The control group did not undergo any training procedures throughout the study period.
Experimental Human Endotoxemia.
Subjects refrained from caffeine- or alcohol-containing substances 24 h before the start of the experiment, and food 10 h before the start of the endotoxemia experiment. The experiments were performed at the research unit of the intensive care department. The procedures on the endotoxemia experiment day are depicted in Fig. S4. Purified lipopolysaccharide (LPS, US Standard Reference Endotoxin Escherichia coli O:113) obtained from the Pharmaceutical Development Section of the National Institutes of Health, supplied as a lyophilized powder, was reconstituted in 5 mL saline 0.9% for injection and vortex mixed for at least 20 min after reconstitution. The LPS solution was administered as an i.v. bolus injection at a dose of 2 ng/kg body weight in 1 min at T = 0 h. A cannula was placed in an antecubital vein to permit infusion of 0.9% NaCl solution; the subjects received 1.5 L 0.9% NaCl during 1 h starting 1 h before endotoxin infusion (prehydration) as part of our standard endotoxemia protocol (29), followed by 150 mL/h until 6 h after endotoxin infusion and 75 mL/h until the end of the experiment. The radial artery was cannulated using a 20-gauge arterial catheter (Angiocath; Becton Dickinson) and connected to an arterial pressure monitoring set (Edwards Lifesciences) to allow the continuous monitoring of blood pressure and blood sampling. Heart rate (three-lead electrocardiogram), blood pressure, respiratory rate, and oxygen saturation (pulse oximetry) data were recorded from a Philips MP50 patient monitor every 30 s by a custom in-house–developed data recording system, starting 1 h before administration of LPS until discharge from the intensive care unit 8 h after LPS administration. Body temperature was measured using an infrared tympanic thermometer (FirstTemp Genius 2; Sherwood Medical). LPS-induced flu-like symptoms (headache, nausea, shivering, muscle and back pain) were scored every 30 min on a six-point Likert scale (0 = no symptoms, 5 = worst ever experienced), resulting in a total score of 0–25.
Thirty minutes before LPS administration (T = −0.5 h), subjects in the trained group started the first breathing technique (hyper/hypoventilation cycles, see Movie S2) until T = 1 h, followed by the second breathing technique (deep inhalation and exhalation in combination with tightening muscles) until T = 2.5 h. Afterward, the subjects stopped practicing all of the techniques. The control group did not practice any techniques throughout the endotoxemia experiment day.
Blood Gas Parameters.
Blood gas parameters were analyzed in lithium heparin anticoagulated arterial blood using CG4+ cartridges and a point-of-care i-STAT blood gas analyzer (Abbott).
Catecholamines.
Blood was collected into chilled lithium-heparin tubes and were immediately placed on ice and centrifuged at 2,000 × g for 10 min at 4 °C after which plasma was stored at −80 °C until analysis. Plasma norepinephrine, epinephrine, and dopamine concentrations were measured using routine analysis methods also used for patient samples (HPLCy with fluorometric detection, as described previously) (30).
Cortisol.
Blood was collected in serum-separating tubes and was allowed to clot at room temperature for a minimum of 30 min. Subsequently, samples were centrifuged at 2,000 × g for 10 min at 4 °C, after which serum was stored at −80 °C until analysis. Cortisol levels were determined using a routine analysis method also used for patient samples (electrochemiluminescent immunoassay on a Modular Analytics E170 (Roche Diagnostics).
Leukocyte Counts and Differentiation.
Analysis of leukocyte counts and differentiation was performed in EDTA anticoagulated blood using routine analysis methods also used for patient samples (flow cytometric analysis on a Sysmex XE-5000.
Plasma Cytokines.
EDTA anticoagulated blood was centrifuged immediately at 2,000 × g for 10 min at 4 °C after which plasma was stored at −80 °C until analysis. Concentrations of TNF-α, IL-6, IL-8, and IL-10 were measured using a simultaneous Luminex assay according to the manufacturer’s instructions (Milliplex; Millipore). IL-1β, TGF-β, and leptin were measured using ELISAs according to the manufacturer’s instructions (IL-1β and TGF-β, Quantikine and leptin, Duoset; both R&D Systems).
Calculations and Statistical Analysis.
Data are represented as median and interquartile range/range or mean and SEM based on their distribution (calculated by the Shapiro–Wilk test). Statistical tests used are indicated in the figure/table legends or text. Spearman’s correlation was used. A P value of <0.05 was considered statistically significant. Statistical calculations were performed using Graphpad Prism version 5.0 (GraphPad Software).
Supplementary Material
Acknowledgments
The authors thank K. Mostard, K. Pijper, D. Bernard, and E. Hof for help during the training sessions; the Radboud University Nijmegen Sports Centre for providing space for training days in The Netherlands; and the research nurses of the Radboud University Medical Centre Intensive Care Unit for help during the endotoxemia experiments. This study was supported by a Serendipity Grant from Reumafonds (www.reumafonds.nl).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. T.L.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1322174111/-/DCSupplemental.
References
- 1.Buch MH, Emery P. New therapies in the management of rheumatoid arthritis. Curr Opin Rheumatol. 2011;23(3):245–251. doi: 10.1097/BOR.0b013e3283454124. [DOI] [PubMed] [Google Scholar]
- 2.Cheifetz AS. Management of active Crohn disease. JAMA. 2013;309(20):2150–2158. doi: 10.1001/jama.2013.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJ. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov. 2010;9(4):325–338. doi: 10.1038/nrd3003. [DOI] [PubMed] [Google Scholar]
- 4.Marra CA, Bansback N, Anis AH, Shojania K. Introduction to economic modeling for clinical rheumatologists: Application to biologic agents in rheumatoid arthritis. Clin Rheumatol. 2011;30(Suppl 1):S9–S18. doi: 10.1007/s10067-010-1635-8. [DOI] [PubMed] [Google Scholar]
- 5.Siegmund B, Eigler A, Hartmann G, Hacker U, Endres S. Adrenaline enhances LPS-induced IL-10 synthesis: Evidence for protein kinase A-mediated pathway. Int J Immunopharmacol. 1998;20(1–3):57–69. doi: 10.1016/s0192-0561(98)00014-9. [DOI] [PubMed] [Google Scholar]
- 6.van der Poll T, Jansen J, Endert E, Sauerwein HP, van Deventer SJ. Noradrenaline inhibits lipopolysaccharide-induced tumor necrosis factor and interleukin 6 production in human whole blood. Infect Immun. 1994;62(5):2046–2050. doi: 10.1128/iai.62.5.2046-2050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Poll T, Coyle SM, Barbosa K, Braxton CC, Lowry SF. Epinephrine inhibits tumor necrosis factor-alpha and potentiates interleukin 10 production during human endotoxemia. J Clin Invest. 1996;97(3):713–719. doi: 10.1172/JCI118469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alvarez SM, et al. Low-dose steroid alters in vivo endotoxin-induced systemic inflammation but does not influence autonomic dysfunction. J Endotoxin Res. 2007;13(6):358–368. doi: 10.1177/0968051907086465. [DOI] [PubMed] [Google Scholar]
- 9.van der Poll T, Barber AE, Coyle SM, Lowry SF. Hypercortisolemia increases plasma interleukin-10 concentrations during human endotoxemia—a clinical research center study. J Clin Endocrinol Metab. 1996;81(10):3604–3606. doi: 10.1210/jcem.81.10.8855809. [DOI] [PubMed] [Google Scholar]
- 10.Kox M, et al. The influence of concentration/meditation on autonomic nervous system activity and the innate immune response: A case study. Psychosom Med. 2012;74(5):489–494. doi: 10.1097/PSY.0b013e3182583c6d. [DOI] [PubMed] [Google Scholar]
- 11.van Eijk LT, et al. Gender differences in the innate immune response and vascular reactivity following the administration of endotoxin to human volunteers. Crit Care Med. 2007;35(6):1464–1469. doi: 10.1097/01.CCM.0000266534.14262.E8. [DOI] [PubMed] [Google Scholar]
- 12.Sayk F, et al. Endotoxemia causes central downregulation of sympathetic vasomotor tone in healthy humans. Am J Physiol Regul Integr Comp Physiol. 2008;295(3):R891–R898. doi: 10.1152/ajpregu.90444.2008. [DOI] [PubMed] [Google Scholar]
- 13.van Westerloo DJ, et al. Acute stress elicited by bungee jumping suppresses human innate immunity. Mol Med. 2011;17(3–4):180–188. doi: 10.2119/molmed.2010.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong DL. Why is the adrenal adrenergic? Endocr Pathol. 2003;14(1):25–36. doi: 10.1385/ep:14:1:25. [DOI] [PubMed] [Google Scholar]
- 15.Kumar A, et al. Interleukin-10 blunts the human inflammatory response to lipopolysaccharide without affecting the cardiovascular response. Crit Care Med. 2005;33(2):331–340. doi: 10.1097/01.ccm.0000152229.69180.2. [DOI] [PubMed] [Google Scholar]
- 16.Pajkrt D, et al. Attenuation of proinflammatory response by recombinant human IL-10 in human endotoxemia: Effect of timing of recombinant human IL-10 administration. J Immunol. 1997;158(8):3971–3977. [PubMed] [Google Scholar]
- 17.Benschop RJ, Rodriguez-Feuerhahn M, Schedlowski M. Catecholamine-induced leukocytosis: Early observations, current research, and future directions. Brain Behav Immun. 1996;10(2):77–91. doi: 10.1006/brbi.1996.0009. [DOI] [PubMed] [Google Scholar]
- 18.Staubli M, Vogel F, Bartsch P, Fluckiger G, Ziegler WH. Hyperventilation-induced changes of blood cell counts depend on hypocapnia. Eur J Appl Physiol Occup Physiol. 1994;69(5):402–407. doi: 10.1007/BF00865403. [DOI] [PubMed] [Google Scholar]
- 19.Huang CJ, Webb HE, Garten RS, Kamimori GH, Acevedo EO. Psychological stress during exercise: Lymphocyte subset redistribution in firefighters. Physiol Behav. 2010;101(3):320–326. doi: 10.1016/j.physbeh.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Schedlowski M, et al. Catecholamines induce alterations of distribution and activity of human natural killer (NK) cells. J Clin Immunol. 1993;13(5):344–351. doi: 10.1007/BF00920243. [DOI] [PubMed] [Google Scholar]
- 21.Laderach H, Straub W. Effects of voluntary hyperventilation on glucose, free fatty acids and several glucostatic hormones. Swiss Med Wkly. 2001;131(1–2):19–22. doi: 10.4414/smw.2001.05735. [DOI] [PubMed] [Google Scholar]
- 22.Mantysaari M, et al. Unaltered blood coagulation and platelet function in healthy subjects exposed to acute hypoxia. Aviat Space Environ Med. 2011;82(7):699–703. doi: 10.3357/asem.3012.2011. [DOI] [PubMed] [Google Scholar]
- 23.Oltmanns KM, et al. Acute hypoxia decreases plasma VEGF concentration in healthy humans. Am J Physiol Endocrinol Metab. 2006;290(3):E434–E439. doi: 10.1152/ajpendo.00508.2004. [DOI] [PubMed] [Google Scholar]
- 24.Krapf R, Caduff P, Wagdi P, Staubli M, Hulter HN. Plasma potassium response to acute respiratory alkalosis. Kidney Int. 1995;47(1):217–224. doi: 10.1038/ki.1995.26. [DOI] [PubMed] [Google Scholar]
- 25.Ijland MM, Heunks LM, van der Hoeven JG. Bench-to-bedside review: Hypercapnic acidosis in lung injury–from 'permissive' to 'therapeutic'. Crit Care. 2010;14(6):237. doi: 10.1186/cc9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cutolo M, et al. The hypothalamic-pituitary-adrenal and gonadal axes in rheumatoid arthritis. Ann N Y Acad Sci. 2000;917:835–843. doi: 10.1111/j.1749-6632.2000.tb05449.x. [DOI] [PubMed] [Google Scholar]
- 27.Granowitz EV, et al. Hematologic and immunomodulatory effects of an interleukin-1 receptor antagonist coinfusion during low-dose endotoxemia in healthy humans. Blood. 1993;82(10):2985–2990. [PubMed] [Google Scholar]
- 28.Suffredini AF, et al. Effects of recombinant dimeric TNF receptor on human inflammatory responses following intravenous endotoxin administration. J Immunol. 1995;155(10):5038–5045. [PubMed] [Google Scholar]
- 29.Dorresteijn MJ, et al. Iso-osmolar prehydration shifts the cytokine response towards a more anti-inflammatory balance in human endotoxemia. J Endotoxin Res. 2005;11(5):287–293. doi: 10.1179/096805105X58715. [DOI] [PubMed] [Google Scholar]
- 30.Willemsen JJ, et al. Highly sensitive and specific HPLC with fluorometric detection for determination of plasma epinephrine and norepinephrine applied to kinetic studies in humans. Clin Chem. 1995;41(10):1455–1460. [PubMed] [Google Scholar]