Abstract
The mechanisms underlying bladder sensation and the way we experience sensations during normal voiding and in pathology is complex and not well understood. During storage and emptying, mechanical changes occurring in number of cell types within the bladder wall (i.e. the uroepithelium and bladder afferents) can have a major influence on our sensory systems. In this review, we discuss bladder sensation with a focus on coding events in the periphery.
Keywords: afferents, sensation, urothelium
Part 1: urothelial signalling
The urothelium, or epithelial lining of the urinary tract, is composed of a number of cell layers (Apodaca et al. 2007, Khanderwal et al. 2009). These include a basal layer, intermediate and a superficial or apical layer, often referred to as ‘umbrella cells’. An important, but not well understood, function of epithelial cells is the ability to sense changes in their extracellular environment and then communicate these changes to the underlying nervous, connective and muscular tissues. For example, during bladder filling and emptying, the urothelium is able to respond to a wide range of physical stimuli including changes in bladder pressure, geometric extension (not necessarily accompanied by large forces), tension (in the urothelium or bladder wall) and ranges of urine composition as well as tonicity. A variety of studies in humans and in animals have reported that urothelial cells express ‘sensor molecules’ capable of responding to physical as well as chemical stimuli (Birder & Groat 2006, Birder et al. 2010). Although the urothelium is able to respond to physical (and chemical) changes by releasing signalling molecules that can impact on afferent activity, the transducer protein (s) and the physiological regulators involved in these functions are not well described.
There is evidence that epithelial cells in different organ systems may express similar receptor subtypes (Folkerts & Nijkamp 1998, Lumpkin & Caterina 2007, Kummer et al. 2008). Accordingly, epithelial cells could use multiple signalling pathways, whose intracellular mechanisms differ according to location and environmental stimuli. This would permit a greater flexibility for the cell to regulate function and respond to complex changes in their surrounding microenvironment. Whether urothelial sensor molecules all feed into a diverse array of signalling pathways or share similarities with systems such as olfaction, whereby hundreds of receptors share identical transduction cascades (Brunet et al. 1996), is yet to be uncovered.
The urothelium: role in volume regulation and afferent activation
As the bladder fills, the superficial or umbrella cell membrane unfolds and expands because of the addition of membrane (Truschel et al. 2002, Born et al. 2003, Apodaca et al. 2007, Kreft et al. 2009). The modulation of the superficial urothelial cell surface is likely to play a role in accommodating variations in urine volume as the bladder fills and empties and also to substitute leaky membrane areas. The mechanisms and factors underlying these processes are complex and not completely understood. There is evidence that ATP released from urothelium and binding in an autocrine manner to urothelial purinergic cell surface receptors can modulate membrane traffic (Wang et al. 2005).
ATP, released from the urothelium following either mechanical or chemical stimulation, can also act in a paracrine manner to stimulate underlying cells within the bladder wall (Burnstock 2001, Ruggieri 2005, Birder & Groat 2006, Apodaca et al. 2007). This can influence bladder sensation by binding to purinergic receptors on underlying nerves and interstitial cells. While evidence supports a role for ATP and purinergic receptors in modulating symptoms in several urologic diseases, the mechanisms underlying activation of the micturition pathway at lower bladder volumes (during urgency) and mediators (amount; type) involved are not understood. In addition, the directionality of transmitter release, the mixture of receptor subtypes in the apical and basolateral domains and interactions between multiple transmitters is likely to affect the nature of the output in both health and disease.
Assuming that signal transfer is not unidirectional, how then might communication occur from the urothelium to the underlying cells within the bladder wall and to the nervous system? Studies carried out using optical imaging approaches have reported a directionality of signal transfer, originating within the urothelium/suburothelial layer and propagating to the detrusor (Kanai et al. 2007, Ikeda & Kanai 2008). Mechanical stretch, exogenous agents as well as pathology were found to enhance this propagation. It remains to be determined as to how signalling occurs between the apical–basal urothelium as well as within the superficial urothelium. It has been suggested that there may be subsets of urothelial cells that could act as a type of pacemaker cell, which may initiate and propagate activity (Shabir & Southgate 2008). It has also been suggested that changes in sensitivity or activity in bladders with damage to the neck/proximal urethra may contribute to urge sensation (de Jongh et al. 2007). In this regard, it seems plausible that the epithelium in this region is well positioned to release excitatory agents that could impact bladder sensation. Whether or not regional variations in innervation may be linked to a specific stimulus modality is not known (though, some clinical observation studies involving peripheral nerve trauma would suggest this).
A heightened perception of sensation (visceral hypersensitivity) is common in various bladder disorders including overactive bladder and bladder pain syndrome. Augmented expression/release of urothelial-derived chemical mediators is likely to reduce the threshold for activation of nearby bladder afferents. Thus, the urothelium has the potential for amplifying signals, both within the urothelium and the bladder wall and contributing to a gain of function in sensory processing.
Stressors that can impact on this ‘gain of function’ include alterations in levels of trophic factors as well as stress and steroid hormones. For example, stress-mediated activation of the hypothalamic–pituitary–adrenal axis can result in increased production of corticotrophin releasing factor, which can regulate neuroendocrine and autonomic responses to stress (Stengel & Tache 2010). Altered levels of circulating estrogens may also play a role in urinary bladder dysfunction, including urgency and frequency and possibly urogenital atrophy (Robinson & Cardozo 2002, Hillard 2010). The resulting structural and functional abnormalities may lead to enhanced signalling between the urothelium and underlying cells.
Part 2: experiencing sensation related to the urinary bladder
Sensation is a perception made by an individual related to a stimulus that occurs in the body. Feeling fullness of the bladder is one such sensation. Filling of the bladder creates afferent potentials that run through afferent labelled nervous pathways which consist in general of different parts: a primary order neuron from receptor/stimulus site, through all peripheral nerves of the bladder to the spinal cord or brainstem, a second order neuron towards the thalamus and a third order neuron towards the sensory cortex. Triggering afferent potentials is through a complex system of different mechanisms, and the urothelium and underlying structures have been extensively studied in this respect and described above.
One technique of gathering information on afferents in their peripheral part is through measuring of the potentials directly at the level of the dorsal root in animal experiments. Several studies have been carried out exploring the differences of potentials in healthy and pathologic conditions as in spinal animals, animals with bladder pain syndrome/interstitial cystitis or in animals with hormonal deficiency (Birder et al. 2004, Iijima et al. 2009, Wyndaele 2010, Aizawa et al. 2011a). The influence of different transmitters and drugs have been studied, with intravesical or parenteral administration, and often, an effect has been found on afferent potentials in the primary order neuron in these settings (De Wachter & Wyndaele 2003a, De Laet et al. 2005, Iijima et al. 2007, Aizawa & Wyndaele 2010a, Aizawa et al. 2010b, 2011b,c). In these uni or multifibre studies, it has been shown over and over again that bladder filling gives higher afferent potential rate related to increasing bladder volume. When voiding occurs, this nerve activity diminishes again to baseline. It has also become clear that different types of afferent fibres exist, and that in every type, there are subtypes more or less responsive to chemical actions Figure 1.
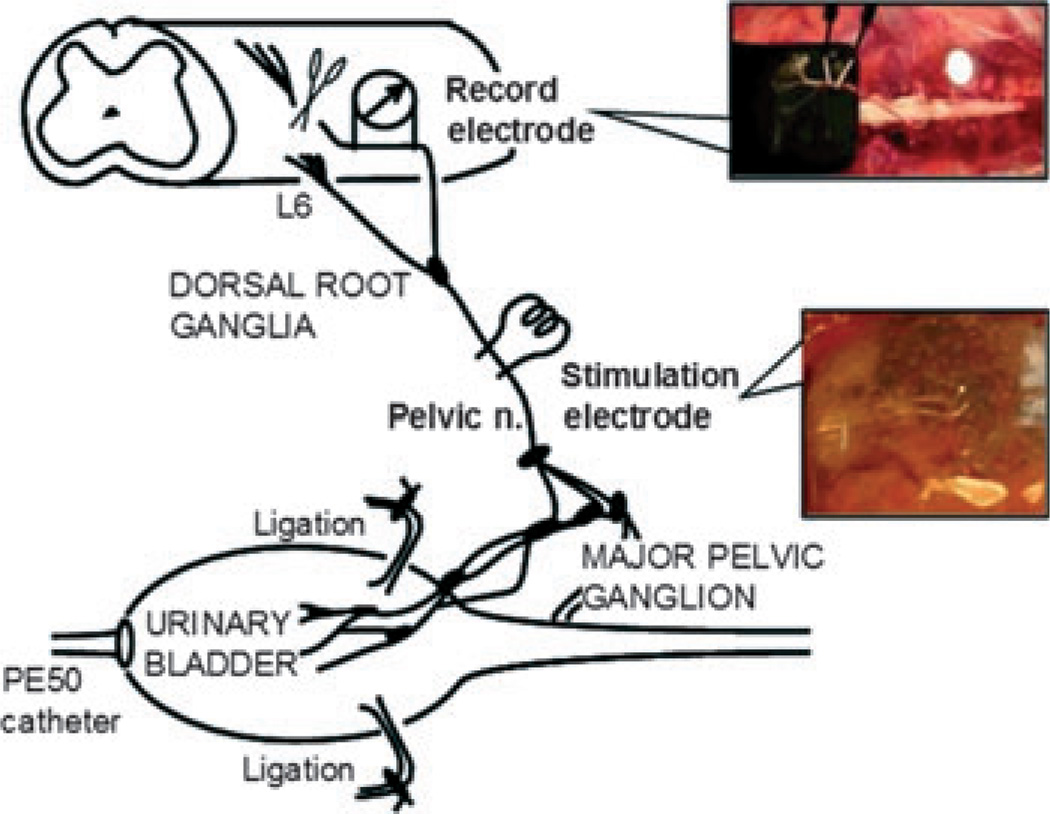
Figure 1.
Diagram of the technical operation procedure (Aizawa, Wyndaele University Antwerp 2011).
This creates the overall image of almost continuous upward flow of large quantities of different bladder nerve potentials elicited by bladder filling and reaching the brain. When these afferent stimuli arrive in the perception areas of the brain, the interpretation and translating of the stimuli into a sensation is carried out through different coding mechanisms: for stimulus type, which relates to the receptors triggered and/or the interpretation by the brain of a combination of potentials if they derive from stimuli for which no specific receptors exist; for stimulus intensity, which depends on number of receptors activated and the frequency of action potentials; for stimulus location. To try and objectivate this afferent inflow, central nervous imaging has been used (Griffiths 2011).
Clinical experience clearly shows that afferent potentials will most of the time not result in conscious sensations but will be hidden unconsciously until certain thresholds are reached. Observations have shown that most of the bladder filling is not felt. In investigation circumstances as with cystometry, individuals, when focussed on the bladder filling and reminded by an investigator or an equipment to try and keep this concentration going for mean 10–20 min, most will report similar sensory events which have been put into a fitting terminology by several investigators over the last decades (Wyndaele 2010). The ICS has then endorsed these results into their terminology report (Abrams et al. 2003), and now often urodynamic equipment and urodynamic evaluation sheets will indicate first sensation of filling, first desire to void and strong desire to void, eventually urgency or pain. Much attention has gone into these semantics and discussions of what is thought correct and what not. It remains semantics, and at the best, a good approach of what human physiology could have as expressions for the individual in such investigating circumstances Figure 2.
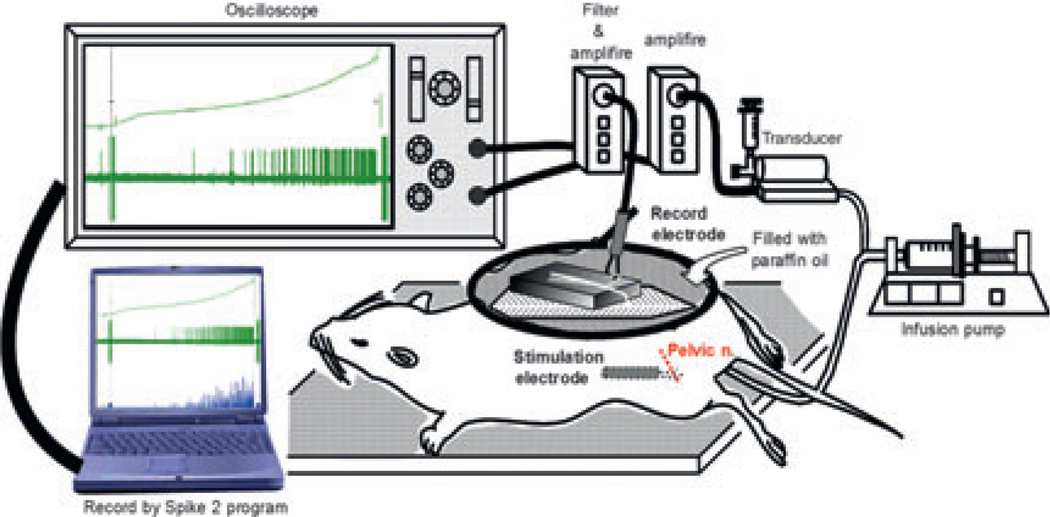
Figure 2.
Diagram of the experimental summary (Aizawa, Wyndaele University Antwerp 2011).
In daily life, the sensory information received from bladder filling, which will on average take 3–4 h during the working day, could be different from sensory data gathered during a focused period of 20-min-urodynamic testing during which filling is performed at an unphysiological speed. However, data from a sensation-related voiding diary, where every void and leakage is reported with the desire that was experienced at the same time, show a correlation with data from urodynamic tests (De Wachter & Wyndaele 2003b, Naoemova et al. 2009). It indicates that first desire to void during cystometry can correspond with bladder sensation in daily life, experienced as that some activities still can be ended before going to empty the bladder. A strong desire to void on cystometry corresponds with a sensation on voiding diary that the individual needs to void and has the impression that he/she can no longer postpone then at the most for 5 min. Activities have to be interrupted. These sensory descriptions relate to events that are most easily recognized by individuals. One can hypothesize that such information is then stored in a sensory database like system in the brain and acts like a trigger as soon as its threshold is reached to let go a sensory warning that requires action. Studies with sensation-related voiding diaries have shown that in consecutive days, the pattern and sensation/volume relations are stable (Naoemova et al. 2008). Studies on fake cystometry have also given interesting data. When an individual is told that a cystometry, fully installed, is starting while no fluid is given, there is still a minority that will report some of previously described filling sensations in the next 15 min (De Wachter et al. 2008). One can hypothesize that these individuals might have the ‘sensory scenario’, painted in their unconsciousness, played off automatically and report back some sensations that should develop in the scenario out of memory and without proper new changes in afferent information at the time. It is remarkable that in such individuals, the time and consecutive sensation ratios are as normal. It is easy to imagine that with sensory events that occur daily, every day, for years, the stored information must be solid and the impact of memory is important.
Afferent information from the bladder would seem to get a favoured handling in the central nervous system. In coma patients, with the exception of the lowest Oxford scale, bladder filling will elicit strong body movements as of unease before ending in a reflex, synergic voiding (Wyndaele 1986). Bladder filling will lower rectal filling sensation (De Wachter & Wyndaele 2003c). When volunteers go to the toilet with the desire to empty the bowel, most will empty the bladder first (De Wachter et al. 2007).
The question remains how, from an almost continuous inflow of afferent potentials, with increasing intensity the further the bladder fills, distinct signs of warning develop. Probably, many different mechanisms will play, including modulation in sensory pathways, memory and emotion, attention and more. Sensation is by definition always subjective, and thus, special for every single individual Figure 3.
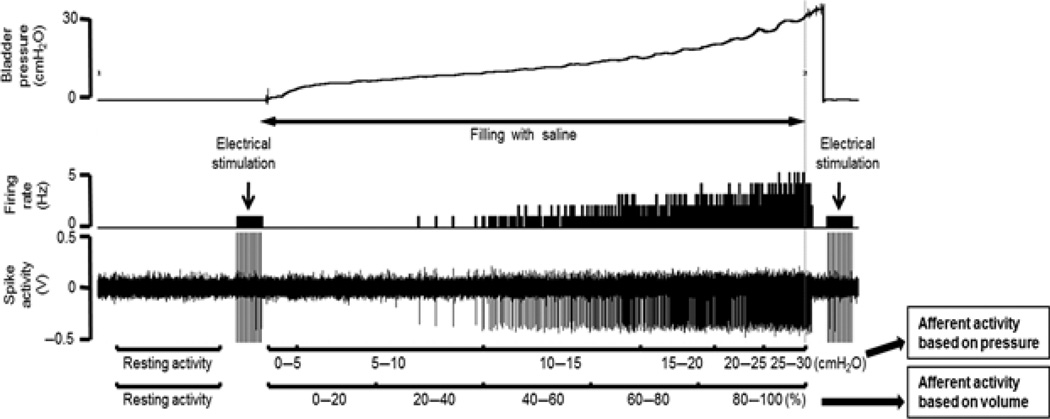
Figure 3.
Typical single afferent activity traces. Recorded afferent activity was averaged at 5-cm H2O interval of pressure or at five equal divisions (0–20, 20–40, 40–60, 60–80 and 80–100%) of volume in the filling phase. Average total unitary activity was also calculated in function of intravesical pressure or volume. Afferent nerve activity is expressed as impulses per second or Hz, which is based on pressure or volume respectively (Aizawa, Wyndaele University Antwerp 2011).
This has to be taken into account and standardizing sensory information will always need to accept large ranges and different expressions between people, a concept also developed as the ‘cognitive voiding’ by J. Gillespie in the State of the art David Rowen lecture during the Annual International Continence Society meeting in Glasgow, UK, August 30 to September 2, 2011.
Another item that needs further developing is the afferent and sensory interaction between pelvic organs. The different pelvic functions are carried out in a coordinated way through complex integrative pathways that may converge peripherally and/or centrally. If pathology occurs, traumatic, irritative or infectious, the shared afferent pathways may produce generalized pelvic sensitization or cross-sensitization to both chemical and mechanical stimuli, dependent on both intact bladder sensory innervation and neuropeptide content as shown bidirectionally for the bladder and bowel in animal models (Ustinova et al. 2010). There are many examples of mutual sensory influences between bladder and bowel, in health and in sickness [Malykhina A, Wyndaele JJ, k Andersson KE, De Wachter S, Dmochowski R.Do the urinary bladder and large bowel interact, in sickness or in health? Accepted for publication NUU 2011].
Sensation can be pathological, either that there is not enough sensory warning either that it becomes too strong and interferes with daily life and quality of life. In such last case, urgency can be experienced or pain and frequency can develop. How in these circumstances the normal handling of afferent potentials gets disturbed needs more study. Is it a breaking through inhibiting mechanisms, or a too high and quick inflow of extra afferent potentials so that thresholds are reached earlier or combinations? What is the role of neuroplasticity? Bladder afferents show plasticity; the total number of active primary afferents is not static but critically depends on the state of the tissue. This may account for the changes in bladder sensation as in BPS-IC and needs further study (Peeker et al. 2000, Nazif et al. 2007).
An important domain for research is the possible influence of treatment modalities on sensation from the lower urinary tract. It can be influenced by behavioural means (Wyndaele et al. 1997), drugs (Finney et al. 2006) and surgery.
Conclusion
Recent research has shown that afferent potentials from the lower urinary tract depend on complex regulating mechanisms. This afferent inflow is transported in a few sensory warnings. How this is processed in healthy and in patients with different bladder conditions needs to be further explored.
Acknowledgments
For Lori Birder, NIH R37 DK54824 and R01 DK57284. For Wyndaele JJ, Urology Research Fund Antwerp University Hospital.
Footnotes
Conflict of interest
None.
References
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, Van Kerrebroeck P, Victor A, Wein A. Standardisation Sub-Committee of the International Continence Society. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37–49. doi: 10.1016/s0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]
- Aizawa N, Wyndaele JJ. Effects of phenazopyridine on rat bladder primary afferent activity, and comparison with lidocaine and acetaminophen. Neurourol Urodyn. 2010a;29:1445–1450. doi: 10.1002/nau.20886. [DOI] [PubMed] [Google Scholar]
- Aizawa N, Igawa Y, Nishizawa O, Wyndaele JJ. Effects of CL316,243, a beta(3)-adrenoceptor agonist, and intravesical prostaglandin E(2) on the primary bladder afferent activity of the rat. Neurourol Urodyn. 2010b;29:771–776. doi: 10.1002/nau.20826. [DOI] [PubMed] [Google Scholar]
- Aizawa N, Iijima K, Rosenbaum JS, Downs TR, Igawa Y, Andersson KE, Wyndaele JJ. Comparison of the effects of oestrogen deficiency and old age on primary bladder afferent activity and voiding behaviour in the ageing female rat. BJU Int. 2011a;108:E10–E16. doi: 10.1111/j.1464-410X.2010.09689.x. [DOI] [PubMed] [Google Scholar]
- Aizawa N, Igawa Y, Andersson KE, Iijima K, Nishizawa O, Wyndaele JJ. Effects of intravesical instillation of ATP on rat bladder primary afferent activity and its relationship with capsaicin-sensitivity. Neurourol Urodyn. 2011b;30:163–168. doi: 10.1002/nau.20940. [DOI] [PubMed] [Google Scholar]
- Aizawa N, Igawa Y, Nishizawa O, Wyndaele JJ. Effects of nitric oxide on the primary bladder afferent activities of the rat with and without intravesical acrolein treatment. Eur Urol. 2011c;59:264–271. doi: 10.1016/j.eururo.2010.10.035. [DOI] [PubMed] [Google Scholar]
- Apodaca G, Balestreire E, Birder LA. The urothelial-associated sensory web. Kidney Int. 2007;72:1057–1064. doi: 10.1038/sj.ki.5002439. [DOI] [PubMed] [Google Scholar]
- Birder LA, de Groat WC. Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nat Clin Urol. 2006;4:46–54. doi: 10.1038/ncpuro0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, Ruan HZ, Chopra B, Xiang Z, Barrick S, Buffington CA, Roppolo JR, Ford AP, de Groat WC, Burnstock G. Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am J Physiol Renal Physiol. 2004;287:F1084–F1091. doi: 10.1152/ajprenal.00118.2004. [DOI] [PubMed] [Google Scholar]
- Birder LA, Kanai AJ, Cruz F, Moore K, Fry CH. Is the urothelium intelligent? Neurourol Urodyn. 2010;29:598–602. doi: 10.1002/nau.20914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born M, Pahner I, Ahnert-Hilger G, Jons T. The maintenance of the permeability barrier of bladder facet cells requires a continuous fusion of discoid vesicles with the apical plasma membrane. Eur J Cell Biol. 2003;82:343–350. doi: 10.1078/0171-9335-00326. [DOI] [PubMed] [Google Scholar]
- Brunet LJ, Gold GH, Ngai J. General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel. Neuron. 1996;17:681–683. doi: 10.1016/s0896-6273(00)80200-7. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci. 2001;22:133–145. doi: 10.1016/s0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- De Laet K, De Wachter S, Wyndaele JJ. Systemic oxybutinin decreases afferent activity of the pelvic nerve of the rat: new insights into the working mechanisms of antimuscarinics. Neurourol Urodyn. 2005;25:156–161. doi: 10.1002/nau.20208. [DOI] [PubMed] [Google Scholar]
- De Wachter S, Wyndaele JJ. Intravesical oxybutinin: a local anesthetic effect on bladder c-afferents. J Urol. 2003a;169:1892–1895. doi: 10.1097/01.ju.0000049903.60057.4b. [DOI] [PubMed] [Google Scholar]
- De Wachter S, Wyndaele JJ. Frequency volume charts: a tool to evaluate bladder sensation? Neurourol Urodyn. 2003b;22:638–642. doi: 10.1002/nau.10160. [DOI] [PubMed] [Google Scholar]
- De Wachter S, Wyndaele JJ. Impact of rectal distension on the results from evaluations of the lower urinary tract sensation. J Urol. 2003c;169:1392–1394. doi: 10.1097/01.ju.0000053393.45026.4d. [DOI] [PubMed] [Google Scholar]
- De Wachter S, de Jong A, Van Dyck J, Wyndaele JJ. Interaction of filling related sensation between anorectum and lower urinary tract and its impact on the sequence of their evacuation. A study in healthy volunteers. Neurourol Urodyn. 2007;26:481–485. doi: 10.1002/nau.20384. [DOI] [PubMed] [Google Scholar]
- De Wachter S, Van Meel T, Wyndaele JJ. Can a faked cystometry deceive patients in their perception of filling sensations? A study on the reliability of spontaneously reported cystometric filling sensations in patients with non-neurogenic lower urinary tract dysfunction. Neurourol Urodyn. 2008;27:395–398. doi: 10.1002/nau.20523. [DOI] [PubMed] [Google Scholar]
- Finney SM, Andersson KE, Gillespie JI, Stewart LH. Antimuscarinic drugs in detrusor overactivity and the overactive bladder syndrome: motor or sensory actions? BJU Int. 2006;98:503–507. doi: 10.1111/j.1464-410X.2006.06258.x. [DOI] [PubMed] [Google Scholar]
- Folkerts G, Nijkamp FP. Airway epithelium: more than just a barrier! Trends Pharmacol Sci. 1998;19:334–341. doi: 10.1016/s0165-6147(98)01232-2. [DOI] [PubMed] [Google Scholar]
- Griffiths D. Use of functional imaging to monitor central control of voiding in humans. Handb Exp Pharmacol. 2011;202:81–97. doi: 10.1007/978-3-642-16499-6_5. [DOI] [PubMed] [Google Scholar]
- Hillard T. The postmenopausal bladder. Menopause Int. 2010;16:74–80. doi: 10.1258/mi.2010.010020. [DOI] [PubMed] [Google Scholar]
- Iijima K, De Wachter S, Wyndaele JJ. Effects of the M3 Receptor Selective Muscarinic Antagonist Darifenacin on Bladder Afferent Activity of the Rat Pelvic Nerve. Eur Urol. 2007;52:842–847. doi: 10.1016/j.eururo.2007.02.057. [DOI] [PubMed] [Google Scholar]
- Iijima K, Igawa Y, De Wachter S, Wyndaele JJ. Mechanosensitive primary bladder afferent activity in rats with and without spinal cord transection. J Urol. 2009;182:2504–2510. doi: 10.1016/j.juro.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Kanai A. Urotheliogenic modulation of intrinsic activity in spinal cord-transected rat bladders: role of mucosal muscarinic receptors. Am J Physiol. 2008;295:F454–F461. doi: 10.1152/ajprenal.90315.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jongh R, van Koeveringe GA, van Kerrebroeck PEV, Merkerink-van Ittersum M, de Vente J, Gillespie JI. Damage to the bladder neck alters autonomous activity and its sensitivity to cholinergic agonists. BJU Int. 2007;100:919–929. doi: 10.1111/j.1464-410X.2007.07129.x. [DOI] [PubMed] [Google Scholar]
- Kanai A, Roppolo J, Ikeda Y, Zabbarova I, Tai C, Birder L, Griffiths D, de Groat W, Fry C. Origin of spontaneous activity in neonatal and adult rat bladders and its enhancement by stretch and muscarinic agonists. Am J Physiol. 2007;292:F1065–F1072. doi: 10.1152/ajprenal.00229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanderwal P, Abraham SN, Apodaca G. Cell biology and physiology of the uroepithelium. Am J Physiol. 2009;297:F1477–F1501. doi: 10.1152/ajprenal.00327.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreft ME, Jezernik K, Kreft M, Romih R. Apical plasma membrane traffic in superficial cells of bladder urothelium. Ann NY Acad Sci. 2009;1152:18–29. doi: 10.1111/j.1749-6632.2008.04004.x. [DOI] [PubMed] [Google Scholar]
- Kummer W, Lips KS, Pfell U. The epithelial cholinergic system of the airways. Histochem Cell Biol. 2008;130:219–234. doi: 10.1007/s00418-008-0455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature. 2007;445:858–865. doi: 10.1038/nature05662. [DOI] [PubMed] [Google Scholar]
- Naoemova I, De Wachter S, Wuyts FL, Wyndaele JJ. Reliability of the 24-h sensation-related bladder diary in women with urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2008;46:513–516. doi: 10.1007/s00192-008-0565-3. [DOI] [PubMed] [Google Scholar]
- Naoemova I, Van Meel T, De Wachter S, Wyndaele JJ. Does sensory bladder function during cystometry differ from that in daily life? A study in incontinent women. Neurourol Urodyn. 2009;28:309–312. doi: 10.1002/nau.20643. [DOI] [PubMed] [Google Scholar]
- Nazif O, Teichman JM, Gebhart GF. Neural upregulation in interstitial cystitis. Urology. 2007;69:24–33. doi: 10.1016/j.urology.2006.08.1108. [DOI] [PubMed] [Google Scholar]
- Peeker R, Aldenborg F, Dahlström A, Johansson SL, Li JY, Fall M. Increased tyrosine hydroxylase immunoreactivity in bladder tissue from patients with classic and nonulcer interstitial cystitis. J Urol. 2000;163:1112–1115. [PubMed] [Google Scholar]
- Robinson D, Cardozo L. Overactive bladder in the female patient: the role of estrogens. Curr Urol Rep. 2002;3:452–457. doi: 10.1007/s11934-002-0096-2. [DOI] [PubMed] [Google Scholar]
- Ruggieri MR. Mechanisms of disease: role of purinergic signalling in the pathophysiology of bladder dysfunction. Nat Clin Prac Urol. 2005;3:206–214. doi: 10.1038/ncpuro0456. [DOI] [PubMed] [Google Scholar]
- Shabir S, Southgate J. Calcium signalling in wound-responsive normal human urothelial cell monolayers. Cell Calcium. 2008;44:453–464. doi: 10.1016/j.ceca.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Stengel A, Tache Y. Corticotropin-releasing factor signalling and visceral response to stress. Ex Biol Med. 2010;2010:1168–1178. doi: 10.1258/ebm.2010.009347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truschel ST, Wang E, Ruiz WG, Leung SM, Rojas R, Lavelle J, Zeidel M, Stoffer D, Apodaca G. Stretch-regulated exocytosis/endocytosis in bladder umbrella cells. Mol Biol Cell. 2002;13:830–846. doi: 10.1091/mbc.01-09-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustinova EE, Fraser MO, Pezzone MA. Crosstalk and sensitization of bladder afferent nerves. Neurourol Urodyn. 2010;29:77–81. doi: 10.1002/nau.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang EC, Lee JM, Ruiz WG, Balestreire EM, von Bodungen M, Barrick S, Cockayne DA, Birder LA, Apodaca G. ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. J Clin Invest. 2005;115:2412–2422. doi: 10.1172/JCI24086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyndaele JJ. Micturition in comatose patients. J Urol. 1986;135:1209–1211. doi: 10.1016/s0022-5347(17)46038-1. [DOI] [PubMed] [Google Scholar]
- Wyndaele JJ. Investigating afferent nerve activity from the lowerurinary tract. Highlighting some basic research techniques and clinical evaluation methods. Neurourol Urodyn. 2010;29:56–62. doi: 10.1002/nau.20776. [DOI] [PubMed] [Google Scholar]
- Wyndaele JJ, Hoekx L, Vermandel A. Bladder biofeedback for the treatment of refractory sensory urgency in adults. Eur Urol. 1997;32:429–432. [PubMed] [Google Scholar]