Abstract
The effects of oral administration of enteric-coated tablets containing lactoferrin (LF; 100 mg/tablet) and heat-killed Lactobacillus brevis subsp. coagulans FREM BP-4693 (LB; 6×109 bacteria/tablet) on fecal properties were examined in 32 Japanese women (20–60 years of age) with a tendency for constipation (defecation frequency at equal to or less than 10 times/2 weeks) by a double-blind placebo-controlled crossover design. A significant increase in defecation days per week was obserbed in the subjects who ingested the tablets containing LF and LB compared with the placebo group. The number of bifidobacteria in feces also significantly increased compared with the placebo group. In an in vitro study, LF and tryptic hydrolysate of LF, but not peptic hydrolysate of LF, upregulated the growth of Bifidobacterium longum ATCC15707 when added to the culture. These results demonstrate the capability of the enteric-coated tablets containing LF and LB in improving intestinal function and suggest that they have a growth promoting function for bifidobacteria.
Keywords: double-blind placebo-controlled trial, lactoferrin, Lactobacillus brevis subsp. coagulans, probiotics, intestinal microbiota, enteric-coated tablets
INTRODUCTION
Lactoferrin (LF) is an iron-binding glycoprotein existing chiefly in milk and found at especially high concentrations in mammary breast milk. Moreover, it is also known to be found in tears and saliva in mammals. LF is a multifunctional protein with antibacterial, antiviral, immunostimulatory and antioxidant activity and has a cancer-preventive potential [1,2,3,4,5]. It has been approved as a food additive in Japan and is included in the “generally recognized as safe” (GRAS) category in the USA.
Recently, we have found that one of the many functions of LF is visceral fat-decreasing action. The ingestion of enteric-coated tablets that contain LF (300 mg/day) for an 8-week period significantly decreased human visceral fat [6]. Moreover, growth-promoting action on bifidobacteria in addition to antibacterial activity is reported for LF [7]. Thus, researchers have been studying the functions of lactoferrin for potential use for health conditions.
Lactobacillus brevis subsp. coagulans FERM BP-4693 (LB) is a lactic acid bacteria isolated from traditional pickles called “Suguki” of Kyoto, Japan. The immunopotentiating action [8, 9] and probiotic function [10] of L. brevis have been reported, and it has been used as an active ingredient of a sour milk beverage and supplement.
A randomized double-blind placebo-controlled crossover trial was conducted to elucidate the effects of LF and LB on the intestinal function and microbiota in Japanese women with a tendency for constipation.
MATERIAL AND METHODS
Preparation of lactoferrin hydrolysates
Tryptic and peptic hydrolysates of LF were prepared as described previously [11]. Briefly, tryptic hydrolysates of LF were prepared by digestion with trypsin (trypsin diphenyl carbamyl chloride-treated type XI from bovine pancreas, T1005-250MG, Sigma-Aldrich Japan, Tokyo, Japan) at 37°C for 24 hr with the pH adjusted to 7.0 with NaOH. The reaction was terminated by heating at 80°C for 15 min. Peptic hydrolysates of LF were prepared by digestion with pepsin (pepsin 1:10,000, from porcine stomach mucosa, 162-20 752; Wako Pure Chemical Industries, Limited, Tokyo, Japan) at 37°C for 24 hr with the pH adjusted to 2.5 with HCl. The reaction was terminated by heating at 80°C for 15 min, and the pH was adjusted to 7.0 by the addition of NaOH.
Strain of bifidobacterium, culture conditions and measurement of growth
The bifidobacterial strain used in this study was Bifidobacterium longum ATCC 15707. Prior to use, the bifidobacterial strain was cultured for 24 hr anaerobically at 37°C in MRS broth using an anaerobic chamber (Anaerobic Box ANK-1, Hirasawa Works, Tokyo, Japan). Ten microliters of activated culture (5.0×108 cfu/ml) were freshly inoculated into 3 mL of MRS broth. Fermentations in the test tubes were performed anaerobically using an anaerobic chamber at 37°C. LF and hydrolysates of LF were filter sterilized (pore size 0.22 μm) and added to the autoclaved medium to give a final concentration of 5 mg/ml. Control incubations of bifidobacterium in MRS were performed without adding hydrolysates of LF. The growth of bifidobacteria was monitored by measuring the optical density at 550 nm using a calorimeter (Calorimeter ANA-7S; Tokyo Photo-Electronic Co., Ltd., Tokyo, Japan) at defined time intervals. Values for each sample were determined in triplicate.
Enteric-coated tablets
We used enteric-coated tablets containing LF (100 mg/tablet; LF tablet) and tablets containing both LF (100 mg/tablet) and LB (6×109 bacteria/tablet; LF+LB tablet). The LF tablets contained the same weight of lactose in place of the LB contained in a LF+LB tablet. The placebo tablets contained an amount of lactose equivalent to the weight of the LF plus LB in a LF+LB tablet. The other constituents of each tablet were crystalline cellulose, calcium carboxymethylcellulose, sucrose ester, silicon dioxide, shellac, sorbitol, arginine, and dextrin as described previously [6]. In this formulation, LF molecules are protected from proteolytic digestion in the stomach, since the tablets are coated with an acid-resistant material, shellac, which dissolves easily under the neutral pH conditions in the intestine. The enteric-coated properties of this formulation were checked by the standard disintegration test to satisfy the criterion for the Japanese Pharmacopoeia.
Subjects and study design of a preliminary clinical trial
Fifteen Japanese adults (3 males, 12 females) from 20 to 50 years of age with a tendency for constipation at equal to or less than 10 times of defecation per 2 weeks were selected as subjects under the declaration of Helsinki. Informed consent was obtained from all volunteers before the study began.
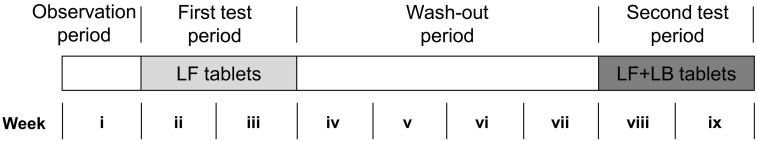
The study was conducted with the open design shown in Figure 1. Following a one-week (week i) observation period, the LF tablets were administered daily for 2 weeks (weeks ii and iii). After a wash-out period of 4 weeks (weeks iv-vii), the LF+LB tablets were administered daily for 2 weeks (weeks viii and ix). The subjects filled out a daily questionnaire on defecation frequency, stool output, defecation days, prescriptions and health remarks throughout the entire study period.
Fig. 1.
Study design of preliminary clinical trial.
Subjects and study design of a randomized placebo-controlled crossover clinical study
Thirty-two Japanese women from 20 to 60 years of age with a tendency for constipation at equal to or less than 10 times/2 weeks were selected as subjects under the declaration of Helsinki. Informed consent was obtained from all volunteers before the study began.
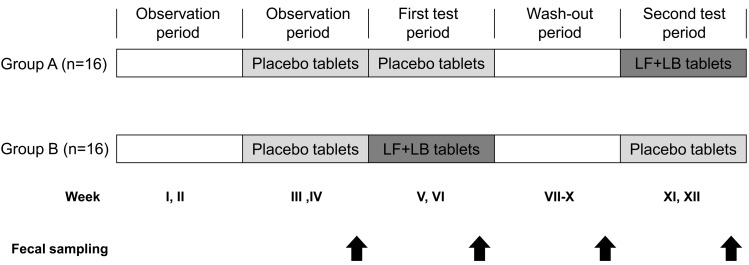
The study was conducted with a double-blind placebo-controlled crossover design shown in Fig. 2. The subjects were randomly divided into two groups. Following the observation period for 2 weeks from week I to week II, the placebo tablets were administered daily for 2 weeks, from week III to week IV. Then either the LF+LB or placebo tablets were administered daily for two weeks from week V to week VI. After a 4-week wash-out period, from week VII to week X, we replaced the LF+LB tablets with the placebo tablets and vice versa, which were administered daily for two weeks from week XI to week XII. All subjects were requested to stop consuming other lactoferrin supplements, other lactic acid bacteria supplements and dietary supplements that might have effects on the intestinal microbiota, such as oligosaccharides or dietary fiber. Antibiotics, cathartics, antidiarrheals, enemas and digestive medicines were restricted from being prescribed except at times of necessity. When the subjects used these medicines, the data of the subjects were excluded from the analysis. The subjects filled out a daily questionnaire on defecation frequency, stool output, defecation days, prescriptions and health remarks throughout the entire study period.
Fig. 2.
Study design of the randomized double-blind placebo-controlled crossover trial. Group A, n=16; Group B, n=16. Fecal samples were collected at the end of weeks IV, VI, X and XII.
Fecal microbiota analysis by anaerobic culture method
Fecal samples were collected at the end of weeks IV, VI, X and XII. Whole fecal samples were stored anaerobically (AnaeroPack-Anaero; Mitsubishi Gas Chemical Company Inc, Tokyo, Japan) at 4°C and used for fecal microbiota analysis within 24 hr after defecation. Decimal dilution series of fecal samples were prepared by adding the samples to the dilution solution. From an appropriate dilution, 50 µL of samples were plated onto the media, and the bacteria were analyzed according to Mitsuoka’s method [12]. Two nonselective media (blood liver, CDC anaerobe sheep blood agar) and three selective media (bifidobacteria-selective, cycloserine cefoxitin fructose agar, CW Egg-yolk agar with kanamycin) were used. Bacterial species were identified by colonization, Gram staining, morphology, lecithinase reaction, aerobic growth and sporulation. The bacteria were incubated for 3 to 7 days at 35°C.
Terminal restriction fragment length polymorphism analysis (T-RFLP analysis)
Fecal samples were kept in guanidine thiocyanate solution (100 mM Tris-HCl [pH 9.0],40 mM EDTA [pH 8.0],4 M guanidine thiocyanate) in sample tubes (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). Fecal microbiota analysis by the T-RFLP method was performed by Techno Suruga Laboratory Co., Ltd. T-RFLP analysis was performed as described in previous reports [13, 14]. The length of the terminal restriction fragments was determined using an ABI PRISM 3130xl genetic analyzer (Applied Biosystems,Carlsbad, CA, USA) and GeneMapper (Applied Biosystems).
Fecal color score
During the study, fecal color at every defecation was recorded in journals by the subjects. Fecal color was determined by the subjects themselves, who compared the fecal color with the tones corresponding to five stages on a card. The scores for the tones of the five stages were 1, yellow (No. 241): 2, ocher (No. 321): 3, pale ocher (No. 308): 4, brown (No. 311): and 5, dark brown (No. 647), according to a color sample book (Color Guide, 19th edition, Dainippon Ink and Chemicals, Inc., Tokyo, Japan). The difference between the mean fecal color score during two weeks of the LF+LB tablet ingestion period (weeks XI and XII for group A and weeks V and VI for group B) and that during the two weeks before the ingestion period (weeks IX and X group A and weeks III and IV for group B) for each subject was calculated. The mean of the differences with the standard error is indicated in the figure. Similarly, the difference between the mean fecal color score during the placebo tablet ingestion period (weeks V and VI for group A and weeks XI and XII for group B) and that during two weeks before the ingestion period (weeks III and IV for group A and weeks IX and X for group B) was calculated, and the mean with the standard error is shown.
Fecal water content
Fecal water content was calculated as the difference between the weights before and after freeze-drying a portion of the stool.
Statistical analysis
The results are expressed as mean values and standard errors. Data were analyzed with the SPSS 15.0 J software (SPSS Japan, Tokyo, Japan). Statistical differences between the optical densities of bifidobacteria cultures were analyzed by the Tukey-Kramer test. Statistical differences between the test and placebo groups and between before and after administration were examined with the Student’s t-test. Statistical differences between before and after 1 week or 2 week-administration were examined with the Dunnett’s test. Statistical differences in the appearance ratio of fecal bacteria were examined with the Student’s t-test. The difference between means was considered to be significant at p<0.05.
RESULTS
Growth of bifidobacteria in MRS broth supplemented with LF or LF hydrolysates
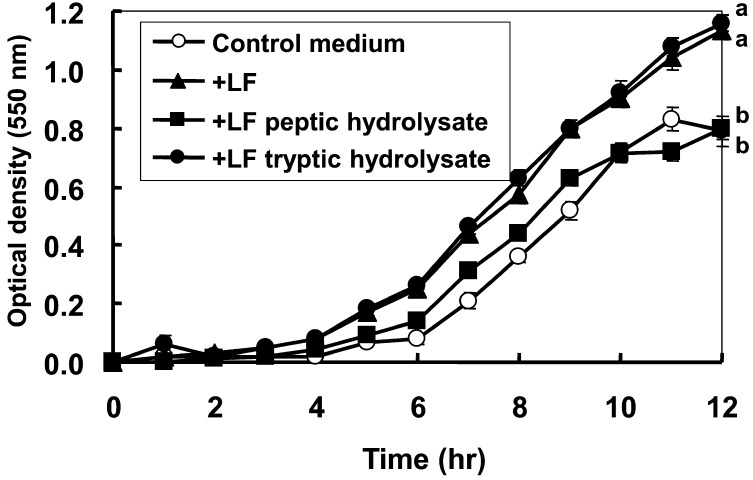
Fig. 3 shows the growth of B. longum in MRS broth supplemented with LF, LF hydrolysates produced by pepsin digestion and those produced by trypsin digestion. In contrast to the control medium and the medium containing peptic hydrolysates of LF, the medium containing LF and that containing tryptic hydrolysates of LF showed a greater growth promotional effect on B. longum. The optical density of the culture at 12 hr was significantly higher for the culture containing LF or the tryptic hydrolysate of LF compared with the control culture or the culture containing the peptic hydrolysate of LF.
Fig. 3.
Changes in optical density during the growth of Bifidobacterium longum ATCC 15707 in MRS broth supplemented with LF or LF hydrolysates. B. longum (5 ×106 cfu) was inoculated into 3 mL of MRS broth containing 5 mg/ml of LF (triangles), peptic hydrolysate of LF (squares) or tryptic hydrolysate of LF (closed circles) and cultured anaerobically at 37 °C. Statistical differences at 12 hr were analyzed by the Tukey-Kramer test. Data are statistically different among the points with different letters. p<0.01 between tryptic hydrolysate of LF and the control or peptic hydrolysate of LF, and p<0.05 between LF and the control or peptic hydrolysate of LF.
Effect on defecation frequency in the preliminary clinical trial
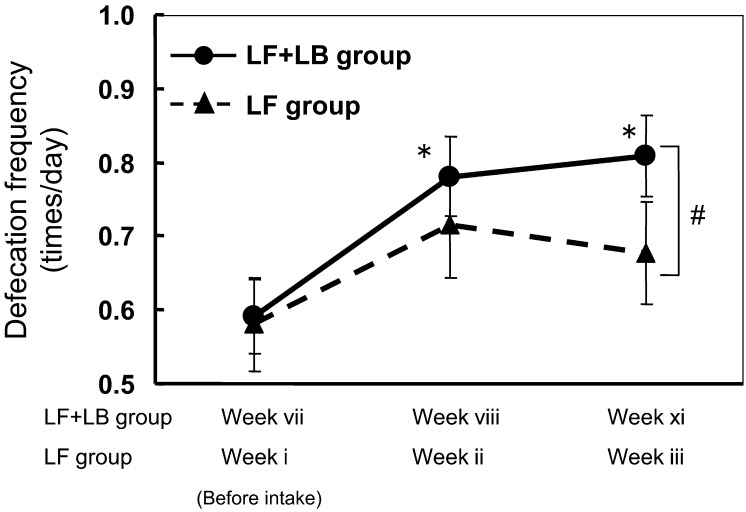
Fig. 4 shows the effect of ingestion of the LF+LB tablets and LF tablets on defecation frequency during the test period. During the LF tablet ingestion period, defecation frequency in the subjects tended to increase from 0.58 times/day (week i) to 0.71 times/day (week ii) and then to 0.68 times/day (week iii), but the differences were not statistically significant. During the LF+LB tablet ingestion period, defecation frequency in the subjects significantly increased from 0.59 times/day (week vii) to 0.78 times/day (week viii, p<0.05, Dunnett’s test) and then 0.81 times/day (week ix, p<0.05, Dunnett’s test). The frequency during the LF+LB tablet ingestion period (week ix) was significantly higher than that during the LF tablet ingestion period (week iii, Student’s t-test, p<0.05).
Fig. 4.
Effect of the LF tablets and the LF+LB tablets on the defecation frequency of the subjects in the preliminary clinical trial. Data are presented as means with standard errors represented by vertical bars. (*p<0.05, Dunnett’s test, #p<0.05, Student’s t-test)
Effect on intestinal microbiota in the randomized placebo-controlled crossover clinical study
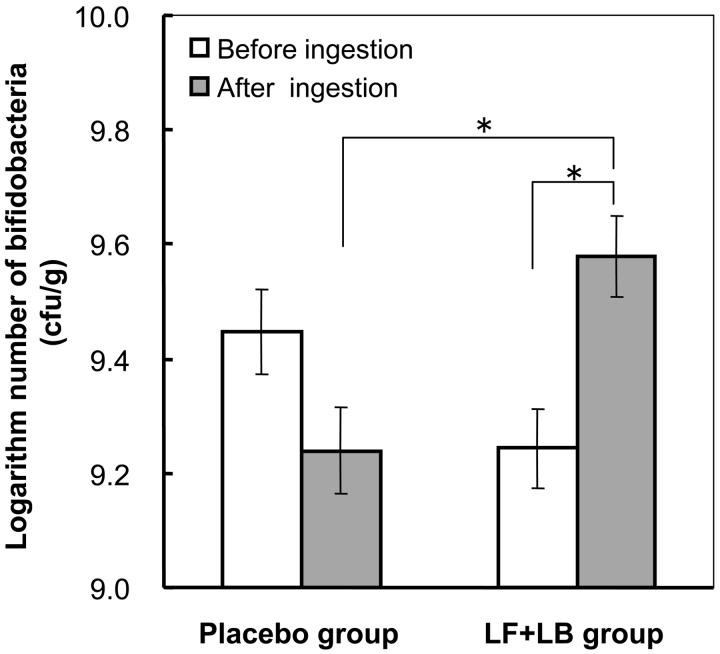
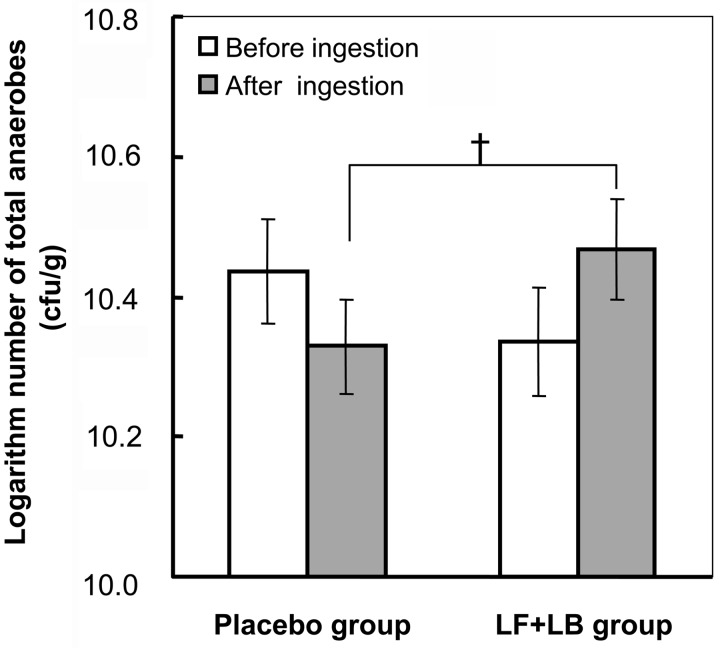
Figs. 5 and 6 show the effect of the LF+LB tablets on the intestinal microbiota. During the LF+LB tablet ingestion period (from the start of week XI to the end of week XII in group A and from the start of week V to the end of week VI in group B), the average number of bifidobacteria in the feces significantly increased from 109.24 (SE 100.13) cfu/g to 109.58 (SE 100.13) cfu/g (Student’s t-test, p<0.05). However, during the placebo tablet ingestion period (from the start of week V to the end of week VI in group A and from the start of week XI to the end of week XII in group B), it decreased from 109.45 (SE 100.14) cfu/g to 109.24 (SE 100.13) without statistical significance (Fig. 5). The average number of bifidobacteria at the end of the LF+LB tablet ingestion period was significantly higher than that at the end of the placebo tablet ingestion period (Student’s t-test, p<0.05). Also, the average change in the number of bifidobacteria after the LF+LB tablet ingestion period compared with that before ingestion was significantly higher than that after the placebo tablet ingestion period (Student’s t-test, p<0.05, data not shown). As shown in Fig. 6, during the LF+LB tablet ingestion period, the average number of total anaerobes in the feces increased from 1010.34 (SE 100.08) cfu/g to 1010.47 (SE 100.07) cfu/g, but the number decreased during the placebo tablet ingestion period from 1010.44 (SE 100.07) cfu/g to 1010.33 (SE 100.07) cfu/g. However, these changes were not statistically significant. The average number of anaerobes at the end of the LF+LB tablet ingestion period tended to be higher than that at the end of the placebo tablet ingestion period (Student’s t-test, p<0.1). The average change during the LF+LB tablet ingestion period also tended to be higher than that during the placebo tablet ingestion period (Student’s t-test, p<0.1, data not shown).
Fig. 5.
Number of bifidobacteria in feces determined by the anaerobic culture method in the randomized placebo-controlled crossover clinical study. The numbers of bifidobacteria before and after the LF+LB or placebo tablet ingestion period are shown as the mean +/− SE. *p<0.05 in the Student’s t-test.
Fig. 6.
Number of total anaerobes in feces determined by the anaerobic culture method in the randomized placebo-controlled crossover clinical study. The numbers of anaerobes before and after the LF+LB or placebo tablet ingestion period are shown as the mean +/− SE. †p<0.1 in the Student’s t-test.
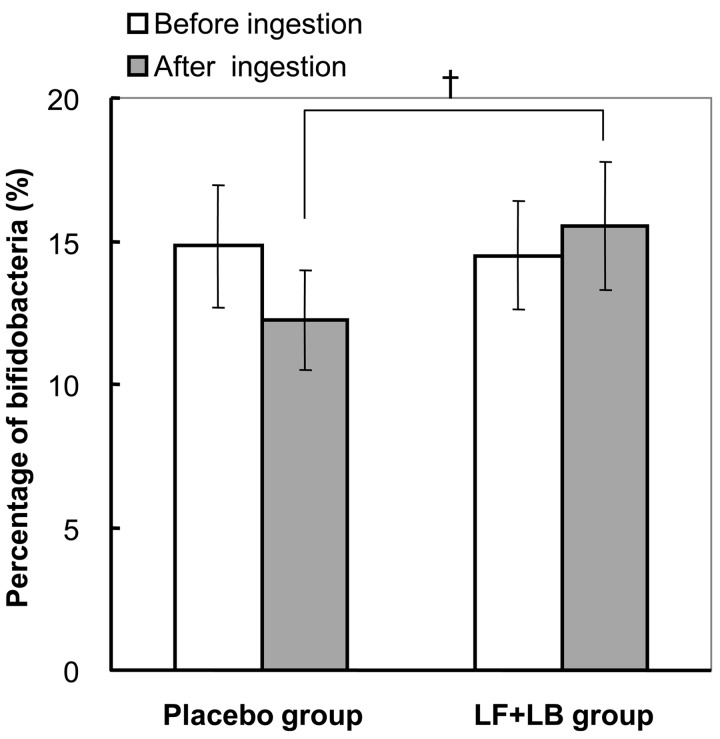
Fig. 7 shows the effect of the LF+LB tablets on the intestinal microbiota analyzed by T-RFLP. During the LF+LB tablet ingestion period, the percentage of bifidobacteria to the total bacteria in the feces increased from 14.5% (SE 1.9) to 15.5% (SE 2.2). In contrast, during the placebo tablet ingestion period, it decreased from 14.8% (SE 2.1) to 12.2% (SE 1.8). However, these changes were not statistically significant. The average percentage of bifidobacteria at the end of the LF+LB tablet ingestion period tended to be higher than that at the end of the placebo tablet ingestion period (Student’s t-test, p<0.1). The average change during the LF+LB tablet ingestion period also tended to be higher than that during the placebo tablet ingestion period (Student’s t-test, p<0.1, data not shown).
Fig. 7.
Occupancy of bifidobacteria in intestinal microbiota analyzed by T-RFLP analysis in the randomized placebo-controlled crossover clinical study. The percentages of bifidobacteria to the total bacterial cells before and after the LF+LB or placebo tablet ingestion period are shown as the mean +/− SE. †p<0.1 in the Student’s t-test.
Fecal color and water content
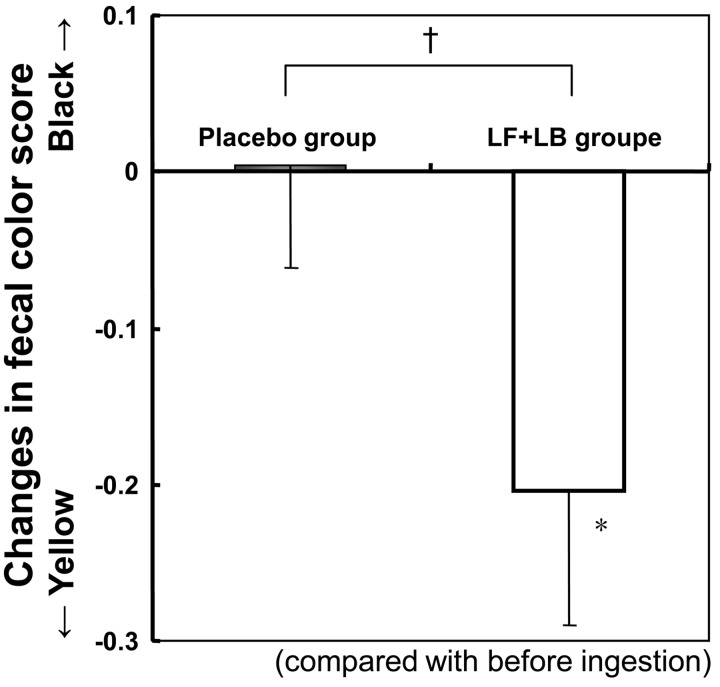
Fig. 8 shows the effect of the LF+LB tablets on fecal color score. During the test period, the fecal color score decreased significantly compared with that before ingestion (Student’s t-test, p<0.05), indicating that the fecal color changed, becoming yellowish. In contrast, during the placebo period, the fecal color score did not change significantly.
Fig. 8.
Changes in the fecal color score in the randomized placebo-controlled crossover clinical study. Fecal color was recorded daily by the subjects, who compared the color with the tones of five color stages on a card. The scores for the tones of the five stages were from 1, light yellow, to 5, dark brown, in incremental steps. Average changes in the fecal color score during the LF+LB or placebo tablet ingestion period are shown. *p<0.05 in the Student’s t-test for the fecal color score before and after the tablet ingestion period. †p=0.1 in the Student’s t-test between the average difference during the LF+LB tablet ingestion period and that during the placebo tablet ingestion period.
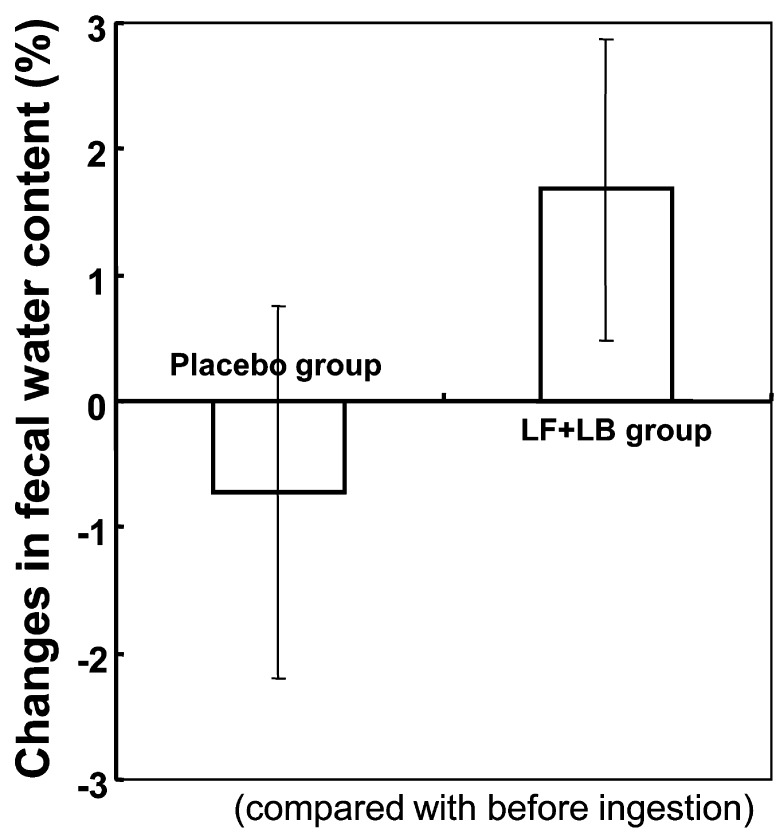
Fig. 9 shows the effect of the LF+LB tablets on fecal water content. During the test period, the fecal water content appeared to increase compared with that before ingestion, while during the placebo period, the fecal water content appeared to decrease slightly. However, the differences were not statistically significant.
Fig. 9.
Changes in the fecal water content in the randomized placebo-controlled crossover clinical study. Average changes in fecal water content during the LF+LB or placebo tablet ingestion period are shown.
Defecation frequency
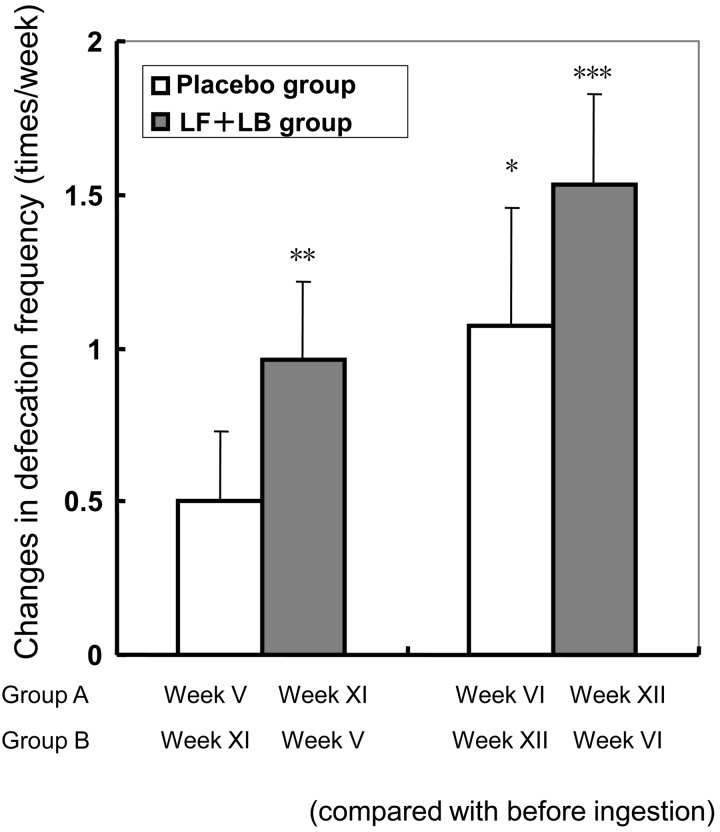
Fig. 10 shows the effect of the LF+LB tablets on the defecation frequency. During the LF+LB tablet ingestion period (weeks XI and XII for group A and weeks V and VI for group B), defecation frequency in the subjects increased by 0.96 (SE 0.26) times/week in the first week (week 1 was week XI for group A and week V for group B, Dunnett’s test, p<0.01) and by 1.54 (SE 0.29) times/week in the second week (week 2 was week XII for group A and week VI for group B, Dunnett’s test, p<0.001) compared with that before ingestion. During the placebo tablet ingestion period (weeks V and VI for group A and weeks XI and XII for group B), defecation frequency in the subjects increased by 1.07 (SE 0.38) times/week in the second week (week 2 was week VI in group A and week XII in group B, Dunnett’s test, p<0.05). For week 1 (week V in group A and week XI in group B) of the placebo period, the frequency appeared to increase slightly, but there was no significant difference.
Fig. 10.
Changes in the defecation frequency of the subjects in the randomized placebo-controlled crossover clinical study. Average increases in defecation frequency (times/week) during the LF+LB or placebo tablet ingestion period (with the standard error represented by vertical bars) are shown. Data are shown as mean frequency (times/week) for the first or second week of the tablet ingestion period with the standard error represented by vertical bars. *p<0.05, **p<0.05, ***p<0.001 (Dunnett’s test compared with the defecation frequency before ingestion period).
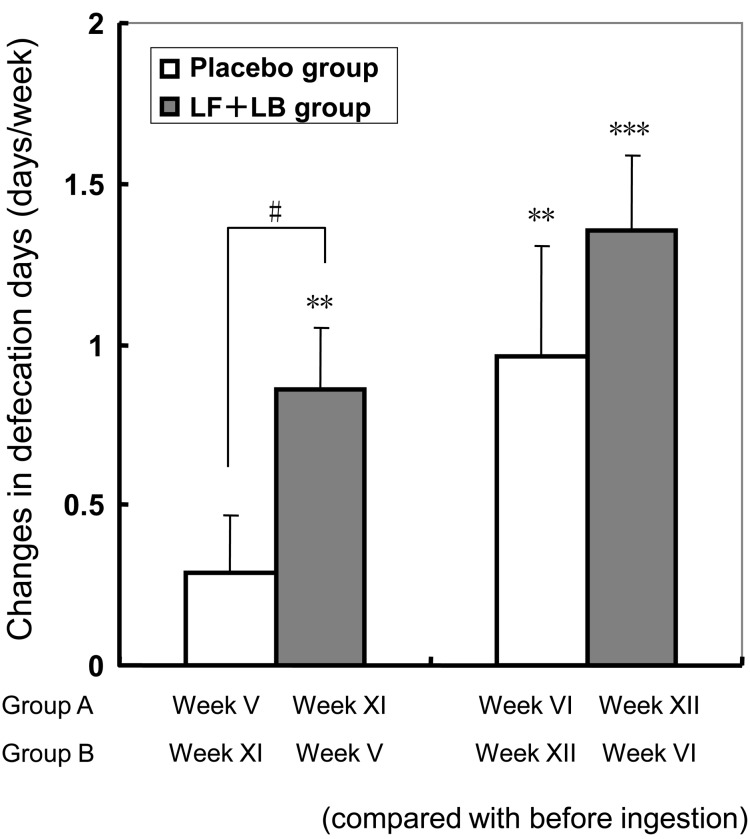
Fig. 11 shows the effect of the LF+LB tablets on the defecation days. During the LF+LB tablet ingestion period, the defecation days in the subjects increased by 0.86 (SE 0.20) times/week in the first week (Dunnett’s test, p<0.01) and by 1.36 (SE 0.23) times/week in the second week (Dunnett’s test, p<0.001) compared with that before ingestion. During the placebo tablet ingestion period, defecation frequency in the subjects increased by 0.96 (SE 0.34) times/week in the second week (Dunnett’s test, p<0.01), compared with that before ingestion. During the first week of the placebo tablet ingestion period, weekly defecation days tended to increase, but not significantly. The average difference in the frequency during the first week in the LF+LB tablet ingestion period was significantly higher than that in week 1 in the placebo tablet ingestion period (Student’s t-test p<0.05).
Fig. 11.
Changes in the defecation days per week of the subjects in the randomized placebo-controlled crossover clinical study. Average increases in defecation days per week during the LF+LB or placebo tablet ingestion period (with the standard error represented by vertical bars) are shown. *p<0.05, **p<0.05, ***p<0.001 (Dunnett’s test compared with the defecation days before the ingestion period). #p<0.05 in the Student’s t-test between the average difference during the LF+LB tablet ingestion period and that during the placebo tablet ingestion period.
DISCUSSION
In the present study, we demonstrated in a double-blind placebo-controlled crossover trial that ingestion of enteric-coated tablets containing LF and LB promoted the intestinal health of Japanese women with a tendency for constipation, in terms of an increase in defecation frequency and the increased number of bifidobacteria in feces. Since LF and LF tryptic fragments promoted the growth of bifidobacteria in vitro, it was suggested that growth of bifidobacteria was promoted in the intestine by ingesting the tablets, which possibly caused the improvement of the defecation function of the intestines.
We paid attention to the antibacterial activity [15] of LF on harmful bacteria and growth promotion effects on typical good bacteria, bifidobacteria. There are reports that peptic and tryptic hydrolysates of LF promoted the growth of B. bifidum, B. breve and B. infantis in vitro [16, 17], and for B. bifidum in particular, the growth stimulatory effects of the tryptic hydrolysate of LF were higher than those of the peptic hydrolysates; no effects of LF and the tryptic hydrolysate on the growth promotion of B. longum were observed. However, afterwards, growth promotion effects were reported for B. longum by the same research group [18]. These growth promotion effects of LF were dose and strain dependent. In the present study, we observed that LF and the tryptic hydrolysate of LF, but not the peptic hydrolysate of LF, promoted the growth of B. longum. The possible reason was presumed to be that the concentration of LF and hydrolysate of LF in culture media was higher than reported previously [16, 17]. In addition, there is a report that the peptic hydrolysate of LF was degraded to many small peptides but that the trypsin hydrolysate of LF was degraded to four fragments that included full-length LF when checked by SDS-PAGE [11]. These seem to be the reasons why the trypsin hydrolysate maintained the growth promoting function. There are many reports concerning the bifidobacterium growth-promoting effects of LF in vitro, but research results for humans are limited to the studies performd for infants [19, 20]. The reason for this is presumed to be because intact LF reaches the intestines in infants with undeveloped digestive functions of the stomach but not in adults. Balmar et al. [20] reported that administering LF to infants for 14 days did not influence the pattern of fecal microbiota. However, it was presumed that the administration period was too short for the effect to be observed. In the present study, it was possible to make intact LF reach the intestines in adults using the enteric-coated LF. Moreover, it is presumed that the LF or hydrolysate of LF that reached the intestines activated bifidobacterium inhabiting the large intestine. However, ingestion of the tablet containing LF but not LB did not significantly improve defecation frequency in humans (Fig. 4).
There are reports that L. brevis improves the immunostimulatory action by increasing interferon (IFN)-α secretion and natural killer (NK) activity [8, 9], ameliorates the development of dermatitis atopic dermatitis [21] and exerts phylactic action [22]. In addition to these reports, L. brevis was shown to be a promising probiotic candidate by an in vitro and in vivo feeding trial study [10]. As shown in Fig. 4, oral administration of enteric-coated tablets containing LF and L. brevis improved the fecal properties of adults with a tendency for constipation compared with enteric-coated tablets containing LF without L. brevis. The number of fecal bifidobacteria significantly increased in the LF+LB tablet intake group compared with the placebo tablet intake group. The number of fecal bifidobacteria significantly increased during the LF+LB tablet ingestion period. It seems that LF and the tryptic digest of LF escape digestion in the stomach due to the enteric coating, and promote the growth of bifidobacteria in the intestines. The defecation frequency and defecation days per week increased in the LF+LB tablet intake group compared with the placebo tablet intake group. Accurate consideration of these results cannot be done with the present study. Ogata et al. [23] reported that the defecation frequency increased even when there was no drastic change in the microbiota. They consider that improvement of the total intestinal environment, which is influenced by fecal microbiota including bifidobacteria, putrefactive substances, enzyme activities and other characteristics comprising water content, organic acids and acetic acid, is possibly effective for easing defecation.
Recent reports clearly demonstrated that ingested bifidobacteria [24] or lactobacilli [25] reached and increased in the intestine using a polymerase chain reaction (PCR) method in addition to a culturing method. In these reports, the total number of intestinal bifidobacteria was shown to increase 2–3-folds, which was considered to result in an increased defecation frequency. Similar to these previous reports, we observed an increase in the number of bifidobacteria in feces as a result of ingestion of LF+LB tablets. Therefore, it can be presumed that the increased defecation frequency observed in this study was the consequence of the increase in bifidobacteria in the intestines.
In conclusion, the results obtained in this study suggest that enteric-coated LF tablets containing Lactobacillus brevis subsp. coagulans have an efficacy for improving the intestinal microbiota and intestinal function by the bifidobacterium growth-promoting effects of LF and some effects of L. brevis.
REFERENCES
- 1.Tomita M, Bellamy W, Takase M, Yamauchi K, Wakabayashi H, Kawase K. 1991. Potent antibacterial peptides generated by pepsin digestion of bovine lactoferrin. J Dairy Sci 74: 4137–4142 [DOI] [PubMed] [Google Scholar]
- 2.Harmsen MC, Swart PJ, de Béthube MP, Pauwels R, De Clercq E, The TH, Meijer DK. 1995. Antiviral effects of plasma and milk proteins: lactoferrin shows potent activity against both human immunodeficiency virus and human cytomegalovirus replication in vitro. J Infect Dis 172: 380–388 [DOI] [PubMed] [Google Scholar]
- 3.Zimecki M, Wlaszczyk A, Cheneau P, Brunel AS, Mazurier J, Spik G, Kübler A. 1998. Immunoregulatory effects of a nutritional preparation containing bovine lactoferrin taken orally by healthy individuals. Arch Immunol Ther Exp (Warsz) 46: 231–240 [PubMed] [Google Scholar]
- 4.Shoji H, Oguchi S, Shinohara K, Shimizu T, Yamashiro Y. 2007. Effects of iron-unsaturated human lactoferrin on hydrogen peroxide-induced oxidative damage in intestinal epithelial cells. Pediatr Res 61: 89–92 [DOI] [PubMed] [Google Scholar]
- 5.Sekine K, Ushida Y, Kuhara T, Iigo M, Baba-Toriyama H, Moore MA, Murakoshi M, Satomi Y, Nishino H, Kakizoe T, Tsuda H. 1997. Inhibition of initiation and early stage development of aberrant crypt foci and enhanced natural killer activity in male rats administered bovine lactoferrin concomitantly with azoxymethane. Cancer Lett 121: 211–216 [DOI] [PubMed] [Google Scholar]
- 6.Ono T, Murakoshi M, Suzuki N, Iida N, Ohdera M, Iigo M, Yoshida T, Sugiyama K, Nishino H. 2010. Potent anti-obesity effect of enteric-coated lactoferrin: decrease in visceral fat accumulation in Japanese men and women with abdominal obesity after 8-week administration of enteric-coated lactoferrin tablets. Br J Nutr 104: 1688–1695 [DOI] [PubMed] [Google Scholar]
- 7.Petschow BW, Talbott RD. 1991. Response of bifidobacterium species to growth promoters in human and cow milk. Pediatr Res 29: 208–213 [DOI] [PubMed] [Google Scholar]
- 8.Kishida T, Uno K, Kishi A, Onishi T, Matsubara Y. 1993. Enhancement of immunological functions by Lactobacillus brevis subsp. coagulans. Clin Rep 27: 3701–3707 [Google Scholar]
- 9.Kishi A, Uno K, Matsubara Y, Okuda C, Kishida T. 1996. Effect of the oral administration of Lactobacillus brevis subsp. coagulans on interferon-α producing capacity in humans. J Am Coll Nutr 15: 408–412 [DOI] [PubMed] [Google Scholar]
- 10.Rönkä E, Malinen E, Saarela M, Rinta-Koski M, Aarnikunnas J, Palva A. 2003. Probiotic and milk technological properties of Lactobacillus brevis. Int J Food Microbiol 83: 63–74 [DOI] [PubMed] [Google Scholar]
- 11.Ono T, Morishita S, Fujisaki C, Ohdera M, Murakoshi M, Iida N, Kato H, Miyashita K, Iigo M, Yoshida T, Sugiyama K, Nishino H. 2011. Effect of pepsin and trypsin on the anti-adipogenic action of lactoferrin against pre-adipocytes derived from mesenteric fat. Br J Nutr 105: 200–211 [DOI] [PubMed] [Google Scholar]
- 12.Mitsuoka T. 1980. A color atlas of anaerobic bacteria, Sobun-Press, Tokyo, pp 51–92. [Google Scholar]
- 13.Nagashima K, Hisada T, Satou M, Mochizuki J. 2003. Application of new primer-enzyme combinations to terminal restriction fragment length polymorphism profiling of bacterial populations in human feces. Appl Environ Microbiol 69: 1251–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagashima K, Mochizuki J, Hisada T, Suzuki S, Shimomura K. 2006. Phylogenetic analysis of 16S ribosomal RNA gene sequences from human fecal microbiota and improved utility of terminal restriction fragment length polymorphism profiling. Biosci Microflora 25: 99–107 [Google Scholar]
- 15.Saito H, Miyakawa H, Ishibashi N, Tamura Y, Hayasawa H, Shimamura S. 1996. Effects of iron-free and metal-bound forms of lactoferrin on the growth of bifidobacteria, E. coli and S. aureus. Biosci Microflora 15: 1–7 [Google Scholar]
- 16.Kim WS, Ohashi M, Tanaka T, Kumura H, Kim GY, Kwon IK, Goh JS, Shimazaki K. 2004. Growth-promoting effects of lactoferrin on L. acidophilus and Bifidobacterium spp. Biometals 17: 279–283 [DOI] [PubMed] [Google Scholar]
- 17.Kim WS, Rahman M, Kumura H, Shimazaki K. 2005. Comparison of growth promoting effects on Bifidobacterium spp. by bovine lactoferrin hydrolysates. Biocience Microflora 24: 119–123 [Google Scholar]
- 18.Rahman MM, Kim WS, Ito T, Kumura H, Shimazaki K. 2009. Growth promotion and cell binding ability of bovine lactoferrin to Bifidobacterium longum. Anaerobe 15: 133–137 [DOI] [PubMed] [Google Scholar]
- 19.Roberts AK, Chierici R, Sawatzki G, Hill MJ, Volpato S, Vigi V. 1992. Supplementation of an adapted formula with bovine lactoferrin: 1. Effect on the infant fecal flora. Acta Paediatr 81: 119–124 [DOI] [PubMed] [Google Scholar]
- 20.Balmer SE, Scott PH, Wharton BA. 1989. Diet and fecal flora in the newborn: lactoferrin. Arch Dis Child 64: 1685–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Segawa S, Hayashi A, Nakakita Y, Kaneda H, Watari J, Yasui H. 2008. Oral administration of heat-killed Lactobacillus brevis SBC8803 ameliorates the development of dermatitis and inhibits immunoglobulin E production in atopic dermatitis model NC/Nga mice. Biol Pharm Bull 31: 884–889 [DOI] [PubMed] [Google Scholar]
- 22.Yajima N, Yakabe T, Fukui Y, Suzuki S, Arakawa C, Nobuta Y, Fukao M. 2007. The physiological characters and functions of Lactobacillus brevis KB290(Labre). Milk Sci 55: 151–156 [Google Scholar]
- 23.Ogata T, Nakamura T, Anjitsu K, Yaeshima T, Takahashi S, Fukuwatari Y, Ishibashi N, Hayasawa H, Fujisawa T, Iino H. 1997. Effect of Bifidobacterium longum BB536 administration on the intestinal environment, defecation frequency and fecal characteristics of human volunteers. Biosci Microflora 16: 53–58 [Google Scholar]
- 24.Ishizuka A, Tomizuka K, Aoki R, Nishijima T, Saito Y, Inoue R, Ushida K, Mawatari T, Ikeda T. 2012. Effect of administration of Bifidobacterium animalis subsp. lactis GCL2505 on defection frequency and bifidobacterial microbiota composition in humans. J Biosci Bioeng 113: 587–591 [DOI] [PubMed] [Google Scholar]
- 25.Yamano T, Iino H, Takada M, Blum S, Rochat F, Fukushima Y. 2006. Improvement of the human intestinal flora by ingestion of the probiotic strain Lactobacillus johnsonii La1. Br J Nutr 95: 303–312 [DOI] [PubMed] [Google Scholar]