Abstract
We examined the inhibitory effect of the extract of kakrol extracted by 3 types of solvent (water, 50% and 100% ethanol) on histamine release in human basophilic KU812 cells. The water extract of kakrol flesh showed the strongest inhibitory effect on histamine release as compared with the other extracts. Therefore, we evaluated whether water extract of kakrol flesh had a suppressive effect on development of atopic dermatitis-like lesions in picryl chloride-treated NC/Nga mice. The dietary kakrol flesh water extract alleviated the development of skin lesions in ears accompanied by lower IgE levels and inflammatory cytokines levels in serum. These results indicate that the water extract of kakrol flesh might have therapeutic potential for allergic responses in vitro and in vivo.
Keywords: Kakrol, Momordica dioica Roxb., histamine, atopic dermatitis
INTRODUCTION
Atopic dermatitis (AD) is a chronically relapsing inflammatory skin disease that is characterized by pruritic and eczematous skin lesions. Although genetic factors play an important role in development of AD, the pathogenesis of AD is attributable to abnormal immunological and inflammatory pathways that include defective skin barrier, exposure to environmental agents and neuropsychological factors [1, 2]. The pathogenic features of AD have revealed that activated mast cells and excess differentiated Th2 cells might play major roles in the development of dermatitis and the increase in immunoglobulin (Ig) E in serum through chemical mediators and cytokines [3, 4]. Although several treatment methods such as topical steroids, emollients and oral antihistamines are used for management of AD, many patients are still dissatisfied with the effectiveness of the treatments and are worried about side effects of chronic use. If dietary intervention could inhibit the production of chemical mediators and IgE resulting from the allergy response, it may be one of the strategies for therapy and prevention of AD.
Momordica dioica Roxb., known as kakrol in Japan belongs to the family Cucurbitaceae and is a climbing creeper. It is a species related to bitter gourd (Momordica charantia Linn), which is nigauri in Japan. Kakrol, rather than nigauri, has generally been used in dishes because it has a sweet taste and contains a larger content of ascorbic acid than nigauri [5, 6].
It have been reported that kakrol flesh extract effectively inhibits triacylglycerol absorption and lowers the risk of development of fatty liver in rats compared with the bitter gourd [5]. In addition, ethanol and water extracts of kakrol fruit not only have nephroprotective activity [6, 7] but also have hepatoprotective activity [8]. However, there is no study about the anti-allergy activity of kakrol extract.
In this study, we evaluated which type of extract (water, 50% ethanol or 100% ethanol) and which part of kakrol (rind or flesh) had an inhibitory effect on histamine release and then performed an experiment to determine whether it alleviated development of AD induced by hapten picryl chloride (PiCl) treatment.
MATERIALS AND METHODS
Preparation of kakrol (Momordica dioica Roxb.) extract
The kakrol used in this study was developed and cultivated at the Ishigaki branch of the Okinawa Prefectural Agricultural Research Center (Okinawa, Japan). Flesh or rind of kakrol was extracted by placing kakrol in distilled water at 4°C overnight. The suspension was filtered through filter paper. After centrifugation at 3000 rpm for 20 min at 4°C, the supernatant of the extract was freeze-dried. It was resuspended with distilled water at a concentration of 5 mg/ml and then filtered with a 0.2 μm filter. For the ethanol extract; flesh or rind of kakrol was extracted by placing kakrol in 50% or 100% ethanol at 4°C overnight. The extract was evaporated under reduced pressure until it was dry. It was then resuspended with distilled water at a concentration of 5 mg/ml and then filter with a 0.2 μm filter.
Cell culture and stimulation
KU812 cells, a human basophilic cell line, were obtained from the Japanese Cancer Resources Bank (Tokyo, Japan) and were maintained in RPMI 1640 (Nissui, Tokyo, Japan) supplemented with 10% fetal bovine serum (Intergen, Purchase, NY, USA), 100 units/ml penicillin G, 100 μg/ml streptomycin and 10 mM HEPES buffer. KU812 cells were cultured at 37°C in a humidified atmosphere with 5% CO2.
Measurement of histamine release
KU812 cells treated with or without extract of kakrol for 20 min were suspended in histamine release buffer (pH 7.4) containing 137 mM NaCl, 2.7 mM KCl, 1.8 mM CaCl2, 1.1 mM MgCl2, 11.9 mM NaHCO3 and 0.4 mM NaH2PO4 and then stimulated with 5 μM A23187 at 37°C for 30 min. The reaction was terminated by cooling for 5 min at 4°C. After centrifugation at 300 g for 5 min, the histamine content of the supernatant was measured by a fluorometric assay.
Experimental animals and diets
Male 5-week-old NC/Nga mice bred under specific pathogen-free (SPF) conditions were purchased from Charles River Laboratories Japan (Yokohama, Japan). The mice were kept at the Biotron Institute of Kyushu University with a 12-hr light/12-hr dark cycle (light on from 8 a.m.-8 p.m.) in an air-conditioned room (20°C and 60% humidity under SPF conditions). This experiment was carried out according to the guidelines for animal experiments of the Faculty and Graduate School of Agriculture, Kyushu University, and Law No. 105 and Notification No. 6 of the Japanese government. During a 1-week acclimation period, all mice were provided the control diet (MF diet) at 5 g/day. Then, the mice were divided in two groups and were provided either of the following diets: control diet or kakrol diet (MF diet containing 0.5% kakrol flesh water extract) at 5 g/day for 46 days. Body weight was measured before the experimental feed started and every fourth day during the experimental period. Blood samples were collected from the abdominal aorta under light anesthesia with diethyl ether at the end of the feeding period. Serum was obtained by centrifugation at 1000 g for 15 min at 4°C and stored at –80°C until use.
Induction and observation of AD
Dermatitis induced by PiCl was developed in NC/Nga mice according to standard instructions as previously described [9, 10]. Briefly, the back and ears were sensitized with 5% PiCl dissolved in an ethanol and acetone mixture (4:1, v/v). Four days after sensitization, the back and ears were challenged with 1% PiCl dissolved in olive oil. This challenge was repeated six times at 1-week intervals. The observation items were the following four symptoms: flare and hemorrhage; edema, neurosis and incrustation; excoriation; and erosion. The dermatitis scores were expressed as the sum of the individual score graded from 0 to 3 (no sign, 0; mild, 1; moderate, 2; severe, 3) for each item.
Measurement of serum Igs and cytokines
Serum levels of total IgG1 and IgE were measured by using a mouse IgG1 and IgE enzyme-linked immunosorbent assay (ELISA) Quantitation Kits (Bethyl Laboratories, Montgomery, TX, USA) according to the manufacturer’s protocol. As described in our previous paper [11], 20 cytokine proteins in mice sera were assessed using a commercially available RayBio® Mouse Cytokine Antibody Arrays (RayBiotech Inc., Norcross, GA, USA). They were measured in pooled serum from 6 mice. The density of each band was quantified using a program obtained from the US National Institutes of Health.
Statistical analysis
Data were analyzed using the Student’s t-test. Mean values were significantly different from those of the control group at p<0.05.
RESULTS
Water extract of kakrol flesh inhibits release of histamine
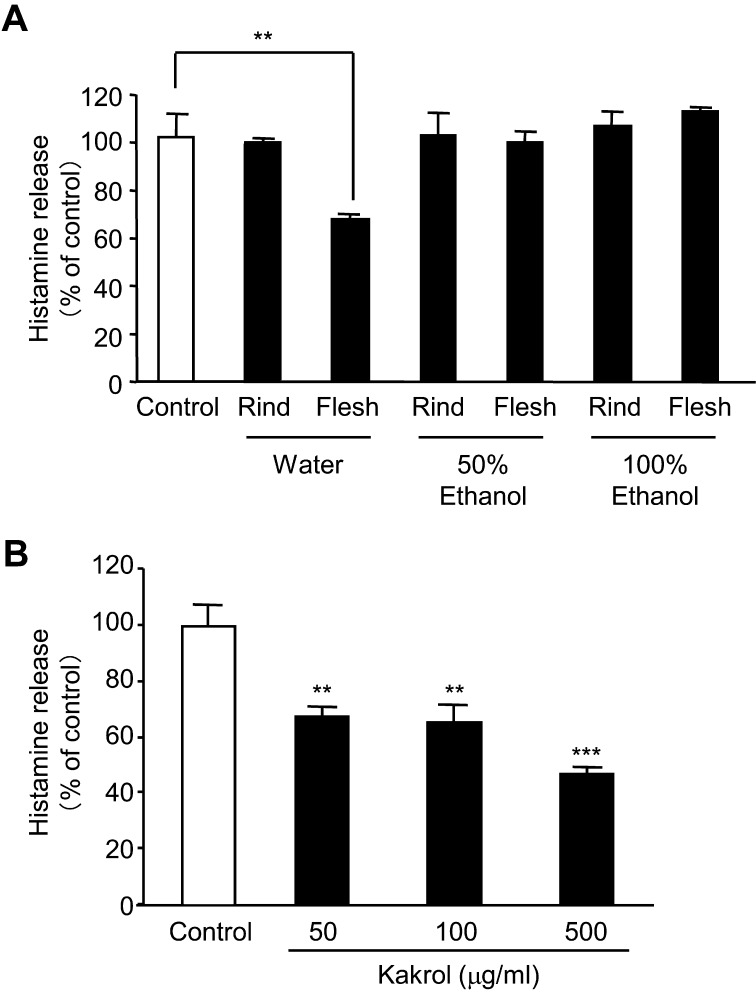
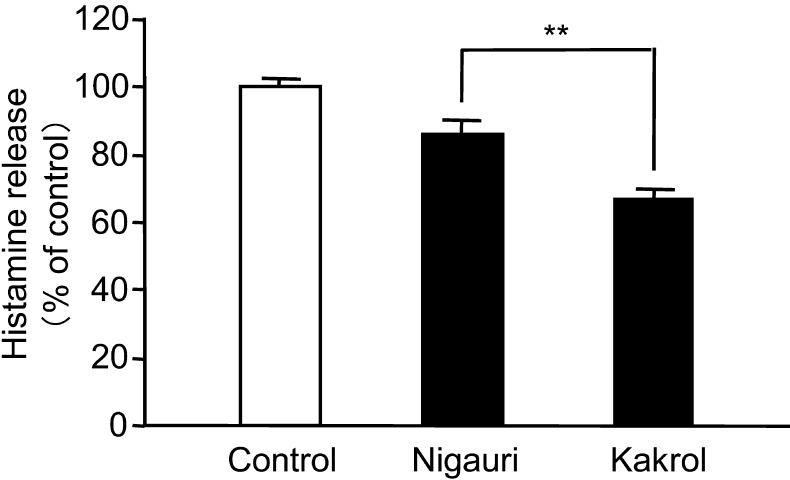
To examine which extract types of rind or flesh of kakrol have an inhibitory effect on histamine release from A23187-treated basophilic KU812 cells, the cells were treated with water, 50% ethanol or 100% ethanol extract of rind or flesh of kakrol. Of the extracts tested, only the water extract of kakrol flesh showed an inhibitory effect on histamine release from A23187-treated KU812 cells in a dose-dependent manner (Fig. 1A, B). This water extract of kakrol flesh had a much stronger inhibitory effect than that of nigauri (Fig. 2).
Fig. 1.
Effect of various extract types of kakrol rind and flesh on the calcium ionophore A23187-induced histamine release from KU812 cells. Fig. 1A represents the effect of several extracts of kakrol rind and flesh. Fig. 1B shows the dose-dependent effect of water extract of kakrol flesh (50, 100 and 500 μg/ml). KU812 cells were pretreated with each extract for 20 min. Then, the cells were stimulated with 5 μM A23187 for 30 min. The concentration of histamine in the supernatant was measured by a fluorometric assay. Data are shown as means ± SD (n=3) and expressed as percentages of control cells treated with A23187 in the absence of extract of kakrol. Statistical analysis was performed using the Student’s t-test. ** and ***: Mean values are significantly different from those of the control group (p<0.01 and p<0.001, respectively).
Fig. 2.
Comparison of the inhibitory effect between nigauri and kakrol flesh water extract on calcium ionophore A23187-induced histamine release from KU812 cells. KU812 cells were pretreated with each extract for 20 min. Then, the cells were stimulated with 5 μM A23187 for 30 min. The concentration of histamine in the supernatant was measured by a fluorometric assay. Data are shown as means ± SD (n=3) and expressed as percentages of control cells treated with A23187 in the absence of water extract of nigauri or kakrol flesh. Statistical analysis was performed using the Student’s t-test. **: Mean values are significantly different from those of the nigauri group (p<0.01).
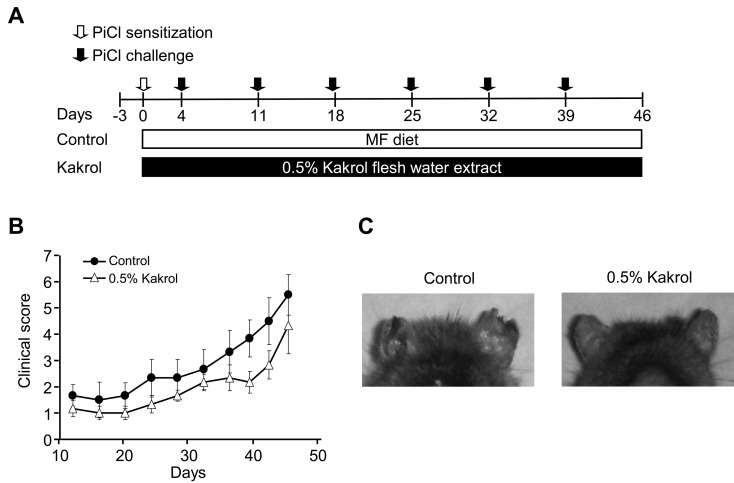
Dietary kakrol suppresses PiCl-induced AD-like skin lesions in NC/Nga mice
To examine whether kakrol diet could attenuate the development of dermatitis symptoms, kakrol extract diet was provided for 8 weeks (Fig. 3A). The clinical scores for the ears of mice fed the kakrol diet showed a tendency to decrease compared with the control (Fig. 3B). As shown in Fig. 3C, skin lesions of the ears were suppressed in the 0.5% kakrol diet group compared with the control. These results suggest that dietary kakrol may be beneficial for attenuating the development of dermatitis symptoms.
Fig. 3.
Effect of water extract of kakrol flesh on clinical scores of skin symptoms in PiCl-induced atopic dermatitis. Schematic representation of the experiment (A). Data are shown as means ± SE for six mice in each group. Statistical analysis was performed using the unpaired t-test. Mean values are significantly different from those of the control group (B). Representative photographs at day 46 (C).
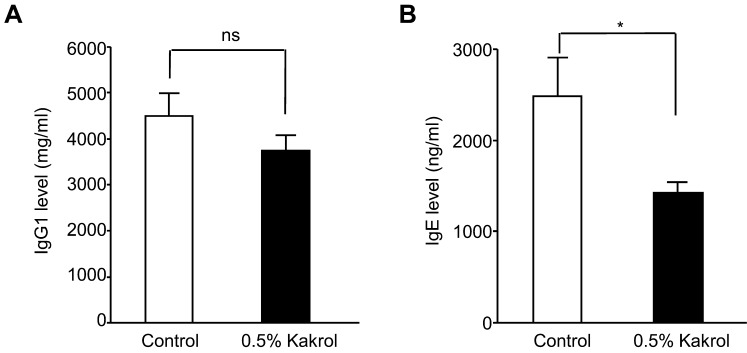
Effect of kakrol diet on Igs and cytokines levels in serum
To investigate whether the kakrol diet could affect the Igs levels in NC/Nga mice sensitized by PiCl, we measured total IgG1 and IgE levels in serum from mice. Although there was no difference on IgG1 level between the control and 0.5% kakrol groups, the kakrol diet significantly decreased IgE levels as compared with the control (Fig. 4). Next, to clarify the mechanisms involved in the suppression of IgE production in mice fed the 0.5% kakrol flesh extract diet, we evaluated the Th1 and Th2 cytokines levels using a Mouse Cytokine Array (Table 1). Among the 20 cytokines assessed, no cytokines were upregulated or downregulated over 2 times in the kakrol flesh extract diet group as compared with the control. However, the kakrol flesh diet had an effect not only on increasing the levels of IL-2 and TNF-α, which are Th1 cytokines, but also on decreasing inflammatory cytokines such as GM-CSF, MCP-1 and MCP-5 (Table 1). These data suggest that the decrease in IgE levels caused by the kakrol diet may result in part from the increase in Th1 cytokines.
Fig. 4.
Total serum IgG1 and IgE levels in NC/Nga mice. Serum concentrations of IgG1 (A) and IgE (B) at the end of the experiment were measured by ELISA. Data are menas ± SE for six mice in each group. Statistical analysis was performed using the unpaired t-test. *: Mean values are significantly different from those of the control group (p<0.05). n.s: Not significant.
Table 1. Effects of dietary kakrol on cytokine levels of NC/Nga mice in sera.
| Cytokines | Kakrol/control | Cytokines | Kakrol/control |
| GCSF | 0.70 | IL-12p70 | 0.86 |
| GM-CSF | 0.85 | IL-13 | 0.86 |
| IL-2 | 1.46 | IL-17 | 1.28 |
| IL-3 | 1.14 | IFN-γ | 1.10 |
| IL-4 | 1.18 | MCP-1 | 0.77 |
| IL-5 | 1.71 | MCP-5 | 0.72 |
| IL-6 | 1.14 | RANTES | 0.89 |
| IL-9 | 0.81 | sTNFR1 | 0.83 |
| IL-10 | 0.84 | TNF-α | 1.23 |
| IL-12p40p70 | 0.90 | Thrombopoietin | 0.67 |
The cytokine levels were measured in pooled serum from 6 mice using a cytokine micro array. The relative levels of cytokines were determined by intensity. The densities of signals were normalized against the background and positive control. Data are represented by relative intensity compared with the control group.
GCSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; MCP, monocyte chemoattractant protein; RANTES, regulated on activation, normal T cell expressed and secreted; sTNFR, soluble tumor necrosis factor receptor.
DISCUSSION
In this study, we demonstrated that water extract of kakrol flesh inhibited histamine release from human basophilic KU812 cells and alleviated development of AD in NC/Nga mice resulting from PiCl treatment.
The NC/Nga mice are the most extensively studied animal model for AD. Antigen-specific IgE is preferentially produced in mice repeatedly painted with a hapten [12]. In this model, immune responses initially induced by epicutaneous introduction of a hapten through normal skin shift to those induced by chronic introduction of the hapten into damaged skin as the antigen-induced hypersensitivity response progresses into chronicity [13]. When sequential cytokine mRNA expression after hapten application is assessed in acute versus chronic lesions, the former is driven by the production of Th1 cytokines, while the latter is driven by the production of Th2 cytokines [14]. This indicates that chronic epicutaneous exposure to a hapten, which can predominantly induce Th1 cytokines during the primary response, induces a shift in the pattern of antigen-induced cytokine expression toward the induction of Th2 cytokines in a site-restricted fashion. The inflammatory response in chronic lesions shares many of the histopathological, immunological and clinical features of human AD [15]. Approximately 70–80% of AD patients have elevated serum IgE levels with IgE antibodies to environmental and/or food allergens [16, 17]. Because a high serum IgE concentration is one of the critical characteristics of AD and clinical severity of the disease has been suggested to be correlated with serum IgE levels, the IgE level is an important therapeutic target for AD. In this report, we showed that dietary kakrol suppresses increase of serum IgE levels induced by PiCl. This result suggests that the kakrol diet inhibits development of AD through suppression of IgE production.
It has been suggested that T cells, mast cells, monocytes/macrophages and eosinophils infiltrated into AD-like skin lesions play important roles in the development of AD by releasing various cytokines/chemokines. Cytokines/chemokines produced by these cells regulate the recruitment and trafficking of immune cells into AD-like skin lesions and modulate IgE synthesis [1, 18]. In AD patients, increases in mast cell number and mast cell activation have been shown in skin lesions [19, 20]. Mast cells stimulated by antigen-specific IgE produce inflammatory cytokines such as GM-CSF and MCP-1 acting on dendritic cells to enhance antigen presentation, adhesion and migration into lymph nodes [21, 22]. Therefore, regulation of the production of these cytokines/chemokines by therapeutic agents is a beneficial target for cure of AD skin lesions. Dietary kakrol flesh not only increases Th1 cytokines such as IL-2 and TNF-α but also decreases inflammatory cytokines like GM-CSF, MCP-1 and MCP-5. These changes in the cytokine profile caused by dietary kakrol might contribute to alleviate AD.
It has been reported that the contents of ascorbic acid and the ratio of unsaturated fatty acid in kakrol flesh are higher than in the bitter gourd [5]. However, these differences were small in the diets. Until now, there have been no papers about the active compounds in kakrol extract. Further studies are needed to identify the active compounds in the water extract of kakrol flesh.
In conclusion, the results obtained in this study suggest that intake of water extract of kakrol flesh might be an effective way to prevent allergic responses.
REFERENCES
- 1.Liu FT, Goodarzi H, Chen HY. 2011. IgE, Mast cells, and Eosinophils in atopic dermatitis. Clin Rev Allergy Immunol 41: 298–310 [DOI] [PubMed] [Google Scholar]
- 2.Novak N, Bieber T, Leung DY. 2003. Immune mechanisms leading to atopic dermatitis. J Allergy Clin Immunol 112: S128–S139 [DOI] [PubMed] [Google Scholar]
- 3.Cooper KD. 1994. Atopic dermatitis: recent trends in pathogenesis and therapy. J Invest Dermatol 102: 128–137 [DOI] [PubMed] [Google Scholar]
- 4.Grewe M, Bruijnxeel-Koomen CA, Schopf E, Thepen T, Langevel-Wildschut AG, Ruzicka T. 1998. A role for Th1 and Th2 cells in immunopathogenesis of atopic dermatitis. Immunol Today 19: 359–361 [DOI] [PubMed] [Google Scholar]
- 5.Sato M, Ueda T, Nagata K, Shiratake S, Tomoyori H, Kawakami M, Ozaki Y, Okubo H, Shirouchi B, Imaizumi K. 2011. Dietary kakrol (Momordica dioica Roxb.) flesh inhibits triacylglycerol absorption and lowers the risk for development of fatty liver in rats. Exp Biol Med 236: 1139–1146 [DOI] [PubMed] [Google Scholar]
- 6.Jain A, Singhai AK. 2010. Nephroprotective activity of Momordica dioica Roxb. in cisplatin-induced nephrotoxicity. Nat Prod Res 24: 846–854 [DOI] [PubMed] [Google Scholar]
- 7.Jain A, Singhai AK. 2010. Effect of Momordica dioica Roxb on gentamicin model of acute renal failure. Nat Prod Res 24: 1379–1389 [DOI] [PubMed] [Google Scholar]
- 8.Kushawa SK, Jain A, Jain A, Gupta VB, Patel JR, Dubey PK. 2005. Hepatoprotective activity of fruits of Mormordica dioica Roxb. Plant Arch 5: 613–616 [Google Scholar]
- 9.Taniguchi Y, Kohno K, Inoue S, Koya-Miyata S, Okamoto I, Arai N. 2003. Oral administration of royal jelly inhibits the development of atopic dermatitis-like skin lesions in NC/Nga mice. Int Immunopharmacol 3: 1313–1324 [DOI] [PubMed] [Google Scholar]
- 10.Ohmura T, Tsunenari I, Hayashi T, Satoh Y, Konomi A, Nanri H. 2004. Role of substance P in NC/Nga mouse model of atopic dermatitis-like disease. Int Arch Allergy Immunol 133: 389–397 [DOI] [PubMed] [Google Scholar]
- 11.Yano S, Umeda D, Maeda N, Fujimura Y, Yamada K, Tachibana H. 2006. Dietary apigenin suppresses IgE and inflammatory cytokines production in C57BL/6N mice. J Agric Food Chem 54: 5203–5207 [DOI] [PubMed] [Google Scholar]
- 12.Matsuda H, Watanabe N, Geba GP, Sper J, Tsudzuki M, Hiroi J, Matsumoto M, Ushio H, Saito S, Askenase PW, Ra C. 1997. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int Immunol 9: 461–466 [DOI] [PubMed] [Google Scholar]
- 13.Suto H, Matsuda H, Mitsuishi K, Hira K, Uchida T, Unno T, Ogawa H, Ra C. 1999. NC/Nga mice: a mouse model for atopic dermatitis. Int Arch Allergy Immunol 120: 70–75 [DOI] [PubMed] [Google Scholar]
- 14.Kitagaki H, Fujisawa S, Watanabe K, Hayakawa K, Shiohara T. 1995. Immediate-type hypersensitivity response followed by a late reaction is induced by repeated epicutaneous application of contact sensitizing agents in mice. J Invest Dermatol 105: 749–755 [DOI] [PubMed] [Google Scholar]
- 15.Kitagaki H, Ono N, Hayakawa K, Kitazawa T, Watanabe K, Shiohara T. 1997. Repeated elicitation of contact hypersensitivity induces a shift in cutaneous cytokine milieu from a T helper cell type 1 to a T helper cell type 2 profile. J Immunol 159: 2484–2491 [PubMed] [Google Scholar]
- 16.Shiohara T, Hayakawa J, Mizukawa Y. 2004. Animal models for atopic dermatitis: are they relevant to human disease? J Dermatol Sci 36: 1–9 [DOI] [PubMed] [Google Scholar]
- 17.Leung DYM, Boguniewicz M, Howell MD, Nomura I, Hamid OA. 2004. New insights into atopic dermatitis. J Clin Invest 113: 651–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burton OT, Oettgen HC. 2011. Beyond immediate hypersensitivity: evolving roles for IgE antibodies in immune homeostasis and allergic diseases. Immunol Rev 242: 128–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gangur V, Oppenheim JJ. 2000. Are chemokines essential or secondary participants in allergic responses? Ann Allergy Asthma Immunol 84: 569–581 [DOI] [PubMed] [Google Scholar]
- 20.Soter NA. 1989. Morphology of atopic eczema. Allergy 44: 16–19 [DOI] [PubMed] [Google Scholar]
- 21.Irani AM, Sampson HA, Schwartz LB. 1989. Mast cells in atopic dermatitis. Allergy 44: 31–34 [PubMed] [Google Scholar]
- 22.Biedermann T, Kneilling M, Mailhammer R, Maier K, Sander CA, Kollias G, Kunkel SL, Hültner L, Röcken M. 2000. Mast cells control neutrophil recruitment during T cell-mediated delayed-type hypersensitivity reactions through tumor necrosis factor and macrophage inflammatory protein 2. J Exp Med 192: 1441–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon JR. 2000. Monocyte chemoattractant peptide-1 expression during cutaneous allergic reactions in mice is mast cell dependent and largely mediates the monocyte recruitment response. J Allergy Clin Immunol 106: 110–116 [DOI] [PubMed] [Google Scholar]