Abstract
Poly-trans-[(2-carboxyethyl) germasesquioxane] (Ge-132) is the most common organic germanium compound. The ingestion of Ge-132 promotes bile secretion. We assessed the rat caecal characteristics after the administration of Ge-132 and raffinose, a prebiotic oligosaccharide, because both Ge-132 and some prebiotics can change the fecal color to yellow. We also compared the changes in the caecal flora caused by the two compounds. In addition, we evaluated the simultaneous administration of Ge-132 and raffinose and their effects on β-glucuronidase activity, which is known to be a factor related to colon cancer. Male Wistar rats (three weeks old) were given one of the following diets: 1) a control diet (control group), 2) a diet containing 0.05% Ge-132 (Ge-132 group), 3) a diet containing 5% raffinose (RAF group) or 4) a diet containing 0.05% Ge-132 + 5% raffinose (GeRAF group). The Bifidobacterium, Lactobacillus and total bacteria counts were significantly increased by the dietary raffinose, and Ge-132 did not suppress this increase. The raffinose intake increased caecal acetic acid production significantly. The activity of β-glucuronidase in the caecal contents was increased by dietary Ge-132, whereas dietary raffinose decreased the β-glucuronidase activity significantly. These results indicate that the simultaneous intake of dietary raffinose and Ge-132 does not inhibit the effects of either compound on intestinal fermentation and bile secretion. Additionally, the simultaneous intake of both raffinose and Ge-132 could abrogate the increase in β-glucuronidase activity induced by Ge-132 alone.
Keywords: Ge-132, organic germanium, raffinose, caecal flora, Bifidobacterium, β-glucuronidase
INTRODUCTION
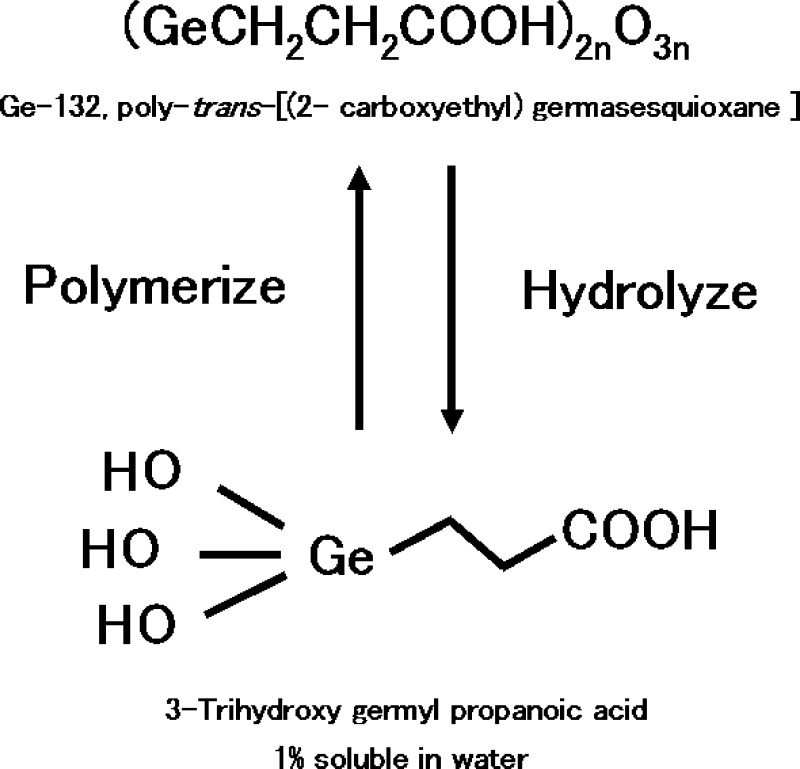
Poly-trans-[(2-carboxyethyl) germasesquioxane] (Ge-132) is the most common organic germanium compound. It was first synthesized by Oikawa et al., and the structure has been previously reported [1]. Ge-132 is water soluble, and its safety has been confirmed [2]. The structures of Ge-132 and trihydroxy germyl propanoic acid, a product of the hydrolysis of Ge-132, are shown in Figure 1. Ge-132 has many therapeutic effects including anticarcinogenic and antihypertensive effects, the alleviation of rheumatism, and the prevention of hepatic disease [3,4,5]. Ge-132 also induces γ-interferon secretion and activates both natural killer cells and macrophages [6]. Therefore, the activation of the immune system by dietary Ge-132 can contribute to disease protection by modulating immune functions or the response to viral infections. In contrast to the benefits of Ge-132, the toxicity of germanium dioxide (GeO2), which is an inorganic germanium compound, has been described. Significant oral intake of GeO2 results in renal insufficiency due to accumulation in the kidney [7], which often causes fatal damage. We have verified that dietary Ge-132 does not accumulate or become distributed in the body [8]. Currently, germanium lactate-citrate is sold as an organic germanium formulation, but this formulation does not contain a C-Ge bond (and thus is actually inorganic germanium); therefore, it is hydrolyzed in the human body. This hydrolysis has caused fatalities after ingestion of germanium lactate-citrate [9, 10] in Europe. Because of the lack of the C-Ge bond, consumers must be careful to choose a reliable manufacturer.
Fig. 1.
The structures of Ge-132 and its hydrolysis to 3-trihydroxy germyl propanoic acid.
Recently, Ge-132 has been used as an ingredient in health foods and supplements in Japan, Korea and the U.S.A. We observed two typical changes in the characteristics of feces after administration of Ge-132 in humans: the fecal color changed from brown to yellow, and the feces sometimes softened. The same results were observed when oligosaccharides such as raffinose (RAF), which is a bifidus factor that acts as a prebiotic, are administrated. These changes induce by nondigestible oligosaccharide intake are caused by modification of the microflora composition in the intestine, with the microflora becoming dominated by Bifidobacterium [11]. Furthermore, the isomerization of saccharides increases after the addition of the germanium compound [12]. Thus, the isomerization of saccharides into a prebiotic form in the presence of Ge-132 in the intestine was expected. It is known that starch is hydrolyzed to oligo or disaccharide like maltotriose, maltose and α-limit dextrins by α-amylase in the intestine and that maltotriose is then hydrolyzed to glucose by maltase. Glucose is transportable through the cytoplasm of the intestinal epithelium into the interstitial space by SGLT1 and GLUT5 [13]. We hypothesized that evaluation of the effects of the prebiotic RAF and Ge-132 on the caecal flora could reveal the ability of Ge-132 to promote the isomerization of some sugars, for example, hydrolysed oligo saccharides of starch, into a prebiotic sugar like fructooligosaccharide. Additionally, our collaborator manufactures dietary supplements that contain a combination of Ge-132 and RAF. Thus, we were also interested in the effect of simultaneous ingestion of Ge-132 and RAF on nutritional function.
In this study, we compared the changes in caecal characteristics after modification of the microflora composition in rats given RAF, Ge-132 or RAF and Ge-132 and evaluated prebiotic activity. Simultaneous ingestion of Ge-132 and RAF caused a decrease in the β-glucuronidase activity which occurred due to enhancement of bilirubin glucuronide by Ge-132 intake [12].
MATERIALS AND METHODS
Chemicals
Ge-132 (Lot 006316A) was synthesized by the Asai Germanium Research Institute Co., Ltd. (Hokkaido, Japan), and its purity was over 99%. Raffinose (RAF) was purified to commercial grade from beet sugar by Nippon Beet Sugar Mfg. Co., Ltd. (Hokkaido, Japan). Solvents and chemicals were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Agar media and its components were purchased from Difco of Becton, Dickinson and Company (Franklin Lakes, NJ, U.S.A.), Nissui Pharmaceuticals Co., Ltd. (Tokyo, Japan) and Kyokuto Pharmaceutical Industrial Co., Ltd. (Tokyo, Japan). P-nitrophenyl-β-D-glucuronide was purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.).
Animals and diets
Male Wistar rats (three weeks old, Japan SLC, Shizuoka, Japan) were housed in a room with controlled temperature (22-25°C) and light (08:00 to 20:00) conditions. After the rats were acclimated for 1 week, they were randomly divided into four groups (5 rats each) for two weeks and maintained on one of the following diets: a control diet (Control group), a 0.05% Ge-132 diet (Ge-132 group), a 5% RAF diet (RAF group) or a 0.05% Ge-132 + 5% RAF diet (GeRAF group). The number of rats per group was based on that used in a previous study at our laboratory (not published), which showed significant differences in the prebiotic effect of RAF administration. The rats could eat and drink water freely throughout the experiment, which lasted 2 weeks. The composition of each diet is shown in Table 1. The diets were prepared based on the AIN-76 composition. The dosage of Ge-132 was determined based on the daily intake of Ge-132 (500 mg/adult) that causes changes in the fecal color of adult humans, while the dosage of RAF was determined based on the amount needed to beneficially enhance Bifidobacterium levels. These components took the place of cornstarch as the primary ingredient or carbohydrate. On the final day, the animals were anaesthetized using pentobarbital and euthanized by bleeding from the abdominal aorta. The major organs and the caecum were then excised and used for immediate analysis.
Table 1. Composition of the Diets for Ge-132 and RAF Administration.
| Control | Ge-132 | RAF | GeRAF | |
| Casein | 23 | 23 | 23 | 23 |
| Corn starch | 61.5 | 61.45 | 56.5 | 56.45 |
| Corn oil | 5 | 5 | 5 | 5 |
| DL-methionine | 0.3 | 0.3 | 0.3 | 0.3 |
| Vitamin mixture (AIN76) | 1 | 1 | 1 | 1 |
| Mineral mixture (AIN76) | 4 | 4 | 4 | 4 |
| Cellulose | 5 | 5 | 5 | 5 |
| Choline chloride | 0.2 | 0.2 | 0.2 | 0.2 |
| Ge-132 | - | 0.05 | - | 0.05 |
| Raffinose | - | - | 5 | 5 |
| Total | 100 | 100 | 100 | 100 |
(%)
Ethics
This animal experiment was conducted at the Asai Germanium Research Institute Co., Ltd., according to the guidelines provided by the ethics committee for experimental animal care, which are based on the public guidelines set by the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Caecal microflora analysis
Fresh caecal contents (0.5 g) were collected and transferred to 4.5 g of prereduced phosphate-buffered saline containing 0.1% (w/v) purified agar and 0.05% (w/v) cysteine-HCl. Samples were suspended in dilution buffer, and 10-fold serial dilutions from 10-1 to 10-8 were prepared. From the appropriate dilutions, 0.05 ml aliquots were spread onto agar plates. The methods for bacterial analysis of the caecal microflora content have been previously reported [14, 15]. The following 3 nonselective and 6 selective agar media were used to isolate the caecal microflora: modified Eggerth-Gagnon (EG) agar or glucose-liver-blood (BL) agar with 5% horse blood for facultative anaerobes, Trypticase soy (TS) agar for total aerobes, Bifidobacterium-selective (BS) agar for Bifidobacterium, Eubacterium-selective (ES) agar for eubacteria, modified Veillonella-selective (VS) agar for Veillonella, modified Lactobacillus-selective (LBS) agar for Lactobacillus, deoxycholate-hydrogen sulphide-lactose (DHL) agar for enteric bacteria and triphenyltetrazolium chloride-acridine orange-thallous sulphate-esculin-crystal violet (TATAC) agar for Enterococcus and Streptococcus. The aerobes were cultured for 24 hr at 37°C, and the anaerobes were cultured for 72 hr at 37°C in anaerobic jars using the steel wool method [16]. The reduced steel wool was placed in a jar with the culture plates, and the atmosphere was replaced with CO2. Each colony was separated from the culture and spread onto a glass slide for microscopic examination. The slides were stained with Gram stain solution. The log numbers of bacteria in the caecal contents were calculated.
Analysis of organic acids in caecal contents
The caecal contents (0.2 g) were weighed and mixed with 1 ml of Sorensen’s phosphate buffer (pH 7.4), and 0.1 ml of 0.1 N HCl was then added to the mixture. Pyroglutamic acid was used as the internal standard. Prepared samples were analyzed by HPLC with UV detection after filtration. The analytical column was a two-jointed Shodex Ionpak KC-811 column (4.6 × 150 mm, 5 μm, Showa Denko K.K., Tokyo, Japan). The mobile phase was 2 mM perchloric acid, and the flow rate was 1 ml/min. The post-column method was used with bromothymol blue as the pH indicator at a flow rate of 0.5 ml/min [11]. The eluted sample was analyzed at 445 nm.
Assay of β-glucuronidase activity in caecal contents
The activity of β-glucuronidase was measured as previously reported by Ishikawa et al. [17]. An eighty-fold dilution of the caecal contents was used as the enzyme solution. The substrate solution (180 μl), which consisted of 0.1 M phosphate buffer (pH 7.4) containing 4 mM p-nitrophenyl-β-D-glucuronide (Sigma- Aldrich, St. Louis, MO, U.S.A.) and 0.133 mM Na-EDTA, was added to the enzyme solution (60 μl). The reaction mixture was incubated for 20 min at 37°C, and the reaction was stopped by the addition of 240 μl Na2CO3. The mixture was centrifuged, and the amount of p-nitrophenol in the supernatant was quantitated by measuring the absorption at 415 nm.
Data analysis
All values are presented as the means and standard deviations of 5 rats. Statistical comparisons among the groups were performed using analysis of variance (ANOVA). Data were processed with two-way factorial ANOVA. Statistical significance was defined as p<0.05. Data for bacterial numbers with a detection rate of 100% were processed with ANOVA. The Statcel-The Useful Addin Forms on Excel software, 2nd edition (published by OMS Ltd., Saitama, Japan), was used for all statistical analyses.
RESULTS
Effects on the fecal characteristics and the caecal flora composition
The weights of the major organs are shown in Table 2. Dietary Ge-132 had no effect on the tissue weights, whereas dietary RAF increased the weights of both the caecal contents (p= 5.7 × 10–9) and the caecal wall (p= 5.7 ×10–6). The caecum was expanded by dietary RAF. The components of the caecal microflora from rats that received the four different diets are shown in Table 3. The RAF-containing diet resulted in a significantly higher (p= 2.6 × 10–6) total bacterial number in the rats. The total bacterial number was greater in the GeRAF rats (10.2 log10 bacteria/g of caecal content) and RAF (10.3) rats than in the control (9.8) rats, whereas dietary Ge-132 did not change the number of bacteria (9.6). When the rats ingested dietary RAF, the frequency of detection of Bifidobacterium changed from 20% to 100%. The detection frequencies were calculated based on the detection of colonies forming on cultured agar plates; a lack of colony formation from a 10-6 diluted solution was treated as not detected (N.D.). Dietary RAF increased the number of Bifidobacterium cells relative to the control (control=9.0 vs. RAF=10.1 and GeRAF=10.1); in contrast, the numbers of other species of microorganisms, especially Bacteroidaceae (p=0.026) and Enterobacteriaceae (p=0.001), decreased. The number of total aerobes was significantly decreased by the RAF (p=0.027). Some anaerobes, including Bifidobacterium, Lactobacillus and Veillonella, increased in number with dietary RAF treatment, and the detection frequencies of Clostridium changed from 20% to 100% in rats that ingested RAF. Lactobacillus was increased by the RAF-containing diet (p= 0.018).
Table 2. Effects of Ge-132 and RAF Administration on Rat Organ Weight.
| Control | Ge-132 | RAF | GeRAF | P value | |||
| (g) | GE | RAF | GExRAF | ||||
| Liver | 7.7 ± 1.39 | 8.1 ± 0.62 | 8.7 ± 1.48 | 8.1 ± 0.89 | 0.789 | 0.328 | 0.346 |
| Kidney | 1.5 ± 0.19 | 1.5 ± 0.07 | 1.5 ± 0.15 | 1.5 ± 0.18 | 0.572 | 1.000 | 0.572 |
| Caecal content | 2.2 ± 0.23 | 2.0 ± 0.14 | 5.4 ± 0.70 | 5.6 ± 1.16 | 0.977 | 5.7E-09 | 0.523 |
| Caecal wall | 0.4 ± 0.10 | 0.3 ± 0.07 | 0.6 ± 0.05 | 0.7 ± 0.11 | 0.793 | 5.7E-06 | 0.057 |
Values are means ± SD.
P values were obtained from two-way factorial ANOVA (p<0.05).
GE, RAF and GexRAF show the main effect of Ge-132, main effect of RAF and interaction of Ge-132 and RAF, respectively.
Table 3. Effects of Ge-132 and RAF administration on rat caecal flora.
| Control | Ge-132 | RAF | GeRAF | P value | ||||
| (log10 no./ g caecal contents) | GE | RAF | GExRAF | |||||
| Total bacteria | 9.8 ± 0.2 | 9.6 ± 0.2 | 10.3 ± 0.0 | 10.2 ± 0.1 | 0.090 | 2.6E-06 | 0.646 | |
| Bifidobacterium | 9.0 (20) | N.D. (0) | 10.1 ± 0.3 (100) | 10.1 ± 0.1 (100) | - | - | - | |
| Bacteroidaceae | 9.7 ± 0.2 (100) | 9.6 ±0 .2 (100) | 9.5 ± 0.3 (100) | 9.4 ± 0.1 (100) | 0.243 | 0.026 | 0.758 | |
| Eubacterium | 7.1 ± 0.3 (60) | 8.0 ± 0.6 (80) | 8.3 ± 0.0 (60) | 7.9 ± 1.3 (80) | - | - | - | |
| Peptococaceae | 8.7 ± 0.8 (80) | 7.7 ± 0.3 (60) | 9.0 ± 0.8 (100) | 8.3 ± 0.6 (100) | - | - | - | |
| Clostridium | 8.3 (20) | 7.0 (40) | 7.9 ± 0.4 (100) | 8.2 ± 0.5 (60) | - | - | - | |
| Veillonella | N.D. (0) | N.D. (0) | 8.9 ± 0.2 (60) | 9.0 ± 0.3 (60) | - | - | - | |
| Lactobacillus | 6.7 ± 0.7 (100) | 5.9 ± 0.5 (100) | 7.2 ± 0.8 (100) | 7.0 ± 0.7 (100) | 0.109 | 0.018 | 0.470 | |
| Total aerobes | 7.0 ± 0.5 (100) | 7.2 ± 0.3 (100) | 6.6 ± 0.4 (100) | 6.7 ± 0.3 (100) | 0.444 | 0.027 | 0.881 | |
| Enterobacteriaceae | 6.8 ± 0.6 (100) | 6.7 ± 0.5 (100) | 5.9 ± 0.4 (100) | 5.7 ± 0.6 (100) | 0.576 | 0.001 | 0.948 | |
| Streptococcus | 6.6 ± 0.4 (100) | 6.9 ± 0.5 (100) | 6.5 ± 0.5 (100) | 6.7 ± 0.2 (100) | 0.193 | 0.437 | 0.740 | |
Values are means ± SD. The detection rates of colonies on five culture dishes are shown in parentheses. The species that formed no colonies on cultured plates are shown as not detected (N.D.). Each species with no N.D. data was subjected to statistical analysis by two-way factorial ANOVA (p< 0.05). GE, RAF and GexRAF show the main effect of Ge-132, main effect of RAF and interaction of Ge-132 and RAF, respectively.
Apparent change in the caecum induced by the intake of RAF and Ge-132
Images of the rat caeca after administration of each diet for 2 weeks are shown in Fig. 2. The caecal sizes of the rats in the RAF-containing diet groups (RAF and GeRAF) were larger than the caecal sizes of the rats in the control and Ge-132 groups. In contrast, rats that were fed the diet containing Ge-132 exhibited no change in the size of the caecum. In addition, the caeca of the rats treated with Ge-132 (Ge-132 and GeRAF) turned yellow. Dietary RAF alone had a slight influence on the color of the caecum, making the color slightly yellowish.
Fig. 2.
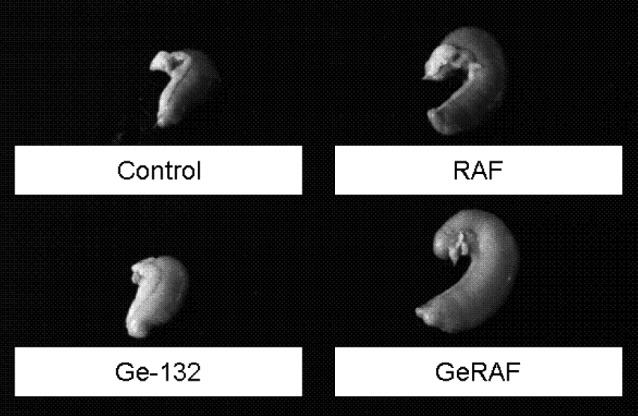
The rat caeca after dietary Ge-132 and raffinose intake. The samples were obtained from the rats after two weeks on each diet. The groups are follows: control AIN-76; Ge-132, 0.05% Ge-132 diet; RAF, 5% raffinose diet; and GeRAF, both 0.05% Ge-132 and 5% raffinose diet.
Caecal pH and organic acids in the caecal contents
The caecal pH and organic acids in the caecal contents are shown in Table 4. Dietary Ge-132 did not change the pH; however, dietary RAF significantly (p= 2.8 × 10-5) reduced the pH relative to the control: control pH = 7.5, RAF pH=6.2 and GeRAF pH=6.1. The intake of Ge-132 decreased lactic acid from 0.430 to 0.192 (p=0.036). However, there was no effect on the level of any other organic acid or on the pH in rats fed a diet with Ge-132. In contrast, the caeca of rats fed a diet containing RAF showed an increase (p= 0.040) in the concentration of acetic acid (1.9 to 2.8 μg/g caecal content) relative to the caeca of rats fed other diets, and the levels of formic acid were increased from 0.003 to 0.062 μg/g caecal content (p= 0.035) by the RAF. On the other hand, n-butyric acid, i-valeric acid and n-valeric acid were decreased from 0.513 to 0.330 μg/g caecal content (p=0.001), from 0.154 to 0.081 μg/g caecal content (p=0.007) and from 0.193 to 0.032 μg/g caecal content (p=1.6 × 10–6) by the RAF, respectively.
Table 4. Effects of Ge-132 and RAF Administration on Caecal pH and Organic Acids in Rats.
| Control | Ge-132 | RAF | GeRAF | P value |
|||
| GE | RAF | GExRAF | |||||
| pH | 7.5 ± 0.2 | 7.2 ± 0.3 | 6.2 ± 0.6 | 6.1 ± 0.6 | 0.331 | 2.8E-05 | 0.742 |
| Organic Acids (microgram/g caecal content) | |||||||
| Succinic Acid | 0.412 ± 0.273 | 0.319 ± 0.100 | 0.370 ± 0.227 | 0.563 ± 0.539 | 0.789 | 0.475 | 0.365 |
| Lactic Acid | 0.430 ± 0.080 | 0.192 ± 0.338 | 1.059 ± 0.467 | 0.392 ± 0.556 | 0.036 | 0.063 | 0.289 |
| Formic Acid | 0.003 ± 0.007 | n. d. | 0.062 ± 0.060 | 0.045 ± 0.081 | 0.673 | 0.035 | 0.771 |
| Acetic Acid | 1.884 ± 0.449 | 1.898 ± 0.657 | 2.751 ± 0.938 | 2.940 ± 1.460 | 0.815 | 0.040 | 0.841 |
| Propanoic Acid | 0.943 ± 0.177 | 0.803 ± 0.206 | 1.038 ± 0.369 | 1.051 ± 0.320 | 0.617 | 0.189 | 0.549 |
| n-Butylic Acid | 0.513 ± 0.101 | 0.534 ± 0.160 | 0.330 ± 0.128 | 0.277 ± 0.104 | 0.775 | 0.001 | 0.515 |
| i-Valeric Acid | 0.154 ± 0.016 | 0.131 ± 0.043 | 0.081 ± 0.053 | 0.090 ± 0.046 | 0.714 | 0.007 | 0.410 |
| n-Valeric Acid | 0.193 ± 0.035 | 0.155 ± 0.032 | 0.032 ± 0.034 | 0.056 ± 0.053 | 0.704 | 1.6E-06 | 0.101 |
Values are means ± SD. P values were obtained from two-way factorial ANOVA (p<0.05). GE, RAF and GexRAF show the main effect of Ge-132, main effect of RAF and interaction of Ge-132 and RAF, respectively.
β-Glucuronidase activities of the caecal contents
The β-glucuronidase activities in the caecal contents of the rats that were fed the four diets are shown in Table 5. Dietary Ge-132 marginal significantly (p=0.051) enhanced the β-glucuronidase activity from 1.6 to 2.6 units/g caecal content, but the two RAF-containing diets, RAF and GeRAF, resulted in significantly reduced the β-glucuronidase activity (p=6.4 × 10–7), to 0.4 and 0.3 units/g. Additionally, the activities measured in the other two units (units/caecum and units/1010 bacterium) showed no significant differences for the rats fed diets with Ge-132. The activities, measured in units/caecum, were 3.6 for the control diet, 5.2 for the Ge-132 diet, 2.2 for the RAF diet and 2.0 for the GeRAF diet, whereas the activities as measured in units/1010 bacteria were 3.3 for the control diet, 7.0 for the Ge-132 diet, 0.2 for the RAF diet and 0.2 for the GeRAF diet. RAF significantly decreased the activity measured by both methods (p= 0.001). The interaction of GE × RAF was evaluated. A significant difference (p=0.032< 0.05) was found in units / g caecal content.
Table 5. Effects of Ge-132 and RAF Administration on β-Glucronidase Activities.
| Control | Ge-132 | RAF | GeRAF | P value |
|||
| GE | RAF | GExRAF | |||||
| β-Glucronidase activity in | |||||||
| Units/g caecal content | 1.6 ± 0.8 | 2.6 ± 0.6 | 0.4 ± 0.2 | 0.3 ± 0.2 | 0.051 | 6.4E-07 | 0.032 |
| Units/caecum | 3.6 ± 1.6 | 5.2 ± 1.0 | 2.2 ± 1.0 | 2.0 ± 1.3 | 0.228 | 0.001 | 0.130 |
| Uunits/1010 bacteria | 3.3 ± 2.8 | 7.0 ± 4.5 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.139 | 0.001 | 0.165 |
Values are means ± SD. P values were obtained from two-way factorial ANOVA (p<0.05). GE, RAF and GexRAF show the main effect of Ge-132, main effect of RAF and interaction of Ge-132 and RAF, respectively.
DISCUSSION
In this study, we evaluated the effects of Ge-132 and RAF on caecal characteristics. We confirmed that RAF can abrogate the high β-glucuronidase activity induced by Ge-132 intake.
We previously reported that dietary Ge-132 promotes bile secretion, and we demonstrated that the fecal color change due to dietary Ge-132 was induced by bile pigment and porphyrin [18, 19]. However, we did not determine the pigments present in the caecal contents in this study because the change in the microflora composition is more important than the color change. We examined the composition of caecal microorganisms to determine if RAF can promote an increase in Bifidobacterium populations. Additionally, we evaluated the effect of Ge-132 intake on the caecal flora. RAF is one of the most common bifidus factors, and our collaborators observed this result in previous studies of RAF intake in humans and mice [11, 20, 21]. In this study, RAF enhanced the detection frequency of Bifidobacterium; this phenomenon was not unexpected because the bacterial number was enhanced by fermentation with RAF, and the increase in the volume of the caecal contents increased the size of the caeca, as shown in Fig. 2 and Table 2. In contrast, dietary Ge-132 did not change the composition of the caecal flora. One of aim of this study was to evaluate if the sugar isomerization induced by Ge-132 affects caecal fermentation. The results show that Ge-132 did not affect the isomerization of saccharides that originated from hydrolysis of starch and have a prebiotic effect in the intestine. However, the fecal color change induced by Ge-132 intake was not related to the differences in the microflora (between the control group and the Ge-132 group). This result is in agreement with a previous report [18]. It suggests that fecal color change caused by the Ge-132 intake did not occurr due to the prebiotic effect but from bile pigment induction.
Bifidobacterium was increased predominantly by the RAF treatments. The same prebiotic effect has been previously reported in different animal species [11, 21]. Additionally, the predominant species changed from Bacteroidaceae to Bifidobacterium with decrease in Bacteroidaceae due to the RAF intake. Lactobacillus also increased, while Enterobacteriaceae were decreased by the RAF intake. In contrast, the frequencies of the detection of Clostridium and Veillonella were higher in the RAF group than in the control group. Generally, Clostridium and Veillonella are categorized as bad or harmful bacteria. Infection with them sometimes triggers diseases. In this study, we observed an increase in them on intake of RAF and GeRAF. The frequencies of detection of Clostridium increased from 0% to 100 and 60%, respectively. However, the number of bacteria for the Control, RAF and GeRAF were similar. Some strains of Clostridium can utilize the RAF [22]; therefore, they might grow as a result of RAF intake. On the other hand, these changes in Clostridium were not found in a human study of RAF intake [11]. Furthermore, the availability of raffinose for some strains of Veillonella was negative according to in vitro studies [22, 23]. In this study, the detection rates of Veillonella were increased by RAF and GeRAF intake. These increases might not have occurred due to direct utilization of RAF. This is probably a reason why the number of Veillonella is low compared with the total number of bacteria or Bifidobacterium cells in the RAF and GeRAF groups. However, this may suggest that some strains of Veillonella can utilize RAF. Dietary RAF intake decreased Bacteroidaceae and Enterobacteriaceae, while the populations of Bifidobacterium and Lactobacillus increased. The prebiotic effect of RAF was confirmed. In this study, there was an apparent difference in the effects of Ge-132 intake and RAF intake on microflora. Therefore, we think that the fecal color change resulting from Ge-132 intake was not influenced by microflora. However, the frequencies of detection in some species were low, and they demand a detailed experiment with a large number of animals per group.
RAF is fermented by Bifidobacterium and Lactobacillus, and the caecal pH was lowered by RAF and GeRAF. Generally, fermentation produces organic acids, and this increase in organic acids lowers the pH of the caecal contents. In this study, a significant increase in the amounts of acetic acid and formic acid were observed. We found an approximately 1.6-fold increase in acetic acid production in the caecal contents of the RAF-treated groups (Table 4). Acetic acid was the most abundant organic acid and showed a significant increase in both RAF-treated groups; thus, the lower pH may be the result of the increase in the amount of acetic acid. In recent studies, probiotics have been shown to protect the intestinal tract from lethal infectious diseases [24]. Some probiotics can produce large amounts of acetic acid, and this ability is critical for the defence against pathogens. In this study, the increase in the populations of Bifidobacterium and Lactobacillus caused by RAF and GeRAF increased the levels of acetic acid in the intestine; thus, we are interested in a resistant effect against infection. To test this hypothesis, future experiments should be performed with more animals per group and with a pathogen infection model.
We previously described the increase in bilirubin glucuronide in the intestine induced by Ge-132 and a corresponding increase in β-glucuronidase activity [18]. β-glucuronidase activity is known to be a risk factor for colon cancer [25,26,27]; therefore, the enhancement of β-glucuronidase activity is not a desirable result. Some prebiotic oligosaccharides can reduce β-glucuronidase activity [28,29,30,31]. Therefore, we wanted to determine if the simultaneous intake of RAF could suppress the increasing levels of β-glucuronidase activity induced by Ge-132. Except for an increase in the amount of Bifidobacterium, RAF reduced the amount of microorganisms in the caeca (Table 3); thus, the β-glucuronidase activity was low (Table 5). RAF increased the volume of the intestinal contents and the caecal contents, as shown in Fig. 2 and Table 3. The enzyme was diluted by the increased content of the microorganisms, as demonstrated by the lack of a decreased in the activity as measured relative to the whole caecum (Table 5). Some probiotics such as the lactic acid bacteria Bifidobacterium and Lactobacillus can reduce intestinal β-glucuronidase activity [32,33,34]. It has been reported that some strains of Bacteroides and E. coli lead to higher levels of β-glucuronidase activity [35, 36]. In this study, RAF acted as a prebiotic and enhanced the lactic acid bacteria while suppressing the enterobacteriaceae, and β-glucuronidase activity might be reduced. In contrast, Ge-132 enhanced the activity of β-glucuronidase in the caecal contents (Table 5). However, the populations of intestinal bacteria did not change. Thus, the increase in β-glucuronidase activity induced by Ge-132 is not related to the bacterial balance. Moreover, there was a significant difference in interaction caused by the GeRAF intake. The data suggest that if glucuronidase does increase the risk of cancer, simultaneous intake of RAF could abrogate this risk.
In this study, Ge-132 and RAF did not inhibit each other’s functions. The effect of the hypothesized promotion of the isomerisation of saccharides into prebiotics in the intestine by Ge-132 seems too little to change the microflora. However, the evaluation of this conversion requires examination in a study that does not need to consider intestinal absorption, such as an analysis of saccharides for culture of cecal contents with Ge-132. In contrast, enhanced acetic acid production and lower pH in the caecal contents due to RAF intake were confirmed. This change may protect against pathogenic infections. Moreover, the simultaneous intake of Ge-132 and RAF suppressed β-glucuronidase activity, and this activity increased when the rats were fed a diet containing only Ge-132. In this study, this was the only beneficial effect found for simultaneous intake of Ge-132 and RAF. We recently revealed a symbiotic effect of Lactobacilli, oligosaccharide and Ge-132 on immune response in mice [37]. Both Ge-132 and RAF have immune activation activity; therefore, the potential for immune modulation by this combination of supplements is interesting. Further research is needed to test this speculation.
Acknowledgments
We are very grateful to Dr. Taro Kishida, Department of Applied Biosciences at Ehime University, for technical guidance on the rat experiments, and we thank Dr. Mitsuo Akiba, Tokyo University of Pharmacy and Life Sciences, for his beneficial advice concerning this study.
REFERENCES
- 1.Tsutsui M, Kakimoto N, Axtell D, Oikawa H, Asai K. 1976. Crystal structure of “carboxyethylgermanium sesquioxide”. J Am Chem Soc 98: 8287–8289 [Google Scholar]
- 2.Sugiya Y, Sakamaki S, Sugita T, Abo Y, Satoh H. 1986. Subacute oral toxicity of carboxyethylgermanium sesquioxide (Ge-132) in rats. Ouyou Yakuri 31: 1181–1190 (in Japanese). [Google Scholar]
- 3.Aso H, Suzuki F, Ebina T, Ishida N. 1989. Antiviral activity of carboxyethylgermanium sesquioxide (Ge-132) in mice infected with influenza virus. J Biol Resp. Modif 8: 180–189 [PubMed] [Google Scholar]
- 4.Pr—nai L, Arimori S. 1991. Prótective effect of carboxyethylgermanium sesquioxide (Ge-132) on superoxide generation by 60Co-irradiated leukocytes. Biotherapy, 3: 273–279. [DOI] [PubMed] [Google Scholar]
- 5.Sato I, Nishimura T, Kakimoto N, Suzuki H, Tanaka N. 1988. Prevention of pulmonary metastasis of Lewis Lung carcinoma and activation of murine macrophages by a novel organic germanium compound. PCAGeS J Biol Resp Modif 7: 1–5 [PubMed] [Google Scholar]
- 6.Aso H, Suzuki F, Yamaguchi T, Hayashi Y, Ebina T, Ishida N. 1985. Induction of interferon and activation of NK cells and macrophages in mice by oral administration of Ge-132, an organic germanium compound. Microbiol Immunol 29: 65–74 [DOI] [PubMed] [Google Scholar]
- 7.Sanai T, Okuda S, Onoyama K, Oochi N, Takaichi S, Mizuhira V, Fujishima M. 1991. Chronic tubulointerstitial changes induced by germanium dioxide in comparison with carboxyethylgermanium sesquioxide. Kidney Int 40: 882–890 [DOI] [PubMed] [Google Scholar]
- 8.Kagoshima M, Ohnishi T, Suguro N, Tomizawa S. 1986. Metabolic fate of 2-carboxygermanium sesquioxide (1) oral administration. Ouyou Yakuri 32: 71–79 (in Japanese). [Google Scholar]
- 9.Hess B, Raisin J, Zimmermann A, Horber F, Bajo S, Wyttenbach A, Jaeger P. 1993. Tubulointerstitial Nephropathy persisting 20 months after discontinuation of chronic intake of germanium lactate citrate. Am J Kidney Dis 21: 548–552 [DOI] [PubMed] [Google Scholar]
- 10.Krapf R, Schaffner T, Iten P. 1992. Abuse of germanium associated with fatal lactic acidosis. Nephron 62: 351–356 [DOI] [PubMed] [Google Scholar]
- 11.Fujisaki H, Nagura T, Kawamoto T, Sayama K. 1994. The effects of raffinose administration on the fecal flora, organic acids and putrefactive products in humans. Bifidus 8: 1–5 (in Japanese). [Google Scholar]
- 12.Barker S, Pelmore H, Somers P. 1983. Effect of oxyanions on the D-glucose isomerase catalysed equilibrium: 2. Effect of germanate on the equilibrium of D-glucose and D-fructose with immobilized D-glucose isomerase. Enzyme Microb Technol 5: 121–124 [Google Scholar]
- 13.Boron W, Boulpaep E. 2009. Medical Physiology 2nd ed., Saunders Elsevier, Philadelphia, pp. 949–955. [Google Scholar]
- 14.Mitsuoka T, Ohno K, Benno Y, Suzuki K, Namba K.1967. Die faekalflora bei menschen. V. Mitteilung: Vorgleich des neu entwickelten verfahrens mit dem bisherigen üblichen verfahren zur darmfloraanalyse. Zentralbl Bakteriol Mikrobiol Hyg 1 Abt Orig A, 234: 219–333. [PubMed] [Google Scholar]
- 15.Mitsuoka T, Sega T, Yamamoto S.1965. Eine verbesserte methodik der qualitativen und quantitativen analyse der darmflora von menschen und tieren. Zentralbl. Bakteriol. Mikrobiol. Hyg. 1 Abt Orig. A, 195: 455–469. [PubMed] [Google Scholar]
- 16.Gorbach S, Nahas L, Lerner P, Weinstein L. 1967. Studies of intestinal microflora. I. Effects of diet, age, and periodic sampling on numbers of fecal microorganisms in man. Gastroenterology 53: 845–855 [PubMed] [Google Scholar]
- 17.Ishikawa F, Takayama H, Matsumoto K, Ito M, Chonan O.1995. Effects of β 1-4 linked galactooligosaccharides on human fecal microflora Bifidus. 9: 5–18(in Japanese). [Google Scholar]
- 18.Nakamura T, Nagura T, Akiba M, Sato K, Tokuji Y, Ohnishi M, Osada K. 2010. Promotive effects of the dietary organic germanium poly-trans-[(2-carboxyethyl) germasesquioxane] (Ge-132) on the secretion and antioxidative activity of bile in rodents. J Health Sci 56: 72–80 [Google Scholar]
- 19.Nakamura T, Saito M, Shimada Y, Fukaya H, Shida Y, Tokuji Y. 2011. Induction of aminolevulinic acid synthase gene expression and enhancement of metabolite, protoporphyrin IX, excretion by organic germanium. Eur J Pharmacol 653: 75–81 [DOI] [PubMed] [Google Scholar]
- 20.Benno Y, Endo K, Shiragami N, Sayama K, Mitsuoka T. 1987. Effects of raffinose intake on human fecal microflora. Bifidobact. Microflora 6: 59–63 [Google Scholar]
- 21.Nagura T, Hachimura S, Kaminogawa S, Aritsuka T, Itoh K. 2005. Characteristic intestinal microflora of specific pathogen-free mice bred in two different colonies and their influence on postnatal murine immunocyte profiles. Exp Anim 54: 143–148 [DOI] [PubMed] [Google Scholar]
- 22.Hayakawa K, Mizutani J, Wada K, Masai T, Yoshihara I, Mitsuoka T. 1990. Effects of soybean oligosaccharides on human fecal flora. Microb Ecol Health Dis 3: 293–303 [Google Scholar]
- 23.Jumas-Bilak E, Carlier JP, Jean-Pierre H, Teyssier C, Gay B, Campos J, Marchandin H. 2004. Veillonella montpellierensis sp. nov., a novel, anaerobic, Gram-negative coccus isolated from human clinical samples. Int J Syst Evol Microbiol 54: 1311–1316 [DOI] [PubMed] [Google Scholar]
- 24.Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Opping DL, Suzuki T, Taylor TD, Itoh K, Kikuchi J, Morita H, Hattori M, Ohno H. 2011. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469: 543–537 [DOI] [PubMed] [Google Scholar]
- 25.Takada H, Hirooka T, Hiramatsu Y, Yamamoto M. 1982. Effect of beta glucuronidase inhibitor on azoxymethane-induced colonic carcinogenesis in rats. Cancer Res 42: 331–334 [PubMed] [Google Scholar]
- 26.Nakamura J, Kubota Y, Miyaoka M, Saitoh T, Mizuno F, Benno Y. 2002. Comparison of four microbial enzymes in Clostridia and Bacteroides isolated from human feces. Microbiol Immunol 46: 487–490 [DOI] [PubMed] [Google Scholar]
- 27.de Moreno de LeBlanc A, Perdigon G. 2005. Reduction of β-glucuronidase and nitroreductase activity by yoghurt in a murine colon cancer model. Biocell 29: 15–24 [PubMed] [Google Scholar]
- 28.Buddington K, Williams H, Chen C, Witherly A. 1996. Dietary supplement of neosugar alters the fecal flora and decreases activities of some reductive enzymes in human subjects. Am J Clin Nutr 63: 709–716 [DOI] [PubMed] [Google Scholar]
- 29.Kleessen B, Sykura B, Zunft J, Blaut M. 1997. Effects of inulin and lactose on fecal microflora, microbial activity, and bowel habit in elderly constipated persons. Am J Clin Nutr 65: 1397–1402 [DOI] [PubMed] [Google Scholar]
- 30.Gudiel-Urbano M, Goni I. 2002. Effect of short-chain fructooligosaccharides and cellulose on cecal enzyme activities in rats. Ann Nutr Metab 46: 254–258 [DOI] [PubMed] [Google Scholar]
- 31.Juskiewicz J, Zdunczyk Z. 2002. Lactulose-induced diarrhoea in rats: effects on caecal development and activities of microbial enzymes. Comparative biochemistry and physiology. Part A. Mol Integr Physiol 133: 411–417 [DOI] [PubMed] [Google Scholar]
- 32.Spanhaak S, Havenaar R, Schaafsma G. 1998. The effect of consumption of milk fermented by Lactobacillus casei strain Shirota on the intestinal microflora and immune parameters in humans. Eur J Clin Nutr 52: 899–907> [DOI] [PubMed] [Google Scholar]
- 33.Park Y, Bae A, Han J, Choi C, Kim H. 1998. Inhibitory effects of Bifidobacterium spp. isolated from a healthy Korean on harmful enzymes of human intestinal microflora. Arch Pharm Res 21: 54–61 [DOI] [PubMed] [Google Scholar]
- 34.Han Y, Huh S, Ahn T, Lim S, Baek J, Kim H. 2005. Hepatoprotective effect of lactic acid bacteria, inhibitors of beta-glucuronidase production against intestinal microflora. Arch Pharm Res 28: 325–329 [DOI] [PubMed] [Google Scholar]
- 35.Chang W, Brill J, Lum R. 1989. Proportion of β-D-glucuronidase-negative Escherichia coli in human fecal samples. Appl Environ Microbiol 55: 335–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McBain J, Macfarlane T. 1998. Ecological and physiological studies on large intestinal bacteria in relation to production of hydrolytic and reductive enzymes involved in formation of genotoxic metabolites. J Med Microbiol 47: 407–416 [DOI] [PubMed] [Google Scholar]
- 37.Nakamura T, Saito M, Aso H. 2012. Effects of lactobacilli, oligosaccharide and organic germanium intake on the immune responses of mice. Biosci Biotechnol Biochem 76: 375–377 [DOI] [PubMed] [Google Scholar]