Significance
Potent antiviral CD4 Th1 responses generated at the onset of persistent infection are lost as infection progresses. However, it is unknown how CD4 T cell responses are mounted in the midst of an established persistent infection to restore the diminishing Th1 response. We report that an established persistent virus infection suppresses the induction and distribution of new virus-specific CD4 Th1 cells. The failure to generate new Th1 responses is mediated by chronic type I interferon (IFN-I) signaling, and its blockade effectively restored de novo Th1 development. Our study identifies a mechanism of immunosuppression and a method to restore Th1 generation during persistent infection.
Keywords: immunosuppression, LCMV
Abstract
CD4 T cells are central to orchestrate, sustain, and potentially regenerate antiviral immunity throughout persistent viral infections. Although the evolving immune environment during persistent infection reshapes established CD4 T-cell responses, the fate of naïve CD4 T cells primed in the midst of persistent infection is unclear. We demonstrate that, in marked contrast to the onset of infection, virus-specific CD4 T cells primed during an established persistent infection have diminished ability to develop Th1 responses, to efficiently accumulate in peripheral tissues, and almost exclusively differentiate into T follicular helper cells. Consistent with suppressed Th1 and heightened Tfh differentiation, virus-specific CD4 T cells primed during the established persistent infection provide help to B cells, but only limited help to CD8 T cells. The suppression of de novo Th1 generation and tissue distribution was mediated by chronic type I IFN (IFN-I) production and was effectively restored by blocking IFN-I signaling during CD4 T-cell priming. Thus, we establish a suppressive function of chronic IFN-I signaling and mechanism of immunoregulation during an established persistent virus infection.
The majority of viruses stimulate robust and effective T-cell responses that efficiently eliminate the infection; however, certain viruses are able to subvert host T-cell control of viral replication and generate a persistent infection. Sustained CD4 T helper (Th) cell responses are a strong correlate of control and clearance of multiple persistent virus infections, including HIV and hepatitis C virus infection in humans and lymphocytic choriomeningitis virus (LCMV) infection in mice (1). CD4 Th cells are central orchestrators of the immune response and differentially activate diverse branches of innate and adaptive immunity to guide the appropriate response to an invading pathogen. In response to viral infections, CD4 T cells predominately develop into Th1 or T follicular helper (Tfh) cells (2, 3). CD4 Th1 immunity is critical to sustain residual CD8 T-cell activity to control infection during persistent infection and is characterized in CD4 T cells by the secretion of IFN-γ, TNF-α, and IL-2 (1, 4). Tfh cells localize to the follicle via C-X-C chemokine receptor type 5 (CXCR5) expression to direct B-cell differentiation and antibody production through cell surface interactions and secreted cytokines such as IL-21 (2). Ultimately, control of infection is critically dependent upon the correct Th-mediated orchestration of these diverse responses.
At the onset of what will become a persistent LCMV infection, CD4 T cells initially generate a Th1 response, but these Th1 cells progressively develop into Tfh as infection progresses (3), indicating that CD4 T-cell differentiation is continually modulated by infection. The Th1-to-Tfh transformation as persistent infection progresses also suggests that CD4 T cells primed in an established persistent infection may develop differently than those activated at the onset of infection, thus affecting the ability to replenish the diminishing antiviral Th1 response. Although CD4 T cells can be primed during persistent infection (5, 6), it is still unclear how the ongoing infection alters de novo CD4 T-cell differentiation and function. Importantly, a naïve T cell activated in an established persistent infection will encounter a substantially different immunologic environment than one primed at the onset of infection, most notably characterized by the disruption of lymphatic organ architecture, the immediate exposure to high levels of antigen and inflammatory and suppressive factors, as well as changes in the type and functional quality of antigen-presenting cells (APCs) (1, 7). Biologically, de novo T-cell activation will be required for diverse needs of the immune response during viral persistence, such as to balance attrition in response to lifelong persistently replicating infections and to control escape mutations that arise as infection progresses (6, 8, 9). Therapeutically, activation of naïve T cells will be required to stimulate de novo immunity through therapeutic vaccination and production of virus-specific T cells by means of hematopoietic stem cell engineering (10).
Given the broad immunologic implications that alterations in CD4 T-cell differentiation could have on the antiviral immune response, we sought to understand the molecular, cellular, and effector development of CD4 T-cell responses primed in the midst of persistent infection. Herein, we demonstrate that type I IFN (IFN-I) signaling in the persistently infected immune environment suppresses the generation of de novo Th1 but not Tfh responses, and blockade of IFN-I signaling effectively restores de novo Th1 differentiation. Ultimately, the failure to form Th1 coupled with exclusive Tfh formation has important implications toward the long-term breadth of the CD4 Th response and the resultant control of persistent viral infections.
Results
Virus-Specific CD4 T Cells Primed in an Established Persistent Infection Experience an Initial Defect in Effector Differentiation.
To investigate the dynamics of virus-specific CD4 T-cell priming in the midst of viral persistence, we used the LCMV system. Infection with LCMV-Armstrong (Arm) induces robust CD4 and CD8 T-cell responses that clear the virus within 8–10 d after infection. Conversely, the LCMV-clone 13 (Cl13) variant replicates to substantially higher titers and rapidly elicits the expression of multiple host immunoregulatory factors that suppress the immune response to generate a persistent infection (11, 12). To determine how the environment during an established persistent infection affects de novo virus-specific CD4 T-cell priming and differentiation, we transferred naïve LCMV-specific T-cell receptor (TCR)-transgenic CD4 (SMARTA) T cells into mice that had been infected 21 d earlier with LCMV-Cl13. In parallel, naïve SMARTA T cells were transferred into naïve mice that were then infected with LCMV-Cl13, thus allowing a direct comparison of CD4 T-cell priming at the onset and during an established persistent infection. Importantly, SMARTA transgenic cells behave similarly to their endogenous (i.e., host-derived LCMV-GP66 tetramer+) CD4 T-cell counterparts (13, 14). Herein, T cells primed at the onset of infection are termed “early primed,” whereas T cells transferred into an established persistent infection are referred to as “late primed.”
Sixty hours after transfer, early- and late-primed virus-specific CD4 T cells up-regulate the activation marker CD44, proliferate, and expand to similar levels (Fig. 1A), indicating priming and activation of naïve virus-specific CD4 T cells in the midst of a persistent infection. Depletion of dendritic cells (DCs) in CD11c-DTR mice (15) before SMARTA transfer greatly reduced late-primed CD4 T-cell proliferation and expansion, indicating that DCs are necessary for priming during persistent infection (Fig. S1). Following activation, early-primed CD4 T cells down-regulate the lymph node retention molecule CD62L and up-regulate the IL-2 receptor α- (IL-2Rα, CD25) and β-chains (IL-2Rβ, CD122), Granzyme B, and the Th1 and Tfh fate determining transcriptional regulators T-box 21 (Tbet) and B-cell lymphoma 6 (Bcl6), whereas this was not observed in late-primed cells (Fig. 1B). Late-primed virus-specific CD4 T cells did not develop into either of the Th1 [signaling lymphocytic activation molecule (SLAM)hi, IL-2Rβhi, CXCR5−] or Tfh (SLAMlo, IL-2Rβlo, CXCR5+) precursor populations evident in early-primed CD4 T cells (Fig. 1C), nor do they produce the critical antiviral/immunostimulatory cytokines IFN-γ, TNF-α, or IL-21 in response to antigen stimulation (Fig. 1D). IL-2 is produced at low, but similar levels by both groups [9.3 ± 1.0% of SMARTA (early priming) vs. 13.4 ± 2.3% of SMARTA (late priming); P = 0.16]. These differences in differentiation were also observed 24 h after priming, indicating the failure to undergo this initial differentiation program as opposed to accelerated kinetics of differentiation. Early- and late-primed CD4 T cells expressed the same levels of the transcription factor FoxP3 and Grail, indicating that they are not instead forming Tregs or becoming anergic. Thus, despite activation and proliferation, virus-specific CD4 T cells primed during an established persistent infection initially undergo an attenuated Th differentiation program.
Fig. 1.
Late-primed CD4 T cells are activated and proliferate, but undergo a delay in differentiation. (A) CFSE-labeled virus-specific CD4 SMARTA T cells were transferred into recipient mice and spleens isolated 60 h after transfer. Early priming (E) (white histogram); late priming (L) (red histogram); CFSE-labeled SMARTA cells injected into naïve recipients that were not infected with LCMV (gray histogram). (B) Expression of the indicated protein and Blimp1 mRNA on early-primed (E) (white) and late-primed (L) (red) virus-specific CD4 SMARTA T cells 60 h after priming. Endogenous CD4 T cells are shown (gray). Numbers on plots quantify percentage of cells within gate (where applicable) or geometric fluorescence intensity of the population. (C) Expression of Th1 (SLAMhi, CXCR5lo) and Tfh (SLAMlo, CXCR5hi) phenotypic proteins at 60 h after priming. (D) Percentages indicate IFN-γ+, IL-21+, or IFN-γ+/IL-21+ double-positive SMARTA cells following ex vivo peptide stimulation. *P < 0.05. Data are representative of five independent experiments with three to five mice per group.
Virus-Specific CD4 T Cells Primed in the Midst of an Established Persistent Infection Fail to Generate Th1 Cells.
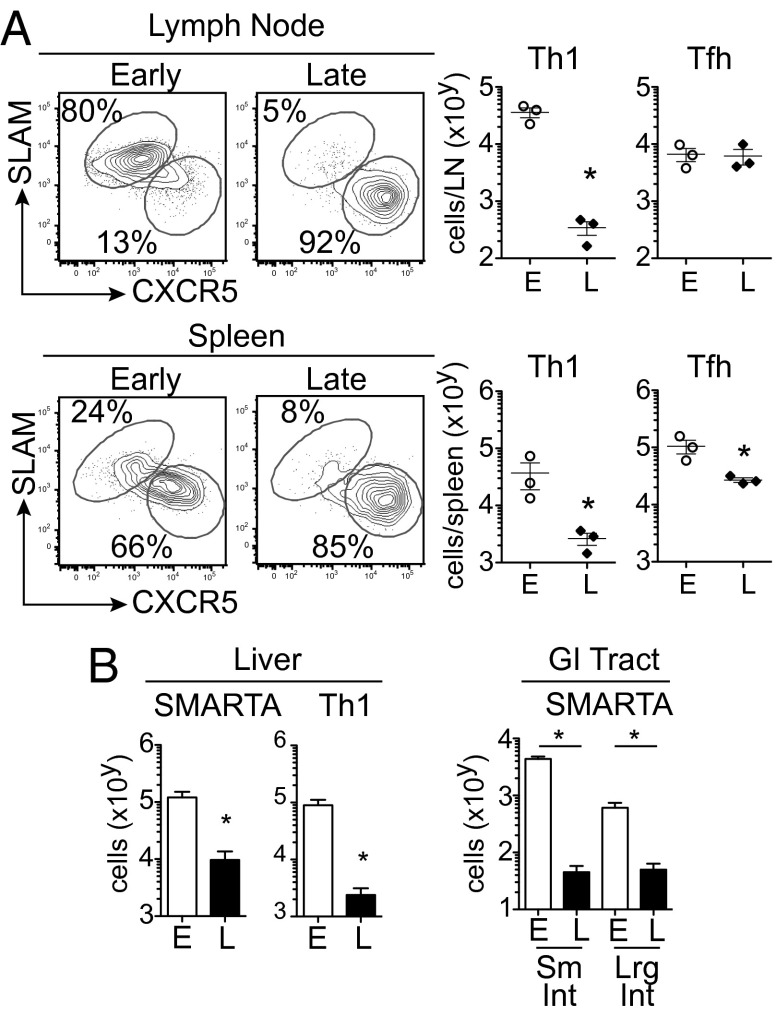
To determine whether priming in the midst of persistent infection continues to inhibit Th differentiation, we sorted and performed microarray analysis on early- and late-primed virus-specific CD4 T cells at 8 d after priming, a time point coinciding with the peak of the early-primed effector response (13). At the population level, Tfh-associated genes were increased in late priming, whereas the majority of Th1-associated genes were highly expressed in early-primed cells (Fig. S2A). Consistent with the RNA analysis, the late-primed virus-specific CD4 T-cell response within the mesenteric, inguinal, and brachial/axillary lymph nodes and spleen was predominately Tfh (Fig. 2A and Fig. S2 B and C). Furthermore, with exception of the inguinal lymph node, the absolute number of Tfh cells formed in each organ was not increased compared with early priming (Fig. 2A and Fig. S2 B and C). However, the number of Th1 cells was markedly reduced compared with early priming. Thus, our data indicate that an established persistent infection does not skew toward de novo Tfh formation per se, but instead that de novo Th1 development is not supported when priming is initiated in the midst of an ongoing persistent infection.
Fig. 2.
Late-primed CD4 T cells generate Tfh but have greatly diminished Th1 responses. (A) Th1 (SLAMhi, CXCR5lo) and Tfh (SLAMlo, CXCR5hi) development in the mesenteric lymph node and spleen 8 d after priming. The graphs demonstrate total number of virus-specific Th1 and Tfh SMARTA cells. (B) Total number of SMARTA cells within the liver, and the small and large intestine 8 d after early or late priming. *P < 0.05. Data are representative of six independent experiments with three to five mice per group.
Transfer of physiologic numbers of virus-specific CD4 T cells in the midst of persistent infection did not accelerate viral control (Fig. S2D), indicating that the lack of Th1 generation is not a result of accelerated viral clearance. Importantly, efficient activation and proliferation of naïve CD4 SMARTA T cells occurred when mice received a second transfer 8 d after the first late-priming transfer, demonstrating that the lack of Th1 formation is not due to viral escape (Fig. S2E). Interestingly, the failure to generate Th1 cells was not observed when virus-specific CD4 T cells were transferred into an established acute LCMV-Arm infection (day 4 after infection; Fig. S2F), indicating that the inability to efficiently generate Th1 cells is not simply due to viral infection, but rather is a property of the environment during an established persistent infection.
Functionally, late-primed CD4 T cells produced decreased IFN-γ and increased IL-21 compared with early-primed CD4 T cells (Fig. S3A) and expressed the master Tfh transcriptional regulator Bcl6 (Fig. S3B), consistent with the formation of a Tfh response and decreased Th1 differentiation (14). Unlike cells primed at the onset of acute LCMV-Arm infection, late-primed Tfh differentiation did not require LCMV-specific B cells (2), or IL-6, as has been reported in other situations (16) (Fig. S3 C and D). Although it is possible that late-primed cells receive autocrine IL-6 signals because the transferred cells were not IL-6 deficient, we did not detect increased IL-6 mRNA by microarray analysis or IL-6 protein secretion after peptide stimulation. These data further support that virus-specific CD4 T cells form predominately a Tfh instead of Th1 response following priming during established persistent infection through mechanisms distinct from the Th1-to-Tfh transition that occurs by early-primed virus-specific CD4 T cells (3).
Because alterations in Th differentiation affect CD4 T-cell homing and distribution (17, 18), we next assessed whether the diminished Th1 formation in late-primed virus-specific CD4 T cells led to changes in their tissue distribution. By day 8 after transfer, late-primed CD4 T cells had down-regulated the lymph tissue homing/retention molecule CD62L. However, corresponding to the lack of Th1 generation, the number of late-primed virus-specific CD4 T cells in the liver were greatly reduced, and although they were present in the mesenteric lymph nodes, they were almost entirely absent from the gastrointestinal (GI) tract despite high virus titers in all organs (Fig. 2 A and B and Fig. S4). Thus, consistent with the lack of Th1 differentiation, virus-specific CD4 T cells primed in an established persistent infection were absent from multiple tissues and almost entirely fail to accumulate in the GI tract.
Late-Primed CD4 T Cells Help B-Cell Responses.
Tfh cells provide signals to B cells to mediate antibody secretion and direct cellular differentiation (2). To test whether late-primed CD4 T cells can help virus-specific B cells in vivo, we developed a system to introduce a traceable LCMV-specific B-cell response into persistent infection. B cells from TgKL25 mice transgenically express the heavy chain of the KL25 antibody, and endogenous light chain rearrangement generates ∼7–10% of naïve B cells expressing the KL25 antibody (19). The KL25 antibody efficiently binds LCMV-WE (20), but not LCMV-Cl13 (Fig. S5). To use the TgKL25 transgenic mice with LCMV-Cl13, we used reverse genetics to produce two recombinant Cl13 viruses containing mutations within its GP1 coding region facilitating recognition by the KL25 antibody (20). One viral variant termed LCMV-M1 is neutralized by KL25 and another termed LCMV-M2 is bound but not neutralized by KL25 (Fig. S5). None of the mutations are in the LCMV-GP61–80 CD4 T-cell epitope and they do not affect SMARTA cell recognition. Both LCMV-M1 and M2 replicate in vivo and suppress Th1 formation in the late-priming situation analogous to WT LCMV-Cl13.
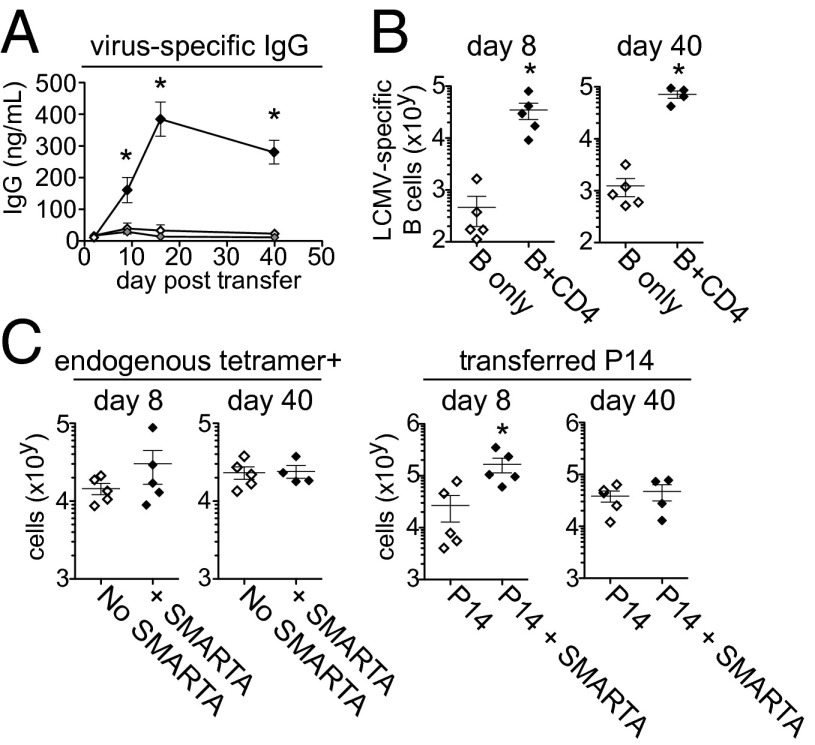
To determine the CD4 Th capacity of late-primed cells in vivo, we transferred transgenic LCMV-specific B cells (from TgKL25 mice) and/or transgenic LCMV-specific CD8 T cells (P14 cells) into mice persistently infected with LCMV-M2 and then with or without LCMV-specific CD4 SMARTA T cells. In these experiments, mice were CD4 depleted before infection to generate a lifelong viremic infection lacking endogenous LCMV-specific CD4 T cells and ensuring that all help is derived from the transferred virus-specific CD4 T cells. In the CD4-depleted model, late-primed CD4 T cells failed to form Th1 cells or distribute to nonlymphoid organs (Fig. S6). Late-primed CD4 T cells did expand to greater levels in lymphoid organs (likely due to a larger available niche), although they did not lead to enhanced viral control (Fig. S6). Importantly, transferred TgKL25+ B cells only expanded, differentiated into plasma cells, and produced antibody when cotransferred with SMARTA cells (Fig. 3A), consistent with the lack of preexisting virus-specific CD4 T cells in CD4-depleted mice, and indicating that late-primed CD4 T cells are capable of providing help to B cells in vivo. Similar results were observed using LCMV-M1. In the presence of late-primed virus-specific CD4 T cells, TgKL25+ B cells and antibody production were maintained at least up to 40 d after transfer (Fig. 3 A and B). However, B cells from TgKL25 mice that were not specific for the viral glycoprotein were not enhanced long-term (Fig. S6C), indicating that the sustained helper effect of late-primed CD4 T cells on B cells is exerted via virus-specific interactions.
Fig. 3.
Virus-specific CD4 T cells primed during established persistent infection help B-cell responses. (A) Plasma LCMV-specific IgG levels on the indicated day following transfer of SMARTA cells alone (gray), TgKL25 B cells alone (white), or SMARTA cells and TgKL25 B cells (black). Cells were transferred into mice infected for 30 d with LCMV M2. Mice were CD4 depleted before infection. (B) The graphs demonstrate the expansion of adoptively transferred transgenic KL25+ B cells (with or without SMARTA cell transfer) 8 and 40 d after transfer. (C) The graphs indicate the number of endogenous (preexisting) LCMV-GP33–41 tetramer+ CD8 T cells and transferred virus-specific CD8 P14 T cells 8 and 40 d after transfer with or without SMARTA cell cotransfer. *P < 0.05. Data are representative of two independent experiments with four to five mice per group.
Although late-primed CD4 T cells provided help for B cells, they did not increase the level of endogenous preexisting LCMV-GP33-41 tetramer+ CD8 T cells by 8 d after transfer (in either undepleted or mice CD4 depleted before infection) and only induced a modest but unstained increase in cotransferred late-primed virus-specific CD8 P14 T cells (Fig. 3C). Furthermore, transfer of virus-specific CD4 T cells did not enhance the cytokine expression of endogenous or cotransferred virus-specific CD8 T cells (Fig. S6D). Taken together, these data indicate that, upon transfer of physiologic numbers, late-primed CD4 T cells are capable of providing B-cell help but exert only minimal, short-term effects on the established or de novo CD8+ T-cell response, consistent with their Tfh differentiation.
Type I IFN Signaling Inhibits de Novo Virus-Specific Th1 Formation During an Established Persistent Infection.
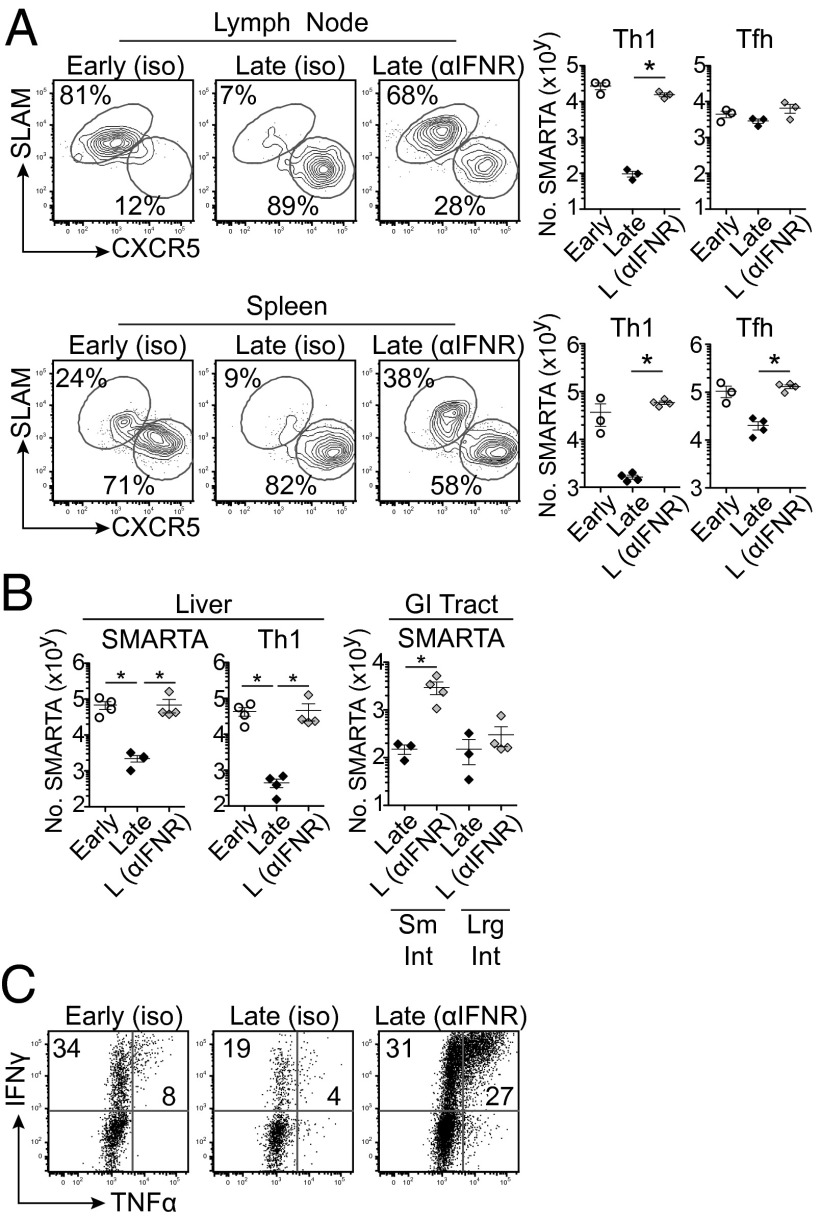
IFN-I signaling remains active throughout persistent infection and chronic IFN-I signaling can suppress antiviral CD4 T-cell responses (21, 22). To evaluate the role of IFN-I in modulating Th1 differentiation during persistent infection, we treated animals with an antibody that blocks IFN-I receptor (IFNR) signaling in vivo (21, 22). Sixty hours after transfer, late-primed CD4 T cells in anti-IFNR–treated mice now down-regulated CD62L and up-regulated IL-2Rα similar to levels observed in early priming. By day 8 after transfer, anti-IFNR blockade restored Th1 differentiation and the absolute number of late-primed virus-specific CD4 T cells to the same level observed in early priming (Fig. 4A). However, anti-IFNR blockade did not impair Tfh differentiation in late-primed virus-specific CD4 T cells (Fig. 4A), supporting that IFN-I signaling inhibits Th1 differentiation as opposed to skewing otherwise Th1 cells into Tfh.
Fig. 4.
IFNR blockade restores Th1 differentiation during late priming. (A–C) Early- and late-primed conditions were treated with isotype antibody or with anti-IFNR blocking antibody. Antibody treatment was initiated 2 d before SMARTA cell transfer and then every 2 d through day 6 after transfer. (A) The flow plots illustrate the frequency and number of Th1 and Tfh SMARTA cells in the mesenteric lymph node and spleen 8 d after priming in the presence of the indicated antibody treatment. The graphs quantify total number of SMARTA cells and number of Th1 and Tfh SMARTA cells within the organs. (B) The graphs quantify total SMARTA cell number and number of Th1 SMARTA cells within the liver and the total SMARTA cell number within the GI tract after indicated antibody treatment. (C) IFN-γ and TNF-α production following ex vivo peptide stimulation by brachial/axillary lymph node-derived early- and late-primed SMARTA cells 8 d after transfer. *P < 0.05. Data are representative of four independent experiments with three to four mice per group.
Consistent with the restoration of Th1 immunity in the lymphoid organs, anti-IFNR blockade restored the frequency and number of late-primed virus-specific CD4 T cells and Th1 development within the liver and small intestine to the same levels observed in early priming (Fig. 4B and Fig. S7). Anti-IFNR blockade also enhanced the capacity of late-primed cells to produce IFN-γ and TNF-α, and did so to levels well above the exhausted virus-specific CD4 T-cell responses observed at the onset of infection (Fig. 4C). Unlike during the established persistent infection, anti-IFNR blockade during priming at the onset of LCMV-Cl13 infection (i.e., early priming) did not increase the magnitude of the virus-specific CD4 Th1 response (Fig. S8), indicating that IFN-I signaling plays temporally disparate roles in modulating virus-specific CD4 T-cell differentiation as persistent infection progresses, and that it can be blocked to systemically restore de novo Th1 differentiation and cytokine expression.
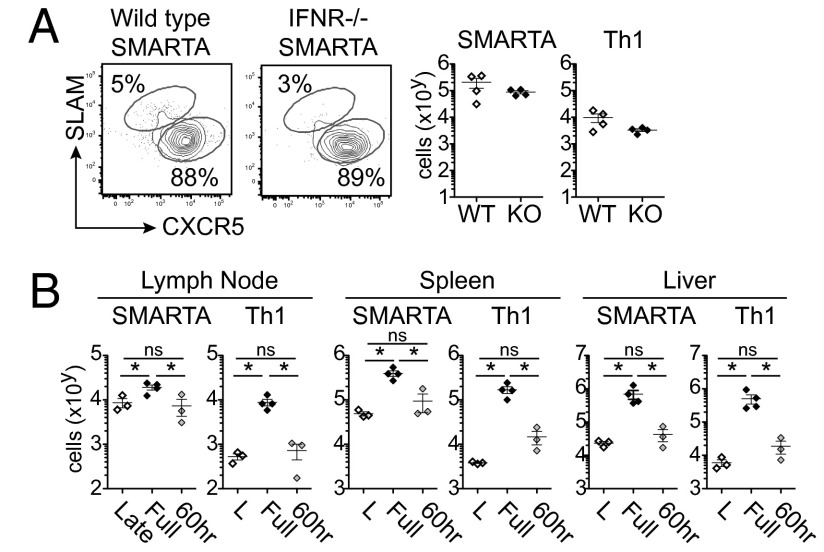
To determine whether IFN-I acts directly on late-primed CD4 T cells to inhibit Th1 formation, we transferred IFNRα-deficient virus-specific CD4 T cells into persistently infected animals. In this experiment, all components of the persistently infected immune environment except for the transferred virus-specific T cells are able to respond to IFN-I signaling. IFNR deficiency on virus-specific CD4 T cells did not increase Th1 differentiation (Fig. 5A), indicating that IFN-I does not act directly on virus-specific CD4 T cells to repress the Th1 response. Interestingly, unlike at the onset of infection wherein direct IFN-I signaling is critical for the survival of virus-specific CD4 T cells (Fig. S9A compared with Fig. 5A), late-primed virus-specific CD4 T cells were present at equal amounts regardless of IFNR expression, thus again highlighting the disparate role of direct IFN-I signaling on CD4 T cells at the onset and in the midst of persistent infection.
Fig. 5.
Indirect IFN-I signals suppress CD4 Th1 differentiation at the time of late priming. (A) The flow plots and graphs demonstrate Th1 and Tfh differentiation by late-primed WT and IFNR−/− SMARTA cells 8 d after late priming. WT, wild-type SMARTA cells; IFNR KO, IFNR−/− SMARTA cells. Cells were transferred into animals infected 21 d prior with LCMV Cl13. (B) Starting 1 d before SMARTA transfer, animals were treated with either isotype antibody, anti-IFNR blocking antibody or isotype antibody followed by anti-IFNR blocking antibody at 60 h after transfer. Cells were transferred into animals infected 21 d prior with LCMV Cl13. The graphs illustrate number of Th1 and Tfh SMARTA cells in the mesenteric lymph nodes, spleen, and liver 8 d after late priming. *P < 0.05. ns, not significant. Data are representative of two independent experiments with three to four mice per group.
To determine whether IFN-I is acting during virus-specific CD4 T-cell priming and programming to repress Th1 formation or whether ongoing IFN-I signaling after priming continues to suppress Th1 differentiation, we blocked IFNR signaling 60 h after cell transfer (once priming has already occurred; Fig. 1). Anti-IFNR treatment rapidly curtails IFN-I signaling and within 1 d IFN-I gene expression was inhibited (Fig. S9B). Whereas blocking IFN-I signaling before priming fully facilitated Th1 differentiation, anti-IFNR blockade 60 h after transfer did not significantly increase Th1 differentiation in lymphoid or peripheral organs (Fig. 5B). Thus, our data demonstrate that IFN-I signaling represses de novo virus-specific CD4 Th1 generation during the priming interactions in an established persistent infection.
Discussion
It is becoming clear that, in addition to its critical antiviral functions, ongoing IFN-I signaling can be detrimental to the immune response, potentiating many of the immune dysfunctions associated with persistent virus infections (21–23). In this report, we now identify a suppressive mechanism associated with chronic IFN-I signaling specifically during an established persistent virus infection. Unlike at the onset of what will become a persistent LCMV infection where IFN-I signaling does not affect Th1 differentiation, in the established persistent infection, IFN-I signaling specifically prevents de novo Th1 generation. The suppression of Th1 development is not mediated by direct IFN-I signaling by the CD4 T cells themselves and similar numbers of IFNR−/− and WT virus-specific CD4 T cells are generated. However, at the onset of infection, IFNR signaling directly by CD4 T cells is critical for their survival and IFNR−/− virus-specific CD4 T cells are almost entirely deleted by 8 d of infection. Thus, our data establish a bifurcation in the role of IFN-I signaling on the immune environment and CD4 T cells themselves as persistent infection progresses.
IFN-I signaling at the time of priming in the established persistent infection rapidly suppresses Th1 differentiation and Th1 markers are never observed (i.e., 60 h after transfer). However, the initial expansion and survival (i.e., total number) of de novo primed virus-specific CD4 T cells observed 60 h after transfer is the same at the onset and in the established persistent infection, demonstrating that the cells that would become Th1 are initially activated and present in early and late priming. However, by day 8, cells that would have become Th1 fail to continue to differentiate and are absent, whereas Tfh differentiation is not dramatically affected. Thus, IFN-I signaling during an established persistent virus infection does not block activation of cells that would become Th1 or skew cells that would become Th1 into Tfh cells, but instead inhibits the differentiation of activated T cells into Th1 effectors. Moreover, the ability to restore Th1 differentiation by blocking IFN-I is lost when anti-IFNR blockade is initiated 60 h after virus-specific CD4 T-cell transfer, confirming that IFN-I signals suppress the differentiation of Th1 cells in the initial priming interactions, without affecting their initial expansion. An alteration in CD8 T-cell activation and differentiation has also been observed when they are initiated a few days into the established acute LCMV (24, 25) or during persistent polyoma virus infection (26). Interestingly, exposure to IFN-I under similar circumstances induces the differentiation of bystander CD8 T cells into a memory-like state displaying some effector properties (27, 28). As in our system, this effect on differentiation was not due to direct IFN signaling to the T cell, but rather was mediated indirectly, potentially through interactions with the priming DC or other APC, indicating that the prepresence of IFN-I modulates the immune environment to control subsequent differentiation of multiple aspects of adaptive immunity. Ultimately, modulating secondary mechanisms downstream of IFN-I signaling may enable restoration of Th1 potential without entirely abolishing the IFN-I system.
CD4 T-cell responses established at the onset of persistent viral infection rapidly develop both Th1 and Tfh immunity. However, as infection progresses, the virus-specific CD4 Th1 cells are redirected toward Tfh (3). In addition to this, our data indicate that the suppression of de novo Th1 differentiation coupled with ongoing de novo Tfh generation may also explain the enlarged Tfh effector pool observed during many established persistent infections (3, 29–31). The sustained ability to continue to produce Tfh responses likely has benefits for the host, as Tfh are necessary to control a persistent infection (3) and the new Tfh are able to sustain B-cell responses. However, an expanding Tfh accumulation may ultimately lead to dysregulation of B-cell development, hypergammaglobulinemia, and the formation of autoantibodies associated with persistent virus infections (32, 33). Ultimately, because a balanced CD4 Th response is likely optimal to sustain the multiple and diverse immunologic needs during persistent virus infection, a progressive differentiation of Th1 into Tfh coupled with diminished ability to generate new Th1 cells could lead to focusing of the immune response and decreased ability to appropriately fight infection.
Th1 cells are associated with enhanced control of multiple persistent viral infections (34–39). Recently, Aubert et al. (5) demonstrated that transfer of high numbers (4 × 106) of naïve virus-specific CD4 T cells into persistent LCMV infection could enhance the preexisting (exhausted) virus-specific CD8 T-cell and B-cell responses. However, in our experiments, we only observed a moderate initial increase in previously established (exhausted) CD8 T-cell responses that was not sustained and no decrease in virus titers. Differences in the amount of transferred virus-specific CD4 T cells (5,000 vs. 4 million) likely account for this discrepancy and suggests that endogenously generated de novo CD4 T-cell immunity may not produce a sufficiently strong Th1 response to help dysfunctional or de novo-primed virus-specific CD8 T cells during viral persistence.
Consistent with the diminished ability to generate de novo Th1 immunity during an established persistent LCMV infection distribution of newly primed CD4 T cells in nonlymphoid sites of virus replication is greatly limited, particularly in the GI tract. The reduced ability of de novo-primed virus-specific CD4 T cells to reach peripheral tissues, could lead to the failure to reconstitute these sites as an infection progresses, thus compounding immunodeficiency and creating viral sanctuaries during persistent infections. As a result, persistently infected individuals may become more susceptible to virus-escape variants, secondary infections, and reinfection in those organs. Ultimately, if new CD4 T-cell responses recruited to balance CD4 T-cell attrition or combat viral escape mutants could not generate a new Th1 component and distribute to tissue reservoirs of infection, it would leave a hole in the CD4 T-cell response and further debilitate control of viral replication. Because CD4 T cells have the potential to direct and sustain multiple types of immune responses in multiple tissues, future therapeutic strategies should consider the alterations in de novo CD4 T-cell differentiation and how to appropriately overcome them.
Materials and Methods
Mice and Virus.
C57BL/6 (WT) mice were purchased from The Jackson Laboratory or the rodent breeding colony at University of California, Los Angeles. B-cell–deficient μMT, hen egg lysozyme transgenic (Hel-tg), and CD11c-DTR mice were purchased from The Jackson Laboratory. Transgenic KL25 mice were provided by Daniel Pinschewer (University of Geneva, Geneva, Switzerland). LCMV-GP61–80–specific CD4 TCR transgenic (SMARTA) and LCMV-GP33–specific CD8 TCR transgenic (P14) mice have been described previously (40, 41). SMARTA mice deficient for the type I IFN receptor were generated by crossbreeding SMARTA mice with IFNR−/− mice (provided by Dorian McGavern, National Institutes of Health, Bethesda). All mice were housed under specific pathogen-free conditions. Mouse handling conformed to the experimental protocols approved by the University of California, Los Angeles, Animal Research Committee. In all experiments, the mice were infected i.v. via the retroorbital sinus with 2 × 106 PFU of LCMV-Armstrong, LCMV-clone 13, LCMV-M1, or LCMV-M2. Virus stocks were prepared and viral titers were quantified as described previously (41).
To generate an LCMV-Cl13 variant that could be recognized by the KL25 antibody, we used reverse-genetics approaches to rescue a recombinant Cl13 virus containing mutations within the GP1 coding region at I118L and S119N for LCMV-M1 and I118L, S119N, and N121K for LCMV-M2.
Isolation and Adoptive Transfer of Virus-Specific T and B Cells.
LCMV-specific SMARTA cells, P14 cells, or TgKL25 B cells were isolated from the spleens of respective transgenic mice by negative selection (StemCell Technologies). All cell transfers were performed i.v. in the retroorbital sinus. To assess priming and differentiation of virus-specific CD4 T cells in the midst of persistent infection, we transferred 5,000 SMARTA cells into either naïve mice that were infected with LCMV-Cl13 1 h later (early priming) or into mice that had been infected with LCMV-Cl13 21 d earlier. For experiments in which the mice were killed at 60 h after transfer, 250,000 SMARTA cells were transferred to enable detection at this early time point. For late priming during acute infection, 5,000 SMARTA cells were transferred into mice infected for 4 d with LCMV-Armstrong.
To assess viral escape from late-primed responses, 5,000 SMARTA cells were transferred into mice 21 d after LCMV-Cl13 infection. Eight days later (day 29 after infection) 5,000 carboxyfluorescein succinimidyl ester (CFSE)-labeled SMARTA cells were transferred into the same mice (i.e., a second SMARTA cell transfer) or into infection-matched mice that did not previously receive SMARTA cells at day 21 (control for effective proliferation). Proliferation was then assessed by CFSE dilution 60 h after the second transfer.
To assess how late-primed virus-specific CD4 T cells help B cells and CD8 T-cell responses, 2–5 × 106 B cells from TgKL25 mice (containing ∼1–3 × 105 KL25+ B cells) and/or 5,000 P14 cells were transferred i.v. with or without 50,000 SMARTA cells. Cells were transferred into mice infected 30–45 d previously and that were CD4 depleted before LCMV infection.
In Vivo CD4 Depletion and Type I IFN Receptor Blockade.
To deplete CD4 T cells before LCMV infection, mice were treated i.v. with 500 μg of anti-CD4 antibody (clone GK1.5; BioXCell) 4 d before infection and again on the day of infection. Thirty to 45 d were allowed to pass before further experiments were performed to allow for the reconstitution of the endogenous CD4 T-cell compartment.
To block IFN-I signaling in vivo during persistent infection, mice were treated i.v. with 500 μg of anti-type I IFN receptor (IFNR1) blocking antibody (clone MAR1-5A3; Leinco Technologies) or isotype control antibody 1 or 2 d before SMARTA cell transfer (on day 19 or 20 of infection), and every 48 h subsequently through day 27 or 28 after infection. For experiments where IFN-I signaling was blocked after virus-specific T-cell priming, mice received isotype control antibody on days 20 and 22 of infection, and IFNR1 blocking antibody starting on day 24 of infection (60 h after SMARTA cell transfer) and every 2 d subsequently. To block IFN-I signaling at the onset of infection, animals were treated with isotype antibody or with anti-IFNR blocking antibody starting 1 d before LCMV-Cl13 infection and SMARTA cell transfer. Antibody treatment was continued every 2 d through day 7 after infection.
Statistical Analysis.
Student t tests (two-tailed, unpaired) and Mann–Whitney nonparametric tests (two-tailed, unpaired) were performed using GraphPad Prism 5 software (GraphPad Software).
Supplementary Material
Acknowledgments
We thank all the members of the Brooks Laboratory for discussions and technical assistance. Our work was supported by National Institutes of Health Grants AI085043 and AI082975 (to D.B.), Microbial Pathogenesis Training Grant T32-AI07323 (to I.O.), Virology and Gene Therapy Training Grant T32AI060567 (to C.R.C.), a Training grant from Fonds de la Recherche en Santé du Québec (to L.M.S.), the Stein Oppenheimer Endowment Award (to D.B.), University of California, Los Angeles (UCLA), Clinical and Translational Science Institute UL1TR000124 Award (to D.B.), and the UCLA Center for AIDS Research (Grant P30 AI028697).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1401662111/-/DCSupplemental.
References
- 1.Ng CT, Snell LM, Brooks DG, Oldstone MB. Networking at the level of host immunity: Immune cell interactions during persistent viral infections. Cell Host Microbe. 2013;13(6):652–664. doi: 10.1016/j.chom.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 3.Fahey LM, et al. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J Exp Med. 2011;208(5):987–999. doi: 10.1084/jem.20101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 5.Aubert RD, et al. Antigen-specific CD4 T-cell help rescues exhausted CD8 T cells during chronic viral infection. Proc Natl Acad Sci USA. 2011;108(52):21182–21187. doi: 10.1073/pnas.1118450109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin E, et al. Heterogeneity among viral antigen-specific CD4+ T cells and their de novo recruitment during persistent polyomavirus infection. J Immunol. 2010;185(3):1692–1700. doi: 10.4049/jimmunol.0904210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson EB, et al. Emergence of distinct multiarmed immunoregulatory antigen-presenting cells during persistent viral infection. Cell Host Microbe. 2012;11(5):481–491. doi: 10.1016/j.chom.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moir S, Chun TW, Fauci AS. Pathogenic mechanisms of HIV disease. Annu Rev Pathol. 2011;6:223–248. doi: 10.1146/annurev-pathol-011110-130254. [DOI] [PubMed] [Google Scholar]
- 9.Petrovic D, Dempsey E, Doherty DG, Kelleher D, Long A. Hepatitis C virus—T-cell responses and viral escape mutations. Eur J Immunol. 2012;42(1):17–26. doi: 10.1002/eji.201141593. [DOI] [PubMed] [Google Scholar]
- 10.Kitchen SG, Zack JA. Stem cell-based approaches to treating HIV infection. Curr Opin HIV AIDS. 2011;6(1):68–73. doi: 10.1097/COH.0b013e3283412370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160(2):521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fahey LM, Brooks DG. Opposing positive and negative regulation of T cell activity during viral persistence. Curr Opin Immunol. 2010;22(3):348–354. doi: 10.1016/j.coi.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks DG, Teyton L, Oldstone MB, McGavern DB. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J Virol. 2005;79(16):10514–10527. doi: 10.1128/JVI.79.16.10514-10527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston RJ, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325(5943):1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Probst HC, van den Broek M. Priming of CTLs by lymphocytic choriomeningitis virus depends on dendritic cells. J Immunol. 2005;174(7):3920–3924. doi: 10.4049/jimmunol.174.7.3920. [DOI] [PubMed] [Google Scholar]
- 16.Harker JA, Lewis GM, Mack L, Zuniga EI. Late interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science. 2011;334(6057):825–829. doi: 10.1126/science.1208421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cucak H, Yrlid U, Reizis B, Kalinke U, Johansson-Lindbom B. Type I interferon signaling in dendritic cells stimulates the development of lymph-node-resident T follicular helper cells. Immunity. 2009;31(3):491–501. doi: 10.1016/j.immuni.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Hale JS, et al. Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity. 2013;38(4):805–817. doi: 10.1016/j.immuni.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hangartner L, et al. Antiviral immune responses in gene-targeted mice expressing the immunoglobulin heavy chain of virus-neutralizing antibodies. Proc Natl Acad Sci USA. 2003;100(22):12883–12888. doi: 10.1073/pnas.2135542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hangartner L, et al. Nonneutralizing antibodies binding to the surface glycoprotein of lymphocytic choriomeningitis virus reduce early virus spread. J Exp Med. 2006;203(8):2033–2042. doi: 10.1084/jem.20051557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teijaro JR, et al. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science. 2013;340(6129):207–211. doi: 10.1126/science.1235214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson EB, et al. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science. 2013;340(6129):202–207. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson EB, Brooks DG. Decoding the complexity of type I interferon to treat persistent viral infections. Trends Microbiol. 2013;21(12):634–640. doi: 10.1016/j.tim.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fousteri G, et al. Increased memory conversion of naïve CD8 T cells activated during late phases of acute virus infection due to decreased cumulative antigen exposure. PLoS One. 2011;6(1):e14502. doi: 10.1371/journal.pone.0014502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mueller SN, et al. Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science. 2007;317(5838):670–674. doi: 10.1126/science.1144830. [DOI] [PubMed] [Google Scholar]
- 26.Vezys V, et al. Continuous recruitment of naive T cells contributes to heterogeneity of antiviral CD8 T cells during persistent infection. J Exp Med. 2006;203(10):2263–2269. doi: 10.1084/jem.20060995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marshall HD, Urban SL, Welsh RM. Virus-induced transient immune suppression and the inhibition of T cell proliferation by type I interferon. J Virol. 2011;85(12):5929–5939. doi: 10.1128/JVI.02516-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welsh RM, Bahl K, Marshall HD, Urban SL. Type 1 interferons and antiviral CD8 T-cell responses. PLoS Pathog. 2012;8(1):e1002352. doi: 10.1371/journal.ppat.1002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong JJ, Amancha PK, Rogers K, Ansari AA, Villinger F. Spatial alterations between CD4+ T follicular helper, B, and CD8+ T cells during simian immunodeficiency virus infection: T/B cell homeostasis, activation, and potential mechanism for viral escape. J Immunol. 2012;188(7):3247–3256. doi: 10.4049/jimmunol.1103138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindqvist M, et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest. 2012;122(9):3271–3280. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrovas C, et al. CD4 T follicular helper cell dynamics during SIV infection. J Clin Invest. 2012;122(9):3281–3294. doi: 10.1172/JCI63039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Recher M, et al. Deliberate removal of T cell help improves virus-neutralizing antibody production. Nat Immunol. 2004;5(9):934–942. doi: 10.1038/ni1102. [DOI] [PubMed] [Google Scholar]
- 33.Hangartner L, Zinkernagel RM, Hengartner H. Antiviral antibody responses: The two extremes of a wide spectrum. Nat Rev Immunol. 2006;6(3):231–243. doi: 10.1038/nri1783. [DOI] [PubMed] [Google Scholar]
- 34.Grakoui A, et al. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302(5645):659–662. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 35.Thimme R, et al. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194(10):1395–1406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vingert B, et al. HIV controllers maintain a population of highly efficient Th1 effector cells in contrast to patients treated in the long term. J Virol. 2012;86(19):10661–10674. doi: 10.1128/JVI.00056-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324(5934):1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fröhlich A, et al. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324(5934):1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- 39.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324(5934):1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oxenius A, Bachmann MF, Zinkernagel RM, Hengartner H. Virus-specific MHC-class II-restricted TCR-transgenic mice: Effects on humoral and cellular immune responses after viral infection. Eur J Immunol. 1998;28(1):390–400. doi: 10.1002/(SICI)1521-4141(199801)28:01<390::AID-IMMU390>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 41.Brooks DG, McGavern DB, Oldstone MB. Reprogramming of antiviral T cells prevents inactivation and restores T cell activity during persistent viral infection. J Clin Invest. 2006;116(6):1675–1685. doi: 10.1172/JCI26856. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.