Significance
Secretion of protein products from cells is crucial during development, as well as in adult tissues, where secreted factors perform specialized functions. Previous studies demonstrated that an essential, conserved protein modification (O-glycosylation) affected secretion in Drosophila and mammalian tissues. However, the mechanisms by which secretion was affected were unknown. Here we demonstrate that this protein modification affects secretory vesicle formation and polarized secretion by protecting an essential endoplasmic reticulum/Golgi transmembrane protein (Tango1) from protease cleavage. Moreover, we identify the specific enzyme (PGANT4) and furin protease (Dfur2) that form this regulatory partnership. Finally, our results have implications for the treatment of human diseases resulting from the loss of this protein modification by modulating the activity of specific proteases in vivo.
Keywords: mucin, peritrophic membrane, proventriculus, Drosophila, familial tumoral calcinosis
Abstract
Polarized secretion is crucial in many tissues. The conserved protein modification, O-glycosylation, plays a role in regulating secretion. However, the mechanisms by which this occurs are unknown. Here, we demonstrate that an O-glycosyltransferase functions as a novel regulator of secretion and secretory vesicle formation in vivo by glycosylating the essential Golgi/endoplasmic reticulum protein, Tango1 (Transport and Golgi organization 1), and conferring protection from furin-mediated proteolysis. Loss of the O-glycosyltransferase PGANT4 resulted in Tango1 cleavage, loss of secretory granules, and disrupted apical secretion. The secretory defects seen upon loss of pgant4 could be rescued either by overexpression of Tango1 or by knockdown of a specific furin (Dfur2) in vivo. Our studies elucidate a novel regulatory mechanism whereby secretion is influenced by the yin/yang of O-glycosylation and proteolytic cleavage. Moreover, our data have broader implications for the potential treatment of diseases resulting from the loss of O-glycosylation by modulating the activity of specific proteases.
Regulation of secretory vesicle formation and polarized secretion in vivo is crucial to ensure the proper deposition of signaling molecules, morphogens, and matrix components that mediate growth and differentiation. Polarized secretion is also required in many differentiated tissues, such as the digestive tract, where secreted components along the apical surface form the mucous membrane that confers protection from mechanical and microbial insults (1) and provides immunoregulatory signals (2). Indeed, disruptions in the secreted mucous membrane are associated with diseases of the digestive tract, such as colitis and colon cancer (3–6).
Recent studies aimed at identifying the factors that influence secretion have elucidated novel proteins that function in unique aspects of secretion, including the enzymes responsible for the addition of sugars to mucins and other proteins (mucin-type O-glycosylation) (7). O-glycosylation is an essential, evolutionarily conserved protein modification (8, 9) that has direct medical relevance, as aberrations are responsible for the human diseases familial tumoral calcinosis (10, 11) and Tn syndrome (12). Loss of this protein modification affected constitutive secretion and Golgi apparatus structure in Drosophila cell culture (7, 13) and secretion in the developing respiratory system in vivo (14). Additionally, loss of O-glycosylation also disrupted secretion of an extracellular matrix protein (Tiggrin) in the developing wing, resulting in aberrant basement membrane formation and disrupted integrin-mediated cell adhesion (15). Mammalian studies have confirmed the effects of O-glycosylation on secretion, as loss of a glycosyltransferase (ppGalNAcT-1) disrupted secretion of laminin and collagen during mammalian organogenesis, altering the composition of the basement membrane and disrupting proper FGF signaling and organ growth (16). Although these studies all point to a role for O-glycosylation in secretion, the mechanisms by which this protein modification affects secretion are currently unknown. Here, we demonstrate that O-glycosylation influences secretion and secretory vesicle formation by glycosylating the essential endoplasmic reticulum (ER)/Golgi protein Tango1, and conferring protection from furin-mediated proteolysis. Interestingly, secretory defects caused by the loss of O-glycosylation could be rescued by reducing the activity of a specific furin (Dfur2) in vivo. Our results elucidate a novel regulatory paradigm whereby the competing activities of an O-glycosyltransferase and a furin control secretion and secretory vesicle formation. Moreover, this finding offers a potential treatment for disorders of glycosylation by modulating the activity of specific proteases in vivo.
Results
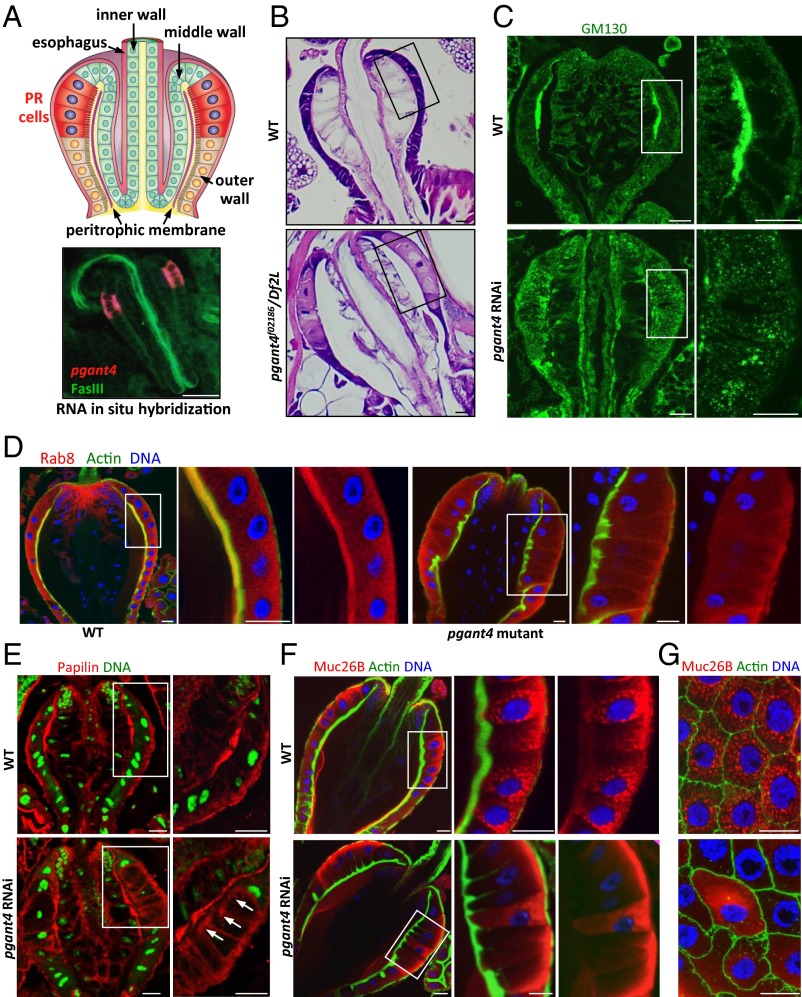
To address how O-glycosylation is affecting polarized secretion in vivo, we examined mutations in an essential gene controlling the initiation of O-glycosylation (pgant4; polypeptide GalNAc transferase 4) (17) in specialized secretory cells of the Drosophila digestive tract (Fig. 1 and Fig. S1). The secretory cells (PR cells) of the Drosophila proventriculus (PV) are responsible for the synthesis and secretion of the peritrophic membrane that lines and protects the larval digestive tract (Fig. 1), similar to the mucous lining of the mammalian digestive tract. PR cells contain large secretory granules and have a highly polarized secretory apparatus (18) (Fig. 1 C, D, F, and G). Like goblet cells of the mammalian digestive tract, PR cells also contain mucins (e.g., Muc26B) (19) (Fig. 1 F and G). Loss of pgant4 function, via in vivo RNA interference (RNAi) or conventional mutations (pgant4f02186/Df2L) (Fig. S2), disrupted PR cell morphology and secretion. PR cells were enlarged and displayed loss of apical secretory components (the apical GTPase Rab8 and the Golgi marker GM130), loss of apical secretion of peritrophic membrane components (papilin and chitin), and loss of secretory granules (Fig. 1 and Fig. S1). Secretory apparatus structure and secretion were restored upon expression of a catalytically active version of pgant4 (pgant4OE), but not a catalytically inactive version (pgant4m2) in the mutant background (Fig. S2E), indicating that the catalytic O-glycosyltransferase activity of PGANT4 is required for proper polarized secretion and secretory vesicle formation in PR cells.
Fig. 1.
Loss of pgant4 affects secretion and secretory vesicle formation in PR cells of the digestive tract. (A) Schematic diagram of the larval PV with PR cells (responsible for secretion of the peritrophic membrane) shown in red. (Lower) pgant4 gene expression (red) specifically in PR cells, as detected by RNA in situ hybridization. Fasciclin III (FasIII) is shown in green. (B) PR cells (boxed areas) in pgant4 mutants (pgant4f02186/Df2L) are enlarged and irregular in morphology relative to wild-type (WT) as seen by H&E staining. Loss of pgant4 results in loss of apical GM130 (C), loss of apical Rab8 (D), reduced secretion of the ECM protein papilin (arrows) (E), and loss of secretory vesicles containing Muc26B (F). (G) Lateral section of PV showing loss of secretory vesicles in PR cells of pgant4 mutants. Magnified views of insets are shown to the right of each figure. (Scale bars, 20 μm.)
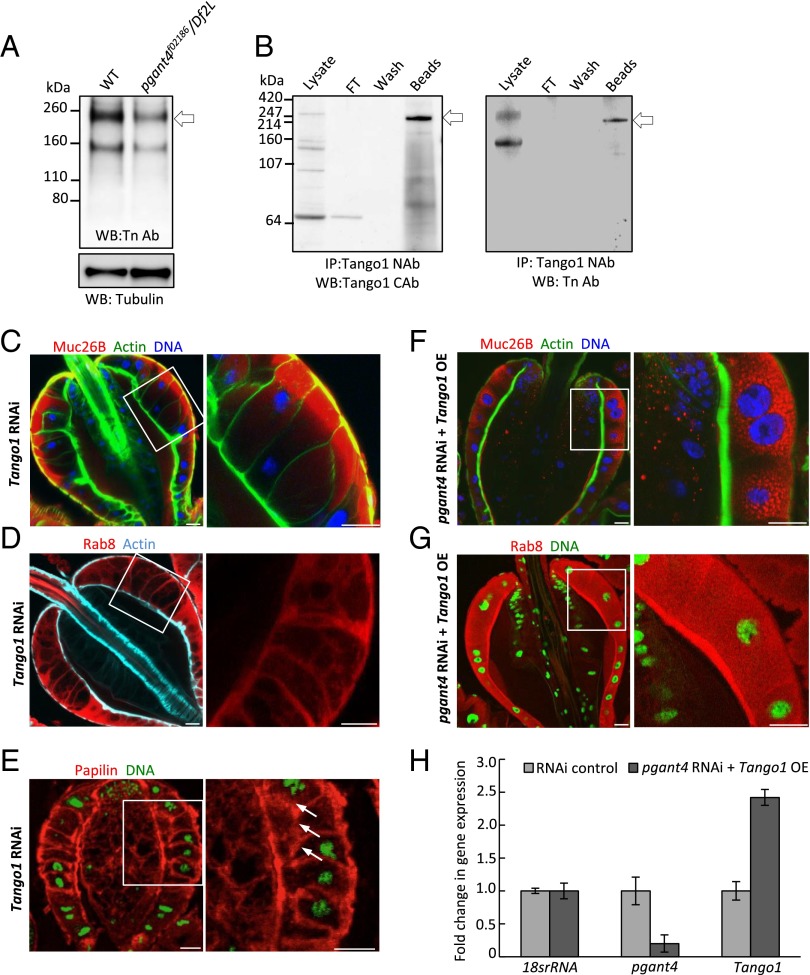
To determine how PGANT4 is mediating effects on secretion, we performed Western blots probed with an antibody and lectins that detect O-linked glycoproteins to identify direct targets of PGANT4. The Tn antibody (Tn Ab), which detects GalNAc linked to serine or threonine, revealed specific decreases in the intensity of bands upon loss of pgant4. Comparison of O-glycoproteins from wild-type and pgant4 mutant proventriculi, revealed a decrease in intensity of a band the approximate size (∼250 kDa) of Tango1 (Transport and Golgi organization 1), suggesting that Tango1 may be a direct target of PGANT4 (Fig. 2A). Tango1 is an essential ER/Golgi transmembrane protein originally identified in a Drosophila cell culture screen for genes that influence constitutive secretion and Golgi apparatus structure (7); the mammalian ortholog of Tango1 (Mia3) is essential for the vesicular packaging and secretion of collagen and other high molecular weight proteins (20–22). We next made antibodies to the C-terminal (CAb) and N-terminal (NAb) regions of Tango1 (Fig. S3B) using the peptides described in SI Materials and Methods. Immunoprecipitation of Tango1 using the NAb followed by Western blotting with the Tn Ab demonstrated that full-length Tango1 is normally O-glycosylated in the wild-type Drosophila PV (Fig. 2B).
Fig. 2.
Tango1 is normally O-glycosylated and loss of Tango1 phenocopies loss of pgant4. (A) Western blot of extracts from proventriculi using an antibody that specifically detects O-linked glycosylation (Tn Ab) shows a decrease in an ∼250-kDa band in pgant4 mutants (pgant4f02186/Df2L) relative to wild-type. Tubulin loading control is shown (Lower). (B) Immunoprecipitated Tango1 from wild-type PR cells (Left, arrow) is O-glycosylated (Right, arrow), as detected by the Tn Ab. Loss of Tango1 (Tango1 RNAi) causes loss of secretory vesicles (C), loss of apical Rab8 staining (D), intracellular accumulation of papilin (white arrows) (E), and enlarged PR cells. Overexpression of Tango1 in a pgant4 RNAi background rescues secretory vesicle formation (F) and apical localization of secretory components (Rab8) (G). (H) Quantitative PCR shows levels of expression of pgant4 and Tango1 in the rescue experiments. (Scale bars, 20 μm.)
To investigate whether PGANT4 may be exerting its effects through Tango1, we next examined secretion and secretory vesicle formation in PR cells in which Tango1 had been knocked down by RNAi (Fig. S3A). Loss of Tango1 resulted in phenotypes indistinguishable from those seen upon loss of pgant4, including loss of secretory vesicles, mislocalization of apical secretory components (Rab8), and decreased apical secretion (Fig. 2 C–E). To directly address whether the effects of pgant4 loss are mediated through Tango1, we next performed genetic rescue experiments. We found that phenotypes resulting from the loss of pgant4 could be rescued by overexpression of Tango1 in PR cells (Fig. 2 F–H). These results demonstrate that the secretory defects seen in pgant4 mutants are the result of its effect on Tango1. Moreover, these results demonstrate that PGANT4 and Tango1 operate together in regulating secretion and secretory vesicle formation within these specialized secretory cells.
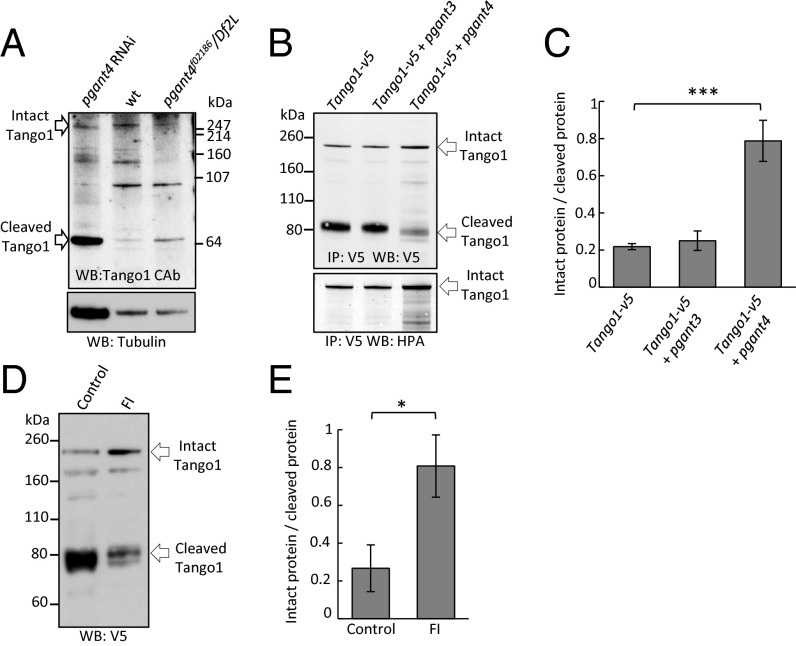
To investigate how PGANT4 affects Tango1 function, we next examined Tango1 stability in the absence of PGANT4. Antibodies to Tango1 revealed a reduction in full-length Tango1 protein and a prominent Tango1 cleavage fragment in pgant4 mutant proventriculi (Fig. 3A and Fig. S4). Additionally, increased expression of pgant4 was able to increase the glycosylation of Tango1 [as shown by increased helix pomatia agglutinin (HPA) lectin reactivity] as well as increase the ratio of intact/cleaved Tango1 in Drosophila cell culture (Fig. 3 B and C). The effects of pgant4 on Tango1 were specific, as expression of another pgant family member (pgant3) neither increased glycosylation of Tango1 nor protected it from cleavage (Fig. 3 B and C). These results suggest that glycosylation of Tango1 by PGANT4 confers protection from proteolysis.
Fig. 3.
O-glycosylation of Tango1 by PGANT4 protects it from furin-mediated cleavage. (A) Western blots show increased cleavage of Tango1 in proventriculi upon loss of pgant4 (pgant4f02186/Df2L or pgant4 RNAi) relative to wild-type. Intact and cleaved Tango1 are denoted at left. (B) Western blots from Drosophila cells expressing Tango1 show increased glycosylation (using the O-glycan-specific lectin HPA) and decreased cleavage of Tango1 in the presence of pgant4. (C) Ratios of intact to cleaved Tango1 for multiple cell culture experiments are shown. ***P < 0.001. (D) Furin inhibitors (FI) decrease cleavage of Tango1 in Drosophila cells. (E) Quantitation of the effects of furin inhibition on the ratio of intact to cleaved Tango1. *P < 0.05.
Examination of the Tango1 amino acid sequence revealed numerous potential dibasic proprotein convertase cleavage sites flanked by potential sites of O-glycosylation (Fig. S5A). To directly test whether Tango1 is subject to proprotein convertase-mediated cleavage, we next examined ratios of full-length/cleaved Tango1 in the presence or absence of furin inhibitors. Treatment of Drosophila S2R+ cells with furin inhibitors dramatically increased the ratio of full-length/cleaved Tango1 relative to untreated cells, indicating that Tango1 normally undergoes some degree of furin-mediated proteolysis (Fig. 3 D and E). Construction of a truncated Tango1 construct beginning at one of the putative furin cleavage sites near the transmembrane domain (amino acid 751) was in agreement with the size of the cleaved fragment observed in Drosophila cell culture (Fig. S5D), suggesting that Tango1 cleavage occurs at or near this site. We next examined regions of Tango1 that are O-glycosylated when pgant4 is expressed in Drosophila cell culture. Mass spectroscopy identified at least one region (near the transmembrane domain) that is O-glycosylated only when pgant4 is coexpressed (Fig. S5 B and C), further suggesting that glycosylation by PGANT4 may be conferring protection from proteolysis occurring within or near this region.
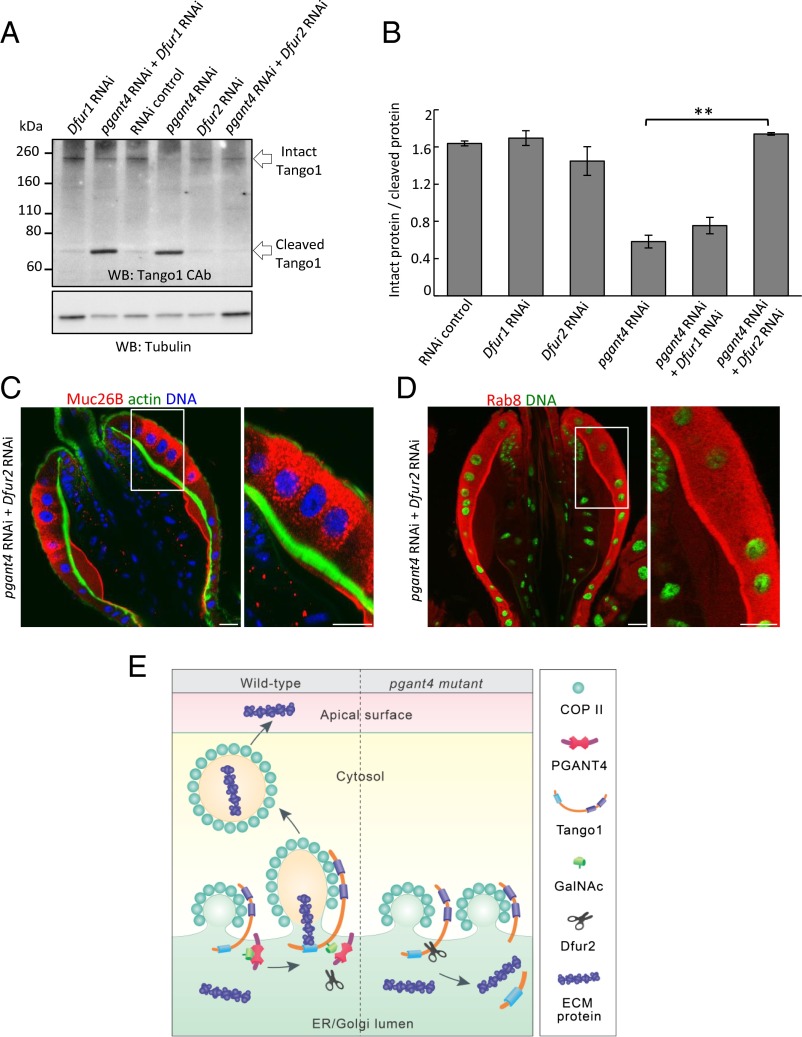
To directly address the relationship between furin-mediated cleavage and PGANT4-mediated protection of Tango1 in vivo, we altered levels of the furin genes normally expressed in the PV (Dfur1 or Dfur2) (23) to test whether a decrease in furin activity could rescue Tango1 stability and function in the pgant4-deficient background. RNAi-mediated knockdown of Dfur2 in the pgant4 RNAi background increased the ratio of full-length/cleaved Tango1 (in the PV) and rescued the PR cell phenotypes (Fig. 4 A–D and Fig. S6). RNAi to Dfur1 neither rescued Tango1 stability nor the PR cell phenotypes in the pgant4 RNAi background (Fig. 4 and Fig. S6). Taken together, our results indicate that PGANT4-directed glycosylation of Tango1 protects it specifically from cleavage by the furin Dfur2, thus allowing proper secretory vesicle formation and polarized secretion to occur (Fig. 4E). Moreover, the phenotypes seen upon loss of pgant4 can be rescued by modulating the activity of Dfur2 in vivo.
Fig. 4.
RNAi to Dfur2 can rescue Tango1 cleavage and PR cell phenotypes seen upon loss of pgant4 in vivo. (A) Western blots of extracts from proventriculi show decreased Tango1 cleavage in the pgant4 RNAi background when Dfur2 is also knocked down. Tubulin control is shown (Lower). (B) Ratios of intact to cleaved Tango1 for the in vivo experiments shown in A. **P < 0.01. PR cell secretory vesicles (C) and apical Rab8 localization (D) are restored in flies expressing dsRNA to both pgant4 and Dfur2. (Scale bar, 20 μm.) (E) Model depicting how O-glycosylation of Tango1 by PGANT4 confers protection from Dfur2-mediated cleavage, thus allowing formation of secretory vesicles carrying cargo. In pgant4 mutants, unglycosylated Tango1 is cleaved by Dfur2, thus abrogating secretory vesicle formation and secretion.
Discussion
Here, we demonstrate that O-glycosylation regulates polarized secretion and secretory vesicle formation in vivo by modulating the stability of an essential component of the secretory apparatus. While previous studies have documented the effects of loss of O-glycosylation on constitutive secretion (7, 13) and secretion of extracellular matrix (ECM) proteins in vivo (15, 16), our studies elucidate a mechanism by which these effects may occur. Here, we show that Tango1 stability is modulated by the presence of O-glycans, which serve to protect it from furin-mediated cleavage (Fig. 4E). To our knowledge, this represents the first example of O-glycans modulating secretion by stabilizing an essential component of the secretory apparatus.
Roles for Tango1 in constitutive secretion, Golgi structure, and COPII vesicle formation have been identified previously (7, 20–22), but the factors that regulate its activity and stability are not completely understood. Here, we demonstrate that Tango1 stability is modulated by the competing activities of a specific O-glycosyltransferase (PGANT4) and a specific furin (Dfur2) in secretory cells of the Drosophila digestive tract. Tango1 is a ubiquitously expressed protein that regulates not only constitutive secretion, but also the formation of large secretory vesicles that transport bulky ECM proteins in certain cells (7, 20–22). Tango1/Mia3 is proposed to bind secretory cargo via its luminal domain and COPII coat subunits via its cytoplasmic domain, thereby coordinating the size of secretory vesicles to accommodate large ECM proteins (20–22). However, not all cells have the same secretory demands nor produce large vesicular carriers. Here we demonstrate that the expression of PGANT4 specifically in the secretory PR cells of the digestive tract confers increased stability to Tango1 by protecting it from Dfur2-mediated cleavage, thereby allowing the formation of large mucin-containing secretory vesicles within these cells. As the enzymes controlling the initiation of O-glycosylation are typically abundantly expressed in cells under high secretory burden (8, 24), it raises the possibility that O-glycosylation may modulate the stability of Tango1 in other tissues, ensuring that Tango1 activity is commensurate with the secretory demands of the cell.
How are O-glycans specifically influencing Tango1 stability? Based on in vitro studies, O-glycans may influence protease sensitivity by blocking access to vicinal protease cleavage sites (25). Additionally, O-glycans may affect protein conformation, thus altering protease sensitivity at more distant sites. Finally, O-glycosylation may also influence the binding of partner or cargo proteins, thereby affecting protease access. Although our data suggest that O-glycans added by PGANT4 may influence vicinal protease cleavage, we cannot rule out the possibility that O-glycans may influence Tango1 conformation and/or cargo binding. Future studies mapping all sites of glycosylation of Tango1 by PGANT4 will aid in determining the mechanisms involved.
PGANT4 is one member of a large family of enzymes (PGANTs in Drosophila, GALNTs in humans, and ppGalNAcTs in mice) that control the initiation of O-glycosylation. These family members are expressed in unique spatial and temporal patterns during development and in adult tissues (8, 9). Additionally, these enzymes display unique substrate specificities, with some members preferring to add the initial GalNAc (peptide transferases) and others preferring to add GalNAc to previously glycosylated substrates (glycopeptide transferases). The concerted activity of these many members is thought to result in the elaborate glycosylation patterns typically seen in mucin-like molecules. As members of this family are abundantly expressed in many secretory cells and tissues (8, 24), it raises the possibility that the yin/yang provided by the opposing forces of O-glycosylation and proteolytic cleavage may serve as a more widespread, dynamic system to regulate the stability and bioactivity of many proteins. In support of this theory, recent glycoproteomic studies performed in mammalian cell culture have mapped sites of O-glycosylation to be in close proximity to potential furin cleavage sites on many proteins (26). Additionally, these studies also identified O-glycans on mammalian Tango1/Mia3 (26, 27), suggesting that O-glycans may perform similar protective functions to control secretion in mammalian systems. Indeed, alterations in both Tango1/Mia3 and O-glycosylation have been associated with diseases of the mammalian gastrointestinal tract (5, 6, 28, 29), which are typically characterized by loss of secretion, loss of mucous membrane formation, and increased diffusion barrier permeability. Interestingly, PGANT4 is most similar to the mammalian ppGalNAc-T10 (8), which is abundantly expressed in the digestive system (30). It will be interesting to determine if mammalian Tango1/Mia3 is similarly regulated by O-glycosylation and proteolytic cleavage.
More broadly, our results provide in vivo evidence for models where competing activities of O-glycosyltransferases and proteases may modulate protein stability, with imbalances in these activities contributing to disease. Genome-wide association studies demonstrating a link between O-glycosylation and blood lipid levels have led to the proposal that O-glycans on proteins involved in lipid metabolism may confer protection from proteolysis, thereby influencing HDL-cholesterol and triglyceride levels, and thus cardiovascular disease risk (31–34). Similarly, in the case of familial tumoral calcinosis, it is proposed that mutations in an O-glycosyltransferase (GALNT3 in humans and Galnt3 in mice) may lead to decreased FGF23 glycosylation, increased FGF23 cleavage, abnormal phosphate levels, and calcified tumor development in patients (10, 11). However, although cell culture and in vitro assays have shown that FGF23 can be glycosylated by Galnt3 (25), the glycosylation status of endogenous FGF23 in the presence or absence of Galnt3 has not been examined (in mice or humans). Similarly, although O-glycans can confer protection from protease cleavage of peptides in vitro (25), a demonstration that cleavage of endogenous FGF23 can be rescued by altering levels of an endogenous protease in vivo has not been previously shown. Here, our results provide in vivo evidence for the interplay between O-glycosylation and protease sensitivity, supporting the model of how loss of O-glycosylation may contribute to human disease and disease associations. More importantly, our ability to rescue the effects of loss of O-glycosylation by altering the levels of a specific furin in vivo suggests that inhibition/modulation of specific proteases may be a viable treatment option for certain diseases associated with aberrant O-glycosylation.
Materials and Methods
Antibody Preparation.
Two Tango1 polyclonal antibodies were raised in rabbits using the keyhole limpet hemocyanin-conjugated N-terminal peptide (EQIDQKEFPKQVLDA-C) and C-terminal peptide (C-HRGSYSHSPRTYRSL). One Muc26B antibody was raised in rabbits using the keyhole limpet hemocyanin-conjugated peptide RPVRPAVRPALEIDE-C (amino acids 82–96). The antibodies were affinity purified on a peptide column (Covance). Additional information is found in SI Materials and Methods.
Fly Strains and Genetics.
RNA interference (RNAi) lines containing UAS-inducible inverted repeats (IR) from the Vienna Drosophila RNAi Center (VDRC) (35) were stocks #7286 (pgant4IR), 21594 (Tango1IR), 22853 (Dfur1IR), 101242 (Dfur2IR). Additionally, the w1118; UAS-pgant4IR#7 stock was constructed as described previously (17, 36). Additional information is found in SI Materials and Methods.
Quantitative Real-Time PCR.
Primers used are as described previously (17) and additional information is in SI Materials and Methods.
In Situ Hybridization.
pgant4 RNA probes were prepared as described previously (24) for in situ hybridization. Additional information is found in SI Materials and Methods.
Staining of Proventriculi.
Detailed methods are described in SI Materials and Methods. The anti-Papilin antibody (1:500) (37) was the kind gift of L. and J. Fessler (University of California, Los Angeles).
Immunoprecipitation and Western Blotting.
Details are described in SI Materials and Methods. The Tn antibody (1:500) was the kind gift of Richard Cummings (Emory University, Atlanta) (38).
Bioinformatic Predictions and Statistics.
Details are described in SI Materials and Methods.
Detection of O-Linked Glycosylation Sites in Tango1 by LC-MS/MS.
Details are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank the members of our laboratory for comments and feedback; Drs. L. Tabak, L. Angerer, and R. Angerer for many helpful discussions and insight; Drs. R. Cummings and T. Ju for the kind gift of the Tn antibody; Drs. L. Fessler and J. Fessler for the kind gift of the papilin antibody; and Drs. J. Kennison and D. Andrew, the Vienna Drosophila RNAi Center, the Bloomington Stock Center, and the Developmental Studies Hybridoma Bank for fly stocks and other reagents. This work was supported by the Intramural Research Program of the National Institute of Dental and Craniofacial Research, National Institutes of Health Grant Z01-DE-000713 (to K.G.T.H.); National Institutes of Health/National Institute of General Medical Sciences (National Center for Biomedical Glycomics, Grant P41GM103490, L.W. Senior Investigator); and the Basic Science Research Program and the Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology Grants NRF 2011-0013961 and NRF 2010-0029634 (to J.-M.L).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1322264111/-/DCSupplemental.
References
- 1.Johansson ME, Sjövall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol. 2013;10(6):352–361. doi: 10.1038/nrgastro.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shan M, et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science. 2013;342(6157):447–453. doi: 10.1126/science.1237910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Velcich A, et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295(5560):1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 4.Van der Sluis M, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131(1):117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 5.An G, et al. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J Exp Med. 2007;204(6):1417–1429. doi: 10.1084/jem.20061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guda K, et al. Inactivating germ-line and somatic mutations in polypeptide N-acetylgalactosaminyltransferase 12 in human colon cancers. Proc Natl Acad Sci USA. 2009;106(31):12921–12925. doi: 10.1073/pnas.0901454106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bard F, et al. Functional genomics reveals genes involved in protein secretion and Golgi organization. Nature. 2006;439(7076):604–607. doi: 10.1038/nature04377. [DOI] [PubMed] [Google Scholar]
- 8.Bennett EP, et al. Control of mucin-type O-glycosylation: A classification of the polypeptide GalNAc-transferase gene family. Glycobiology. 2012;22(6):736–756. doi: 10.1093/glycob/cwr182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran DT, Ten Hagen KG. Mucin-type O-glycosylation during development. J Biol Chem. 2013;288(10):6921–6929. doi: 10.1074/jbc.R112.418558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topaz O, et al. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet. 2004;36(6):579–581. doi: 10.1038/ng1358. [DOI] [PubMed] [Google Scholar]
- 11.Ichikawa S, et al. Ablation of the Galnt3 gene leads to low-circulating intact fibroblast growth factor 23 (Fgf23) concentrations and hyperphosphatemia despite increased Fgf23 expression. Endocrinology. 2009;150(6):2543–2550. doi: 10.1210/en.2008-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ju T, Cummings RD. Protein glycosylation: Chaperone mutation in Tn syndrome. Nature. 2005;437(7063):1252. doi: 10.1038/4371252a. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Ten Hagen KG. Dissecting the biological role of mucin-type O-glycosylation using RNA interference in Drosophila cell culture. J Biol Chem. 2010;285(45):34477–34484. doi: 10.1074/jbc.M110.133561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian E, Ten Hagen KG. A UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase is required for epithelial tube formation. J Biol Chem. 2007;282(1):606–614. doi: 10.1074/jbc.M606268200. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Tran DT, Ten Hagen KG. An O-glycosyltransferase promotes cell adhesion during development by influencing secretion of an extracellular matrix integrin ligand. J Biol Chem. 2010;285(25):19491–19501. doi: 10.1074/jbc.M109.098145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian E, Hoffman MP, Ten Hagen KG. O-glycosylation modulates integrin and FGF signalling by influencing the secretion of basement membrane components. Nat Commun. 2012;3:869. doi: 10.1038/ncomms1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tran DT, et al. Multiple members of the UDP-GalNAc: Polypeptide N-acetylgalactosaminyltransferase family are essential for viability in Drosophila. J Biol Chem. 2012;287(8):5243–5252. doi: 10.1074/jbc.M111.306159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rizki MT. The secretory activity of the proventriculus of Drosophila melanogaster. J Exp Zool. 1956;131(2):203–221. [Google Scholar]
- 19.Syed ZA, Härd T, Uv A, van Dijk-Härd IF. A potential role for Drosophila mucins in development and physiology. PLoS ONE. 2008;3(8):e3041. doi: 10.1371/journal.pone.0003041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson DG, et al. Global defects in collagen secretion in a Mia3/TANGO1 knockout mouse. J Cell Biol. 2011;193(5):935–951. doi: 10.1083/jcb.201007162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saito K, et al. TANGO1 facilitates cargo loading at endoplasmic reticulum exit sites. Cell. 2009;136(5):891–902. doi: 10.1016/j.cell.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 22.Saito K, et al. cTAGE5 mediates collagen secretion through interaction with TANGO1 at endoplasmic reticulum exit sites. Mol Biol Cell. 2011;22(13):2301–2308. doi: 10.1091/mbc.E11-02-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Bie I, et al. Processing specificity and biosynthesis of the Drosophila melanogaster convertases dfurin1, dfurin1-CRR, dfurin1-X, and dfurin2. J Biol Chem. 1995;270(3):1020–1028. doi: 10.1074/jbc.270.3.1020. [DOI] [PubMed] [Google Scholar]
- 24.Tian E, Ten Hagen KG. Expression of the UDP-GalNAc: Polypeptide N-acetylgalactosaminyltransferase family is spatially and temporally regulated during Drosophila development. Glycobiology. 2006;16(2):83–95. doi: 10.1093/glycob/cwj051. [DOI] [PubMed] [Google Scholar]
- 25.Kato K, et al. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J Biol Chem. 2006;281(27):18370–18377. doi: 10.1074/jbc.M602469200. [DOI] [PubMed] [Google Scholar]
- 26.Steentoft C, et al. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 2013;32(10):1478–1488. doi: 10.1038/emboj.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steentoft C, et al. Mining the O-glycoproteome using zinc-finger nuclease-glycoengineered SimpleCell lines. Nat Methods. 2011;8(11):977–982. doi: 10.1038/nmeth.1731. [DOI] [PubMed] [Google Scholar]
- 28.Fu J, et al. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. J Clin Invest. 2011;121(4):1657–1666. doi: 10.1172/JCI45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arndt S, Bosserhoff AK. Reduced expression of TANGO in colon and hepatocellular carcinomas. Oncol Rep. 2007;18(4):885–891. [PubMed] [Google Scholar]
- 30.Ten Hagen KG, et al. Cloning and characterization of a ninth member of the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase family, ppGaNTase-T9. J Biol Chem. 2001;276(20):17395–17404. doi: 10.1074/jbc.M009638200. [DOI] [PubMed] [Google Scholar]
- 31.Holleboom AG, et al. Heterozygosity for a loss-of-function mutation in GALNT2 improves plasma triglyceride clearance in man. Cell Metab. 2011;14(6):811–818. doi: 10.1016/j.cmet.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schjoldager KT, et al. O-glycosylation modulates proprotein convertase activation of angiopoietin-like protein 3: Possible role of polypeptide GalNAc-transferase-2 in regulation of concentrations of plasma lipids. J Biol Chem. 2010;285(47):36293–36303. doi: 10.1074/jbc.M110.156950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kathiresan S, et al. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet. 2008;40(2):189–197. doi: 10.1038/ng.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willer CJ, et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet. 2008;40(2):161–169. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dietzl G, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448(7150):151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 36.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118(2):401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 37.Kramerova IA, et al. Papilin in development; a pericellular protein with a homology to the ADAMTS metalloproteinases. Development. 2000;127(24):5475–5485. doi: 10.1242/dev.127.24.5475. [DOI] [PubMed] [Google Scholar]
- 38.Avichezer D, Springer GF, Schechter B, Arnon R. Immunoreactivities of polyclonal and monoclonal anti-T and anti-Tn antibodies with human carcinoma cells, grown in vitro and in a xenograft model. Int J Cancer. 1997;72(1):119–127. doi: 10.1002/(sici)1097-0215(19970703)72:1<119::aid-ijc17>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.