Abstract
We previously observed that gut colonization by Candida albicans promoted serum antibody response to orally administered ovalbumin in mice. We therefore postulated that C. albicans affects oral tolerance induction. The present study tested this idea. BALB/c mice were intragastrically administered with either C. albicans (1 × 107) or vehicle, and the colonization was confirmed by weekly fecal cultures. Mice were further divided into two subgroups and intragastrically administered with either ovalbumin (20 mg) or vehicle for five consecutive days. Thereafter, all mice were intraperitoneally immunized with ovalbumin in alum. In mice without C. albicans inoculation, ovalbumin feeding prior to immunization significantly suppressed the increase in ovalbumin-specific IgE, IgG1 and IgG2a in sera, suggesting oral tolerance induction. In C. albicans-inoculated mice, however, the antibody levels were the same between ovalbumin- and vehicle-fed mice. In contrast, ovalbumin feeding significantly suppressed cellular immune responses, as evidenced by reduced proliferation of splenocytes restimulated by ovalbumin ex vivo, in both C. albicans-inoculated and uninoculated mice. Ex vivo supplementation with neither heat-killed C. albicans nor the culture supernatant of C. albicans enhanced the production of ovalbumin-specific IgG1 in splenocytes restimulated by the antigen. These results suggest that gut colonization by C. albicans inhibits the induction of humoral immune tolerance to dietary antigen in mice, whereas C. albicans may not directly promote antibody production. We therefore propose that C. albicans gut colonization could be a risk factor for triggering food allergy in susceptible individuals.
Keywords: Candida albicans, oral tolerance, ovalbumin, mice
Candida albicans is a member of the indigenous microbial flora in the gut of healthy humans [1]. However, it is also a potential pathogen and a frequent cause of complicating systemic infections and mortality in patients undergoing chemotherapy for cancer, immunosuppressive therapy or prolonged antibiotic therapy [2, 3]. In addition, it has been postulated that the excessive colonization by C. albicans in the gut may be responsible for a variety of hypersensitivity diseases including allergy, whereas more research is needed to understand its cause-and-effect relationships [4].
We have developed a model of sustained C. albicans gut colonization by a single intragastric inoculation of C. albicans in healthy adult mice without administration of antibiotics or immunosuppresants [5]. Although these mice appear healthy despite lifelong C. albicans gut colonization under immunocompetent conditions, disseminated infection by C. albicans in visceral organs including the spleen, kidneys, liver, and lungs is induced upon treatment with immunosuppressive agents. Thus, these mice are useful as an animal model mimicking immunocompetent humans with chronic and latent gut colonization by C. albicans. Using this model, we demonstrated that serum antibody responses to repeated oral administration of ovalbumin (OVA) were enhanced by C. albicans gut colonization, suggesting that C. albicans gut colonization is likely to promote sensitization against dietary antigens and increase the risk for food allergy [6].
Exposure to soluble antigens in the gut leads to a systemic unresponsiveness to the same antigens subsequently delivered systemically, a phenomenon named oral tolerance [7, 8]. This mechanism presumably prevents the development of food allergy. Oral tolerance induction can be abrogated under some conditions, and this can be considered equivalent to the promotion of sensitization, which may trigger food allergy in susceptible individuals. During viral or bacterial infections in the gut, intestinal permeability to dietary antigens generally increases, because of alterations in the intestinal epithelium caused by infectious agents and by the inflammatory reaction [9]. In the context of such an inflammatory environment, local antigen-presenting cells (mainly dendritic cells) switch from a tolerogenic to an immunogenic state [10]. Indeed, some reports suggest a positive association between Helicobacter pylori infection and food allergy [11, 12] and atopic dermatitis [13]. Matysiak-Budnik et al. described that H. pylori increases the absorption of antigens across the gastric mucosa [14] and inhibits the development of oral tolerance to dietary antigens in mice [15]. Similar to H. pylori, therefore, gut colonization by C. albicans may disturb oral tolerance induction. The present study aimed to test this possibility.
MATERIALS AND METHODS
Animals
Five-week-old female specific pathogen-free BALB/c mice, which were purchased from Japan SLC (Hamamatsu, Japan), were housed in a temperature-controlled (23 ± 2°C) room with a dark period from 20:00 to 8:00 hr and allowed free access to sterile water and a purified diet prepared according to AIN-93G [16].
This study was approved by the Hokkaido University Animal Use Committee (approval no. 08-0139), and the animals were maintained in accordance with the guidelines for the care and use of laboratory animals of Hokkaido University.
Experimental design
After acclimatizing for two weeks, twenty-four mice were divided into two groups and then administered intragastrically either phosphate-buffered saline (PBS) containing C. albicans or PBS alone as described below. Weekly fecal samples were collected and cultured for C. albicans as described below. At three weeks after inoculation, mice in each group were further divided into two groups (six mice per group) and then administered intragastrically with either PBS containing OVA (20 mg/mouse, 5 × crystallized, Seikagaku Corporation, Tokyo, Japan) or PBS alone for five consecutive days. At one week after completion of intragastric administration, all mice were intraperitoneally immunized with OVA (40 μg) in alum. Thereafter, blood samples were obtained from the tail vein at weekly intervals and subjected to ELISA for measurement of OVA-specific IgG and IgE titers as described below. At three weeks after immunization, mice were anesthetized by diethyl ether and euthanized by exsanguination from the carotid artery. The blood samples were subjected to ELISA for measurement of OVA-specific IgG1 and IgG2a titers as described below. Following laparotomy, the spleen was aseptically excised and then subjected to an ex vivo antibody production assay and cell proliferation assay as described below.
Inoculation and enumeration of C. albicans
C. albicans (JCM 1542) was obtained from the Japan Collection of Microorganisms of the Institute of Physical and Chemical Research (Saitama, Japan) and maintained as previously described [5]. For inoculation, mice were acclimatized to a purified diet for two weeks before being deprived of the diet overnight. Mice were then inoculated intragastrically with 0.2 ml of PBS containing 1 × 107 cells of C. albicans. Uninoculated control mice were administered with 0.2 ml of PBS. Fecal specimens were quantitatively cultured using a standard pour plate technique as previously described [5].
Ex vivo experiments of splenocytes
Single-cell suspensions were prepared from spleens in RPMI 1640 (Gibco-BRL, Tokyo, Japan) supplemented with 2% heat inactivated fetal calf serum (FCS). Red blood cells were lysed with 0.83% ammonium chloride in 10 mM Tris-HCl (pH 7.4). Cell debris and clumps were removed by filtering through nylon mesh (100 μm), and cell suspensions were then made up in RPMI 1640 containing 5% heat inactivated FCS, 100 units/ml penicillin, 100 μg/ml streptomycin, 50 μg/ml gentamicin, and 50 μM 2-mercaptoethanol. Cells were aliquoted at 2 × 105 cells/well in 96-well round-bottom plates along with different concentrations of OVA (0, 100 and 1,000 μg/ml). All cultures were then incubated at 37°C in a humidified 5% CO2 atmosphere for 72 hr and pulsed with 100 μM 5-bromo-2’-deoxyuridine (BrdU) for the last 6 hr of culture. Proliferative responses were assayed using a Cell Proliferation ELISA BrdU kit (Roche, Tokyo, Japan) according to the manufacturer’s recommendations. OVA-specific cell proliferation was presented as a stimulation index calculated with the following equation: stimulation index = (BrdU incorporation in the cells with OVA)/(BrdU incorporation in the cells without OVA).
For the antibody production assay, splenocytes isolated from individual mice were pooled in each group and suspended in RPMI 1640 containing 10% heat inactivated FCS, 100 units/ml penicillin, 100 μg/ml streptomycin, 50 μg/ml gentamicin, 50 μM 2-mercaptoethanol, and 25 mM Hepes. Cells were aliquoted at 5 × 106 cells/well in 24-well plates along with or without OVA (100 μg/ml) and heat-killed C. albicans (105 cells/ml) and the supernatant of C. albicans culture medium prepared as described below. After 3 d of incubation at 37°C in a humidified 5% CO2 atmosphere, cells were washed with PBS and then incubated in the medium without OVA supplementation for further 7 d. The concentration of OVA-specific IgG1 in the culture supernatant was determined by ELISA as described below. The culture was performed in triplicate.
Preparation of C. albicans culture supernatant and heat-killed C. albicans
C. albicans was maintained as described above. Cells grown in the yeast form were suspended in PBS and then incubated at 37°C for 2.5 hr. The culture supernatant was obtained by centrifugation at 3,000 × g for 10 min, and the cell pellet was suspended in PBS and autoclaved at 121°C for 0.5 hr. Because C. albicans shows hyphal growth when it colonizes in the gut mucosa [5], the culture supernatant and heat-killed cells were also prepared from hyphally grown C. albicans. Hyphal growth was induced by culturing the yeast cells (106 cells/ml) of C. albicans in PBS supplemented with 10% FCS at 37°C for 2.5 hr. The culture supernatant and heat-killed cells were obtained as described above.
ELISA for anti-OVA antibodies
OVA-specific antibodies in the culture supernatant of splenocytes and serum were determined by ELISA as previously described [6]. In brief, 96-well microtiter plates were coated with 200 μg/ml OVA, and culture supernatant and serially diluted sera were then added and incubated. For OVA-specific IgG, IgG1 and IgG2a, HRP-conjugated goat anti-mouse IgG, IgG1 and IgG2a (Zymed, San Francisco, CA, USA) diluted 1:2,000, 1:1,000 and 1:1,000, respectively, were added and incubated. Plates were developed after addition of o-phenylenediamine (0.4 mg/ml) and hydrogen peroxide (0.012%). Finally, 1 M H2SO4 was added, and absorbance was measured at 490 nm. For OVA-specific IgE, 96-well microtiter plates were coated with 10 μg/ml anti-mouse IgE monoclonal antibody (LO-ME-2, Zymed), and serially diluted sera were then added and incubated. After the incubation, OVA-DIG conjugate (20 ng/ml) was added and incubated. Coupling of DIG to OVA was performed using a DIG Protein Labeling Kit (Roche) according to the manufacturer’s instructions. HRP-conjugated sheep anti-DIG Fab fragments (Roche) diluted 1:4,000 were then added and incubated. Plates were developed at room temperature after addition of 3,3’,5,5’-tetramethylbenzidine (0.123 mg/ml, Sigma, St. Louis, MO, USA) and hydrogen peroxide (0.012%). Finally, 1 M H2SO4 was added, and the absorbance was measured at 450 nm. Pre-immunized serum was used as a negative control. The average absorbance in the negative control wells, to which three times the standard deviation was added, provided the reference for determination of the titer in the test sera. Antibody titers were expressed as the reciprocal of the last dilution yielding an extinction value higher than the reference value. For OVA-specific IgG1 in the culture supernatant of splenocytes, the absorbance was shown as an indicator of antibody concentrations.
STATISTICS
Results are presented as means ± SEM. The Tukey-Kramer test following one-way or two-way ANOVA was used to compare mean values. GraphPad Prism for Macintosh (version 5.0, GraphPad Software, Inc., La Jolla, CA, USA) was used for the analysis.
Results
To study the effect of chronic gut colonization by C. albicans on oral tolerance induction, BALB/c mice were intragastrically inoculated with C. albicans. By weekly enumeration in fecal specimens after noculation, a high fecal recovery of C. albicans was observed in all mice throughout the experimental period (approx. 1 × 107 colony forming units/g feces). In addition, enumeration of organisms in gut tissues by quantitative culture after euthanasia of animals revealed that the colonization occurred in the stomach, small intestine, and large intestine in all mice (data not shown). No organism was detected in the feces and tissues of uninoculated control mice. All mice showed a good health appearance. These results indicate that chronic asymptomatic colonization by C. albicans was established in the gut of mice after a single inoculation of C. albicans.
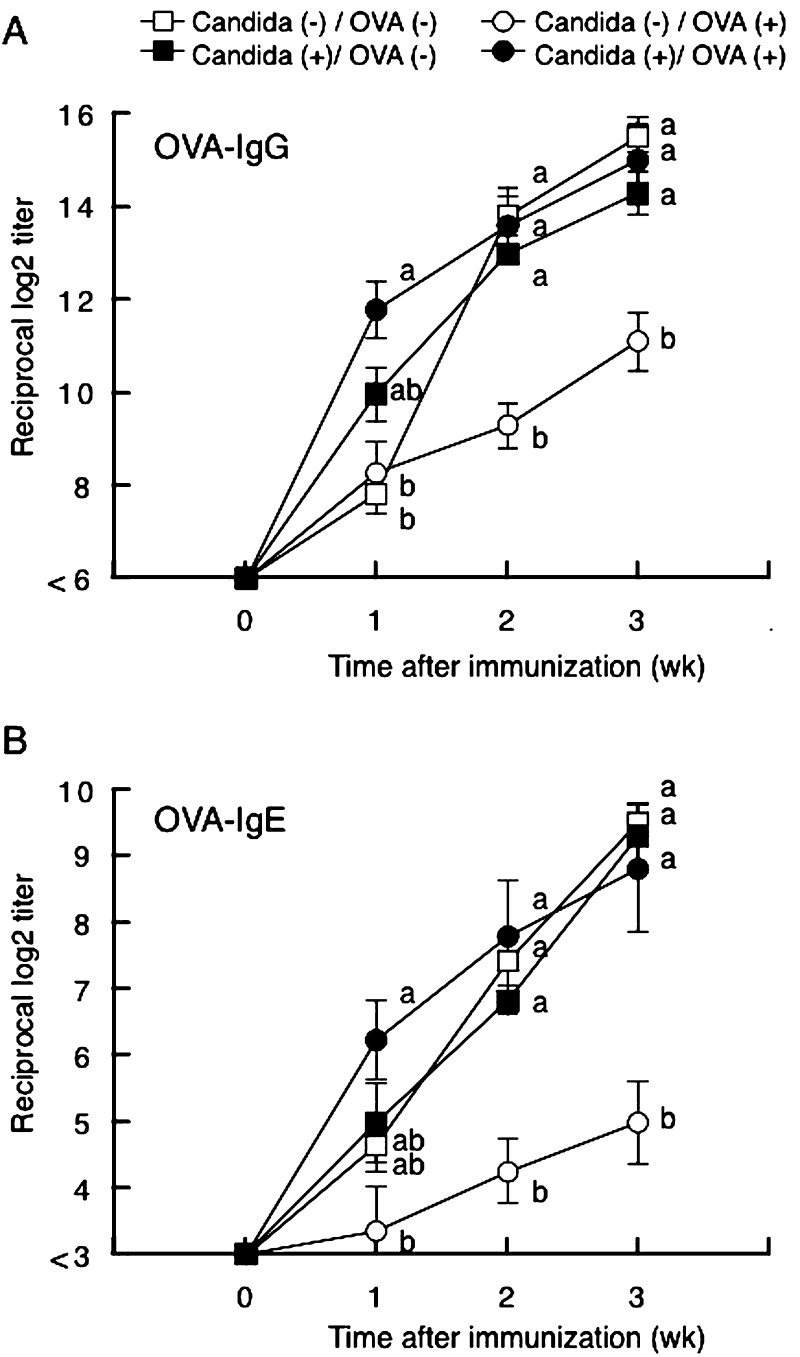
Figure 1 shows the time course of changes in OVA-specific antibody titers in sera after intraperitoneal immunization. OVA-specific IgG and IgE began increasing at one week after eimmunization (Fig. 1A and 1B, respectively). At two and three weeks after immunization, the OVA-specific IgG and IgE were significantly lower in those fed OVA than in those fed PBS. In C. albicans-inoculated mice, however, there were no significant differences in OVA-specific IgG and IgE titers between OVA-fed and PBS-fed mice. In PBS-fed mice, there were no significant differences in OVA-specific IgG and IgE titers between C. albicans-inoculated and uninoculated mice.
Fig. 1.
Time course of changes in OVA-specific IgG (panel A) and IgE (panel B) titers in sera of BALB/c mice with (closed symbols) or without (open symbols) C. albicans gut colonization. Mice fed OVA (closed and open circles) or PBS (closed and open triangles) were intraperitoneally immunized with OVA in alum, and weekly blood samples were then subjected to ELISA. Values are presented as means ± SEM of six mice per group. Values not sharing the same letters at each time point are significantly different at p < 0.05.
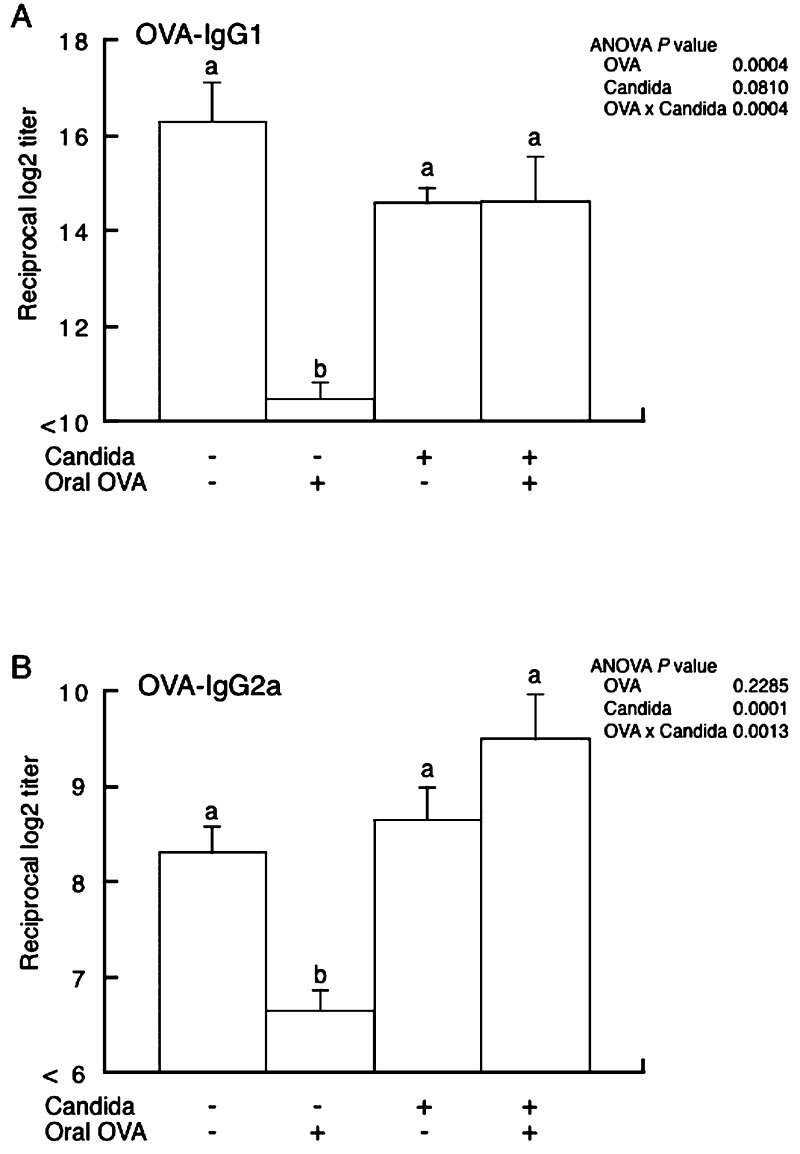
OVA-specific IgG1 and IgG2a (Fig. 2A and 2B, respectively) titers were determined in sera obtained on the last day of the experiment. In uninoculated mice, IgG1 and IgG2a titers were significantly lower in mice fed OVA than in mice fed PBS, whereas there was no significant difference between OVA-fed and PBS-fed mice in the C. albicans-inoculated group. In mice fed PBS, there were no significant differences in OVA-specific IgG1 and IgG2a titers between C. albicans-inoculated and uninoculated mice.
Fig. 2.
OVA-specific IgG1 (panel A) and IgG2a (panel B) titers in sera of BALB/c mice with or without C. albicans gut colonization. Mice fed OVA (OVA (+)) or PBS (OVA (–)) were intraperitoneally immunized with OVA in alum. Blood samples three weeks after immunization were subjected to ELISA. Values are presented as means ± SEM of six mice per group. p values for two-way ANOVA are shown. Values not sharing the same letters are significantly different at p < 0.05 as estimated by a post-hoc Tukey-Kramer test.
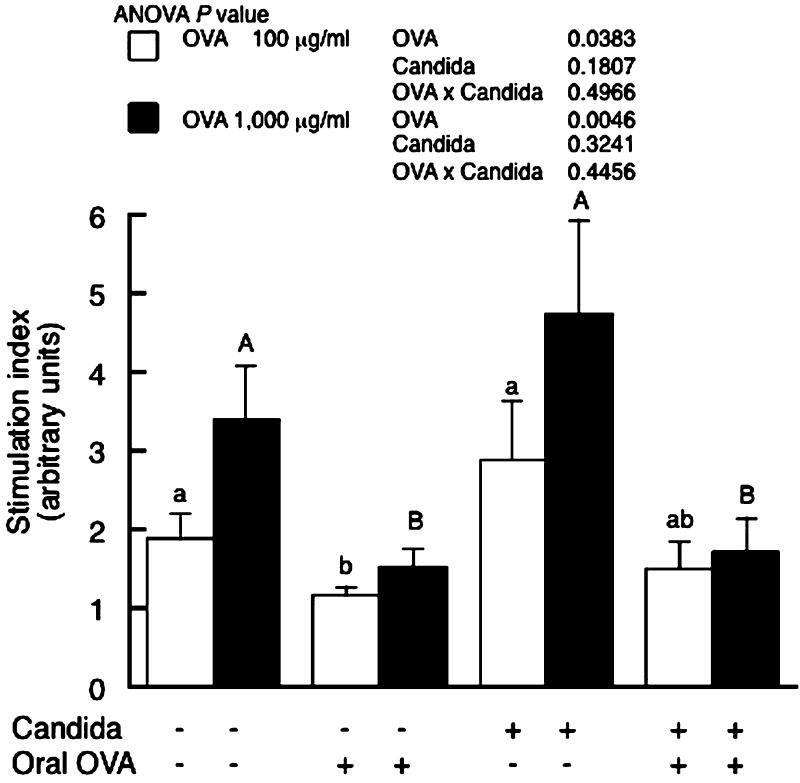
Oral tolerance induction was also examined ex vivo by assessing antigen-specific proliferation of splenocytes. OVA feeding significantly reduced the OVA-specific cell proliferation measured by BrdU incorporation in both C. albicans-inoculated and uninoculated mice (Fig. 3). C. albicans-inoculation had no influence on cell proliferation.
Fig. 3.
OVA-specific splenocyte proliferation in BALB/c mice with or without C. albicans gut colonization. Mice were fed either OVA (OVA (+)) or PBS (OVA (–)) and then intraperitoneally immunized with OVA in alum. The spleen was removed three weeks after immunization, and splenocytes were then cultured with 100 and 1,000 μg/ml OVA (open and closed columns, respectively). Cell proliferation was assessed by BrdU incorporation and presented as a stimulation index as described in the Materials and Methods section. Values are presented as means ± SEM of six mice per group. p values for two-way ANOVA are shown. Values not sharing the same letters are significantly different at p < 0.05 as estimated by a post-hoc Tukey-Kramer test.
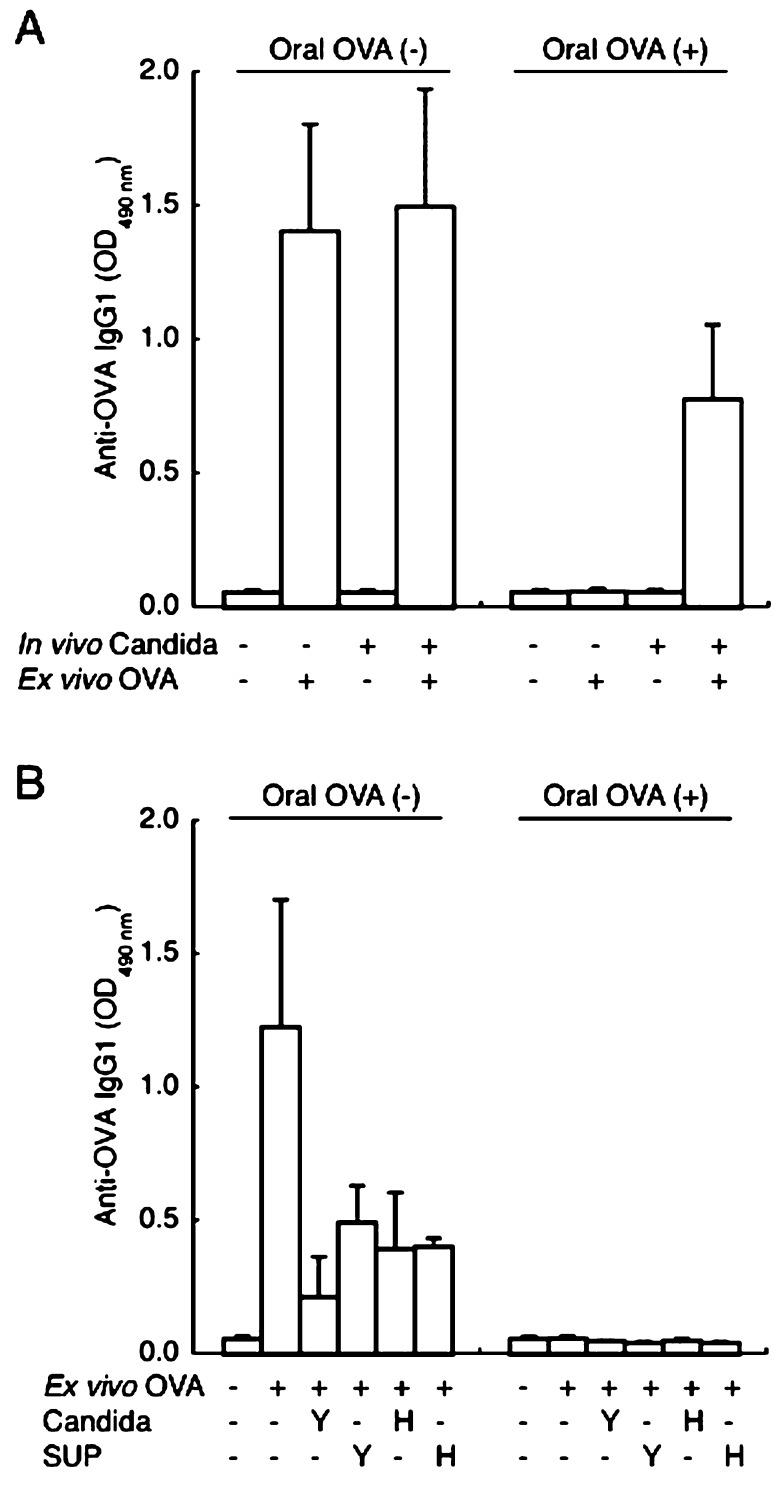
In our preliminary experiments, ex vivo stimulation by OVA induced production of OVA-specific IgG1 but not IgE and IgG2a in splenocytes under our experimental conditions. Therefore, we measured OVA-specific IgG1 in order to test whether ex vivo supplementation with C. albicans promotes antibody production in splenocytes. In the splenocytes isolated from C. albicans-uninoculated mice, ex vivo stimulation by OVA promoted production of OVA-specific IgG1 in PBS-fed mice but not in OVA-fed mice (Fig. 4A). In contrast, OVA-specific IgG1 production was increased by OVA stimulation in the splenocytes isolated from C. albicans-inoculated and OVA-fed mice. However, ex vivo supplementation with neither heat-killed C. albicans nor the culture supernatant of C. albicans grown in the yeast and hyphal form increased the IgG1 production in the splenocytes isolated from uninoculated and OVA-fed mice (Fig. 4B). In fact, ex vivo supplementation with C. albicans tended to reduce the IgG1 production in the splenocytes isolated from PBS-fed mice.
Fig. 4.
OVA-specific IgG1 levels in the culture supernatant of splenocytes isolated from BALB/c mice with or without C. albicans gut colonization. Mice fed OVA (Oral OVA (+)) or PBS (Oral OVA (–)) were intraperitoneally immunized with OVA in alum. The spleen was removed three weeks after immunization, and splenocytes isolated from individual mice were pooled in each group and then subjected to an ex vivo antibody production assay. Panel A shows the effect of ex vivo stimulation by OVA (100 μg/ml), and panel B shows the effect of ex vivo supplementation with heat-killed C. albicans (Candida) and the culture supernatant of C. albicans (SUP) prepared in the yeast (Y) and hyphal (H) forms. Values are presented as means ± SEM of three wells.
DISCUSSION
The present study investigated the development of oral immune tolerance to dietary antigens in a mouse model for chronic and asymptomatic colonization by C. albicans in the gut. Our data clearly showed that C. albicans gut colonization inhibits antigen feeding-induced suppression of serum antibody responses to systemic immunization, suggesting the inhibition of oral tolerance induction by C. albicans gut colonization. We previously reported that C. albicans gut colonization promotes serum IgE and IgG antibody responses to repeated oral administration with OVA in mice [6]. Thus, the inhibition of oral tolerance induction appears to explain the increased responses of serum antibodies to repeated oral antigen exposure in C. albicans-colonized mice.
Gastrointestinal pathogens and their products have been reported to disrupt oral tolerance induction. Cholera toxin has been well known as a mucosal adjuvant that abrogates oral tolerance to an unrelated protein fed simultaneously in mice [17]. In addition, Escherichia coli-heat labile enterotoxin has been reported to disrupt oral tolerance induction in mice [18]. Furthermore, gastric infection with H. pylori reportedly inhibited the development of oral tolerance by preventing anti-OVA IgE suppression in mice [15]. Because oral tolerance to dietary antigens presumably prevents the development of food allergy, these findings suggest that gastrointestinal infections could trigger food allergy in susceptible individuals. Indeed, H. pylori infection has been reported to have a positive association with the development of food allergy [11, 12]. Therefore, the present study suggests that C. albicans colonized in the gut could be a pathogen triggering food allergy through disrupted induction of oral tolerance by preventing dietary-antigen specific antibody suppression.
Cellular and molecular mechanisms for disrupted induction of oral tolerance in C. albicans-colonized mice remain to be elucidated. CD4+ effector T cells can be classified as Th1 and Th2 and, also newly identified Th17 cells, according to their profile of cytokine secretion [19, 20]. Because Th1 and Th2 cytokines are responsible for cellular and humoral immune responses, respectively, one may suspect that disruption of oral tolerance induction by preventing antibody suppression is associated with a Th2-skewed condition in C. albicans-colonized mice. In mice, IgG antibodies are classified as Th1-dependent isotype (IgG2a) and Th2-dependent isotypes (IgG1). In the present study, C. albicans colonization inhibited suppression of both IgG2a and IgG1 antibodies in OVA-fed mice. It is therefore unlikely that the inhibition of antibody suppression by C. albicans colonization is due to a Th2-skewed response, although additional studies are needed to show the response of Th1 and Th2 cytokines (e.g., IFN-γ and IL-4, respectively). Alternatively, C. albicans colonization might affect the induction of regulatory T cells (Treg) because Treg cells are involved in the oral tolerance induction by suppressing both Th1 and Th2 responses [21]. We are now investigating whether C. albicans gut colonization influences the responses of Th1, Th2, Th17 and Treg cells under an experimental setting in which oral tolerance is induced.
In the present study, because antigen feeding-induced suppression of splenocyte proliferation was still observed in C. albicans-colonized mice, it appears that the reduced response of cellular immunity in antigen-fed mice is unaffected by C. albicans colonization. Therefore, one may speculate that C. albicans might stimulate plasma cells to produce antibodies. The present study tested whether C. albicans stimulates antibody production in splenocytes ex vivo. In the splenocytes isolated from C. albicans-uninoculated mice, ex vivo stimulation by OVA promoted production of OVA-specific IgG1 in PBS-fed mice, whereas IgG1 production was not promoted by ex vivo OVA in OVA-fed mice. In contrast, in C. albicans-inoculated mice, ex vivo stimulation by OVA promoted IgG1 production in the splenocytes isolated from both PBS- and OVA-fed mice. These results suggest that antibody production in splenocytes restimulated by an antigen ex vivo is inhibited by antigen feeding prior to immunization, and that C. albicans gut colonization inhibits the antigen feeding-induced suppression of antibody production in splenocytes. Thus, these ex vivo observation appear to reflect the in vivo findings. Under these conditions, supplementation with neither heat-killed C. albicans nor the culture supernatant of C. albicans promoted production of OVA-specific IgG1 in the splenocytes isolated from OVA-fed mice. In addition, in the splenocytes isolated from PBS-fed mice, IgG1 production was rather reduced by supplementation with both heat-killed C. albicans and the culture supernatant of C. albicans. To understand the mechanism for these phenomena, we are now investigating cytokine production in splenocytes ex vivo. Based on the above, it is unlikely that the cell constituents of C. albicans directly promote antibody production in splenocytes restimulated by an antigen and abrogate already established oral tolerance in splenocytes. Although Tokunaka et al. reported that intraperitoneal administration of β-D-glucan, a cell-wall constituent of C. albicans, along with bovine serum albumin (BSA) promoted an increase in serum anti-BSA IgG in mice [22], our previous study showed that the serum concentrations of β-D-glucan in C. albicans-colonized mice did not differ from those of the control mice [23]. In our model of C. albicans-colonized mice, therefore, it is unlikely that β-D-glucan released from C. albicans is absorbed in the gut and then contributes to the inhibition of antigen feeding-induced suppression of serum antibodies.
Similar to the present study, Noverr et al. previously described the effect of C. albicans gut colonization on the induction of immune tolerance in mice [24]. The authors observed that repeated intranasal administration with OVA in antibiotics-administered and C. albicans-colonized BALB/c mice without previous systemic immunization resulted in an allergic airway response characterized by increased eosinophils and Th2 cytokines in the lung and increased serum IgE. Because repeated airway exposure to an antigen usually leads to tolerance rather than sensitization [25,26,27], the findings by Noverr et al. suggest disrupted induction of airway mucosal tolerance in C. albicans-colonized mice. In addition, Noverr et al. reported that C. albicans secretes prostaglandin-like oxylipin molecules having immunomodulatory actions [28, 29]. These molecules might be involved in the inhibition of oral tolerance development by C. albicans gut colonization.
Pattern recognition receptors (PRRs), such as C-type lectin receptors (CLRs) and Toll-like receptors (TLRs), are expressed in the host cells and sense pathogen-associated molecular patterns (PAMPs) including β-glucans, chitin, and mannans in C. albicans [30,31,32,33]. CLRs such as dectin-1 are central to the recognition of PAMPs in C. albicans, and TLR2, TLR4 and TLR9 are also involved in sensing the PAMPs. The recognition of PAMPs by PRRs on phagocytes contributes to the antifungal innate immune response through phagocytosis and direct pathogen killing, while uptake of C. albicans by dendritic cells (DCs) promotes the adaptive immune response, i.e., the differentiation of naïve T cells into effector T cell subtypes. Specifically, inflammatory DCs initiate antifungal Th2 and Th17 cell responses, whereas tolerogenic DCs activate Th1 and Treg cell differentiation [34]. Thus, the immune response eliminates the fungus while preventing excessive inflammation. Indeed, C. albicans and the fungal product zymosan reportedly activated a tolerogenic program in the gut, resulting in the activation of Treg cell-dependent immune tolerance [34,35,36]. These findings are in contrast to those of the present study, but whether PAMPs in C. albicans promote the induction of humoral immune tolerance by dietary antigens remains to be elucidated.
In conclusion, the present results suggest that C. albicans gut colonization inhibits oral tolerance induction by preventing antibody suppression in mice. The disrupted induction of oral tolerance would lead to sensitization to repeated antigen exposure in the gut of C. albicans-colonized mice. We therefore propose that C. albicans gut colonization could be a risk factor for triggering food allergy in susceptible individuals.
Acknowledgments
This work was supported in part by grants from the Food Science Institute Foundation and the Akiyama Life Science Foundation.
REFERENCES
- 1.Calderone RA. 2001. Candida and Candidiasis, ASM Press, Washington DC. [Google Scholar]
- 2.Bodey GP. 1984. Candidiasis in cancer patients. Am J Med 77: 13–19 [PubMed] [Google Scholar]
- 3.Verghese A, Prabhu K, Diamond RD, Sugar A. 1988. Synchronous bacterial and fungal septicemia. A marker for the critically ill surgical patient. Am Surg 54: 276–283 [PubMed] [Google Scholar]
- 4.Goldman DL, Huffnagle GB. 2009. Potential contribution of fungal infection and colonization to the development of allergy. Med Mycol 47: 445–456 [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi N, Sonoyama K, Kikuchi H, Nagura T, Aritsuka T, Kawabata J. 2005. Gastric colonization of Candida albicans differs in mice fed commercial and purified diets. J Nutr 135: 109–115 [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi N, Sugita R, Miki A, Takemura N, Kawabata J, Watanabe J, Sonoyama K. 2006. Gastrointestinal Candida colonisation promotes sensitisation against food antigens by affecting the mucosal barrier in mice. Gut 55: 954–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandtzaeg P. 1997. Mechanisms of gastrointestinal reactions to food. Environ Toxicol Pharmacol 4: 9–24 [DOI] [PubMed] [Google Scholar]
- 8.Strobel S, Mowat AM. 1998. Immune responses to dietary antigens: oral tolerance. Immunol Today 19: 173–181 [DOI] [PubMed] [Google Scholar]
- 9.Heyman M, Desjeux JF. 1996. Antigen handling by intestinal epithelial cells. In Antigen Presentation by Intestinal Epithelial Cells, Kaiserlian D (eds), RG Landes Company, Austin, pp. 1–16. [Google Scholar]
- 10.Williamson E, Westrich GM, Viney JL. 1999. Modulating dendritic cells to optimize mucosal immunization protocols. J Immunol 163: 3668–3675 [PubMed] [Google Scholar]
- 11.Corrado G, Luzzi I, Lucarelli S, Frediani T, Pacchiarotti C, Cavaliere M, Rea P, Cardi E. 1998. Positive association between Helicobacter pylori infection and food allergy in children. Scand J Gastroenterol 33: 1135–1139 [DOI] [PubMed] [Google Scholar]
- 12.Figura N, Perrone A, Gennari C, Orlandini G, Bianciardi L, Giannace R, Vaira D, Vagliasinti M, Rottoli P. 1999. Food allergy and Helicobacter pylori infection. Ital J Gastroenterol Hepatol 31: 186–191 [PubMed] [Google Scholar]
- 13.Murakami K, Fujioka T, Nishizono A, Nagai J, Tokieda M, Kodama R, Kubota T, Nasu M. 1996. Atopic dermatitis successfully treated by eradication of Helicobacter pylori. J Gastroenterol 31: 77–82 [PubMed] [Google Scholar]
- 14.Matysiak-Budnik T, Hashimoto K, Heyman M, de Mascarel A, Desjeux JF, Megraud F. 1999. Antral gastric permeability to antigens in mice is altered by infection with Helicobacter felis. Eur J Gastroenterol Hepatol 11: 1371–1317 [DOI] [PubMed] [Google Scholar]
- 15.Matysiak-Budnik T, van Niel G, Megraud F, Mayo K, Bevilacqua C, Gaboriau-Routhiau V, Moreau MC, Heyman M. 2003. Gastric Helicobacter infection inhibits development of oral tolerance to food antigens in mice. Infect Immun 71: 5219–5224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reeves PG, Nielsen FH, Fahey GC., Jr1993. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123: 1939–1951 [DOI] [PubMed] [Google Scholar]
- 17.Elson CO, Ealding W. 1984. Cholera toxin feeding did not induce oral tolerance in mice and abrogated oral tolerance to an unrelated protein antigen. J Immunol 133: 2892–2897 [PubMed] [Google Scholar]
- 18.Clements JD, Hartzog NM, Lyon FL. 1988. Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine 6: 269–277 [DOI] [PubMed] [Google Scholar]
- 19.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. 1986. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 136: 2348–2357 [PubMed] [Google Scholar]
- 20.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. 2006. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity 24: 677–688 [DOI] [PubMed] [Google Scholar]
- 21.Weiner HL, da Cunha AP, Quintana F, Wu H. 2011. Oral tolerance. Immunol Rev 241: 241–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tokunaka K, Ohno N, Adachi Y, Tanaka S, Tamura H, Yadomae T. 2000. Immunopharmacological and immunotoxicological activities of a water-soluble (1→3)-beta-D-glucan, CSBG from Candida spp. Int J Immunopharmacol 22: 383–394 [DOI] [PubMed] [Google Scholar]
- 23.Sonoyama K, Miki A, Sugita R, Goto H, Nakata M, Yamaguchi N. 2011. Gut colonization by Candida albicans aggravates inflammation in the gut and extra-gut tissues in mice. Med Mycol 49: 237–247 [DOI] [PubMed] [Google Scholar]
- 24.Noverr MC, Falkowski NR, McDonald RA, McKenzie AN, Huffnagle GB. 2005. Development of allergic airway disease in mice following antibiotic therapy and fungal microbiota increase: role of host genetics, antigen, and interleukin-13. Infect Immun 73: 30–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrew DK, Schellenberg RR, Hogg JC, Hanna CJ, Pare PD. 1984. Physiological and immunological effects of chronic antigen exposure in immunized guinea pigs. Int Arch Allergy Appl Immunol 75: 208–213 [DOI] [PubMed] [Google Scholar]
- 26.Holt PG, McMenamin C. 1989. Defence against allergic sensitization in the healthy lung: the role of inhalation tolerance. Clin Exp Allergy 19: 255–262 [DOI] [PubMed] [Google Scholar]
- 27.Tsitoura DC, Blumenthal RL, Berry G, Dekruyff RH, Umetsu DT. 2000. Mechanisms preventing allergen-induced airway hyperreactivity: role of tolerance and immune deviation. J Allergy Clin Immunol 106: 239–246 [DOI] [PubMed] [Google Scholar]
- 28.Noverr MC, Phare SM, Toews GB, Coffey MJ, Huffnagle GB. 2001. Pathogenic yeasts Cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect Immun 69: 2957–2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noverr MC, Williams DD, Toews GB, Huffnagle GB. 2002. Production of prostaglandins and leukotrienes by pathogenic fungi. Infect Immun 70: 400–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bourgeois C, Majer O, Frohner IE, Tierney L, Kuchler K. 2010. Fungal attacks on mammalian hosts: pathogen elimination requires sensing and tasting. Curr Opin Microbiol 13: 401–408 [DOI] [PubMed] [Google Scholar]
- 31.Jouault T, Sarazin A, Martinez-Esparza M, Fradin C, Sendid B, Poulain D. 2009. Host responses to a versatile commensal: PAMPs and PRRs interplay leading to tolerance or infection by Candida albicans. Cell Microbiol 11: 1007–1015 [DOI] [PubMed] [Google Scholar]
- 32.Romani L. 2011. Immunity to fungal infections. Nat Rev Immunol 11: 275–288 [DOI] [PubMed] [Google Scholar]
- 33.van de Veerdonk FL, Kullberg BJ, van der Meer JW, Gow NA, Netea MG. 2008. Host-microbe interactions: innate pattern recognition of fungal pathogens. Curr Opin Microbiol 11: 305–312 [DOI] [PubMed] [Google Scholar]
- 34.Bonifazi P, Zelante T, D’Angelo C, De Luca A, Moretti S, Bozza S, Perruccio K, Iannitti RG, Giovannini G, Volpi C, Fallarino F, Puccetti P, Romani L. 2009. Balancing inflammation and tolerance in vivo through dendritic cells by the commensal Candida albicans. Mucosal Immunol 2: 362–374 [DOI] [PubMed] [Google Scholar]
- 35.De Luca A, Montagnoli C, Zelante T, Bonifazi P, Bozza S, Moretti S, D’Angelo C, Vacca C, Boon L, Bistoni F, Puccetti P, Fallarino F, Romani L. 2007. Functional yet balanced reactivity to Candida albicans requires TRIF, MyD88, and IDO-dependent inhibition of Rorc. J Immunol 179: 5999–6008 [DOI] [PubMed] [Google Scholar]
- 36.Dillon S, Agrawal S, Banerjee K, Letterio J, Denning TL, Oswald-Richter K, Kasprowicz DJ, Kellar K, Pare J, van Dyke T, Ziegler S, Unutmaz D, Pulendran B. 2006. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J Clin Invest 116: 916–928 [DOI] [PMC free article] [PubMed] [Google Scholar]