Significance
T lymphocytes are white blood cells that recognize and fight pathogens. Maintenance of sufficient numbers of T cells is essential to prevent susceptibility to infections. Survival of quiescent T cells is maintained, in part, by the interaction between the soluble factor (IL-7 produced by various stromal cells) and the IL-7 receptor (IL-7R) expressed on the surface of T cells. Here, we show that naïve T cells have basal nuclear levels of the transcription factor NF-κB and that is key to maintain IL-7R expression in T cells and for their survival. Our results imply that antiinflammatory therapies targeting NF-κB may affect the pool of naïve T cells required to control infections.
Keywords: IKKβ, IkBaDN, STAT5, Il7r enhancer
Abstract
T cells are essential for immune defenses against pathogens, such that viability of naïve T cells before antigen encounter is critical to preserve a polyclonal repertoire and prevent immunodeficiencies. The viability of naïve T cells before antigen recognition is ensured by IL-7, which drives expression of the prosurvival factor Bcl-2. Quiescent naïve T cells have low basal activity of the transcription factor NF-κB, which was assumed to have no functional consequences. In contrast to this postulate, our data show that basal nuclear NF-κB activity plays an important role in the transcription of IL-7 receptor α-subunit (CD127), enabling responsiveness of naïve T cells to the prosurvival effects of IL-7 and allowing T-cell persistence in vivo. Moreover, we show that this property of basal NF-κB activity is shared by mouse and human naïve T cells. Thus, NF-κB drives a distinct transcriptional program in T cells before antigen encounter by controlling susceptibility to IL-7. Our results reveal an evolutionarily conserved role of NF-κB in T cells before antigenic stimulation and identify a novel molecular pathway that controls T-cell homeostasis.
Survival of naïve quiescent T cells is essential to maintain a pool of polyclonal T cells ready for activation by their cognate antigen. On egress from the thymus, survival of peripheral naïve T cells (CD4+CD44lo and CD8+CD44lo) depends on intermittent tonic engagement of the T-cell receptor (TCR) and signaling by the cytokine IL-7 (1, 2). Tonic TCR engagement is generated by the interaction of the TCR with weakly reactive self-peptides (3). Survival of quiescent CD8 T cells requires MHC class I–TCR engagement, which is indicated by dwindling numbers of naïve CD8 T cells after transfer into MHC class I-deficient mice (4, 5). In addition, long-term (but not short-term) survival of CD4 T cells requires the presence of MHC class II (6).
IL-7 is important for survival and homeostatic proliferation of naïve T cells, which is shown by reduced recovery of naïve T cells transferred into IL-7−/− mice (7, 8) and impaired survival and homeostatic proliferation of T cells from IL-7 receptor α-subunit (IL-7Rα) -deficient mice (9, 10). The receptor for IL-7 is a heterodimer consisting of the IL-7Rα (CD127) and common γ-chain receptor (γc; CD132) subunits. Triggering of IL-7R activates Stat5 through Jak1/Jak3 (11) and the PI3K/Akt/mTOR axis (12). IL-7–mediated survival involves up-regulation of the prosurvival factors Bcl-2 and Mcl-1 as well as reduction of proapoptotic molecules Bax, Bad, and Bim (13). Interestingly, IL-7 negatively regulates the expression of its receptor, promoting endocytosis, degradation, and the transcriptional inhibition of Il7r expression (11, 14). This milieu enables a pool of T cells that have not yet encountered IL-7 to be preferentially responsive to limiting concentrations of this cytokine. Several transcription factors are involved in the control of Il7r expression in T cells, including positive regulation by GA binding protein, glucocorticoid receptor, Ets1, Runx1, Runx3, and Foxo1 and repression by Foxp1 and Gfi1, the latter exclusively in CD8 T cells (15).
The transcription factor NF-κB is critical for T-cell activation, proliferation, and survival after TCR engagement. NF-κB exists mostly as heterodimers between the transactivating proteins RelA, RelB, and c-Rel and their DNA binding partners p50 (p105; NF-κB1) and p52 (p100; NF-κB2) (16). On TCR engagement, the kinase IKKβ, part of the IKK complex, phosphorylates IκBα, the inhibitor of NF-κB, targeting it for degradation and allowing NF-κB to translocate into the nucleus. In activated T cells, NF-κB induces up-regulation of the prosurvival molecules Bcl-xL, A1, A20, and cellular inhibitors of apoptosis (17–19). IκBαΔN mice bear transgenic expression of a nondegradable form of IκBα in early thymocyte development, resulting in NF-κB–impaired T cells (20). These mice have diminished survival of activated mature T cells and reduced numbers of peripheral naïve T cells (20, 21). The mechanism for decreasing survival of IκBαΔN naïve T cells is not clear. Using various genetic mouse models of NF-κB impairment in T cells as well as pharmacological inhibition of NF-κB, our results show that basal NF-κB activity controls survival of naïve quiescent T cells, at least in part, by enhancing Il7r transcription, a mechanism conserved in both mice and humans. Our findings show an essential role of NF-κB in the control of naive T-cell homeostasis.
Results
Basal NF-κB Contributes to the Survival of Quiescent Naïve T Cells.
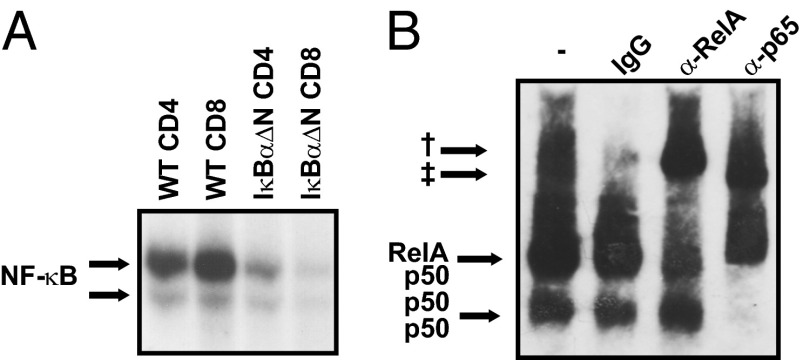
Activation of NF-κB on TCR engagement is essential for survival of activated T cells (22). Basal NF-κB activity has been noted in unstimulated T cells, although at much lower levels than in TCR-stimulated T cells, but its functional significance was unknown (23). To investigate the NF-κB subunits at play in naïve T cells, EMSAs were performed using nuclear extracts from FACS cell-sorted purified CD4+CD44lo and CD8+CD44lo naïve WT and NF-κB–impaired IκBαΔN T cells. NF-κB activity in IκBαΔN naïve T cells was greatly reduced compared with naïve WT T cells (Fig. 1A). Supershift assays revealed that the predominant NF-κB complexes present in quiescent naïve WT T cells were RelA/p50 and p50/p50 dimers (Fig. 1B).
Fig. 1.
Naïve T cells display basal NF-κB activity. (A) EMSA for NF-κB using nuclear extracts of WT and IκBαΔN FACS cell-sorted CD4+CD44lo and CD8+CD44lo T cells. (B) EMSA supershift for NF-κB subunits RelA and p50 performed with nuclear extracts from WT naïve T cells. Results are representative of at least three independent experiments. †Supershifted bands for RelA. ‡Supershifted band for p50.
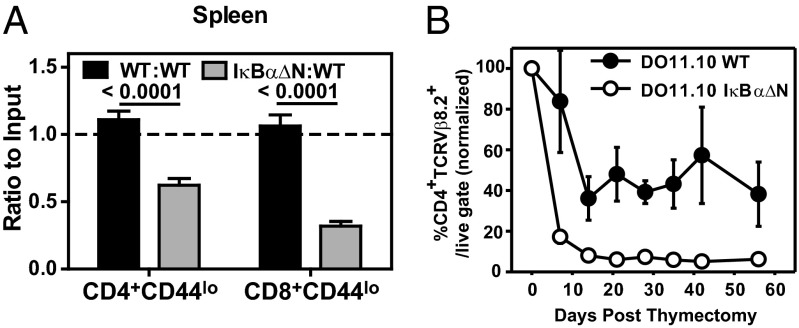
Because NF-κB signaling is required for lymphopenia- and activation-induced proliferation of T cells (21), we tested whether NF-κB drives quiescent naïve T-cell survival in vivo. To this end, equal numbers of WT (CD45.1/2) and IκBαΔN (CD45. 2) CD4+CD44lo and CD8+CD44lo cells were coadoptively transferred into congenic WT recipients (CD45.1). One week later, analyses of spleen (Fig. 2A) and peripheral lymph nodes (Fig. S1) revealed that the ratio of recovered IκBαΔN to WT CD4+CD44lo and CD8+CD44lo T cells was significantly reduced. To rule out a causal role of TCR repertoire differences in developing NF-κB–impaired thymocytes, survival of T cells with a fixed TCR specificity was analyzed. Ovalbumin-specific TCR transgenic DO11.10 WT and DO11.10 IκBαΔN mice were thymectomized, and the half-life of DO11.10 CD4 T cells was assessed over time in peripheral blood cells. The decay of DO11.10 IκBα∆N T cells was faster and more pronounced than that of WT controls (Fig. 2B), further indicating that basal NF-κB activity is necessary for survival of quiescent naïve T cells in vivo.
Fig. 2.
Basal NF-κB is required for T-cell survival in vivo. (A) Equal numbers of CD45.1/2 WT and CD45.2 WT or IκBα∆N CD4+CD44lo and CD8+CD44lo cells were coadoptively transferred into CD45.1 recipients. Seven days later, ratios of IκBαΔN:WT and WT:WT splenic CD4+CD44lo and CD8+CD44lo T cells were assessed as follows: (% CD45.2final/% CD45.1/2final)/(% CD45.2initial/% CD45.1/2initial). The graph represents recipients receiving WT:WT (n = 13) and IκBαΔN:WT (n = 14) cells. Data are pooled from five independent experiments and analyzed by Kruskal–Wallis test with Dunn’s posttest. (B) DO11.10/WT and DO11.10/IκBα∆N mice were thymectomized, and presence of CD4+TCRVβ8.2+ T cells in peripheral blood was assessed weekly by flow cytometry (WT, n = 6; IκΒαΔN, n = 8). Values displayed are percentages of CD4+TCRVβ8.2+ T cells with respect to the live gate and relative to the value obtained at the time of thymectomy (day 0). Data are representative of two independent experiments.
Basal NF-κB Does Not Control Tonic TCR Signaling.
The reduced lifespan of NF-κB–impaired naïve T cells suggests that NF-κB regulates the expression of one or more genes important for naïve T-cell survival. Because tonic TCR signaling is required for survival of naïve T cells, we hypothesized that basal NF-κB activity may control tonic TCR signaling in naïve T cells. At steady state, expression of CD5 in naïve T cells reflects proximal TCR signal strength in response to self-peptide/MHC (24). To test if basal NF-κB controls tonic TCR signals, expression of CD5 was analyzed in peripheral naïve CD4 and CD8 T cells from WT and IκBαΔN mice. Remarkably, CD5 expression in IκBαΔN CD4 and CD8 naïve T cells was comparable with WT T cells (Fig. S2A). To rule out any compensatory effect caused by a potentially disparate TCR repertoire in NF-κB–impaired T cells, expression of CD5 was analyzed in ovalbumin-specific TCR transgenic WT and IκBαΔN OT-II CD4+ naïve T cells. Again, no differences were observed in levels of CD5 expression between the two strains (Fig. S2B), suggesting that basal NF-κB does not control tonic TCR signaling.
Basal NF-κB Activity Controls IL-7–Dependent T-Cell Survival.
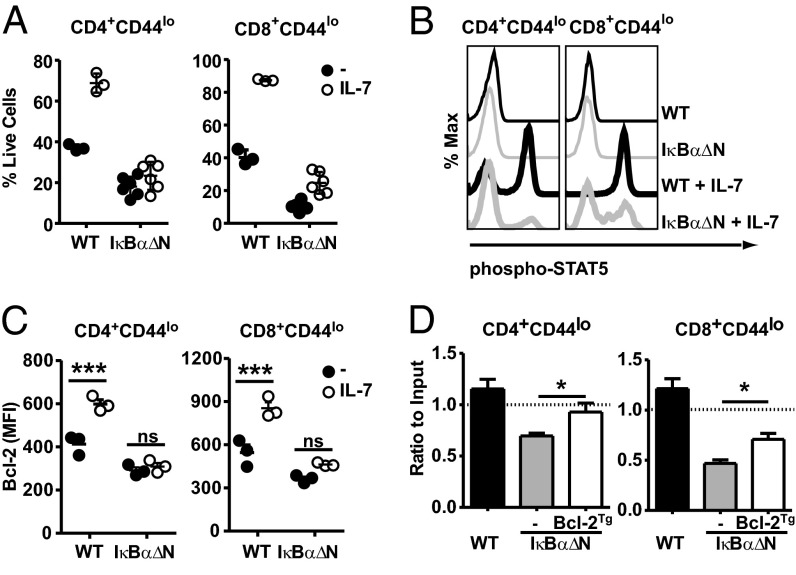
Homeostasis of naïve T cells requires intermittent interaction of IL-7 with its receptor, IL-7R, which in turn, activates the Jak3/Stat5 signaling. This pathway promotes the expression of the prosurvival factor Bcl-2 (1). To test the susceptibility of IκBα∆N T cells to IL-7–mediated survival, WT and IκBα∆N splenocytes were cultured in vitro in the presence of exogenous IL-7, and viability of naïve CD4 and CD8 T cells was analyzed. Surprisingly, addition of IL-7 enhanced survival of WT but not IκBαΔN naïve CD4 and CD8 T cells (Fig. 3A). Consistent with this observation, IL-7–mediated STAT5 phosphorylation (Fig. 3B) and Bcl-2 up-regulation (Fig. 3C) were severely compromised in the majority of IL-7–stimulated IκBαΔN T cells, suggesting that IL-7 signaling in T cells depends on basal NF-κB activity.
Fig. 3.
Basal NF-κB activity is required for IL-7–mediated survival. (A) Splenocytes from WT (n = 3) and IκBα∆N (n = 6) mice were cultured for 3 d in the presence (open symbols) or absence (filled symbols) of 1 ng/mL IL-7. Percentages of CD4+CD44lo and CD8+CD44lo live (7AAD-negative) cells were assessed by flow cytometry. (B) WT and IκBα∆N splenocytes were cultured in vitro for 30 min in the presence or absence of 1 ng/mL IL-7, and (Y694) STAT5 phosphorylation in CD4+CD44lo and CD8+CD44lo was assessed by intracellular flow cytometry. Data are representative of n = 6 mice each. (C) Splenocytes from WT (n = 3) and IκΒαΔN (n = 3) mice were cultured in the presence (open symbols) or absence (filled symbols) of IL-7, and 24 h later, expression of Bcl-2 was assessed by intracellular flow cytometry in CD4+CD44lo and CD8+CD44lo cells. Data were analyzed by two-way ANOVA with Bonferroni posttests. (D) Equal numbers of CD45.1/2 WT, CD45.2 WT, and IκBα∆N or IκBα∆NxBcl-2Tg CD4+CD44lo and CD8+CD44lo cells were coadoptively transferred into CD45.1 recipients. Results were analyzed as in Fig. 2A. The graph represents recipient mice for WT:WT (n = 8), IκBαΔN:WT (n = 9), and IκBαΔNxBcl-2Tg:WT (n = 9). Data are pooled from three independent experiments and analyzed by Kruskal–Wallis test with Dunn’s posttest. All experiments were performed at least three times. MFI, mean fluorescence intensity; ns, not significant. *P < 0.05. ***P < 0.001.
To test if diminished IL-7–mediated Bcl-2 up-regulation was responsible for defective survival of naïve IκBαΔN T cells, IκBαΔN mice overexpressing human Bcl-2 (25) were generated. Indeed, Bcl-2Tg rescued the percentage of CD4 and CD8 naïve T cells in IκBαΔN mice (Fig. S3A), and in vitro, Bcl-2Tg significantly improved viability of IκBαΔN naïve T cells after 3 d of culture with and without IL-7 (Fig. S3 B and C). Finally, coadoptive transfer of CD45.1/2 WT, CD45.2 WT, and IκBαΔN or IκBαΔNxBcl-2Tg CD4 and CD8 naïve T cells into congenic recipients showed that Bcl-2Tg partially restored survival of IκBαΔN naïve T cells in vivo (Fig. 3D). Our data suggest that the defective survival of NF-κB–impaired naïve T cells in response to IL-7 is, at least in part, caused by reduced IL-7–dependent Bcl-2 up-regulation.
NF-κB Is Required for IL-7Rα Expression.
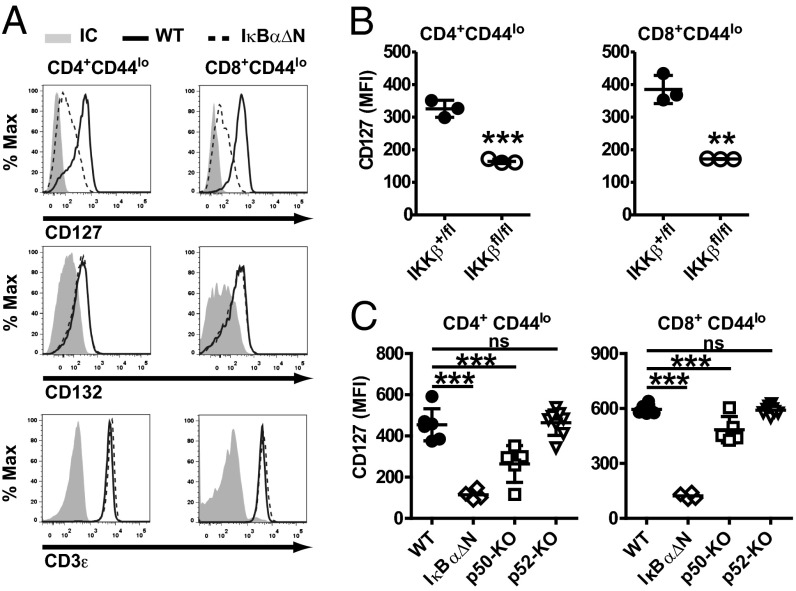
The requirement of NF-κB activity for IL-7R signaling implied that NF-κB might control the expression or activation of components of the IL-7R pathway. To investigate this hypothesis, levels of IL-7Rα (CD127) and the common γc-chain (CD132) subunits of the IL-7R were assessed in WT and IκBαΔN CD4 and CD8 naïve T cells by flow cytometry. Interestingly, levels of IL-7Rα were reduced on IκBαΔN CD4+CD44lo and CD8+CD44lo T cells (25% and 20% of WT, respectively), whereas levels of CD132 and CD3ε were not (Fig. 4A and Fig. S4A), indicating that NF-κB selectively regulates the IL-7Rα chain in T cells. Because of impaired positive and negative selection of CD8SP but not CD4SP IκBαΔN (26), levels of IL-7Rα were also assessed in OT-II IκBαΔN CD4 naïve T cells (Fig. S4B). Similar to results in polyclonal T cells, IL-7Rα levels were diminished in OT-II IκBαΔN cells, suggesting that the impact of NF-κB on IL-7Rα expression is independent of effects on thymic selection.
Fig. 4.
Basal NF-κB is required for IL-7Rα expression. (A) Histograms displaying (Top) IL-7Rα/CD127, (Middle) γc/CD132, and (Bottom) CD3ε as analyzed by flow cytometry in WT (solid line) and IκBαΔN (broken line) CD4+CD44lo (Left) and CD8+CD44lo (Right) T cells. IC, isotype control. (B) Expression of CD127 in (Left) CD4+CD44lo and (Right) CD8+CD44lo splenocytes from CD4CreIKKβ+/fl or CD4CreIKKβfl/fl mice. Data were analyzed by Student t test. (C) Expression of CD127 in WT (n = 4), IκBαΔN (n = 5), p50−/− (n = 5), and p52−/− (n = 8) mice as assessed by flow cytometry and one-way ANOVA with Bonferroni posttests for pairwise comparisons. Results are representative of at least two independent experiments. **P < 0.01. ***P < 0.001.
The IκBαΔN transgene is driven by the proximal Lck promoter; therefore, its expression is turned on early during thymic development (20), which results in reduced IL-7Rα expression in IκBαΔN CD4SP and CD8SP thymocytes (Fig. S5A). CD4Cre IKKβfl/fl mice, which delete IKKβ in double-positive thymocytes and have overall normal thymic development (Fig. S5B) (27), were used to confirm the requirement of NF-κB for IL-7Rα expression. Indeed, IL-7Rα levels on peripheral CD4Cre IKKβfl/fl CD4 and CD8 naïve T cells were one-half of the levels in WT controls (Fig. 4B) and below the levels in IL-7Rα+/− T cells (Fig. S6A). The impact of reduced IL-7Rα expression on CD4CreIKKβfl/fl and IL-7R heterozygous T cells on IL-7–mediated survival was determined in vitro. Survival of IL-7RCre+/− and WT T cells was equivalent, whereas that of CD4CreIKKβfl/fl naïve CD8 T cells was slightly impaired but significantly higher than that of IκBαΔN T cells (Fig. S6B). Thus, these data suggest that a threshold of IL-7Rα is required to confer full survival in the presence of IL-7 and that NF-κB is a limiting factor to attain this threshold.
Two pathways of NF-κB activation have been described: RelA/p50 and cRel/p50 dimers are activated in the canonical (IKKβ and IκBα) pathway, whereas the alternative pathway is dominated by RelB/p52 dimers (18). IL-7Rα expression was reduced in p50-but not p52-deficient CD4 and CD8 naïve T cells (Fig. 4C) to a lesser extent than in IκBαΔN or CD4CreIKKβfl/fl T cells. Our results indicate that the canonical but not the alternative NF-κB pathway regulates IL-7Rα expression.
IL-7 availability is increased in lymphopenic mice and humans (1). To address whether reduced IL-7Rα in IκBαΔN T cells was caused by increased IL-7 availability and consequent receptor down-regulation in vivo, IκBαΔN T cells were harvested from their in vivo environment and cultured in vitro in the absence of IL-7 (18). In contrast to WT T cells that increased their IL-7Rα over a 48-h culture, IκBαΔN T cells were unable to do so (Fig. S7A). Additionally, to avoid potential in vitro artifacts, WT and IκBαΔN T cells were coadoptively transferred into lymphoreplete mice. Similar to the results in vitro, IL-7Rα was not restored in IκBαΔN T cells (Fig. S7B), suggesting that NF-κB is intrinsically required for IL-7Rα expression.
Transcriptional Control of IL-7Rα by NF-κB.
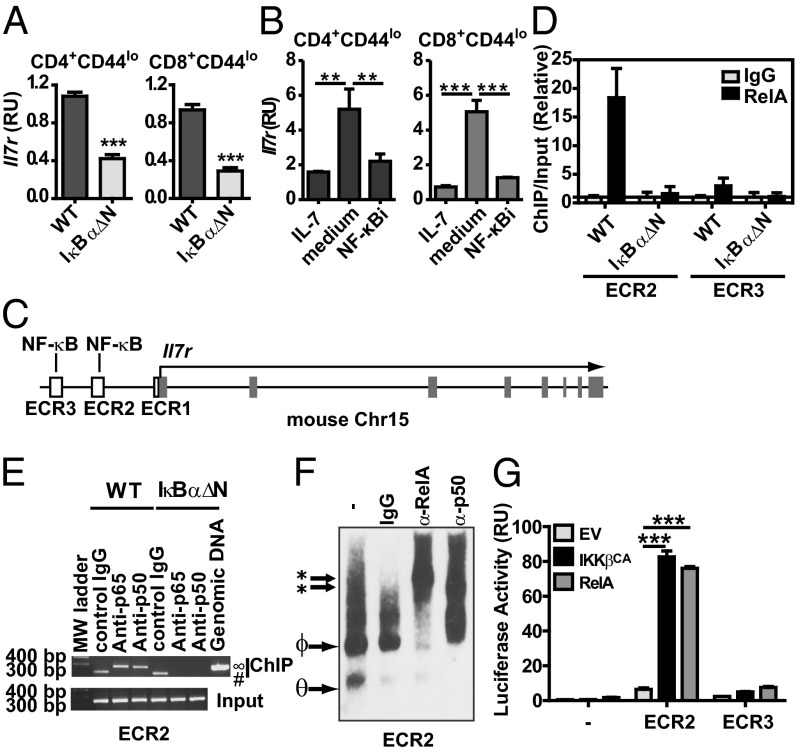
To address whether NF-κB governs IL-7Rα transcription, Il7r mRNA was analyzed by quantitative RT-PCR (RT-qPCR) in WT and IκBαΔN CD4 and CD8 naïve T cells. Levels of Il7r mRNA were reduced in IκBαΔN CD4 and CD8 T cells (Fig. 5A), suggesting that NF-κB is required for Il7r transcription. To test the requirement of NF-κB for de novo IL-7Rα expression in mature T cells, the IKKβ pharmacological inhibitor 6-amino-4–4-phenoxyphenylethylamino-quinazoline (NF-κBi) was used (28). IL-7 inhibits transcription of Il7r, and reexpression of IL-7Rα requires de novo transcription. To assess whether NF-κB controls de novo gene expression of IL-7Rα, WT T cells were incubated for 24 h with IL-7, washed, and cultured alone or in the presence of NF-κBi. CD4+CD44lo and CD8+CD44lo T cells treated with NF-κBi had a substantial reduction in de novo expression of IL-7Rα mRNA (Fig. 5B) and protein (Fig. S8). These data prompted us to investigate whether NF-κB might directly regulate Il7r transcription.
Fig. 5.
Basal NF-κB controls transcription of Il7r in naïve T cells. (A) Il7r mRNA from WT and IκBαΔN CD4+CD44lo and CD8+CD44lo cells was assessed by RT-qPCR, and triplicates were normalized to Actb. RU, relative units. Results are shown as mean ± SD of triplicates analyzed by Student t test, and they are representative of three independent experiments. ***P < 0.001. (B) WT splenocytes were cultured for 24 h with 1 ng/mL IL-7, washed, and incubated for another 24 h with 1 ng/mL IL-7, no IL-7 (medium), or 5 nM NF-κBi. CD127 expression was analyzed by RT-qPCR in FACS cell-sorted CD4+CD44lo live cells with one-way ANOVA and Bonferroni posttests. **P < 0.01. ***P < 0.001. (C) In silico analysis of murine Il7r locus highlighting ECRs upstream of the 5′ TSS. ECR2 (−3.6 kb) and ECR3 (−5.6 kb) contain sequences with putative NF-κB binding sites. (D) ChIP for ECR2 and ECR3 using anti-RelA antibody or isotype control (IgG) in WT and IκBαΔN naïve T cells as assessed by RT-qPCR. (E) Semiquantitative PCR after ChIP for ECR2 using anti-RelA, anti-p50 antibodies or isotype control (IgG) in WT and IκBαΔN naïve T cells. ∞Two hundred eighty-two–base pair expected PCR product. #Nonspecific band. MW, molecular weight. (F) Supershift EMSA for NF-κB binding site contained in ECR2 using antibodies against RelA, p50, or isotype control. *Supershifted bands. ΦRelA/p50. θp50/p50 dimers. **P < 0.01. (G) Dual luciferase assay of lysates of 293T cells transfected for 48 h with the luciferase reporter plasmid pGL4.23 alone or containing ECR2 and ECR3 sequences from Il7r gene plus pRL-TK and control plasmid (EV) or plasmids encoding IKKβ-CA and RelA. Results were analyzed by two-way ANOVA with Bonferroni posttests for pairwise comparisons. Results are representative of at least two independent experiments. ***P < 0.001.
In silico analysis of Il7r genomic DNA in eight mammalian species revealed three evolutionary conserved regions (ECRs) upstream of the Il7r transcription start site (TSS): ECR1 in the proximal Il7r promoter and ECR2 at −3.6 kb and ECR3 at −5.6 kb from the Il7r TSS. ECR2 and ECR3 each contain a potential NF-κB binding site (Fig. 5C). ChIP assays revealed that NF-κB/RelA present in WT T cells bound to ECR2 but marginally bound to ECR3 (Fig. 5D). NF-κB1/p50 was also recruited to ECR2 (Fig. 5E). In contrast, neither subunit was recruited to Il7r ECR2 or ECR3 in IκBαΔN T cells (Fig. 5 D and E). Additionally, EMSA supershift assays confirmed that RelA and p50 bound to this region (Fig. 5F). Finally, to test the capacity of NF-κB to enhance Il7r expression, reporter assays in 293T cells were performed using ECR2 and ECR3 as enhancers of a minimal promoter driving the luciferase gene. Cotransfection with plasmids coding for constitutively active IKKβ (IKKβCA) or RelA showed that only ECR2 possessed a functional NF-κB enhancer (Fig. 5G). Taken together, these data imply that RelA/p50 controls transcription of Il7r through the −3.6-kb ECR2 enhancer.
Forced NF-κB Activity Enhances Il7r Expression.
Given the requirement of canonical NF-κB activation for IL-7Rα expression, we reasoned that manipulations enhancing canonical NF-κB signaling may further augment IL-7Rα levels. To test this possibility, we generated mice expressing IKKβCA specifically in T cells by crossing the Rosa26-StopFLIKKβCA-GFP mice (29) with LckCre transgenic mice (30). The resulting IKKβCA-expressing T cells (LckCreIKKβCA) coexpress the GFP (Fig. 6A) and display enhanced NF-κB activity (31). The penetrance of the LckCre transgene is variable (27), allowing for discrimination of IKKβCA transgenic (GFP+) from nontransgenic (GFP−) cells in the same mouse (Fig. 6A). IKKβCA-GFP+ CD4+CD44lo and CD8+CD44lo T cells had double the amount of IL-7Rα compared with their WT or GFP− counterparts at both the protein (Fig. 6B) and mRNA (Fig. S9) levels. These data also support the conclusion that basal NF-κB activity intrinsically enhances Il7r expression in naïve T cells.
Fig. 6.
Constitutive active IKKβ enhances IL-7Rα expression. (A) Expression of GFP was assessed by flow cytometry in CD4+CD44lo and CD8+CD44lo T cells from WT and LckCre IKKβCA mice. (B) CD127 expression was assessed by flow cytometry in CD4+CD44lo and CD8+CD44lo T cells from WT (n = 4) and LckCre IKKβCA (n = 6; gated on GFP− and GFP+ events) mice. Results were analyzed with one-way ANOVA and Bonferroni posttests. ***P < 0.001.
NF-κB Controls IL-7Rα Expression in Human T Cells.
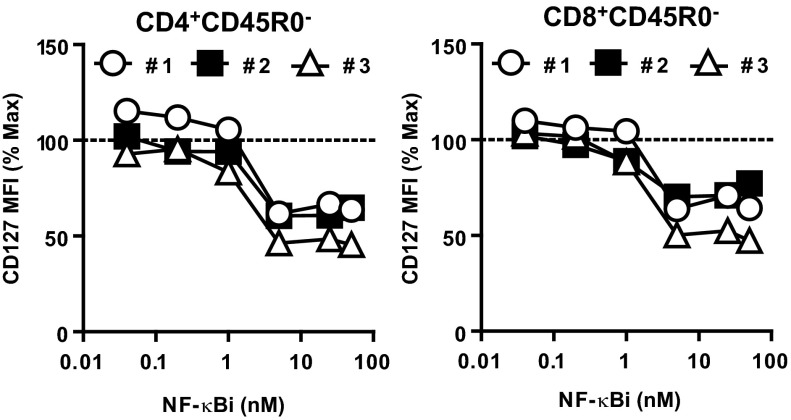
To test whether basal NF-κB activity is important for IL-7Rα expression in human T cells, the IKKβ pharmacological inhibitor (NF-κBi) was used. Human peripheral blood mononuclear cells (PBMCs) from healthy volunteers were subjected to IL-7–mediated IL-7Rα down-regulation and reexpression as described for mouse splenocytes (Fig. 5B). Doses of NF-κBi of 5 nM or greater, although not affecting cell viability (Fig. S10), limited de novo IL-7Rα expression by 35–47% in CD4+CD45RO− and CD8+CD45RO− naïve T cells, which were assessed by flow cytometry in live-gated cells (Fig. 7). These data suggest that NF-κB controls the expression of IL-7Rα in both human and mouse T cells; therefore, therapies targeting NF-κB activity could potentially impact naïve T-cell survival in human patients.
Fig. 7.
Pharmacological inhibition of NF-κB reduces expression of IL-7Rα in human peripheral T cells. PBMCs from healthy individuals were cultured for 24 h with 10 ng/mL recombinant human IL-7, washed, and incubated for 24 h with increasing doses of NF-κBi. Expression of CD127 was assessed in CD3+CD4+CD45R0− and CD3+CD8+CD45R0− live-gated cells and represented compared with maximum (NF-κBi = 0 nM). Results are representative of two independent experiments.
Discussion
Survival of naïve T cells is essential to maintain T-cell homeostasis and prevent lymphopenia and immunodeficiency. Persistence of naïve T cells depends on intermittent signaling through IL-7Rα and tonic TCR stimulation (32). In this study, we show that basal NF-κB activity is essential for the survival of naïve T cells. Rather than controlling homeostatic signals induced by tonic TCR stimulation, basal NF-κB activity in naïve T cells is a limiting factor to enhance transcription of Il7r. In antigen-experienced T cells, NF-κB activity directly induces expression of prosurvival factors, such as c-FLIP and Bcl-xL (21). In contrast, our findings suggest that NF-κB–dependent survival of naïve T cells relies, at least in part, on Bcl-2 up-regulation on IL-7 exposure. Supporting our results, Silva et al. (33) recently reported that IKKβ is required for IL-7Rα expression and homeostatic proliferation. Together with our findings, both studies define a role for NF-κB in the control of naïve T cells homeostasis in both lymphoreplete and lymphopenic hosts.
The signals driving basal NF-κB signaling in quiescent naïve T cells are not known. Several receptors of the TNFR superfamily present in naïve T cells may deliver basal NF-κB signaling. TNFR and CD27 signaling have been shown to promote IL-7Rα expression in an NF-κB–dependent manner (14, 33). Therefore, it is possible that these receptors are part of the physiological network driving basal NF-κB activity in naïve T cells.
The models of NF-κB–deficient T cells used in this study display varying degrees of impairment in IL-7Rα expression, with the lowest in IκBαΔN naïve T cells followed by CD4CreIKKβfl/fl and finally, p50-deficient cells. Differences in the quantity and/or quality of basal nuclear NF-κB in the different NF-κB–impaired T cells may account for their different degrees in reduction of IL-7Rα expression. Although RelA nuclear translocation is mostly impeded in IκBαΔN T cells by virtue of the IκBα superrepressor (20), IKKα partially compensates the canonical NF-κB pathway in IKKβ-deficient T cells (27), and RelA/p52 complexes [generated only in particular circumstances (18)] might suboptimally compensate for the canonical RelA/p50 complexes.
The impact of canonical NF-κB activity in the development and survival of naïve T cells is stronger in CD8 than CD4 T cells, which was observed in several mouse models with defective canonical NF-κB signaling by either overexpression of dominant-negative IκBα proteins in T cells or deletion of either IKKβ or NEMO (20, 27, 34). Given the importance of IL-7Rα in CD8 thymic development (8, 35), our results suggest that the reduced number of CD8SP thymocytes in IκBαΔN mice may be because of their defective IL-7Rα expression (Fig. S5). Deficiencies of other transcription factors that control IL-7Rα expression, such as Foxo1 and Ets1, also lead to a more profound reduction of peripheral naïve CD8 than CD4 T cells (36–38), strengthening reports that IL-7Rα controls development and survival of naïve CD8 T cells more stringently than CD4 naïve T cells (10, 35). Our findings that NF-κB regulates IL-7Rα in both T-cell compartments but has a stronger impact in CD8 T-cell survival further support this theory.
In addition to Foxo1 and Ets1, several other transcription factors have been implicated in the genetic control of Il7r, mostly through the binding to two evolutionary conserved regions upstream of the Il7r. ECR1 is located in the proximal Il7r promoter, spanning 200 bp, and it contains the binding sites for Runx1, PU.1 (active only in B cells), Ets1, and GABPα (38–40). ECR2 is located 3.6 kb upstream of Il7r, and this region was previously described to contain binding sites for NF-κB, GATA3, Foxo1/Foxp1 (36, 37), and glucocorticoid receptor (39). Although the NF-κB binding site in the ECR2 had been previously predicted, we show, for the first time to our knowledge, that RelA/p50 dimers bind to this sequence and confer enhancer characteristics. Interestingly, the NF-κB binding site located in ECR3 (−5.7 kb from Il7r TSS) had marginal RelA binding and enhancer activity, suggesting that NF-κB controls Il7r transcription mainly through ECR2.
Our in vitro data assessing the enhancer capacity of ECR2 correctly predicted that constitutively active IKKβ would potentiate IL-7Rα expression in vivo. Despite higher levels of IL-7Rα, IKKβCA T cells have increased cell death ex vivo (34, 41). Kimura et al. (2) elegantly showed that continuous IL-7 stimulation in T cells with suboptimal TCR triggering promoted cell death in an IFNγ-dependent manner. Interestingly, IKKβCA T cells produce large amounts of IFNγ and express one-half the amount of TCRβ chain compared with WT counterparts (Fig. S11), thus raising the possibility that IKKβCA T cells might die through this mechanism.
Antigen-mediated TCR stimulation, which potently activates NF-κB, also ablates IL- 7Rα expression in T cells. Cekic et al. (42) reported that PI3K activation is partially responsible for IL-7Rα reduction in TCR-stimulated cells, possibly by phosphorylation and inhibition of Foxo1 (36). Transcriptional control of the Il7r ECR2 enhancer likely requires a minimal occupancy by multiple transcription factors. As such, TCR/PI3K-mediated Foxo1 inhibition may deprive ECR2 of a key component for its enhancer. We speculate that, without Foxo1, the Il7r enhancer is not functional, despite abundant active NF-κB in TCR-stimulated T cells. In contrast, in naïve T cells, both Foxo1 and NF-κB are available and required for IL-7Rα expression.
The NF-κB binding site present in the ECR2 is conserved between mice and humans. As in mice, de novo IL-7Rα reexpression in human T cells was impaired by pharmacological inhibition of NF-κB following exposure to IL-7. By contrast, deletion of IKKβ in mouse postthymic T cells did not affect constitutive levels of IL-7Rα (33), suggesting that in mature T cells NF-κB may be required for de novo IL-7Rα reexpression but not for its physiological maintenance. TNF stimulation has been reported to up-regulate IL7R mRNA in human HeLa cells in an NF-κB–dependent manner (43), and chromatin immunoprecipitation and sequencing data from the ENCODE project revealed RelA binding to the homologous IL7R ECR2 sequence in human lymphoblastoid cell lines (44). Because this evidence supports a role for NF-κB in IL-7Rα regulation in human cells, caution should be exercised in therapies designed to inhibit the NF-κB pathway in immunoinflammatory diseases (45), because these regimens may have deleterious effects on the naïve T-cell pool, with potential consequences on immune responses to pathogenic threats.
Materials and Methods
Mice.
C57BL/6 mice were obtained from Harlan Laboratories, whereas LckCre, Rosa26-StopFLIKKβCA-GFP, and CD45.1+ mice (all C57BL/6 background) were purchased from Jackson Laboratories. IκBαΔN mice (C57BL/6 background), with T cells that express a dominant-negative form of IκBα (driven by the proximal Lck promoter and the CD2 enhancer), and DO11.10×IκBαΔN mice generated by breeding IκBαΔN (BALB/c background) to DO11.10 mice, which express an ovalbumin-specific TCR transgene, were a gift from Mark Boothby (Vanderbilt University, Nashville, TN). NF-κB1/p50– and NF-κB2/p52–deficient mice (C57BL/6 background) were provided by Yang-Xin Fu (University of Chicago, Chicago, IL). Bcl-2Tg mice, obtained from Marcus Clark (University of Chicago, Chicago, IL), express human Bcl-2 under control of a Vav promoter (25), and OVA-specific OT-II TCR transgenic mice (Taconic) were crossed to IκBαΔN mice at the University of Chicago. IKKβfl/fl, CD4Cre, and IL-7RCre+/− [a Cre knockin that disrupts normal Il7r mRNA (46)] mice were provided by Michael Karin (La Jolla Institute, La Jolla, CA), Fotini Gounari (University of Chicago, Chicago, IL), and Barbara Kee (University of Chicago, Chicago, IL), respectively. All mice were bred at the University of Chicago specific pathogen free facility in agreement with our Institutional Animal Care and Use Committee and according to the National Institutes of Health guidelines for animal use.
Reagents.
Recombinant mouse and human IL-7 were obtained from Peprotech. 6-amino-4–4-phenoxyphenylethylamino-quinazoline (NF-κBi) was purchased from EMD Millipore.
Splenocytes and Lymph Node Cells Isolation, T-Cell Purification, Adoptive Transfer, and Flow Cytometry.
Cells were isolated, purified, and analyzed as described in SI Materials and Methods.
EMSAs.
Nuclear proteins were extracted and quantified as previously described (31) and used in EMSAs as described in SI Materials and Methods.
In Vivo T-Cell Survival.
Six- to eight-week-old DO11.10/IκBα∆N mice and littermate controls were thymectomized. Approximately 45–50 μL mouse peripheral blood was obtained weekly through the retroorbital plexus using heparin-treated calibrated capillary tubes. Red blood cells were lysed for 5 min in 1 mL ammonium chloride potassium lysis buffer. Peripheral mononuclear cells were analyzed by flow cytometry.
In Silico Analysis.
In silico analysis is described in SI Materials and Methods.
RT-qPCR.
Total RNA was prepared from CD4+CD44lo and CD8+CD44lo T cells and used for RT-qPCR as described in SI Materials and Methods.
Cloning of pGL4.23-ECR2 and pGL4.23-ECR3 and Luciferase Reporter Assays.
The plasmid pGL4.23 (Promega) containing a minimal promoter upstream of firefly luciferase was used to test enhancer activity of the NF-κB–containing sequences present in the ECR2 and ECR3 upstream of mouse Il7r. Details are in SI Materials and Methods.
ChIP.
ChIP was performed following the manufacturer’s instructions (Upstate Biotechnologies). More details are in SI Materials and Methods.
Isolation of PBMCs.
PBMCs were isolated from healthy human volunteers as previously described (47).
Statistical Analyses.
Comparisons of means were performed with GraphPad Prism (GraphPad Software) using the Student t test, one-way ANOVA, or two-way ANOVA, where appropriate, with Bonferroni correction for multiple comparisons (one-way ANOVA). Normality was assessed by Kolmogorov–Smirnov tests, and nonparametric tests, such as Mann–Whitney and Kruskal–Wallis (with Dunn’s test), were used where appropriate. Differences were considered significant for P values < 0.05.
Supplementary Material
Acknowledgments
We thank Shilpa Keerthivasan and Donna Decker for providing the Renilla firefly and RelA expression vectors, respectively, as well as technical advice with the luciferase assay, and Jessalynn Holman for breeding and genotyping all the animals. We also thank the University of Chicago Flow Cytometry core facility for assistance with FACS cell sorting. M.L.M. was supported by a National Institutes of Health T32 Cardiovascular Pathophysiology and Biochemistry Training Grant and an American Heart Association predoctoral fellowship. L.L.M. was supported by an American Heart Association postdoctoral fellowship, and X.Z. and M-L.A. were funded by National Institutes of Health Grants AI065892 (to X.Z.) and AI1052352 (to M-L.A.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.M.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1315398111/-/DCSupplemental.
References
- 1.Mackall CL, Fry TJ, Gress RE. Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol. 2011;11(5):330–342. doi: 10.1038/nri2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kimura MY, et al. IL-7 signaling must be intermittent, not continuous, during CD8+ T cell homeostasis to promote cell survival instead of cell death. Nat Immunol. 2013;14(2):143–151. doi: 10.1038/ni.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29(6):848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Murali-Krishna K, et al. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 1999;286(5443):1377–1381. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- 5.Markiewicz MA, Brown I, Gajewski TF. Death of peripheral CD8+ T cells in the absence of MHC class I is Fas-dependent and not blocked by Bcl-xL. Eur J Immunol. 2003;33(10):2917–2926. doi: 10.1002/eji.200324273. [DOI] [PubMed] [Google Scholar]
- 6.Martin B, Bourgeois C, Dautigny N, Lucas B. On the role of MHC class II molecules in the survival and lymphopenia-induced proliferation of peripheral CD4+ T cells. Proc Natl Acad Sci USA. 2003;100(10):6021–6026. doi: 10.1073/pnas.1037754100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schluns KS, Kieper WC, Jameson SC, Lefrançois L. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat Immunol. 2000;1(5):426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 8.Tan JT, et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci USA. 2001;98(15):8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maraskovsky E, et al. Impaired survival and proliferation in IL-7 receptor-deficient peripheral T cells. J Immunol. 1996;157(12):5315–5323. [PubMed] [Google Scholar]
- 10.Tani-ichi S, et al. Interleukin-7 receptor controls development and maturation of late stages of thymocyte subpopulations. Proc Natl Acad Sci USA. 2013;110(2):612–617. doi: 10.1073/pnas.1219242110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henriques CM, Rino J, Nibbs RJ, Graham GJ, Barata JT. IL-7 induces rapid clathrin-mediated internalization and JAK3-dependent degradation of IL-7Ralpha in T cells. Blood. 2010;115(16):3269–3277. doi: 10.1182/blood-2009-10-246876. [DOI] [PubMed] [Google Scholar]
- 12.Pallard C, et al. Distinct roles of the phosphatidylinositol 3-kinase and STAT5 pathways in IL-7-mediated development of human thymocyte precursors. Immunity. 1999;10(5):525–535. doi: 10.1016/s1074-7613(00)80052-7. [DOI] [PubMed] [Google Scholar]
- 13.Jiang Q, et al. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 2005;16(4-5):513–533. doi: 10.1016/j.cytogfr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Park JH, et al. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: A novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21(2):289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 15.Carrette F, Surh CD. IL-7 signaling and CD127 receptor regulation in the control of T cell homeostasis. Semin Immunol. 2012;24(3):209–217. doi: 10.1016/j.smim.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25(6):280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Wu M, et al. Inhibition of NF-kappaB/Rel induces apoptosis of murine B cells. EMBO J. 1996;15(17):4682–4690. [PMC free article] [PubMed] [Google Scholar]
- 18.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18(18):2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 19.Harlin H, Podack E, Boothby M, Alegre ML. TCR-independent CD30 signaling selectively induces IL-13 production via a TNF receptor-associated factor/p38 mitogen-activated protein kinase-dependent mechanism. J Immunol. 2002;169(5):2451–2459. doi: 10.4049/jimmunol.169.5.2451. [DOI] [PubMed] [Google Scholar]
- 20.Boothby MR, Mora AL, Scherer DC, Brockman JA, Ballard DW. Perturbation of the T lymphocyte lineage in transgenic mice expressing a constitutive repressor of nuclear factor (NF)-kappaB. J Exp Med. 1997;185(11):1897–1907. doi: 10.1084/jem.185.11.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mora AL, et al. Antiapoptotic function of NF-kappaB in T lymphocytes is influenced by their differentiation status: Roles of Fas, c-FLIP, and Bcl-xL. Cell Death Differ. 2003;10(9):1032–1044. doi: 10.1038/sj.cdd.4401257. [DOI] [PubMed] [Google Scholar]
- 22.Molinero LL, Alegre ML. Role of T cell-nuclear factor κB in transplantation. Transplant Rev (Orlando) 2012;26(3):189–200. doi: 10.1016/j.trre.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harlin H, et al. CTLA-4 engagement regulates NF-kappaB activation in vivo. Eur J Immunol. 2002;32(8):2095–2104. doi: 10.1002/1521-4141(200208)32:8<2095::AID-IMMU2095>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 24.Mandl JN, Monteiro JP, Vrisekoop N, Germain RN. T cell-positive selection uses self-ligand binding strength to optimize repertoire recognition of foreign antigens. Immunity. 2013;38(2):263–274. doi: 10.1016/j.immuni.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogilvy S, et al. Constitutive Bcl-2 expression throughout the hematopoietic compartment affects multiple lineages and enhances progenitor cell survival. Proc Natl Acad Sci USA. 1999;96(26):14943–14948. doi: 10.1073/pnas.96.26.14943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voll RE, et al. NF-kappa B activation by the pre-T cell receptor serves as a selective survival signal in T lymphocyte development. Immunity. 2000;13(5):677–689. doi: 10.1016/s1074-7613(00)00067-4. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt-Supprian M, et al. Mature T cells depend on signaling through the IKK complex. Immunity. 2003;19(3):377–389. doi: 10.1016/s1074-7613(03)00237-1. [DOI] [PubMed] [Google Scholar]
- 28.Tobe M, et al. Discovery of quinazolines as a novel structural class of potent inhibitors of NF-kappa B activation. Bioorg Med Chem. 2003;11(3):383–391. doi: 10.1016/s0968-0896(02)00440-6. [DOI] [PubMed] [Google Scholar]
- 29.Sasaki Y, et al. Canonical NF-kappaB activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. Immunity. 2006;24(6):729–739. doi: 10.1016/j.immuni.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Hennet T, Hagen FK, Tabak LA, Marth JD. T-cell-specific deletion of a polypeptide N-acetylgalactosaminyl-transferase gene by site-directed recombination. Proc Natl Acad Sci USA. 1995;92(26):12070–12074. doi: 10.1073/pnas.92.26.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molinero LL, Miller ML, Evaristo C, Alegre ML. High TCR stimuli prevent induced regulatory T cell differentiation in a NF-κB-dependent manner. J Immunol. 2011;186(8):4609–4617. doi: 10.4049/jimmunol.1002361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyman O, Krieg C, Homann D, Sprent J. Homeostatic maintenance of T cells and natural killer cells. Cell Mol Life Sci. 2012;69(10):1597–1608. doi: 10.1007/s00018-012-0968-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silva A, Cornish G, Ley SC, Seddon B. NF-κB signaling mediates homeostatic maturation of new T cells. Proc Natl Acad Sci USA. 2014;111(9):E846–E855. doi: 10.1073/pnas.1319397111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jimi E, Strickland I, Voll RE, Long M, Ghosh S. Differential role of the transcription factor NF-kappaB in selection and survival of CD4+ and CD8+ thymocytes. Immunity. 2008;29(4):523–537. doi: 10.1016/j.immuni.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park JH, et al. ‘Coreceptor tuning’: Cytokine signals transcriptionally tailor CD8 coreceptor expression to the self-specificity of the TCR. Nat Immunol. 2007;8(10):1049–1059. doi: 10.1038/ni1512. [DOI] [PubMed] [Google Scholar]
- 36.Kerdiles YM, et al. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat Immunol. 2009;10(2):176–184. doi: 10.1038/ni.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ouyang W, Beckett O, Flavell RA, Li MO. An essential role of the Forkhead-box transcription factor Foxo1 in control of T cell homeostasis and tolerance. Immunity. 2009;30(3):358–371. doi: 10.1016/j.immuni.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grenningloh R, et al. Ets-1 maintains IL-7 receptor expression in peripheral T cells. J Immunol. 2011;186(2):969–976. doi: 10.4049/jimmunol.1002099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee HC, Shibata H, Ogawa S, Maki K, Ikuta K. Transcriptional regulation of the mouse IL-7 receptor alpha promoter by glucocorticoid receptor. J Immunol. 2005;174(12):7800–7806. doi: 10.4049/jimmunol.174.12.7800. [DOI] [PubMed] [Google Scholar]
- 40.DeKoter RP, et al. Regulation of the interleukin-7 receptor alpha promoter by the Ets transcription factors PU.1 and GA-binding protein in developing B cells. J Biol Chem. 2007;282(19):14194–14204. doi: 10.1074/jbc.M700377200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krishna S, et al. Chronic activation of the kinase IKKβ impairs T cell function and survival. J Immunol. 2012;189(3):1209–1219. doi: 10.4049/jimmunol.1102429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cekic C, Sag D, Day YJ, Linden J. Extracellular adenosine regulates naive T cell development and peripheral maintenance. J Exp Med. 2013;210(12):2693–2706. doi: 10.1084/jem.20130249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tian B, Nowak DE, Jamaluddin M, Wang S, Brasier AR. Identification of direct genomic targets downstream of the nuclear factor-kappaB transcription factor mediating tumor necrosis factor signaling. J Biol Chem. 2005;280(17):17435–17448. doi: 10.1074/jbc.M500437200. [DOI] [PubMed] [Google Scholar]
- 44.Consortium EP. ENCODE Project Consortium A user’s guide to the encyclopedia of DNA elements (ENCODE) PLoS Biol. 2011;9(4):e1001046. doi: 10.1371/journal.pbio.1001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107(2):135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schlenner SM, et al. Fate mapping reveals separate origins of T cells and myeloid lineages in the thymus. Immunity. 2010;32(3):426–436. doi: 10.1016/j.immuni.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Molinero LL, Fuertes MB, Rabinovich GA, Fainboim L, Zwirner NW. Activation-induced expression of MICA on T lymphocytes involves engagement of CD3 and CD28. J Leukoc Biol. 2002;71(5):791–797. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.