Abstract
A double-blind, placebo-controlled, randomized trial was conducted to investigate the beneficial effect of probiotic and prebiotic fermented milk on the skin of healthy adult women. Forty healthy Japanese adult female volunteers with healthy skin randomly received either a bottle of probiotic and prebiotic fermented milk containing Bifidobacterium breve strain Yakult and galacto-oligosaccharides (GOS) (active group) or a non-fermented placebo milk containing neither probiotics nor GOS (placebo group) daily for 4 weeks. Before and after intake, hydration levels and cathepsin L-like activity in the stratum corneum and phenol levels in the serum and urine were determined. After intake, the hydration level of the stratum corneum decreased significantly in only the placebo group and was significantly lower than in the active group (p=0.031). Cathepsin L-like activity, an indicator of keratinocyte differentiation, was significantly increased in the active group (p=0.027). Serum and urine phenol levels decreased significantly in the active group (p=0.014, p=0.002, respectively), and serum phenol levels were significantly lower in the active group compared with the placebo group (p=0.006). The consecutive intake of probiotic and prebiotic fermented milk can benefit skin condition without dryness and decrease the levels of phenol production by gut bacteria in healthy adult women.
Keywords: phenol, prebiotics, probiotics, skin condition, Bifidobacterium, galacto oligosaccharides, skin hydration
INTRODUCTION
Probiotics are live microorganisms such as lactobacilli and bifidobacteria that confer health benefits when administered in adequate amounts [1, 2]. On the other hand, prebiotics are selectively fermented ingredients that alter the composition of intestinal microflora to bring about health benefits [3,4,5,6,7]. Many studies have investigated the effects of probiotic or prebiotic consumption or a combination of the two on intestinal microbial imbalance, suppression of pathogens, prevention and treatment of intestinal and other disorders, inflammatory bowel disease, diarrhea, infection, colon cancer, constipation and atopic diseases [8,9,10,11,12,13].
A survey of 600 Japanese women conducted in 2007 suggested that women who suffer from abnormal bowel movements also experience subjective skin problems such as dryness and dullness (our unpublished data). Indeed, intestinal conditions and/or the environment are thought to be closely associated with skin conditions, although there is limited scientific evidence to support this assumption. However, a small number of non-placebo-controlled studies have shown that the intake of probiotics or yogurt improves skin conditions or constipation in women [14, 15].
We previously showed that phenols (phenol and p-cresol) adversely affected in vitro keratinocyte differentiation and accumulated in murine skin and that intake of prebiotic beverages not only reduced serum phenol levels but also improved skin conditions of female volunteers in an open clinical study [16, 17]. In order to further clarify the relationship between skin condition and the intestinal environment, we conducted a double-blind, placebo-controlled, randomized trial to verify whether consecutive intake of fermented milk containing a probiotic bifidobacteria and a prebiotic galacto-oligosaccharide (GOS) affects skin condition as a primary endpoint and serum and urinary levels of phenols as secondary endpoints in healthy adult female volunteers.
MATERIALS AND METHODS
Subjects
Forty healthy adult female volunteers participated in the trial. Their ages ranged from 23–75 years (mean, 41.9 years), with 9 subjects in their 20s, 12 in their 30s, 8 in their 40s, 7 in their 50s, 2 in their 60s and 2 in their 70s (Table 1). We excluded subjects with food allergies, those who were pregnant or lactating, those attempting to become pregnant, those taking medication known to affect bowel function or skin condition and those who experienced severe bowel movement problems or persistent skin conditions for one month before and during the trial. The purpose and contents of the trial were fully explained to all subjects, whose signed informed consent was obtained before enrollment. The protocol was approved by the Human Studies Committee of the Yakult Central Institute for Microbiological Research, Kunitachi, Tokyo, Japan, in accordance with the Helsinki Declaration and the Committee’s own guidelines.
Table 1. Subject physical characteristics.
| Parameter | Fermented milk | Placebo milk |
| Age (years) | 42.9 ± 13.1 | 41.0 ± 14.0 |
| Body weight (kg) | 51.0 ± 7.8 | 51.8 ± 6.8 |
| Body height (m) | 1.58 ± 0.05 | 1.58 ± 0.06 |
| Body mass index (kg/m2) | 20.4 ± 2.3 | 20.9 ± 3.2 |
Values are expressed as mean ± SD values; active group, n = 20; placebo group, n = 19.
Test Beverage
Fermented milk (active milk) and a placebo milk were compared. The active milk contained GOS, polydextrose, Bifidobacterium breve strain Yakult (YIT 12272), Lactococcus lactis YIT 2027 and Streptococcus thermophilus YIT 2021. These bacteria were obtained from the Culture Collection Research Laboratory of Yakult Central Institute for Microbiological Research, Kunitachi, Tokyo, Japan. Placebo milk was prepared with the same ingredients as active milk and with a similar nutritional content, color, flavor and taste, although it contained lactic acid, acetic acid and dextrin instead of B. breve, L. lactis, S. thermophilus, GOS and polydextrose. Nutritional data of the two milks are shown in Table 2. During the trial, there were 6 × 1010 and 5 × 1010 colony-forming units (cfu) per 100 mL or more of B. breve and other lactic acid bacteria in the active milk, respectively.
Table 2. Nutritional data of fermented milk versus placebo milk.
| Parameter | Fermented milk per 100 mL | Placebo milk per 100 mL |
| Energy (kcal) | 48 | 58 |
| Protein (g) | 3.1 | 3.1 |
| Fat (g) | 0.1 | 0.1 |
| Carbohydrate (g) | 10.5 | 12.7 |
| Galacto-oligosaccharide (g) | 0.6 | 0 |
| Polydextrose (g) | 3.1 | 0 |
| Dextrin (g) | 0 | 4.1 |
Trial Design
The double-blind, placebo-controlled trial comprised a 4-week administration period that followed a 4-week pre-administration period, during which time any probiotics that had been previously consumed were allowed to pass through the body. The lengths of the pre-administration and administration periods were determined from the physiology cycle and epidermal cell turnover rate. During the pre-administration period, the subjects refrained from consuming pro- or prebiotics before being randomly assigned to one of two groups. During the administration period, the subjects ingested 100 mL of active milk or placebo milk after lunch each day.
Hydration level measurements in the stratum corneum and collection of corneocytes, urine and blood (see below) were performed daily after each session. Throughout the trial, the subjects complied with several rules to avoid the intake of other fermented milks, lactic acid bacteria beverages, probiotic and prebiotic products and to not change the use of cosmetic products or alter other lifestyle routines such as diet and exercise habits. Cheese, pickles and pasteurized foods were allowed. The trial was carried out in Tokyo, Japan, between October and December 2009, which is a season of particularly low humidity.
Measurement of Hydration Level
Makeup was removed with a designated cleansing cream, and the subject’s skin was acclimatized for 30 min under standardized conditions (23 ± 1°C, humidity 45 ± 5%). The hydration level of the stratum corneum on the cheek was then measured by high-frequency surface electrical conductance using a Skicon® 200 (IBS Co., Ltd., Shizuoka, Japan) equipped with a probe having a diameter of 2 mm [18]. Measurements were repeated five times on the same site, and the averaged value was used for further analysis.
Collection of Corneocytes and Assay of Cathepsin L-like Protease Activity
As previously reported [17], third-layer corneocytes of the stratum corneum on the inner forearm were gathered by tape stripping (Protect Label typeA; AS ONE, Osaka, Japan). Cathepsin L-like protease activity was then assayed according to previously reported methods [17]. Briefly, tape-attached corneocytes were incubated in a reaction mixture, which comprised 0.2 M Tris-HCl buffer (pH 6.8), 0.5 mM Z-Val-Val-Arg-MCA (synthetic substrate for cathepsin S/L; Peptide Institute, Inc., Osaka, Japan), 2% (v/v) N,N-dimethylformamide and 0.05% (w/v) Triton-X100 (both from Wako Pure Chemical Industries, Osaka, Japan) for 2 hr at 37°C. Fluorescence intensity (excitation wavelength, 380 nm; emission wavelength, 445 nm) of the reaction mixture was measured with a SpectraMax Gemini EM Fluorescence Reader (Molecular Devices, Sunnyvale, CA, USA), while the amount of tape-attached protein was quantified using a BCATM Protein Assay Reagent (Thermo Scientific, Chicago, IL, USA) for 30 min at room temperature; the absorbance at 492 nm was read with a Bio Kinetics Reader (BioTek, Winooski, VT, USA) using a BSA standard curve. The relative activity of cathepsin L-like protease was expressed as fluorescence intensity per mg protein.
Collection of Urine and Blood
Early-morning urine was collected using URO CATCH II® (ATLETA Inc, Osaka, Japan) containing 0.5 mg neomycin trisulfate salt hydrate (Sigma-Aldrich, St. Louis, MO, USA). Blood was collected from an arm vein and centrifuged at 2,000 × g for 20 min at 4°C to prepare serum samples, which were stored at −70°C until used for analysis.
Analysis of Phenols
Levels of phenols (phenol and p-cresol) in serum and urine were analyzed by high-performance lipid chromatography (HPLC) [11, 19] with some modifications. A total of 25 µL serum, 475 µL Milli-Q water, 200 µL concentrated HCl and 10 µL internal standard (1 mg 4-chlorophenol in 300 µL Milli-Q water) were mixed and heated at 100°C for 60 min to hydrolyze the phenol conjugates. After cooling on ice, 2 mL ether was added to the mixture and agitated. Then, 1 mL of ether layer was taken and neutralized with 1 mL 0.05 N NaOH / MeOH. After drying with a N2 purge, the residue was dissolved in 200 µL ethyl acetate and filtered through an 0.45-μm Ultrafree-MC filter unit (Millipore, Billerica, MA, USA) to prepare the HPLC sample.
An HPLC system consisting of an Alliance® 2695 chromatographic system equipped with a 474 fluorescence detector (Waters, Milford, MA, USA) and L-column ODS® (CERI, Tokyo, Japan; 4.6 mm (i.d.) × 150 mm (length) and 5 μm particle size) was used. A 1-μL HPLC sample was automatically injected and eluted with 1% aqueous phosphoric acid solution:acetonitrile (80:20 v/v) at a flow rate of 1 mL/min at 40°C to monitor fluorescence intensity (excitation at 260 nm and emission at 305 nm). Phenol levels were calculated using the standard curve of the peak height ratio between phenol (Wako Pure Chemical Industries) or p-cresol (Tokyo Chemical Industry, Tokyo, Japan) and the internal standard (4-chlorophenol, Tokyo Chemical Industry). Urine creatinine levels were determined with the Creatinine-test Wako (Wako Pure Chemical Industries). Urine phenol levels were corrected according to urine creatinine levels, and the changes were expressed as µM phenols per mM creatinine.
Statistical Analysis
Data were expressed as means ± SD. The paired t-test was used to analyze changes in hydration level, differences in cathepsin L-like activity and levels of phenols before and after the administration period in each group. Hydration level, cathepsin L-like activity and levels of phenols after the administration period were compared between groups using analysis of covariance adjusted for each baseline level. The SAS Preclinical Package software for Windows (version 5.0, SAS Institute Japan Ltd., Tokyo, Japan) and SAS software (version 9.2, SAS Institute Japan Ltd., Tokyo, Japan) were used to perform analyses. p<0.05 was considered to be statistically significant.
RESULTS
Subjects
One subject in the placebo group failed to complete the trial because of personal reasons. Questionnaire (written and oral investigation) results for all other subjects indicated that compliance with trial requirements was over 95%.
Physical characteristics of subjects at baseline are shown in Table 1. Both groups contained two postmenopausal subjects and one subject who smoked. No difference in age, weight, height or body mass index (BMI) was noted between groups.
Hydration Level of the Stratum Corneum
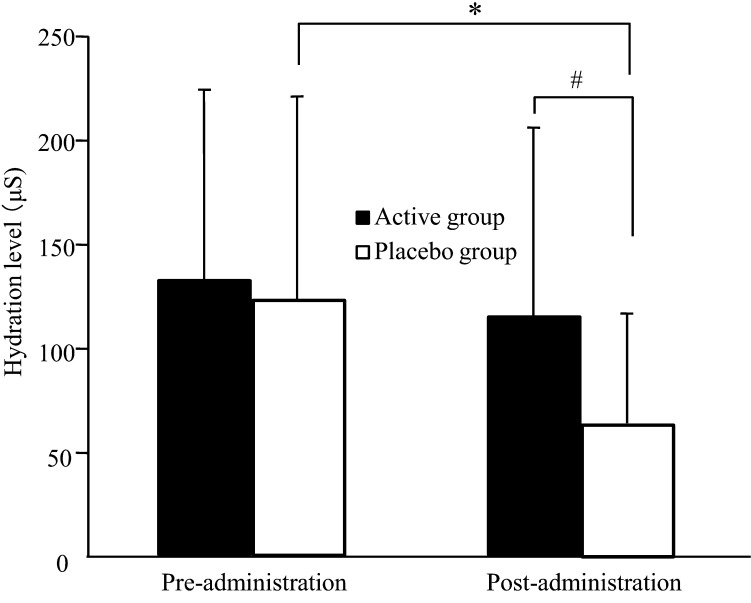
Figure 1 shows changes in the hydration level of the stratum corneum during the trial. In the placebo group, the hydration level was significantly lower after the administration period than after the pre-administration period (p=0.005). In contrast, the active group maintained a constant hydration level during the trial, and there was a significant difference between the active group and the placebo group after the administration period (p=0.031).
Fig. 1.
Hydration level of the stratum corneum as measured by a Skicon in healthy adult women. Values are expressed as mean ± SD values; active group, n = 20; placebo group, n = 19. *p<0.05 comparing pre- and post-administration values by the paired t-test. #p<0.05 comparing the active group with the placebo group by ANCOVA.
Cathepsin L-like Protease Activity in the Stratum Corneum
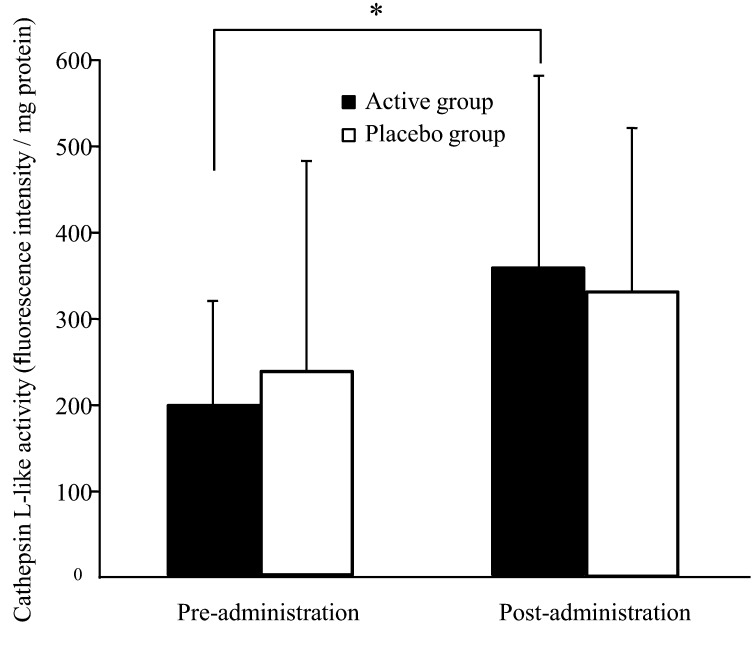
Figure 2 shows changes in cathepsin L-like protease activity in the stratum corneum during the trial. In the active group, cathepsin L-like activity significantly increased after the administration period compared with after the pre-administration period (p=0.027). In contrast, the placebo group showed no significant variations during the trial.
Fig. 2.
Cathepsin L-like protease activity in the stratum corneum of inner forearm skin in healthy adult women. Values are expressed as mean ± SD values; active group, n = 20; placebo group, n = 19. *p<0.05 comparing pre- and post-administration values by the paired t-test.
Serum and Urine Phenol Levels
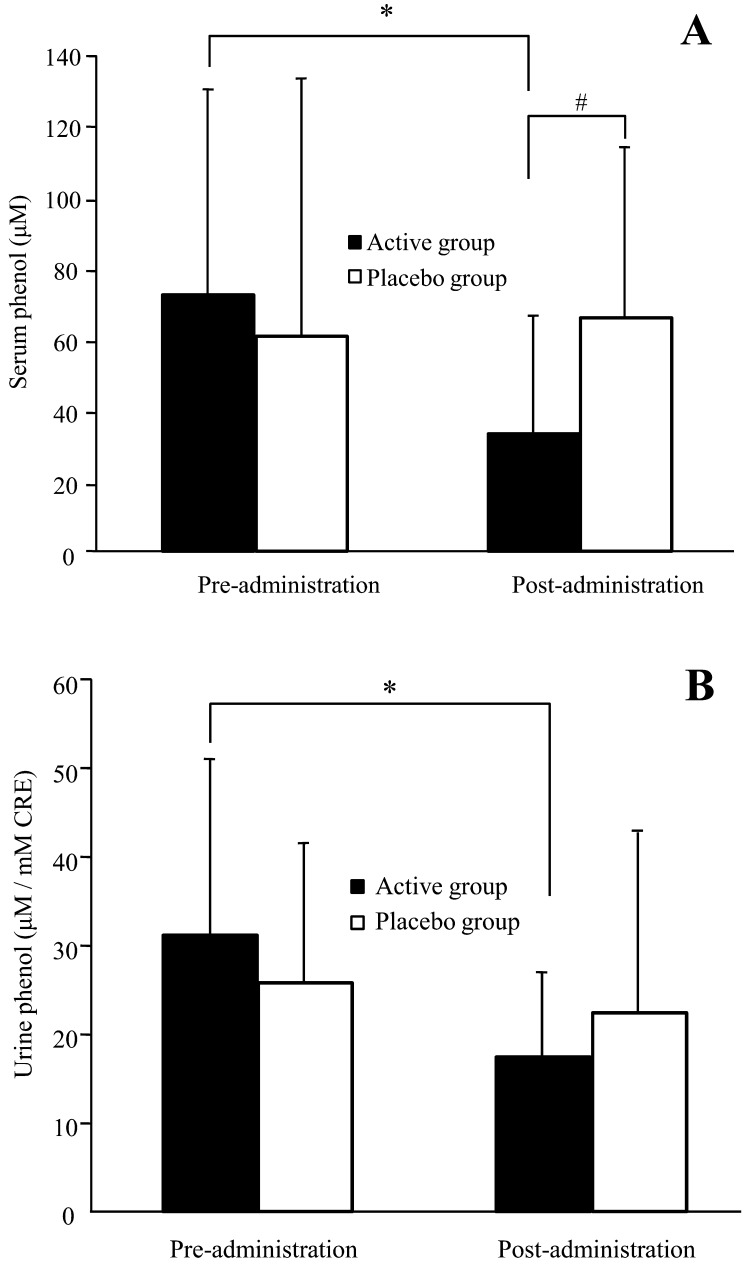
Figure 3 shows variations in phenol levels in the serum and urine of subjects during the trial. In the active group, serum phenol levels were significantly lower after the administration period than after the pre-administration period (p=0.014). On the other hand, the placebo group showed a similar level of serum phenol after the administration period compared with after the pre-administration period. Urinary phenol levels were significantly lower after the administration period than after the pre-administration period in the active group (p=0.002), while there were no significant changes in placebo group urinary phenol levels during the trial. After the administration period, serum phenol levels were significantly lower in the active group compared with the placebo group (p=0.006).
Fig. 3.
Phenol levels in serum (A) and urine (B) in healthy adult women. Values are expressed as mean ± SD values for volunteers with detectable levels; active group, n = 20; placebo group, n = 19. *p<0.05 comparing pre- and post-administration values by the paired t-test. #p<0.05 comparing the active group with the placebo group by ANCOVA.
Serum p-cresol was not detected in all subjects; it was found in 5 and 10 subjects of the active group and in 6 and 8 subjects of the placebo group after the pre-administration and administration periods, respectively. In contrast, urinary p-cresol was detected in all subjects, but neither group showed significant changes (p>0.05) in serum or urinary p-cresol levels during the trial (data not shown).
DISCUSSION
Our previous studies have shown that toxic phenols (phenol and p-cresol) produced from L-tyrosine by cecum bacteria are absorbed from the gut through the bloodstream into the skin in a murine model [16] and that they disturb the differentiation of normal human keratinocytes in a monolayer culture [17]. Moreover, intake of a prebiotic beverage was shown in an open study to decrease serum phenols levels and improve skin markers, corneocyte size and cathepsin L-like protease activity in the stratum corneum of healthy adult women [17].
Phenols can serve as reliable biomarkers of the gut environment because levels of phenolic metabolites are influenced by the frequency and condition of bowel movements. The production of phenols is inhibited at low pH [20, 21]. Therefore, an increase in intestinal organic acid level leads to a reduction in phenol-producing bacteria such as Enterobacteriaceae, Clostridium and Staphylococcus [11].
The present double-blind, placebo-controlled, randomized trial demonstrated that intake of a fermented milk containing B. breve strain Yakult and GOS prevented hydration level decreases in the stratum corneum (Fig. 1), increased cathepsin L-like protease activity (Fig. 2) and reduced phenol levels in serum and urine (Fig. 3) in healthy adult female volunteers. B. breve strain Yakult and GOS, a typical probiotic and prebiotic, respectively, have previously been reported to exhibit beneficial effects (increase in indigenous bifidobacteria, regulation of the functions of the intestines, improvement of intestinal flora and so on) [7, 22,23,24], so their intestinal regulation is believed to have contributed to the current reduction in serum and urine phenol levels.
The fermented milk (active milk) contained L. lactis YIT 2027 and S. thermophilus YIT 2021 at about same cfu level as B. breve strain Yakult, suggesting that these bacteria could have possibly participated in the effect. However, the tolerance of these bacteria to artificial gastric and bile acids was shown to be lower than that of B. breve strain Yakult (unpublished data). Therefore, it is speculated that a less viable number of these bacteria reaches the intestine and that the effect on intestinal environments is less than that of B. breve strain Yakult, although we have not yet examined whether they affect intestinal environments.
The measured serum phenol levels in this study were higher than those reported previously [17], which may reflect the different ratio of deconjugation due to different methods of sample preparation (especially hydrolyzed conditions).
The present findings show that the hydration level of the stratum corneum was maintained at a constant level in the active group but decreased in the placebo group (Fig. 1). As the trial was carried out during the fall and winter months, outdoor temperature and humidity were gradually decreasing, which has previously been demonstrated to be associated with a decrease in hydration level of the stratum corneum and deterioration of skin condition in Tokyo residents [25]. Clarys et al. [26] reported that the hydration levels of dry skin range from 40–50 μS and that those of hydrated skin were about 200 μS as measured by a Skicon®. It therefore appears that the decreased hydration level observed in the placebo group represents dry skin based on seasonal environment changes. On the other hand, the skin of subjects in the active group maintained a higher hydration level, indicating that the skin moisturizing effect was retained.
Cathepsin L activity can be viewed as a good indicator of keratinocyte differentiation conditions, as it is known that proteolysis by cathepsin L activates transglutaminase 3, a key enzyme in the production of stratum corneum [27]. Normal keratinocyte differentiation has been reported to be of great importance in maintaining homeostasis of the epidermal architecture [28], so differentiation disturbances could conceivably reduce the hydration level of the stratum corneum as a result of skin barrier dysfunction. In the present study, reduction of phenol levels is believed to have induced keratinocyte differentiation improvement, leading to increased cathepsin L-like protease activity and the prevention of dryness. However, the results of this study are insufficient to prove a cause-effect relationship between the reduction in phenol level and the effect on the skin hydration level. Further validation is therefore required to fully understand the cause-effect relationship.
Isawa et al. reported in an open study that intake of yogurt is beneficial for skin conditions in adult female volunteers with chronic constipation and dry skin [15]. However, their study was not placebo controlled, and there were no positive data for serum markers associated with skin conditions. Therefore, the present trial is the first double-blind, placebo-controlled, randomized study to verify that the intake of probiotics and prebiotics is beneficial for maintaining healthy skin in healthy adult women. These findings suggest that human skin condition is improved because of the suppression of phenol production by gut bacteria due to the maintenance of a favorable intestinal environment.
CONCLUSIONS
It is generally accepted that several factors such as lifestyle, dietary habits, ultraviolet radiation and the enteral environment are associated with the condition of human skin. In this study, we focused on phenols produced by gut bacteria.
It appears that consecutive intake of fermented milk containing B. breve strain Yakult and GOS maintains and promotes a healthy stratum corneum and reduces the intestinal production of phenols that are potentially harmful to the skin in healthy adult women; this may be involved in the promotion not only of intestinal but also skin health. However, further study is required to clarify the detailed mechanism of the effects of fermented milk on skin health and the relationship between the intestinal environment and skin condition.
Acknowledgments
We thank Mrs. Harue Shibahara-Sone, Mrs. Naomi Harima-Mizusawa, Mr. Atsushi Gomi and Dr. Norihiro Kubota from our laboratory for technical help and discussion. We also thank the staff of the Institute and the Faculty of Research and Development of Yakult Honsha for manufacturing the test beverages.
REFERENCES
- 1.Fuller R. 1989. Probiotics in man and animals. J Appl Bacteriol 66: 365–378 [PubMed] [Google Scholar]
- 2.Reid G. 2005. The importance of guidelines in the development and application of probiotics. Curr Pharm Des 11: 11–16 [DOI] [PubMed] [Google Scholar]
- 3.Tanaka R, Takayama H, Morotomi M, Kuroshima T, Ueyama S, Matsumoto K, Kuroda A, Mutai M. 1983. Effects of administration of TOS and Bifidobacterium breve 4006 on the human fecal flora. Bifidobacteria Microflora 2: 17–24 [Google Scholar]
- 4.Ito M, Deguchi Y, Miyamori A, Matsumoto K, Kikuchi H, Matsumoto K, Kobayashi Y, Yajima T, Kan T. 1990. Effects of administration of galactooligosaccharides on the human faecal microflora, stool weight and abdominal sensation. Microb Ecol Health Dis 3: 285–292 [Google Scholar]
- 5.Gibson GR, Roberfroid MB. 1995. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 125: 1401–1412 [DOI] [PubMed] [Google Scholar]
- 6.Gibson GR, Beatty ER, Wang X, Cummings JH. 1995. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 108: 975–982 [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto K, Takada T, Yuki N, Kawakami K, Sakai T, Nomoto K, Kimura K, Matsumoto K, Iino H. 2004. Effect of transgalactosylated oligosaccharides mixture (N-GOS) in human intestinal microflora. J Intestinal Microbiol 18: 25–35 [Google Scholar]
- 8.Isolauri E, Sutas Y, Kankaanpaa P, Arvilommi H, Salminen S. 2001. Probiotics: effects on immunity. Am J Clin Nutr 73: 444S–450S [DOI] [PubMed] [Google Scholar]
- 9.Guarner F, Malagelada JR. 2003. Gut flora in health and disease. Lancet 361: 512–519 [DOI] [PubMed] [Google Scholar]
- 10.Nomoto K. 2005. Prevention of infections by probiotics. J Biosci Bioeng 100: 583–592 [DOI] [PubMed] [Google Scholar]
- 11.Shioiri T, Yahagi K, Nakayama S, Asahara T, Yuki N, Kawakami K, Yamaoka Y, Sakai Y, Nomoto K, Totani M. 2006. The effects of a symbiotic fermented milk beverage containing Lactobacillus casei strain Shirota and transgalactosylated oligosaccharides on defecation frequency, intestinal microflora, organic acid concentrations, and putrefactive metabolites of sub-optimal health state volunteers: A randomized placebo-controlled cross-over study. Biosci Microflora 25: 137–146 [Google Scholar]
- 12.De Preter V, Vanhoutte T, Huys G, Swings J, De Vuyst L, Rutgeerts P, Verbeke K. 2007. Effects of Lactobacillus casei Shirota, Bifidobacterium breve, and oligofructose-enriched inulin on colonic nitrogen-protein metabolism in healthy humans. Am J Physiol Gastrointest Liver Physiol 292: G358–G368 [DOI] [PubMed] [Google Scholar]
- 13.Miyazaki K, Matsuzaki T. 2008. Health properties of milk fermented with Lactobacillus casei strain Sirota (LcS). In Handbook of Fermented Functional Foods, 2nd ed, Edward RF (ed), CRC Press, Boca Raton, pp. 165–172. [Google Scholar]
- 14.Ara K, Meguro S, Hase T, Tokimitsu I, Otsuji K, Kawai S, Ito S, Iino H. 2002. Effect of spore-bearing lactic acid-forming bacteria (Bacillus coagulans SANK 70258) administration on the intestinal environment, defecation frequency, fecal characteristics and dermal characteristics in humans and rats. Microb Ecol Health Dis 14: 4–13 [Google Scholar]
- 15.Isawa K, Noma T, Yamamoto M, Kimura K, Ito H, Taketomo N, Numano K, Kawashima M. 2008. Verifying the ability of yogurt prepared with LB81 lactic acid bacteria to improve skin function. J Intestinal Microbiol 22: 1–5 [Google Scholar]
- 16.Iizuka R, Kawakami K, Izawa N, Chiba K. 2009. Phenols produced by gut bacteria affect the skin in hairless mice. Microb Ecol Health Dis 21: 50–56 [Google Scholar]
- 17.Iizuka R, Kawakami K, Chiba K. 2009. Gut bacteria producing phenols disturb keratinocyte differentiation in human skin. Microb Ecol Health Dis 21: 221–227 [Google Scholar]
- 18.Tagami H, Ohi M, Iwatsuki K, Kanamaru Y, Yamada M, Ichijo B. 1980. Evaluation of the skin surface hydration in vivo by electrical measurement. J Invest Dermatol 75: 500–507 [DOI] [PubMed] [Google Scholar]
- 19.Niwa T. 1993. Phenol and p-cresol accumulated in uremic serum measured by HPLC with fluorescence detection. Clin Chem 39: 108–111 [PubMed] [Google Scholar]
- 20.Smith EA, Macfarlane GT. 1996. Enumeration of human colonic bacteria producing phenolic and indolic compounds: effect of pH, carbohydrate availability and retention time on dissimilatory aromatic amino acid metabolism. J Appl Bacteriol 81: 288–302 [DOI] [PubMed] [Google Scholar]
- 21.Vince AJ, Burridge SM. 1980. Ammonia production by intestinal bacteria: the effects of lactose and glucose. J Med Microbiol 13: 177–191 [DOI] [PubMed] [Google Scholar]
- 22.Tojo M, Oikawa T, Morikawa Y, Yamashita N, Iwata S, Satoh Y, Hanada J, Tanaka R. 1987. The effects of Bifidobacterium breve administration on campylobacter enteritis. Acta Paediatr Jpn 29: 160–167 [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto K, Deguchi Y, Takada T, Iino T, Osada K, Haga K, Hirano K, Nomoto K, Ishikawa F, Watanuki M, Iino H. 2001. Effects of intake of beverage containing galactooligosaccharides and polydextrose on defecation and fecal microflora. J Nutr Food 3: 1–13 [Google Scholar]
- 24.Kanamori Y, Hashizume K, Sugiyama M, Morotomi M, Yuki N. 2001. Combination therapy with Bifidobacterium breve, Lactobacillus casei, and galactooligosaccharides dramatically improved the intestinal function in a girl with short bowel syndrome: a novel symbiotics therapy for intestinal failure. Dig Dis Sci 46: 2010–2016 [DOI] [PubMed] [Google Scholar]
- 25.Sone T, Ichioka M, Yokokura T. 1991. A study of seasonal changes in skin surface configuration and rough skin by image analysis. J Jpn Cosmet Sci Soc 15: 60–65 [Google Scholar]
- 26.Clarys P, Barel AO, Gabard B. 1999. Non-invasive electrical measurements for the evaluation of the hydration state of the skin: comparison between three conventional instruments - the Comeometer®, the Skicon® and the Nova DPM®. Skin Res Technol 5: 14–20 [Google Scholar]
- 27.Cheng T, Hitomi K, van Vlijmen-Willems IMJJ, de Jongh GJ, Yamamoto K, Nishi K, Watts C, Reinheckel T, Schalkwljk J, Zeeuwen PLJM. 2006. Cystain M/E is a high affinity inhibitor of cathepsin V and cathepsin L by a reactive site that is distinct from the legumain-binding site. A novel clue for the role of cystain M/E in epidermal cornification. J Biol Chem 281: 15893–15899 [DOI] [PubMed] [Google Scholar]
- 28.Ekanayake-Mudiyanselage S, Aschauer H, Schmook F, Jensen JM, Meingassner JG, Proksch E. 1998. Expression of epidermal keratins and the cornified envelope protein involucrin is influenced by permeability barrier disruption. J Invest Dermatol 111: 517–523 [DOI] [PubMed] [Google Scholar]