1. Introduction
Dental composite restoratives comprise a tremendously large segment of the biomaterials market. While these esthetic materials continue to be improved, polymerization shrinkage and the resulting shrinkage stress can be extremely detrimental to the performance of polymer-based dental restoratives [1–5]. The vast majority of these dental restoratives continue to be dimethacrylate-based and the monomers undergo significant shrinkage during polymerization [1,6–9]. In a clinical setting, shrinkage is restricted as the polymerizing composite is strongly attached to the tooth substrate to maintain mechanical integrity and an effective seal between the restoration and tooth. Interfacial failure can result in marginal leakage between the tooth and the restoration further leading to problems such as marginal staining, secondary caries and post-operative sensitivity [10–14]. This constraint placed on shrinkage as the viscous composite paste converts to a glassy, high modulus material is the primary source for the buildup of stress within the composite [5]. The resulting stress is also transferred to the adhesive interface and to the adjacent tooth structure. The magnitude of the stress that develops during polymerization depends on the shrinkage and modulus of the composite [15], the interaction between the reinforcing filler and the developing polymer matrix and the boundary conditions under which the composite is cured [16]. It has also been suggested that the exotherm generated due to the polymerization reaction is an additional factor that affects stress development [17]. All the parameters responsible for stress development are affected by the formulation of the composite, which at the most basic level is composed of an organic resin matrix, reinforcing filler particles, coupling agent between the resin matrix and filler and an initiator system.
Since their introduction in the 1950s, polymeric dental restoratives have undergone significant changes in their formulation [18], along with a steady improvement in performance. Until the 1990s, most of the research contributing towards the evolution of dental composites was in the area of filler development [19]. Filler sizes and composition changed drastically down from dimensions of 10–50 µm or more in the early dental composites to sub-micron range and even extending down to 5 nm in some of the modern nanocomposites [20]. Along with variations in size and composition of the fillers, the filler loading level has also been varied over a considerable range. The volume fraction of fillers in commercial composites can span from about 20 % up to about 75 % [21,22]. Low filled composites typically show lower moduli and strength but exhibit significant flow and adaptability prior to curing. Materials of this type are used in applications where there are limited masticatory forces and as flowable composite liners that produce a somewhat adaptable interface [22]. Highly filled composites are used in posterior restorations where there is a need for sustaining higher recurring loads and greater wear resistance. As shrinkage is one of the main factors leading to stress formation in polymerizing dental restoratives, research over the last few years has focused on developing low shrinkage resins [1,6,23]. The changes in formulations also lead to a range of vitrification-limited double bond conversion levels of the various resin systems that comprise the matrix phase of the composite. The practical variation in polymer conversion is also expanded by differences in photocuring conditions (i.e., wavelength, irradiance, exposure time) as well as differences in light transmission character of composites due to their composition [24,25].
This work examines the development of shrinkage, stress and flexural modulus as a resin converts from the uncured state to a fully cured material, with methacrylate conversion used as a coordinating index to look at interrelationships between these properties as a function of varied filler loading. Several studies have probed the effect of filler loading on shrinkage, modulus and stress in isolation or in some combination [26–33]. However, only a few report the functional group conversion levels at which the property measurements are actually done. The contention here is that an accurate assessment of conversion is necessary to accompany any property measurement that displays conversion dependence to ensure that comparisons between different materials or even within a given material are legitimate. There is a lack of understanding of the effect of filler loading and monomer conversion on shrinkage, modulus and stress as well as how all these factors relate to the development of physical and mechanical properties of composite materials. Given a certain formulation, shrinkage stress would depend on shrinkage and modulus, the C-factor (configuration factor, representation of the constrained or bonded to unbonded surface area), viscoelastic behavior of the material, as well as the curing and boundary conditions. In this study the C-factor and boundary conditions were kept constant for different samples in the same measurement class.
2. Materials and Methods
2.1 Materials
2,2-Bis[4-(2-hydroxy-3-methacrylyloxypropoxy)phenyl]propane (Bis-GMA) and triethylene glycol dimethacrylate (TEGDMA) (both from Esstech, Essington, PA) were used in a 70:30 mass ratio as a model dental resin. A visible light initiating system consisting of 0.3 wt% camphorquinone (CQ) as initiator and 0.8 wt% ethyl 4-dimethylaminobenzoate (EDAB) (Sigma-Aldrich, Milwaukee, WI) as co-initiator was incorporated. Barium glass (mean particle size 0.7 µm) surface treated with a coating of 5 wt% γ-methacryloxypropyltrimethoxysilane (γ-MPS), was used as the inorganic filler (Esstech). The filler was mixed with resin in varying amounts to obtain total final weight percentages of 0, 20, 40, 60 and 70, which correspond to filler volume fractions of 0, 9.4, 21.6, 38.3 and 49.1 respectively, based on densities of filler (2.71 g/cm3) and uncured resin (1.12 g/cm3) [34]. Fillers and resin were blended in a centrifugal mixer (DAC 150 FVZ, Flakteck) to ensure thorough mixing.
2.2 Sample Curing
Visible light irradiation was provided by a halogen dental curing lamp (VIP, Bisco, Schaumburg, IL). Light intensities and irradiation times were varied in order to achieve a wide range of conversions for specimens used in the various testing protocols. A radiometer (Model 100, Demetron Research, Danbury, CT, USA) was used to measure the output light intensity.
2.3 Infrared Spectroscopy
Double bond conversion in the resin and composite specimens was monitored in transmission mode using near-infrared (NIR) spectroscopy (Nexus 670, Nicolet Instruments, Madison, WI). The area of the peak at 6165 cm−1 signifying the =CH2 absorption was used to track the double bond conversion. All NIR measurements were taken at a wavenumber resolution of 4 cm−1. For the static measurements, 64 scans per spectrum were taken for, while 2 scans per spectrum were used for collection of real-time series measurements with approximate 1 Hz sampling temporal resolution.
2.4 Shrinkage Stress Measurement
Shrinkage stress was measured using a cantilever beam-based tensometer, which was designed and fabricated at the Paffenberger Research Center of the American Dental Association Health Foundation (ADAHF–PRC, Gaithersburg, MD, USA). This device measures the dynamic tensile forces generated by a polymerizing sample that causes deflection of the calibrated beam. A more detailed description of the experimental technique has been reported in a previous study [35,36]. Briefly, a composite sample is placed between two cylindrical quartz rods that have been treated with a methacrylate functional silane to promote bonding with the resin in the composite. The lower quartz rod is fixed whereas the upper quartz rod is attached to the cantilever. A curing light is connected by an adapter to the bottom of the lower quartz rod and light is transmitted through the rod to the sample causing polymerization and deflection of the cantilever with system compliance determined by positioning on the beam and designed to generally reflect tooth flexure. An LVDT (linear variable differential transformer) continuously tracks the beam deflection and this data is converted to a force measurement, which when divided by the composite cross-sectional area, provides the stress in the composite. The cavity in which the samples were placed was 6 mm in diameter and 1.5 mm in thickness. For the simultaneous measurement of conversion with shrinkage stress, the NIR signal was guided to/from the sample using optical fiber cables (1 mm diameter).
For the stress measurements, the samples (n=3) were irradiated with the VIP curing light at an incident irradiance of 250 mW/cm2 for 60, 10 and 5 s and also at 35 mW/cm2 for 20, 10 and 5 s, to obtain a wide range of conversion and associated stress values. Irradiance was measured at the end of the quartz rod in contact with the sample. The coupled stress/conversion data was collected continuously for 15 min.
2.5 Shrinkage Measurement
Shrinkage was measured using a linometer (ACTA Foundation, Netherlands). In this method a liquid monomer or uncured composite paste sample was placed between a fixed upper glass plate and a freely moving aluminum disc, both of which were lightly greased to provide radial freedom of movement for the shrinking sample, which allows the linear post-gel shrinkage to be recalculated in terms of volumetric shrinkage. Disc specimens (n=3) of 6 mm in diameter and 1.5 mm in thickness were irradiated from the upper surface and the displacement of the aluminum disc was monitored by a LVDT. Irradiation intensity and times were used to coincide with those used for the stress measurements. Shrinkage was monitored continuously for 10 min. NIR scans of each specimen were taken prior to polymerization and immediately after removal from the linometer to avoid any significant post-cure conversion development.
2.6 Flexure Modulus Measurement
Flexural modulus was measured in three-point bending (MTS 858 Mini Bionix II). The specimen dimensions were 2×2×10 mm. Samples (n=5) were irradiated on one side and immediately inverted and irradiated under equivalent conditions from the opposite side to optimize conversion uniformity throughout the sample. A range of light intensities, from 25 mW/cm2 to 575 mW/cm2, were used. Samples were cured for 30 to 60 s on each side. Conversion was measured by NIR spectroscopy directly from the flexural test specimens before and immediately after curing. As soon as the NIR polymer spectrum was collected, the sample was submitted to mechanical testing. Again this was done to ensure well correlated conversion and mechanical property results.
2.7 Vicker’s Microhardness Test
Disc-shaped specimens (2 mm thickness and 10 mm diameter, n=3) of each formulation were obtained by photopolymerization with the dental light at 700 mW/cm2 for three minutes on each flat surface. The Vicker’s hardness was measured using a microhardness tester (LM700AT, Leco, St. Joseph, MI). The specimens were indented with a diamond tip using a force of 300 gf for 15 s. The length of the indents was measured and the VHN was calculated using the following formula –
| (1) |
Where, F is the applied force in gf and d is the size of the indent in millimeters.
3. Results and Discussion
This work examined the dynamic evolution of material properties including volumetric shrinkage, flexural modulus and stress in photopolymerizing dental composites with respect to methacrylate conversion and filler content as independent parameters. Both the parameters were systematically varied over a wide range to study the changes in the material property values. For each filler loading level, shrinkage, flexural modulus and stress were measured in separate experiments with essentially simultaneous measurement of conversion. The conversion measurements allowed rational comparison of all the interrelated properties and it facilitates understanding of the trends in their conversion-driven development.
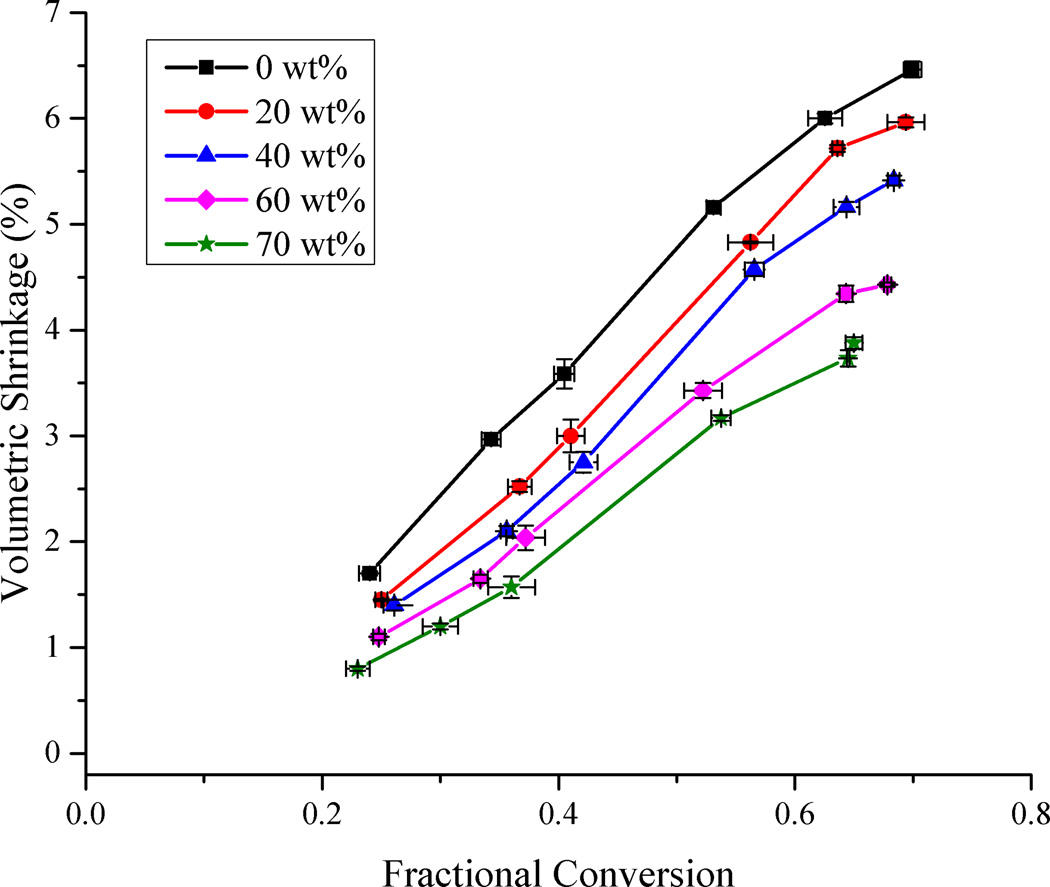
The variation in volumetric shrinkage of the resin system as a function of methacrylate conversion for each filler loading level is represented in Fig. 1. The maximum shrinkage for the unfilled resin was 6.5 ± 0.1 % at 69.9 ± 0.8 % conversion while that for the sample with 70 wt% filler loading was 3.9 ± 0.1 % at 65.0 ± 0.7 % conversion. The extrapolation of the static data in Fig. 1 to zero shrinkage provides a slightly positive conversion intersect that can be expected from this post-gel shrinkage measurement technique. Shrinkage varied linearly with respect to conversion for low to moderate levels of conversion, as has been observed by several authors [8,9,32]. Prior studies have also noted the linear variation of shrinkage with respect to filler loading [26,32,37]. What is not noted normally, however, is a deceleration in the shrinkage rate as the limiting conversion is attained for all the samples. This phenomenon has been reported before in coupled dynamic shrinkage/conversion measurements [1] involving glassy, crosslinked networks. In the vitrified state, the bulk network densification and the continued molecular level reaction of free monomer and pendant methacrylate groups tend to occur on diverging time scales, which results in excess free volume. Based on the broad structural heterogeneity that characterizes dimethacrylate networks, micro-domains containing residual monomer and pendant reactive groups with relatively high mobility (as shown by the DMA-based tan δ peaks that extend to below 0 °C for fully cured dimethacrylate polymers) exist throughout the network that can still undergo polymerization. It is this increase in conversion that is seen to occur without significant change in bulk shrinkage [15,38]. It is expected that the cured composite sample will continue to densify over time and eventually more closely approximate the equilibrium thermodynamic free volume associated with a given conversion.
Figure 1.
Variation of volumetric shrinkage as a function of methacrylate conversion and filler loading – lines connecting data points are provided for visual assistance.
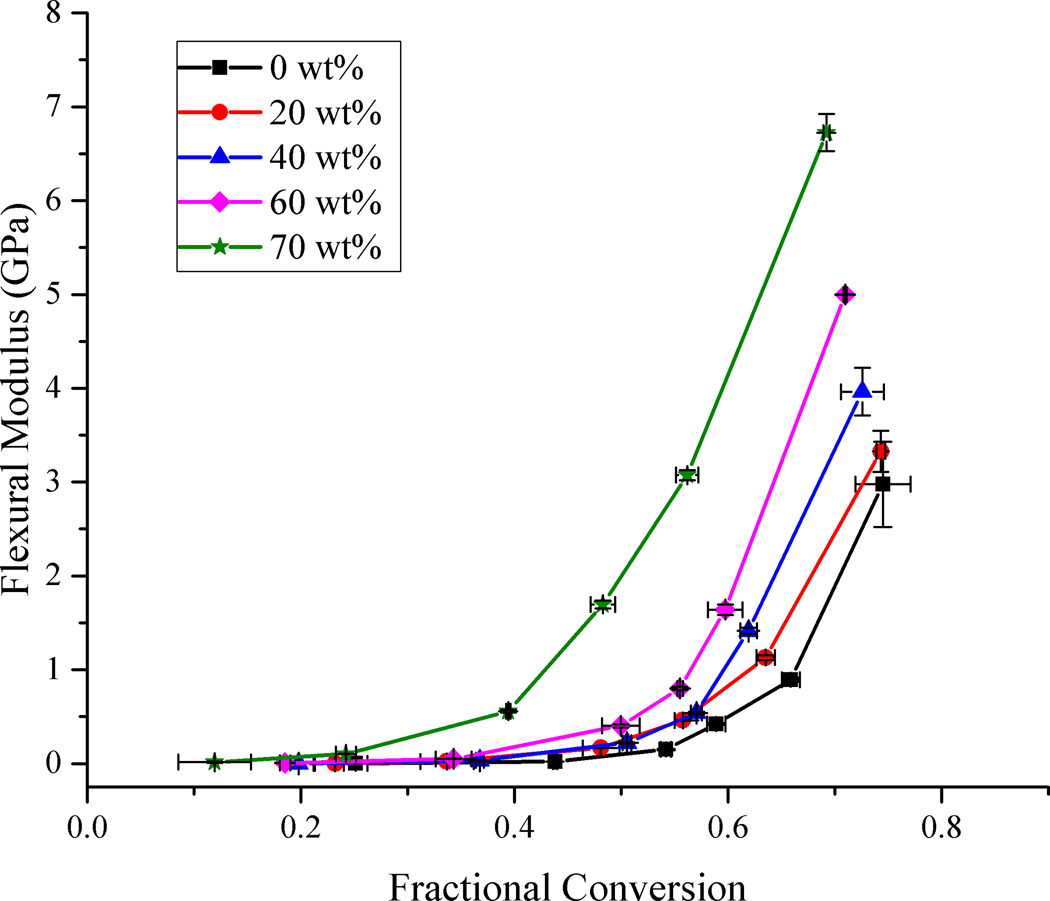
When variation of flexural modulus as a function of filler loading was considered with respect to conversion (Fig. 2), there was a precipitous rise in the modulus for each filler loading level after an initial slow change. This rise in modulus can be associated with the onset of the glassy state of the material [39]. The accelerated rise in modulus occurred at progressively lower values of conversion as the filler content increased. This means that for any given level of conversion, higher filler loading provides a higher modulus value with greater divergence as conversion progresses. Studies measuring modulus of fully cured composites with respect to filler loading show an exponential rise in modulus as the amount of filler increases [40]. The positive effect of the filler on modulus is evident in that even though the limiting conversion is reduced as the filler content rises, the modulus values, which are quite sensitive to small changes in late-stage polymer conversion, still increase significantly. The rationale for the modest reduction in conversion with increasing filler includes a progressive reduction in overall light transmission due to reflection, scattering and absorption of light by filler particles leading to a progressive reduction in light intensity and penetration as composite thickness increases [25,41,42] and a reduction in the reaction exotherm potential due to the reduction in the overall reactive group concentration [34]. Of these factors, the earlier vitrification as implied by the rapid rise in modulus is the most likely cause of the gradual lowering of conversion with increased filler loading. For an equivalent, lower level of conversion within the rubbery regime, there are relatively small differences in the modulus among composites containing 0 to 60 wt% filler fraction. The composite with 70 wt% filler fraction shows significantly higher modulus at all conversion levels compared to the other samples. As the filler fraction increases and the initial interparticle spacing decreases, there is increased potential for interparticle interactions as the matrix contracts further with increasing conversion. As conversion increases, there is progressive covalent attachment between the filler surface with the densifying matrix and this additional reinforcement of the network also contributes to the late stage modulus development in addition to the corresponding increase in the matrix crosslink density. The most significant modulus effect associated with the filler would be expected where the filler surface affected interphase region becomes a continuous phase, which assuming a relatively small hybrid interphase dimension, would be expected only at high filler loading levels.
Figure 2.
Flexural modulus development profiles as a function of methacrylate conversion for different filler loading levels. Lines connecting the data points are provided for visual assistance.
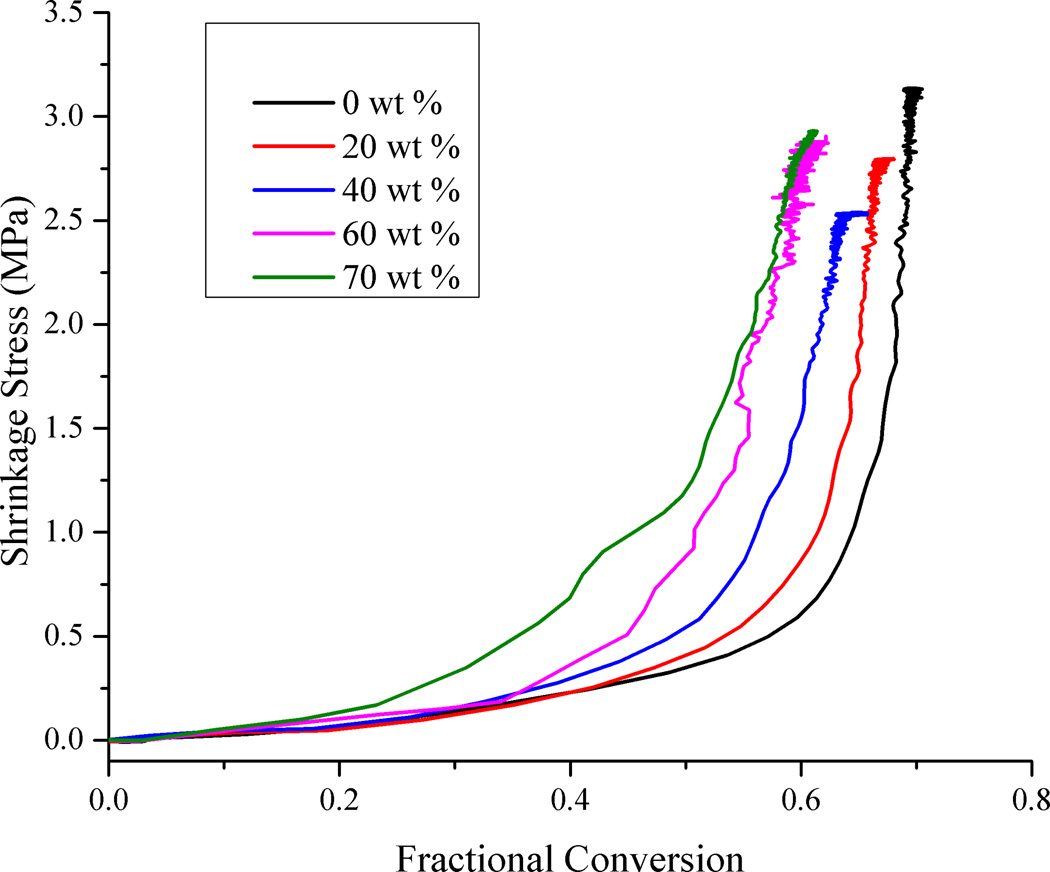
Shrinkage stress and methacrylate conversion were measured simultaneously in real-time under a variety of irradiation conditions. Fig. 3 shows the variation in stress as conversion progresses for various loading levels, for the highest irradiance conditions (250 mW/cm2 for 60 s). At the very early stages of polymerization, as conversion increases there is no corresponding rise in the shrinkage stress level. At less than 5 % conversion, which reasonably approximates the gel point of dimethacrylate systems [43], stress begins to rise slowly. In order for the tensometer to start registering a positive stress value, it is implicit that a continuous load-bearing network form within the sample and thereby covalently attach to both the upper and lower rods in the tensometer. This can only occur once the gel point has been reached along the z axis. Shrinkage that occurs before the gel point does not contribute to stress since this stage of shrinkage is represented as viscous flow that has no elastic memory [44,45]. After a steady increase in stress initially through the rubbery and leathery regimes, as the limiting conversion is reached in the final vitrification stage of polymerization, stress increases rapidly over a fairly narrow range of conversion. Bulk vitrification occurs when a significant portion of the sample’s evolving glass transition temperature exceeds the effective curing temperature. Addition of increasing amounts of filler increases the available surface area in contact with the developing polymer phase. The filler surface places restrictions on the mobility of the polymer in its immediate vicinity [46]. As the volume fraction of fillers increases, this relatively immobile interphase can cause reduced cooperative movement between the polymer chains. In addition, the contribution of the highly rigid filler particles to the overall composite modulus and the covalent linkage with the resin phase, all promote the earlier vitrification of the composite.
Figure 3.
Real-time polymerization shrinkage stress versus methacrylate conversion. Irradiation condition: 250 mW/cm2 for 60 s.
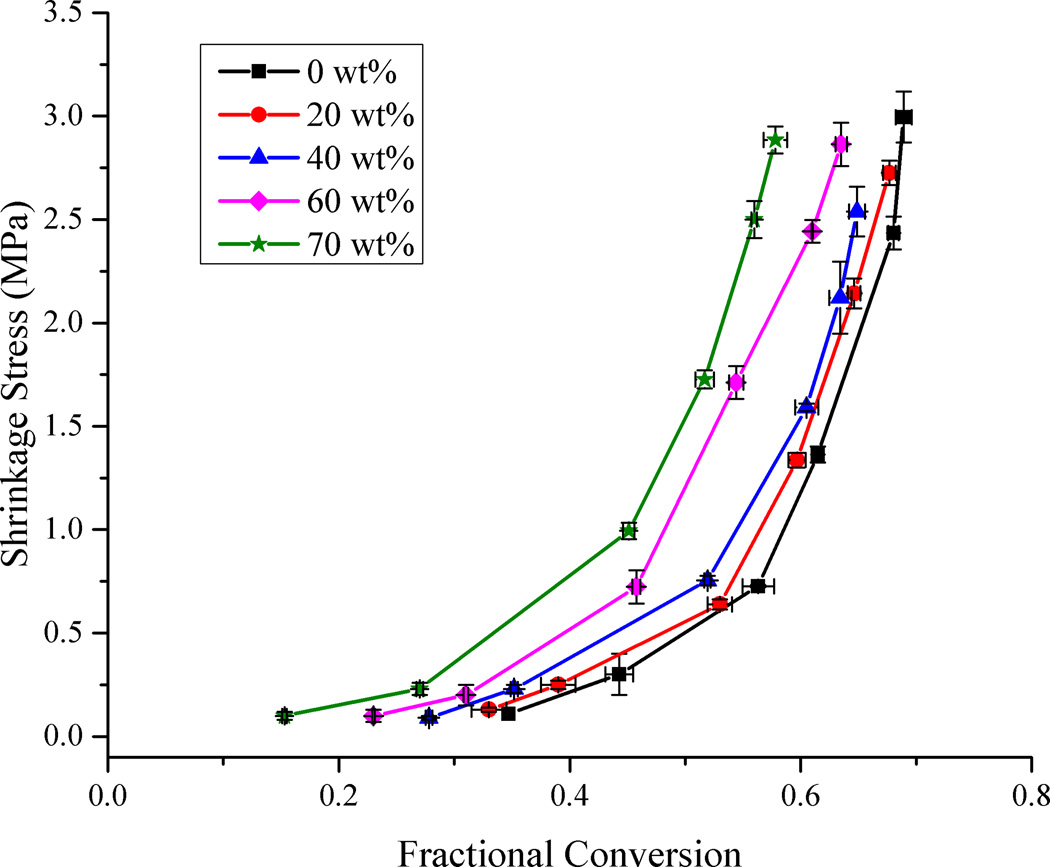
The dynamic stress data presented in Fig. 3 represents just one of the irradiation conditions used during the stress measurements. Fig. 4 was generated using the final values of stress and conversion at the end of each such measurement. It is evident that the progression of data in Fig. 4 is very similar to the analogous dynamic data in Fig. 3. This demonstrates that static measurements can be used to reasonably represent the dynamic progression of property development, as long as accurately correlated conversion data are included. There are a few subtle differences between the two plots. In considering just the end point of the real-time data, the effect of any exotherm during curing is lost. The exotherm contributes to a delay in vitrification with respect to the conversion and also imparts a thermal contraction effect to the dynamic measurement as the system cools beyond the point of the maximum reaction rate. In the static data, the change in the stress with respect to conversion is somewhat more gradual than in the real-time data.
Figure 4.
Plot of shrinkage stress as a function of methacrylate conversion and filler loading. Lines connecting the data points are provided for visual assistance.
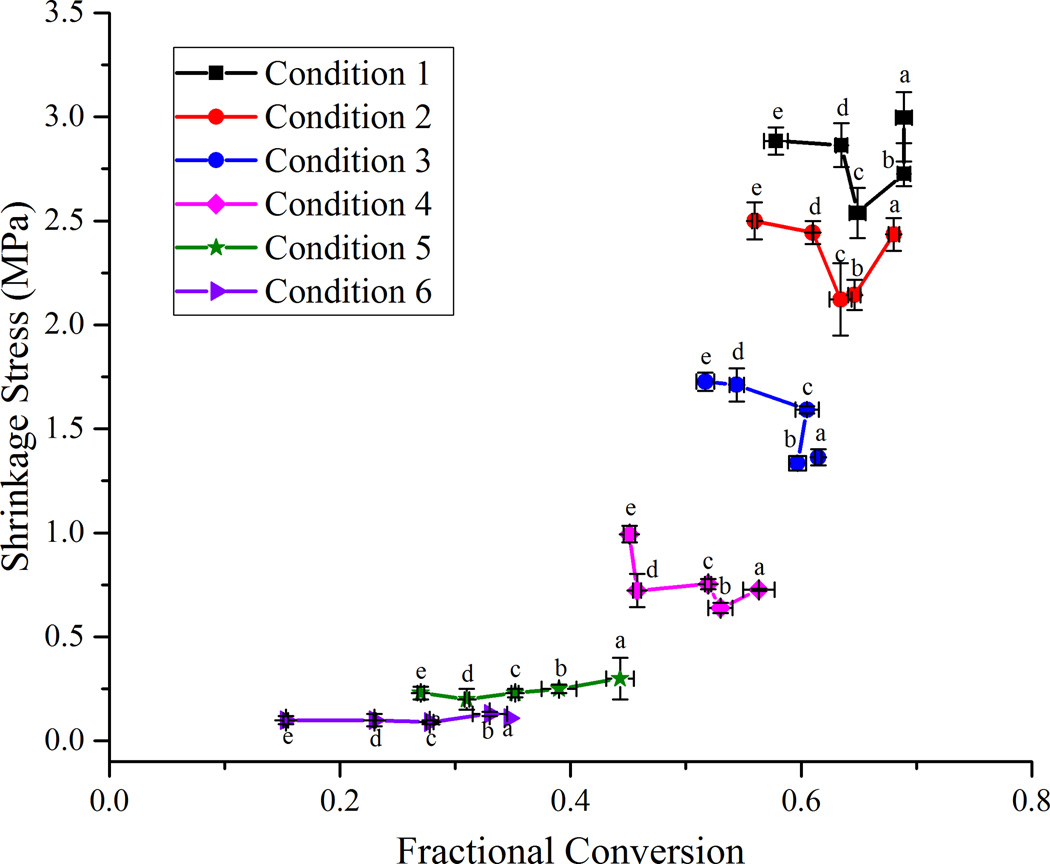
An alternate and instructive way of looking at the stress data is to compare the final stress values when the different samples are subjected to similar irradiation conditions or energy densities (Fig. 5). For the highest irradiance levels (conditions 5 and 6 in Fig. 5) the final shrinkage stress reduces steadily along with a corresponding decrease in conversion as the filler content is increased from 0 to 40 wt%. Above 40 wt% filler loading, the reduction in final conversion continues as additional filler is incorporated, but the final shrinkage stress begins increasing. This trend is most prominent for the two highest irradiance conditions and less obvious or not present at all in the lower ranges of conversion (conditions 1 – 4 in Fig. 5). This has significant implications in a practical situation where a dentist may use a particular curing protocol, regardless of the formulation of the dental composite being used. With the wide variety of filler loading levels and monomer formulations available in current commercial dental restoratives, the same curing protocol used for all of them is certain to result in significant differences in final conversion, shrinkage, modulus and stress. For each particular material, there may exist a region where there is an acceptable target range of conversion that is sufficient for the modulus and other desirable material properties such as fracture toughness to be high and yet have a reasonably lower stress. It would be advantageous to employ a curing protocol that maximizes the benefits across a wide range of properties without overly sacrificing any one of them. Maximizing conversion clearly reduces potential leachable free monomer and may enhance long-term maintenance of polymer mechanical properties, but the current study sheds new light on the complex issues of conversion dependent stress development in composite materials.
Figure 5.
Plot of shrinkage, modulus and stress as a function of methacrylate conversion. Lines connecting the data points are provided for visual assistance. Lines with empty circles, squares and triangles represent the unfilled resin and lines with filled circles, squares and points represent the 70 wt% composite. (Color legend: - Blue = Flexural Modulus; Black = Shrinkage; Red = Shrinkage Stress).
The effect of energy density on polymerization stress in commercial dental composites has been studied previously [47] with conversion and stress values found to generally level off at higher energy densities. Saturation of the conversion is in agreement with our results since the limiting conversion for all loading levels at the higher irradiance conditions is in a fairly narrow range. However, as the limiting conversion is approached, stress values continue increasing significantly. The disagreement can be explained by the fact that the earlier study looked at a narrower range of conversion as compared to the range examined here and the stress actually starts building up most rapidly at latter stages of conversion as vitrification is reached.
Fillers functionalized with a methacrylate silane added to a resin that is based on methacrylates react covalently with the resin upon polymerization. It has been observed that when fillers with non-functional silanes are introduced in a resin matrix, the resulting final stress is lower than composites with fillers that have functional silanes, at the same loading level [48]. This is due to the fact that the reactive surface silanes introduce additional constraints on the polymerizing resin, restricting free shrinkage. At lower levels of conversion, it is likely that the reaction between the surface bound methacrylates and the resin-based methacrylates is incomplete. At this stage the constraints on the shrinking resin are “minimal”. The effect of this can be seen in the flat nature of the modulus (Fig. 2) and stress (Fig. 4) curves at the lower values of conversion (< 30%). As the reaction between the surface-bound methacrylates and the resin continues, the constraints on the densifying resin phase increase and this leads to an increase in modulus and subsequently, shrinkage stress. It can be seen that increasing filler content would enhance the effect of the interface between the fillers and the resin phase. The relatively large (0.7 µm range) fillers considered here are not expected to have a huge effect due to their more limited surface area but introduction of nanofillers would be expected to have larger effects on stress development, as has previously been demonstrated [49], probably due to reduced interparticle spacing that causes an earlier rise in modulus, though not necessarily a higher ultimate modulus as filler dimensions are reduced at a constant loading level. Current commercial dental restoratives use multi-modal fillers [50] with an appreciable amount of nanofillers and it is very likely that the nanofillers will have a significant contribution to the overall property development due to their high surface area.
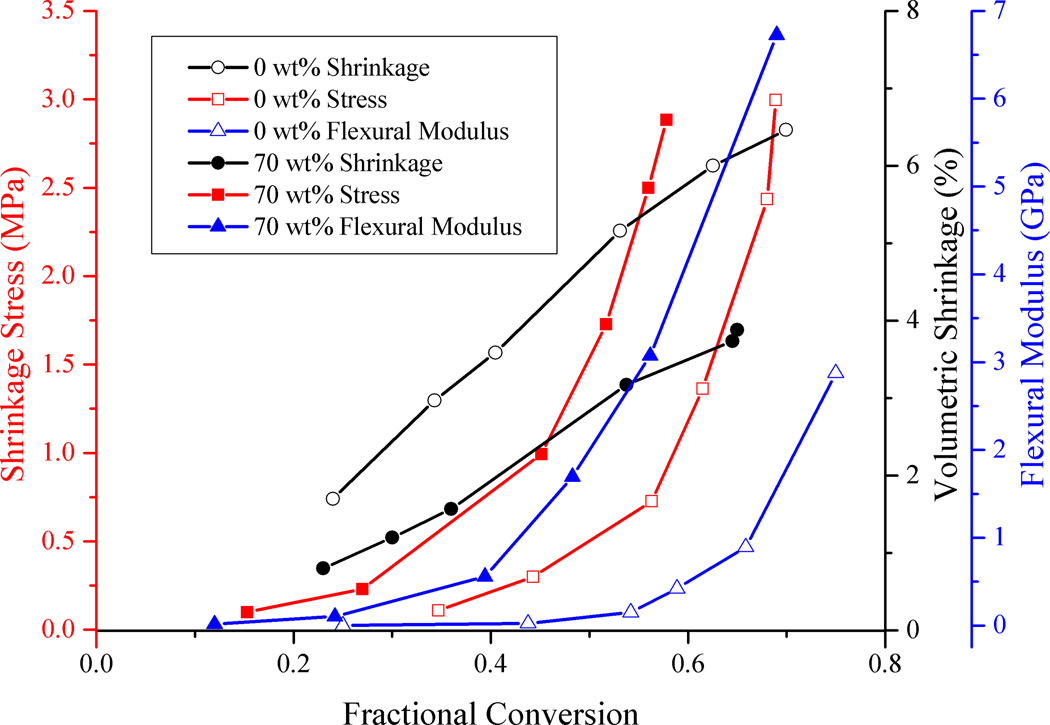
The simultaneous development of shrinkage, modulus and stress as a function of methacrylate conversion is captured in Fig. 6. Only the unfilled resin and 70 wt% samples are compared here for clarity since they represent the filler loading extremes. Shrinkage increases linearly for the two samples, albeit at different rates, with respect to conversion due to the different resin phase volume fractions. Modulus and stress progress along an exponential route and follow each other closely. Overlaying all the property measurements with respect to conversion on a single graph enables us to follow the dynamic trends for each different material and compare them at different conversion points. Fig. 6 brings out the rates of the different material properties and one can qualitatively compare the trends in their development at a glance. The data represented here can also be used as an aid to help with modeling the property development in polymerizing composites. It can be used as a tool for quantitative models to use to iteratively improve on their simulation predictions by comparing answers to experimental results. As a first step in this direction, we introduce a way to compare our experimental stress results with a simple prediction of stress using the modulus and shrinkage measurements as shown in the supplemental information.
Figure 6.
Plot of shrinkage stress versus fractional conversion. Each line represents the same irradiation condition but different filler contents – Condition 1 – 35 mW/cm2 for 5 s; Condition 2 – 35 mW/cm2 for 10 s; Condition 3 – 35 mW/cm2 for 20 s; Condition 4 – 700 mW/cm2 for 5 s; Condition 5 – 700 mW/cm2 for 10 s; Condition 6 – 700 mW/cm2 for 60 s. Letters are used to denote the different filler loading levels – a = 0 wt%; b = 20 wt%; c = 40 wt%; d = 60 wt%; e = 70 wt%. Lines connecting the data points are provided for visual assistance.
The abrupt rise of the modulus of the 70 wt% filled composite for all values of conversion is noteworthy and reinforces the concept of a threshold where particle-particle interactions become predominant. While modulus represents the bulk properties, the surface properties are also important for dental composites as it is the surface that has to withstand masticatory forces and display appropriate wear resistance. Indentation hardness tests give a relevant measure of the surface strength of materials. Vicker’s hardness testing was used to determine the surface hardness of fully cured samples with respect to filler loading. A gradual linear rise in hardness values accompanied the increase of filler loading between 0 and 60 wt% (R2 = 0.99). However, there was an abrupt rise in hardness observed for the 70 wt% sample as demonstrated by the rapid transition from a 1.5-fold to a 2.2-fold increase in surface hardness relative to the unfilled control resin due to the addition of the final 10 wt% of filler (Table 1). This follows the same trend that the modulus values show with respect to filler loading levels and further highlights a transition from particle-resin to particle-particle interactions.
Table 1.
Vicker’s microhardness test values for samples.
| Filler Loading (wt%) | 0 | 20 | 40 | 60 | 70 |
| Hardness Value (HV) | 21.2 (0.4) | 25.3 (0.5) | 27.8 (0.4) | 31.7 (0.6) | 46.4 (2.9) |
Conclusions
The evolution of shrinkage, modulus and shrinkage stress was evaluated as a function of filler loading and methacrylate group conversion. Continuous measurement of conversion during the progressive development of the material properties allowed for their legitimate comparison at specific conversion points to avoid errors or conflicting data that result from measurements taken at different stages of polymer development. It was found that shrinkage varied linearly with conversion, while stress and modulus exhibited related exponential behavior. The value of stress in the highly filled systems, at any point in the conversion profile, appeared lower than what would be expected in a simple case assuming uniaxial composite stress predicted by the corresponding modulus and shrinkage. The disparity can be explained by the compliance of the stress measurement system, which partially accommodates the increase in stress during polymerization of high modulus materials. However, it should be noted that this is not unlike the clinical situation where cuspal deflection or other compliance mechanisms serve to reduce the effective stress levels.
Supplementary Material
Acknowledgements
The authors would like to thank the National Institutes of Health for support of this research through the NIDCR/R01DE014227. We also thank Septodont/Confi-Dental and Evonik for their gracious donation of filler materials and Esstech for the donation of monomers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stansbury JW, Trujillo-Lemon M, Lu H, Ding X, Lin Y, Ge J. Conversion-dependent shrinkage stress and strain in dental resins and composites. Dent Mater. 2005;21:56–67. doi: 10.1016/j.dental.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Schneider LFJ, Cavalcante LM, Silikas N. Shrinkage Stresses Generated during Resin-Composite Applications: A Review. J Dent Biomech. 2010;1:1–15. doi: 10.4061/2010/131630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadowsky SJ. An overview of treatment considerations for esthetic restorations: a review of the literature. J Prosthet Dent. 2006;96:433–442. doi: 10.1016/j.prosdent.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Ferracane JL. Placing Dental Composites—A Stressful Experience. Oper Dent. 2008;33:247–257. doi: 10.2341/07-BL2. [DOI] [PubMed] [Google Scholar]
- 5.Ferracane JL. Developing a more complete understanding of stresses produced in dental composites during polymerization. Dent Mater. 2005;21:36–42. doi: 10.1016/j.dental.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Bowman CN, Cramer NB, Sansbury JW. Recent Advances and Developments in Composite Dental Restorative Materials. J Dent Res. 2010;90:402–416. doi: 10.1177/0022034510381263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stansbury JW, Dickens SH. Determination of double bond conversion in dental resins by near infrared spectroscopy. Dent Mater. 2001;17:71–79. doi: 10.1016/s0109-5641(00)00062-2. [DOI] [PubMed] [Google Scholar]
- 8.Amirouche-Korichi A, Mouzali M, Watts DC. Effects of monomer ratios and highly radiopaque fillers on degree of conversion and shrinkage-strain of dental resin composites. Dent Mater. 2009;25:1411–1418. doi: 10.1016/j.dental.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Dewaele M, Truffier-Boutry D, Devaux J, Leloup G. Volume contraction in photocured dental resins: the shrinkage-conversion relationship revisited. Dent Mater. 2006;22:359–365. doi: 10.1016/j.dental.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Condon JR, Ferracane JL. Assessing the effect of composite formulation on polymerization stress. J Am Dent Assoc. 2000;131:497–503. doi: 10.14219/jada.archive.2000.0207. [DOI] [PubMed] [Google Scholar]
- 11.Sarrett DC. Clinical challenges and the relevance of materials testing for posterior composite restorations. Dent Mater. 2005;21:9–20. doi: 10.1016/j.dental.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Burke FJT, Cheung SW, Mjor IAWN. Restoration longevity and analysis of reasons for the placement and replacement of restorations provided by vocational dental practitioners and their trainers in the United Kingdom. Quintessence Int (Berl) 1999;30:234–242. [PubMed] [Google Scholar]
- 13.Mjör Ia. The reasons for replacement and the age of failed restorations in general dental practice. Acta Odontol Scand. 1997;55:58–63. doi: 10.3109/00016359709091943. [DOI] [PubMed] [Google Scholar]
- 14.Lubisich EB, Hilton TJ, Ferracane JL, Pashova HI, Burton B. Association between caries location and restorative material treatment provided. J Dent. 2011;39:302–308. doi: 10.1016/j.jdent.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abu-elenain DA, Lewis SH, Stansbury JW. Property evolution during vitrification of dimethacrylate photopolymer networks. Dent Mater. 2013;29:1173–1181. doi: 10.1016/j.dental.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boaro LCC, Meira JBC, Ballester RY, Braga RR. Influence of specimen dimensions and their derivatives (C-factor and volume) on polymerization stress determined in a high compliance testing system. Dent Mater. 2013;29:1034–1039. doi: 10.1016/j.dental.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Antonucci JM, Giuseppetti AA, O’Donnell JNR, Schumacher GE, Skrtic D. Polymerization Stress Development in Dental Composites: Effect of Cavity Design Factor. Materials (Basel) 2009;2:169–180. doi: 10.3390/ma2010169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowen RL. Use of Epoxy Resins in Restorative Materials. J Dent Res. 1956;35:360–369. doi: 10.1177/00220345560350030501. [DOI] [PubMed] [Google Scholar]
- 19.Ferracane JL. Resin composite-state of the art. Dent Mater. 2011;27:29–38. doi: 10.1016/j.dental.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 20.Mitra S, Wu D, Holmes B. An application of nanotechnology in advanced dental materials. J Am Dent Assoc. 2003;134:1382–1390. doi: 10.14219/jada.archive.2003.0054. [DOI] [PubMed] [Google Scholar]
- 21.Ferracane JL. Current trends in dental composites. Crit Rev Oral Biol Med. 1995;6:302–318. doi: 10.1177/10454411950060040301. [DOI] [PubMed] [Google Scholar]
- 22.Sakaguchi RL, John M. Craig’s Dental Restorative Mateirals. 13th ed. Elsevier; 2012. Powers. [Google Scholar]
- 23.Moszner N. New developments of polymeric dental composites. Prog Polym Sci. 2001;26:535–576. [Google Scholar]
- 24.Leprince JG, Hadis M, Shortall AC, Ferracane JL, Devaux J, Leloup G, et al. Photoinitiator type and applicability of exposure reciprocity law in filled and unfilled photoactive resins. Dent Mater. 2011;27:157–164. doi: 10.1016/j.dental.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Emami N, Sjödahl M, Söderholm K-JM. How filler properties, filler fraction, sample thickness and light source affect light attenuation in particulate filled resin composites. Dent Mater. 2005;21:721–730. doi: 10.1016/j.dental.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Miki Iga, Fumio Takeshige, Mitsuo Torii YT. The Relationship between Polymerization Shrinkage Measured by a Modified Dilatometer and the Inorganic Filler Content of Light-Cured Composites. Dent Mater J. 1991;10:38–45. [Google Scholar]
- 27.Cavalcanti J, Queiroz G, Monteiro DM, Antonio M, Resende J. Polymerization Shrinkage and Flexural Modulus of Flowable Dental Composites. Mater Res. 2010;13:381–384. [Google Scholar]
- 28.Calheiros FC, Braga RR, Kawano Y, Ballester RY. Relationship between contraction stress and degree of conversion in restorative composites. Dent Mater. 2004;20:939–946. doi: 10.1016/j.dental.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Queiroz G, Monteiro DM, Antonio M, Resende J. Evaluation of Linear Polymerization Shrinkage, Flexural Strength and Modulus of Elasticity of Dental Composites. Spectrum. 2010;13:51–55. [Google Scholar]
- 30.Cadenaro M, Codan B, Navarra CO, Marchesi G, Turco G, Di Lenarda RBL. Contraction stress, elastic modulus, and degree of conversion of three flowable composites. Eur J Oral Sci. 2011;119:241–245. doi: 10.1111/j.1600-0722.2011.00820.x. [DOI] [PubMed] [Google Scholar]
- 31.Masouras K, Silikas N, Watts DC. Correlation of filler content and elastic properties of resin-composites. Dent Mater. 2008;24:932–939. doi: 10.1016/j.dental.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Kleverlaan CJ, Feilzer AJ. Polymerization shrinkage and contraction stress of dental resin composites. Dent Mater. 2005;21:1150–1157. doi: 10.1016/j.dental.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Gonçalves F, Kawano Y, Braga RR. Contraction stress related to composite inorganic content. Dent Mater. 2010;26:704–709. doi: 10.1016/j.dental.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 34.Howard B, Wilson ND, Newman SM, Pfeifer CS, Stansbury JW. Relationships between conversion, temperature and optical properties during composite photopolymerization. Acta Biomater. 2010;6:2053–2059. doi: 10.1016/j.actbio.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu H, Stansbury JW, Dickens SH, Eichmiller FC, Bowman CN. Probing the origins and control of shrinkage stress in dental resin-composites: I. Shrinkage stress characterization technique. J Mater Sci Mater Med. 2004;15:1097–1103. doi: 10.1023/B:JMSM.0000046391.07274.e6. [DOI] [PubMed] [Google Scholar]
- 36.Lu H, Stansbury JW, Dickens SH, Eichmiller FC, Bowman CN. Probing the origins and control of shrinkage stress in dental resin composites. II. Novel method of simultaneous measurement of polymerization shrinkage stress and conversion. J Biomed Mater Res B Appl Biomater. 2004;71:206–213. doi: 10.1002/jbm.b.30088. [DOI] [PubMed] [Google Scholar]
- 37.Atai M, Watts DC. A new kinetic model for the photopolymerization shrinkage-strain of dental composites and resin-monomers. Dent Mater. 2006;22:785–791. doi: 10.1016/j.dental.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Stansbury JW. Dimethacrylate network formation and polymer property evolution as determined by the selection of monomers and curing conditions. Dent Mater. 2012;28:13–22. doi: 10.1016/j.dental.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lange J. Viscoelastic properties and transitions during thermal and UV cure of a methacrylate resin. Polym Eng. 1999;5:1651–1660. [Google Scholar]
- 40.Braem M, Finger W, Vandoren V, Lambrechts P, Vanherle G. Mechanical properties and filler fraction of dental composites. Dent Mater. 1989;5:346–349. doi: 10.1016/0109-5641(89)90128-0. [DOI] [PubMed] [Google Scholar]
- 41.Arimoto A, Nakajima M, Hosaka K, Nishimura K, Ikeda M, Foxton RM, et al. Translucency, opalescence and light transmission characteristics of light-cured resin composites. Dent Mater. 2010;26:1090–1097. doi: 10.1016/j.dental.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 42.Arikawa H, Kanie T, Fujii K, Takahashi H, Ban S. Effect of filler properties in composite resins on light transmittance characteristics and color. Dent Mater J. 2007;26:38–44. doi: 10.4012/dmj.26.38. [DOI] [PubMed] [Google Scholar]
- 43.Pfeifer CS, Wilson ND, Shelton ZR, Stansbury JW. Delayed gelation through chain-transfer reactions: Mechanism for stress reduction In methacrylate networks. Polymer (Guildf) 2011;52:3295–3303. doi: 10.1016/j.polymer.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davidson CL, De Gee AJ, Feilzer A. The competition between the composite-dentin bond strength and the polymerization contraction stress. J Dent Res. 1984;63:1396–1399. doi: 10.1177/00220345840630121101. [DOI] [PubMed] [Google Scholar]
- 45.Feilzer AJ, De Gee AJ, Davidson CL. Quantitative determination of stress reduction by flow in composite restorations. Dent Mater. 1990;6:167–171. doi: 10.1016/0109-5641(90)90023-8. [DOI] [PubMed] [Google Scholar]
- 46.Halvorson RH, Erickson RL, Davidson CL. The effect of filler and silane content on conversion of resin-based composite. Dent Mater. 2003;19:327–333. doi: 10.1016/s0109-5641(02)00062-3. [DOI] [PubMed] [Google Scholar]
- 47.Calheiros FC, Braga RR, Kawano Y, Ballester RY. Relationship between contraction stress and degree of conversion in restorative composites. Dent Mater. 2004;20:939–946. doi: 10.1016/j.dental.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 48.Condon JR, Ferracane JL. Reduction of composite contraction stress through non-bonded microfiller particles. Dent Mater. 1998;14:256–260. doi: 10.1016/s0109-5641(98)00036-0. [DOI] [PubMed] [Google Scholar]
- 49.Satterthwaite JD, Maisuria A, Vogel K, Watts DC. Effect of resin-composite filler particle size and shape on shrinkage-stress. Dent Mater. 2012;28:609–614. doi: 10.1016/j.dental.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 50.Chen M-H. Update on dental nanocomposites. J Dent Res. 2010;89:549–560. doi: 10.1177/0022034510363765. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.