Abstract
We investigated Candida albicans-induced mast cell degranulation in vitro and in vivo. Cell wall fraction but not culture supernatant and cell membrane fraction prepared from hyphally grown C. albicans induced β-hexosaminidase release in RBL-2H3 cells. Cell wall mannan and soluble β-glucan fractions also induced β-hexosaminidase release. Histological examination of mouse forestomach showed that C. albicans gut colonization induces mast cell degranulation. However, intragastric administration of cell wall fraction failed to induce mast cell degranulation. We propose that cell wall polysaccharides are responsible for mast cell degranulation in the C. albicans-colonized gut.
Keywords: Candida albicans, mast cell, degranulation, mannan, β-glucan
Candida albicans is part of the indigenous microbial flora of the human gut [1]. Although it has been postulated that the excessive colonization by C. albicans in the gut may be responsible for a variety of hypersensitivity diseases including allergy, more research is needed to understand its cause-and-effect relationships [2]. We previously observed that serum antibody responses to repeated oral administration of ovalbumin (OVA) and gastrointestinal uptake of orally administered OVA were enhanced by C. albicans gut colonization in mice [3]. In addition, C. albicans gut colonization aggravated OVA-induced allergic diarrhea, 2,4-dinitrofluorobenzene-induced contact skin hypersensitivity, and type-II collagen-induced arthritis in mice [4]. These findings suggest that C. albicans gut colonization is not only a risk factor for food allergy but also an aggravating factor for inflammation in allergic and autoimmune diseases. Mechanistically, mast cell-mediated hyperpermeability of the gut mucosal epithelium is likely to be responsible for the enhanced uptake of orally administered OVA and OVA-induced allergic diarrhea in C. albicans-colonized mice [3, 4]. However, the mechanism by which C. albicans colonization influences mast cell function in the gut mucosa is unclear. The present study examined whether cell constituents of C. albicans stimulate degranulation, a principal function of mast cells, in mast cell-like RBL-2H3 cells and mice.
C. albicans (JCM 1542) was maintained as previously described [5]. Because C. albicans shows hyphal growth when it colonizes in the gut mucosa [5], the cell constituents were prepared from hyphally grown C. albicans. Hyphal growth was induced by culturing the yeast cells (106 cells/ml) of C. albicans in PBS supplemented with 10% fetal calf serum at 37°C for 2.5 hr. The culture supernatant was obtained by centrifugation at 2,000 × g for 10 min, and the cell pellet was subjected to the preparation of cell wall and membrane fractions according to the method of Mizutani et al. [6]. Furthermore, mannan, and soluble and insoluble β-glucan fractions were prepared from the cell wall fraction according to the method of Kocourek and Ballou [7], Miura et al. [8] and Cassone et al. [9], respectively. The carbohydrate content of each fraction was determined by the phenol-sulfuric acid method.
RBL-2H3 cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 μg/ml gentamicin at 37°C in a humidified atmosphere containing 5% CO2. Cells were seeded into each well of 24-well culture plates at 2×105 cells/well and cultured overnight. Adherent cells were washed twice with assay buffer composed of 137 mmol/l NaCl, 2.7 mmol/l KCl, 1.0 mmol/l MgCl2, 1.8 mmol/l CaCl2, 20 mmol/l HEPES, 5.6 mmol/l glucose, and 0.1% BSA (pH 7.4). Cells were then incubated at 37°C for 30 min in 1 ml of assay buffer supplemented with different amounts of either culture supernatant, cell wall, cell membrane, cell wall mannan, or cell wall soluble or insoluble β-glucan fractions prepared from hyphally grown C. albicans. The amounts of each fraction supplemented were equal to equivalent quantities of C. albicans cells (2 × 105, 2 ×106, and 2 ×107 cells). The amounts of mannan, and soluble and insoluble β-glucan prepared from 2 ×107 cells were 39.9, 79.7, and 37.4 μg (glucose equivalent), respectively. Degranulation of RBL-2H3 cells was assessed by monitoring the release of the granular enzyme β-hexosaminidase. Thus, the culture supernatants and cell lysates after incubation were subjected to β-hexosaminidase assay according to the method of Ortega et al. [10].
Female BALB/c mice were randomly separated into three groups: C. albicans-colonized group (n = 6), cell wall-administered group (n = 6) and the control group (n = 6). C. albicans-colonized mice were established by single intragastric inoculation of C. albicans as previously described [5]. Cell wall-administered mice were intragastrically administered with cell wall fraction (equivalent quantity of 1 ×107 C. albicans cells) daily. Control and C. albicans-colonized mice were administered with PBS daily. At 5 weeks after starting the administration, mice were killed by cervical dislocation under anesthesia with diethyl ether. Following a laparotomy, the stomach was excised and subjected to the histological examination (toluidine blue staining). The number of mast cells in the forestomach was counted using a high-power field in a section from each specimen as previously described [5]. A cell with stained material dispersed diffusely was taken as evidence of a degranulated mast cell. The animal study was approved by the Hokkaido University Animal Use Committee (approved no. 08-0139), and animals were maintained in accordance with the guidelines of Hokkaido University for the care and use of laboratory animals.
Results are presented as means ± SEM. An unpaired t-test or Tukey-Kramer test following two-way analysis of variance was used to compare mean values. GraphPad Prism for Macintosh (version 5.0, GraphPad Software, Inc., San Diego, CA, USA) was used for the analyses. Differences were considered significant at p < 0.05.
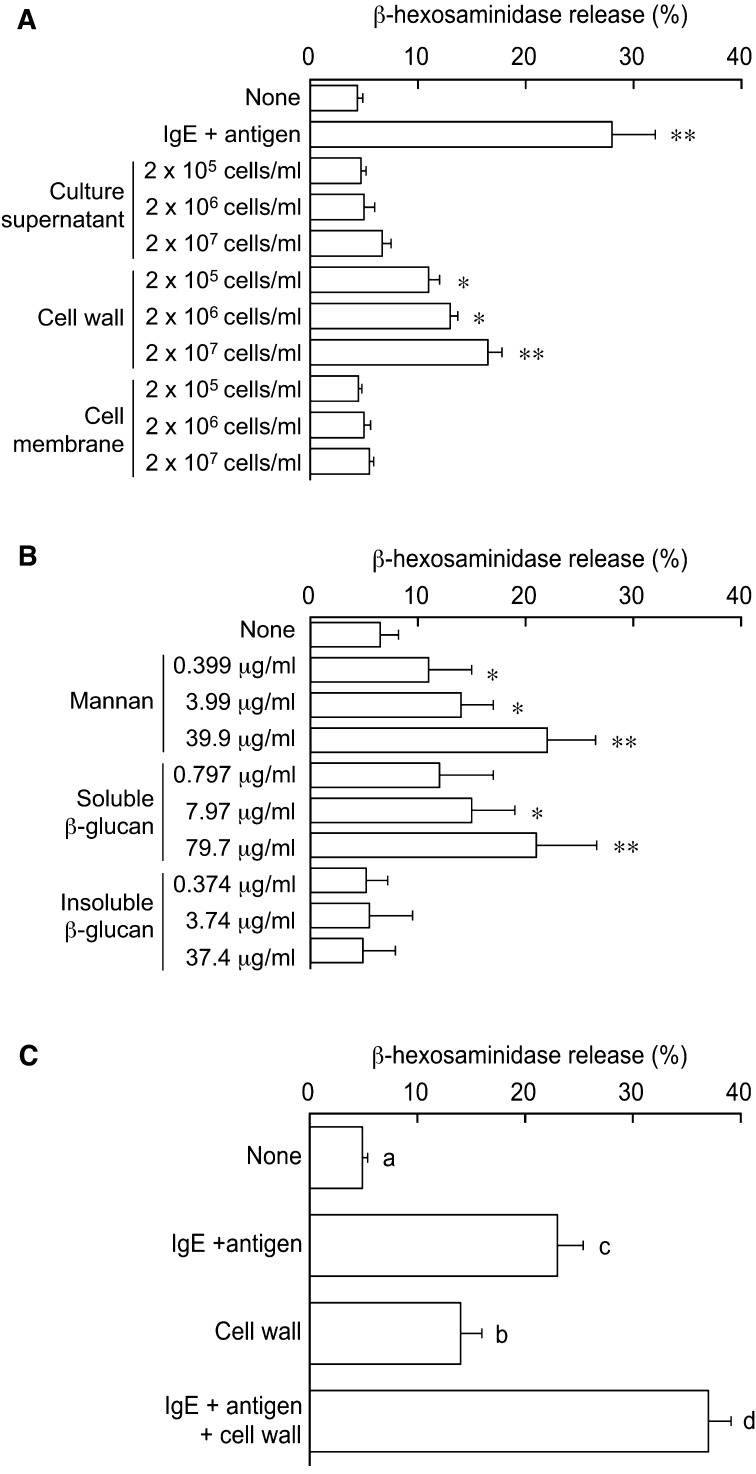
Similar to the treatment with anti-dinitrophenol IgE and its antigen (positive control), the treatment with C. albicans cell wall fraction significantly induced β-hexosaminidase release in RBL-2H3 cells in a dose-dependent manner, whereas culture supernatant and cell membrane fraction had no effects (Fig. 1A), suggesting that cell wall constituents of C. albicans induce mast cell degranulation. C. albicans has a multilayered cell wall composed of an outer layer of proteins glycosylated with mannosyl residues and an inner layer of β-glucans and chitin [11]. In the present study, mannan and soluble β-glucan but not insoluble β-glucan significantly induced β-hexosaminidase release in a dose-dependent manner in RBL-2H3 cells (Fig. 1B). Thus, mannan and soluble β-glucan seem to be responsible for the cell wall-induced mast cell degranulation.
Fig. 1.
Effect of cell constituents of C. albicans on degranulation in RBL-2H3 cells.
RBL-2H3 cells were incubated with C. albicans culture supernatant and subcellular fractions (chart A), cell wall polysaccharide fractions (chart B), and a combination of cell wall fraction and IgE + antigen (chart C), and β-hexosaminidase release was monitored. Cells treated with IgE + antigen were used as a positive control. For antigen activation, after pretreatment with 20 ng/ml mouse anti-dinitrophenol IgE monoclonal antibody (clone SPE-7, Sigma, St. Louis, MO, USA) overnight, cells were incubated with 1 ml of assay buffer supplemented with 100 ng/ml dinitrophenol-conjugated human serum albumin (Sigma). Values are given as means ± SEM from 4 independent assays. * and **, p < 0.05 and 0.01 vs. untreated cells (None). Different letters indicate significant differences (p < 0.05).
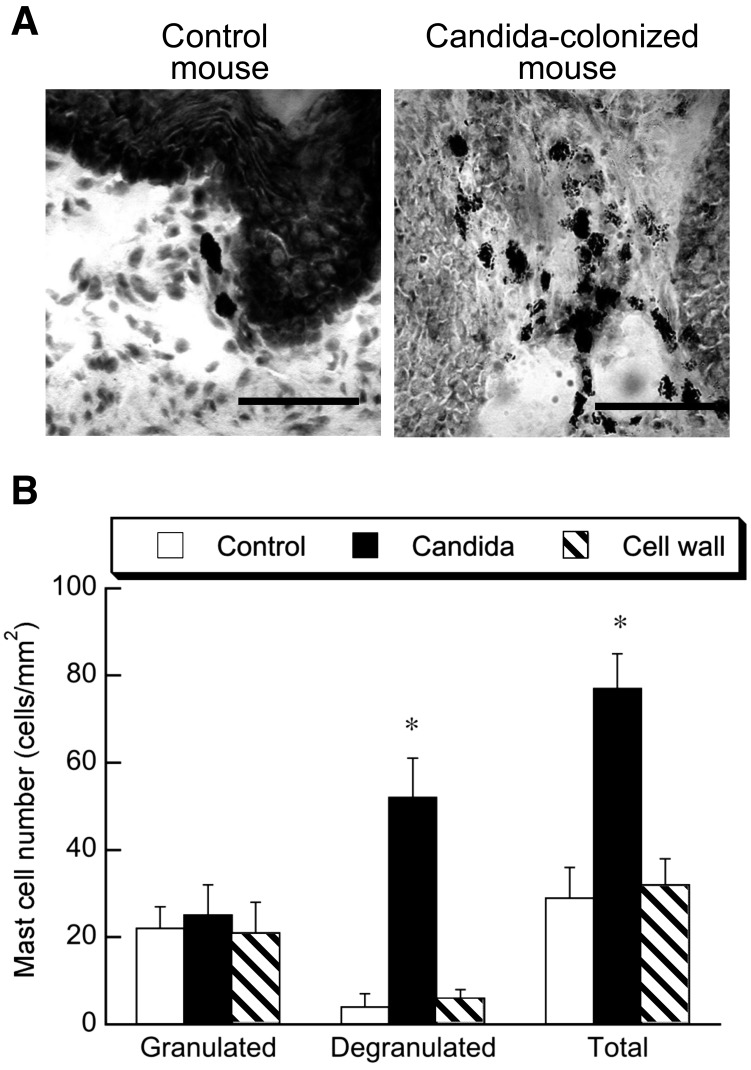
We previously observed that C. albicans gut colonization promoted the infiltration and degranulation of mast cells in the mouse stomach and colon [3, 4]. In the present study, the histochemical examination of gastric mucosa showed infiltration of a number of mast cells in C. albicans-colonized mice and that degranulation was evident in the majority of cells (Fig. 2A). Quantitative examination of mast cells in the histological sections showed that the numbers of granulated cells were the same in the C. albicans-colonized and control mice, whereas the numbers of degranulated and total cells were significantly higher in the C. albicans-colonized mice than in the control mice (Fig. 2B). These results are consistent with our previous observations [3] and suggest that C. albicans colonization stimulates the degranulation of mast cells in the gut. However, daily intragastric administration of cell wall fraction prepared from hyphally grown C. albicans failed to induce any infiltration and degranulation of mast cells (Fig. 2A and 2B). Therefore, the present findings suggest that cell wall mannan and soluble β-glucan released from tissue-colonized C. albicans are absorbed in the gut and subsequently stimulate mast cell degranulation in the gut.
Fig. 2.
Effect of C. albicans gut colonization and cell wall administration on mast cell degranulation in the forestomach of BALB/c mice. Chart A shows a representative section of forestomach tissue stained with toluidine blue. The bars represent 100 μm. Chart B shows the number of granulated and degranulated mast cells and the total number of mast cells in the forestromach. Values are given as means ± SEM of 6 mice per group. *, p < 0.05 vs. control.
β-hexosaminidase release levels were additively increased by concomitant treatment with cell wall fraction of C. albicans and IgE/antigen in RBL-2H3 cells (Fig. 1C), suggesting that the cell wall constituents and IgE/antigen induce mast cell degranulation independently of one another. In our previous study, anti-C. albicans IgG1 antibodies were detected in sera of C. albicans-colonized mice [5]. Considering that non-IgE antibodies such as IgG1 could also induce degranulation via FcγRI receptors [12], it is possible that the Candida antigen-IgG1 antibody complex induces mast cell degranulation via FcγRI receptors in C. albicans-colonized mice. However, further studies are needed to clarify this idea.
Mannan and β-glucan in the cell wall of C. albicans are recognized by toll-like receptor (TLR) 4 and dectin-1/TLR2 complex, respectively [13]. All TLRs except TLR3 require myeloid differentiation primary response protein 88 (MyD88) for signaling function [14]. In the present study, however, RBL-2H3 cells degranulated in response to cell wall constituents of C. albicans, although RBL-2H3 cells reportedly express TLR4 and fail to express MyD88 [15]. Therefore, it remains unclear whether TLR recognition and signaling of cell wall polysaccharides is involved in C. albicans-induced degranulation in RBL-2H3 cells. Further studies are needed to elucidate the molecular mechanisms by which C. albicans induces mast cell degranulation.
REFERENCES
- 1.Calderone RA. 2001. Candida and Candidiasis, ASM Press, Washington DC. [Google Scholar]
- 2.Goldman DL, Huffnagle GB. 2009. Potential contribution of fungal infection and colonization to the development of allergy. Med Mycol 47: 445–456 [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi N, Sugita R, Miki A, Takemura N, Kawabata J, Watanabe J, Sonoyama K. 2006. Gastrointestinal Candida colonisation promotes sensitisation against food antigens by affecting the mucosal barrier in mice. Gut 55: 954–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sonoyama K, Miki A, Sugita R, Goto H, Nakata M, Yamaguchi N. 2011. Gut colonization by Candida albicans aggravates inflammation in the gut and extra-gut tissues in mice. Med Mycol 49: 237–247 [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi N, Sonoyama K, Kikuchi H, Nagura T, Aritsuka T, Kawabata J. 2005. Gastric colonization of Candida albicans differs in mice fed commercial and purified diets. J Nutr 135: 109–115 [DOI] [PubMed] [Google Scholar]
- 6.Mizutani S, Endo M, Inoue T, Kurasawa M, Uno Y, Saito H, Onogi K, Kato I, Takesako K. 2000. CD4(+)-T-Cell-mediated resistance to systemic murine candidiasis induced by a membrane fraction of Candida albicans. Antimicrob Agents Chemother 44: 2653–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kocourek J, Ballou CE. 1969. Method for fingerprinting yeast cell wall mannans. J Bacteriol 100: 1175–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miura NN, Adachi Y, Yadomae T, Tamura H, Tanaka S, Ohno N. 2003. Structure and biological activities of beta-glucans from yeast and mycelial forms of Candida albicans. Microbiol Immunol 47: 173–182 [DOI] [PubMed] [Google Scholar]
- 9.Cassone A, Kerridge D, Gale EF. 1979. Ultrastructural changes in the cell wall of Candida albicans following cessation of growth and their possible relationship to the development of polyene resistance. J Gen Microbiol 110: 339–349 [DOI] [PubMed] [Google Scholar]
- 10.Ortega E, Hazan B, Zor U, Pecht I. 1989. Mast cell stimulation by monoclonal antibodies specific for the Fc epsilon receptor yields distinct responses of arachidonic acid and leukotriene C4 secretion. Eur J Immunol 19: 2251–2256 [DOI] [PubMed] [Google Scholar]
- 11.Chaffin WL. 2008. Candida albicans cell wall proteins. Microbiol Mol Biol Rev 72: 495–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okayama Y, Kirshenbaum AS, Metcalfe DD. 2000. Expression of a functional high-affinity IgG receptor, Fc gamma RI, on human mast cells: Up-regulation by IFN-gamma. J Immunol 164: 4332–4339 [DOI] [PubMed] [Google Scholar]
- 13.Netea MG, Gow NA, Munro CA, Bates S, Collins C, Ferwerda G, Hobson RP, Bertram G, Hughes HB, Jansen T, Jacobs L, Buurman ET, Gijzen K, Williams DL, Torensma R, McKinnon A, MacCallum DM, Odds FC, Van der Meer JW, Brown AJ, Kullberg BJ. 2006. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J Clin Invest 116: 1642–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Neill LA, Bowie AG. 2007. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol 7: 353–364 [DOI] [PubMed] [Google Scholar]
- 15.Passante E, Frankish N. 2010. Deficiencies in elements involved in TLR4-receptor signalling in RBL-2H3 cells. Inflamm Res 59:(Suppl 2): S185–S186 [DOI] [PubMed] [Google Scholar]