Abstract
Gut microbes are present in large concentrations on the human intestinal mucosal surface and play important roles in health and disease of the host. Numerous groups of gut microbes are associated with immunological and metabolic diseases and in maintaining health status of the host. Among these health- and disease-associated gut microbes, Bacteroides, Clostridium and Bifidobacterium appear regularly in the list. Scientific and clinical evidence available to date indicates that diet is a major driving factor for the establishment of the gut microbiome. Slow digestible carbohydrates (human milk glycan, inulin and fructooligosaccharide), insoluble complex carbohydrates and protein diets favor the growth of Bacteroides, Clostridium and Bifidobacterium. Fat on the other hand suppresses the number of Bacteroides, Clostridium and Bifidobacterium; whereas polyphenols in general suppress Bacteroides and Clodtridium but enhance the Bifodobacterium. The implication is that dietary habits could be a major determinant of health and disease susceptibility. Dietary strategies could be an effective means of potentially inducing changes in intestinal microbiota and are certainly achievable, thus facilitating correction of intestinal microbiome aberrations or imbalances to improve our health. Most of the physiological and functional interactions between individual dietary components and the concoction of foods in a meal and gut microbiota have not yet been well studied. A concerted effort is required to acquire better understanding of their interaction in order to rationally maintain our intestinal microbiome homeostasis and general health through dietary intervention.
Keywords: gut microbes, microbiome, microbiota profile, diet
GUT MICROBES AND HEALTH
Gut microbes play important roles in health and disease of the host for the obvious reasons that they are present in large concentrations, closely associated with the host mucosal surface and interact with the host.
It has been widely demonstrated in animal and clinical studies that gut microbiota are involved in maturation and regulation of host immunity and gut functions. Capsular antigen of the human commensal Bacteroides fragilis triggers T cell-dependent immune responses that can affect both the development and homeostasis of the host immune system [1,2,3]. Colonization of the intestinal tract by Lactobacillus and Bifidobacterium exerts a barrier effect and protects the host against pathogens [4,5,6]. Escherichia coli, Klebsiella pneumonia and Streptococcus viridans on the other hand significantly increase intestinal permeability. An increase in permeability can lead to inappropriate immune responses, resulting in diseases like Crohn’s disease [7], Celiac disease and associated type 1 diabetes mellitus [8].
It has been reported that disruption of the microbiome balance can result in overgrowth of Gram-negative enteric bacteria such as Pseudomonas and Staphylococcus aereus and a lower level of Bifidobacterium, Eubacterium rectale/Clostridium coccoides group and Bacteroides-like MIB, resulting in a higher incidence of systemic infection [9] and metabolic disorder [10].
Certain intestinal bacteria such as Bacteroides, Enterobacteriaceae and Clostridium are able to produce mutagens in the presence of dietary precursors, through the actions of β-glucuronidase and nitroreductase [11]. Free radical-producing Enterococcus faecalis, has a positive correlation with human adenomas [12].
Additionally, certain lactic acid bacteria, such as Lactobacillus rhamnosus and Lactobacillus acidophilus, produce bactericidal substances such as bacteriocins, lactic acid and acetic acid [13]. Lower intestinal pH also increases inhibitory activities of other organic acids. Bacterial metabolites such as hydrogen peroxide can also be cytotoxic to other bacteria [5, 14]. Bifidobacterium produces antimicrobial agents that inhibit Gram-positive and Gram-negative organisms [15].
Bacterial fermentation of dietary fiber and slowly digestible carbohydrates forms a range of bacterial metabolites, including short-chain fatty acids (SCFAs), typically acetate, propionate and butyrate. These represent additional energy sources for the host, which would otherwise not available. Acetate is mainly metabolized in the peripheral tissues and is lipogenic, whereas propionate is transported to the liver and is gluconeogenic. Butyrate is the preferred energy source for the colonocytes and has a role in regulating host gene expression [16, 17].
Methanogens comprise up to 10% of all anaerobes in the colons of healthy adults [18,19,20]. The most common methanogenic archaeon found in the gut is Methanobrevibacter smithii, which can reduce CO2 with H2 to methane, allowing an increase in transformation of nutrients into calories [21]. The colonization of Methanobrevibacter in anorexia nervosa patients has been suggested to be an adaptive attempt towards optimization of food transformation in very low calorie diet intake by these patients. The hosts, however, do pay a price in harborng Methanobrevibacter, as it is related to constipation and diverticulosis [22, 23].
The two most abundant bacteria phyla found in healthy individuals are the Bacteroidetes and Firmicutes [24, 25]. The relative abundance of the two phyla in healthy adults appears to be relatively constant, although they do not always comprise the same species. This suggests multiple representatives of the functional groups [26]. The existence of dominant groups of bacteria presumably acts to conserve the metabolic functions essential to gut health, and indeed, a functional gene core has been identified in the gut metagenome [27]. Overall, a balanced gut microbiota composition confers benefits to the host, while microbial imbalances are associated with metabolic and immune-mediated disorders (as summarized in Table 1).
Table 1. Examples of aberrations in the gut microbiota linked to diseases.
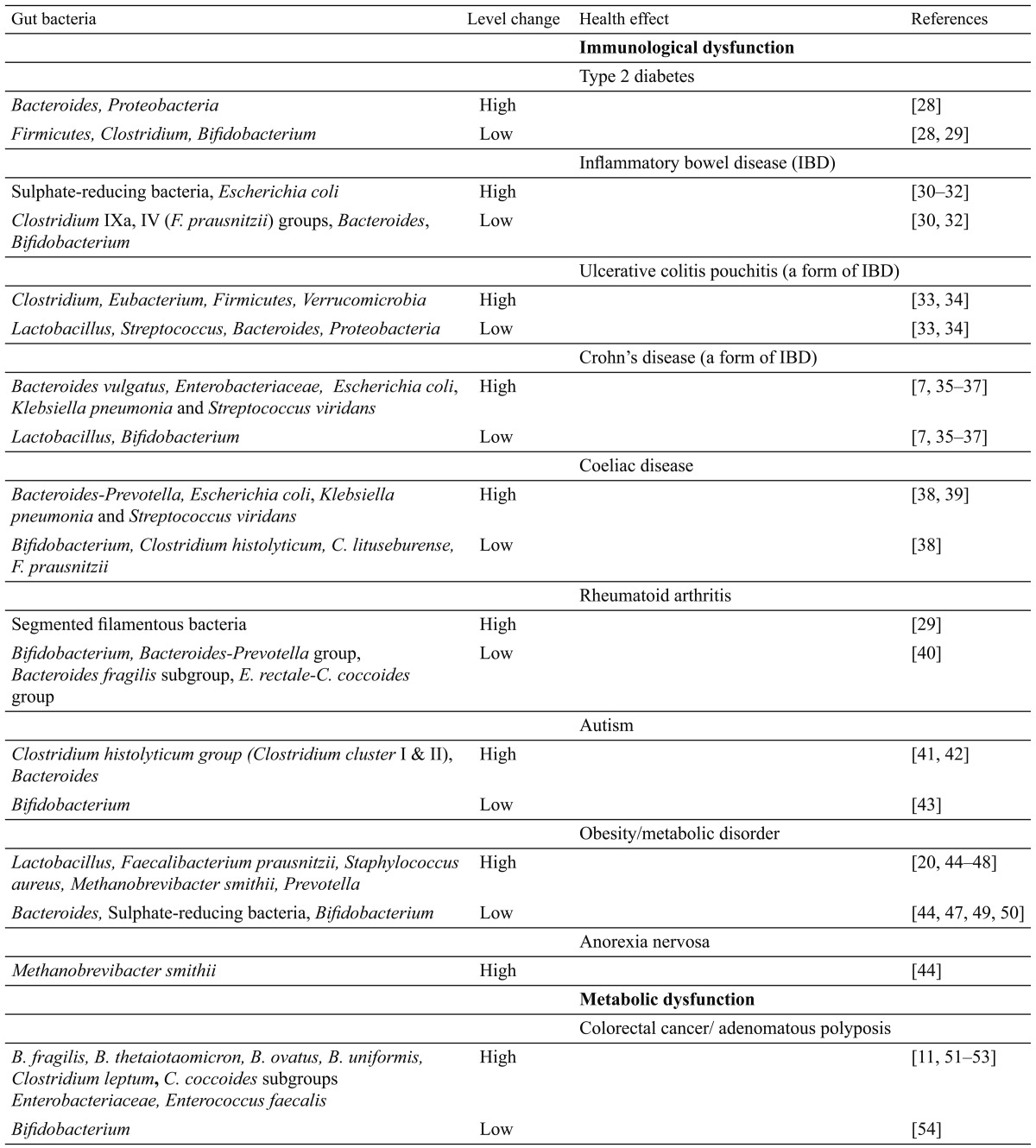
It is interesting to note that Bacteroides among the Bacteroidetes, and lactic acid bacteria (Bifidobacterium) and Clostridium among the Firmicutes consistently appear in diseases involving intestinal microbiome aberrations (in Table 1), and their aberrations could be either higher or lower in abundance. These again support the notion that microbiome homeostasis contributes to gut health and disease.
There is increasing clinical evidence demonstrating that these gut microbiota-linked diseases could be prevented and reverted through microbial (probiotic) intervention. Probiotics are live microorganisms that when administered in adequate amounts confer a health benefit in the host [55]. In clinical studies providing a selected Bifidobacterium or Lactobacillus to mothers with a family history of atopic diseases 2-4 weeks before delivery and to the newborn for 6 months, the incidences of atopic diseases was successfully reduced by half in the infants, and the beneficial effect was still evident after seven years [56, 57]. Lactobacillus, Bifidobacterium, Streptococcus and Escherichia coli showed a protective effect against inflammatory bowel disease in human studies [58, 59]. Lactobacillus and Bifidobacterium improve the clinical conditions in patients with irritable bowel syndrome [60]. In a study on mutagenicity of beef consumption, Lactobacillus casei intake was shown to reduce mutagenicity in urine. This was attributed to reduction in the population of pro-mutagen- forming intestinal bacteria [61]. In patients who underwent surgical treatment for superficial bladder cancer, intake of Lactobacillus prolonged the recurrence- free period [62, 63]. These studies suggest that intestinal microbiota are directly involved in the causation of these diseases, and demonstrate the prospect of balancing gut microbiota for prevention and treatment of these diseases.
Consumption of Lactobacillus-fermented milk resulted in an increase in Lactobacillus and Bifidobacterium counts accompanied by a decrease in Clostridium counts [64]. Other fermented foods, such as Japanese natto and miso, also affect intestinal microbiota. Consumption of these foods resulted in increased levels of Bacillus and Bifidobacterium and decreased levels of Enterobacteriaceae and Clostridium [65].
The probiotic approach, though effective in amending the gut microbiome, is a reactive approach nonetheless, as the gut environment of a diseased individual probably favors the proliferation and establishment of aberrant disease-causing microbes. Maintaining intestinal micro-biome homeostasis is arguably a desirable approach in disease prevention. To achieve intestinal microbiome homeostasis, it is necessary to understand factors that influence microbiome composition.
The composition of the gut microbiota is influenced by endogenous and external factors, such as microbes acquired at birth, diet, host physiology, drug intake and disease [66]. Of these factors, the diet is considered a major driver for changes in gut microbiota diversity, as it provides nutrition and alters the microenvironment for microbes. It could be safely assumed that the distinct differences in microbiota of the adult and infant types are the responses to the host physiological stage and to their different diets. Dietary strategies could be an effective means of potentially inducing changes in intestinal microbiota and are certainly achievable, thus facilitating correction of intestinal microbiome aberrations or imbalances to improve our health.
DIETS AFFECTING THE COMPOSITION OF THE GUT MICROBIOME
It was estimated that about 40% of the microbial genes present in each human individual are shared with at least half of the human individuals in the same geographical cohort [27]. This suggests the existence of a functional core (core microbiome). The functional core may contain shared metabolic functions (e.g., degradation of carbohydrates, production of vitamins) as well as sequential pathways that would, respectively, restrict or expand functional diversity. Several recent reviews have discussed evolutionary and functional aspects of microbial diversity in the human intestine [67,68,69]. The aim of this paper was to extend and exploit new insights, so as to provide better understanding of the responsiveness, variability and resilience of the gut microbiome community with regard to dietary intake and host physiology.
Carbohydrates and sugars
Slow digestible oligosaccharides
The most studied dietary components affecting human health and the gut microbiome are the slowly digestible complex carbohydrates, such as oligosaccharides, which are termed prebiotics [70]. They are slowly digestible by human digestive enzymes but fermentable by some gut microbes, thus selectively stimulating the proliferation and/or activity of selected gut bacterial populations.
The first natural slowly digestible oligosaccharides we encounter are the human milk glycans (HMG), which enrich the gut microbiota that are able to metabolize complex carbohydrates [71]. The ability of certain Bifidobacterium strains (in particular B. infantis) to efficiently use HMG suggests that production of milk oligosaccharides by the mother may be a strategy to ensure the presence of this group of bacteria in the infant gut [72]. The consumption of HMG by Bacteroides species [73] suggests that milk glycans may attract different groups of mutually benefiting microbes to the infant intestine. The colonization of Bacteroides in our intestinal tract is an example of the highly evolved microbe-host interactions in establishing gut microbiome homeostasis. The appearance of Bacteroides thetaiotaomicron in the human gut induces the production of fucα1,2Galβ-containing glycans, which serves as a selective carbon substrate for the bacterium [74].
Consumption of Jerusalem artichoke inulin was reported to increase the fecal Bifidobacterium level and to cause a small though significant increase in the level of the Lactobacillus/Enterococcus group [75]. Besides the Bifidobacterium, two groups of butyrate-producing bacteria, namely Faecalibacterium prausnitzii and a group of clostridial cluster XIVa bacteria, were found to increase following inulin/fructooligosaccharide (FOS) supplementation in a human intervention study, [26, 76]
During a feeding trial in which each subject consumed a GOS-containing product, the fecal microbiota composition was significantly altered and showed an increase in the abundance of Bifidobacterium [77]. The enrichment of Bifidobacterium was generally at the expense of Bacteroides.
Simple sugars
Digestible carbohydrates are eventually broken down into constituent simple sugars. Adhesion of microbes to the gastrointestinal surface is considered a prerequisite for their colonization and modulation of local and systemic physiological (immunological, hormonal) activities of the host and for competitive exclusion of pathogens [78]. The sterospecific adhesion-receptor interaction involves sugar moieties on the intestinal surface and sugar-binding adhesins on the microbial cell surface [79,80,81]. Sugars in food may interfere with the adhesion of intestinal microbes, both probiotics and pathogens, to the intestinal surface [82, 83], leading to an altered intestinal microbiota profile.
The Prevotella human enterotype is associated with a high intake of carbohydrates and simple sugars, indicating an association with a carbohydrate-based diet typical of agrarian societies [84]. Changes in microbiome composition were detectable within 24 hours of initiating controlled feeding [84]. Self-reported vegans were also found to be in the Prevotella enterotype.
Insoluble complex carbohydrates
Plant products are high in slowly digestible and nondigestible carbohydrates. Consumption of 2 apples a day among Japanese resulted in a significant increase in fecal bifidobacteria and clostridia (including the pectinase-positive C. perfringens) after a week [85]. The effect was attributed to apple pectin. Similarly, fecal bifidobacteria and lactobacilli were found to increase significantly during the consumption of kiwifruit (2 a day), and the effect was obvious within a day [86]. The effects were, however, temporary, and the microbiota profile returned to that of the baseline upon cessation of consumption [85, 86].
The energy sources that support the microbial community of the large intestine are dietary components that resist degradation in the upper intestinal tract, together with endogenous products such as mucin. Anaerobic metabolism by the microbiome community in the colon produces short-chain fatty acids together with CO2, H2 and CH4 [87]. These fermentation products have significant effects on the gut environment and on the host, as energy sources, regulators of gene expression and cell differentiation and anti-inflammatory agents. Butyrate, for example, is considered to play a particularly important role as an energy source for colonocytes and in the maintenance of gut health [88, 89].
In an animal study and an in vitro gastrointestinal tract model, cereal cellulose and insoluble non-starch polysaccharides (NSP) were found to increase Ruminococcus flavefaciens-like and Clostridium xylanolyticum-like phylotypes. The cereal amylose content increased the abundance of Clostridium butyricum-like phylotypes, whereas the amylopectin and starch contents increased the abundance of Clostridium ramosum-like phylotypes, members of Clostridium cluster XIVa and Bacteroides-like bacteria [90,91,92,93].
In a human study, the abundance of Ruminococcus bromii and Eubacterium rectale/Roseburia groups showed significant responses to nondigestible carbohydrates [25]. In a separate study, decreasing nondigestible carbohydrate intake significantly decreased both the detectable numbers of the E. rectale/Roseburia group and butyrate production [94]. This strong positive correlation between numbers of the E. rectale/Roseburia group, butyrate detection and nondigestible carbohydrate intake is supported by other human studies [95] and in vitro studies [96].
It was reported that a Japanese diet containing a high level of dietary fiber led to lower counts of anaerobic bacteria such as Clostridium, Bacteroides and Bacillus subtilis, and higher counts in Bifidobacterium and Fusobacterium [97, 98]. A slightly different observation was reported by Finegold et al. [99] in a study comparing the Japanese diet (containing soybean, radishes, cabbage, fish, seaweed and green tea) and Western diet (high in meat); they concluded that the Japanese diet resulted in an intestinal microflora with lower counts of Bacteroides, particularly B. fragilis; higher counts of some facultative or aerobic organisms such as Lactobacillus, E. coli, Proteus, Klebsiella, Staphylococcus, Streptococci and Clostridium; some Gram-positive anaerobic bacilli such as Eubacterium; and some Ruminococcus species.
The African diet consists mainly of cereal (millet, grain, sorghum), legumes (black-eye peas) and vegetables and is generally high in starch, fiber and non-animal protein. African children showed a significant enrichment in Bacteroidetes and depletion in Firmicutes, with a unique abundance of bacteria from the genus Prevotella and Xylanibacter [100]. The latter is known to contain a set of bacterial genes for cellulose and xylan hydrolysis that is not found in EU children.
Bacteroides plays an essential role within the distal gut in the degradation of the fiber consumed by the adult host. Bacteroides use a series of membrane protein complexes, termed Sus-like systems, to catabolize plant cell wall glycans in our diets [101]. It is hypothesized that by providing HMG, the mother ensures the presence of this group of bacteria in the infant intestine, allowing the smooth transition from milk to solid vegetable foods in the postweaning diet. Plants were the main source of foods for humans during the first 40 over million years of human history, that is, before they became omnivorous.
The major nondigestible carbohydrate in wheat is arabinoxylan (represents 50% of the dietary fiber); it is selectively degraded in the colon by xylanases and arabinofuranosidases, producing Bifidobacteria (B. animalis spp lactis), Roseburia and Bacteroides-Prevotella [102].
A consequence of the fermentation of nondigestible carbohydrates in the proximal colon is the production of organic acids and lowering of lumenal pH [103]. Assuming that the increased dietary intake in general of an obese individual resulted in a reduced colonic pH, this pH change could be an important factor in the observed community shift, as different microbes have varying optimal pHs for growth and activities. In a 4-week short-term weight loss program, short-term decreases in Roseburia and Bifidobacterium were observed in response to reduced carbohydrate intake, but no change was observed in Bacteroides [94]. The proportion of Bacteroides only increased gradually during a 52-week weight loss period. This may imply the involvement of some longer-term physiological mechanism (pH change) rather than a short-term response to diet in the abundance of gut Bacteroides.
Fat and fatty acids
Obesity is a major health concern in developed countries. A high-fat (HF) diet in an animal model was found to modulate the dominant intestinal bacterial population; Bacteroides-like bacteria were significantly reduce, and so were the E. rectal-C. coccoides group and Bifidobacterium [10, 104]. The polyunsaturated fatty acid component of fat appears to be a determinant factor for the adherence of intestinal bacteria to the mucosal surface and their growth [105]. In animal models, an HF diet induced pro-inflammatory cytokines such as IL-1, IL-6 and TNF-a, favoring hyperinsulinemia and excessive hepatic and adipose tissue lipid storage, leading to metabolic disorder (type 2 diabetes and insulin resistance) [106,107,108,109,110]. The relationship between HF feeding and the development of a low-grade inflammatory tone and metabolic disease have been attributed to reduced numbers of Bifidobacterium and a higher plasma endotoxin (Gram-negative bacteria-derived lipopolysacchaide) concentration.
Protein
The quantity and quality of protein constituents in food are clearly different in Western and Eastern diets. In an early study based on culturally dependent methods, consumption of meat among human subjects increased the counts of fecal Bacteroides, Bifidobacterium, Peptococcus and anaerobic Lactobacillus species [111]. A recent study by Kabeerdoss et al [112] using molecular techniques for comparison of the fecal microbiota of lacto-vegetarian and omnivorous young women showed that Clostridium cluster XIVa bacteria (also referred to as the Clostridium coccoides group) and butyrate-producing bacteria, specifically Roseburia–E. rectal, were significantly more abundant in the fecal microbiota of omnivores [112]. Functional attributes that have been identified for Clostridium cluster XIVa species include acetogenesis [113], utilization of aromatic compounds from the diet [114], metabolism of linoleic acid [115] and degradation of mucin [116].
Bacteroides enterotype has been proposed to be associated with a diet high in animal protein, a variety of amino acids and saturated fats [84, 100], which suggests that meat consumption as in a Western diet characterized this enterotype. The high protein and fat enterotype appears to be stable; a 10-day dietary intervention (low-fat, high-fiber diet) did not result in alteration of enterotypes [84]. This, however, casts uncertainty on the direct association of Bacteroides and a high animal protein and saturated fat diet. Residual proteins that enter the large intestine are fermented by Bacteroides and Clostridium [117] into a range of products depending on the amino acid composition. For example, branched-chain amino acids yield branched chain fatty acids, including isobutyrate and isovalerate. In the presence of typical colonic concentrations of SCFAs, growth of human colonic Bacteroides was found to be strongly inhibited at a pH of 5.5 (acidic), whereas many Firmicutes, including the most abundant butyrate producers, were less affected. The effect of diet on Bacteroides could be pH dependent.
Nonnutritive dietary components
Antibacterial foods such as Capsicum annuum (red pepper) and Allium sativum (garlic) have been shown to inhibit Bacillus cereus, B. subtilis, and C. tetani, [118] and Helicobacter pylori [119].
Our dietary intake of polyphenols ranges between 0.15 and 1 g/day [120]. The predominant polyphenols in foods and beverages are flavonoids, consisting mainly of catechins, proanthocyanidins, anthocyanidins, flavonols and flavones. A significant portion of dietary polyphenols is not absorbed, and those absorbed into the body are metabolized in the liver, excreted through the bile as glucuronides and accumulated in the ileal and colorectal lumen [121].
Phenolic compounds from olives [122], tea [123], wine [124] and berries [125,126,127,128] have demonstrated antimicrobial properties. Tea polyphenols have been shown to inhibit the growth of Bacteroides, Clostridium (C. perfringens and C. difficile), E. coli and Salmonella typhimurium [123]. Wild blueberries (Vaccinium angustifolium) have been reported to increase the proportion of Bifidobacterium and Lactobacillus acidophilus population after 6 weeks of consumption, but showed no effect on Bacteroides, Prevotella, Enterococcus and C. coccoides [125, 128].
The effects of polyphenols are related to the chemical structure of the compounds and bacterial species. Caffeic acid generally exerted a more significant inhibitory effect on microbial growth than epicatechin, catechin, 3-O-methylgallic acid and gallic acid [123]. Another in vitro study showed that (+)-catechin increased the counts of the C. coccoides–E. rectale group and E. coli, but inhibited those of C. histolyticum [129]. The effects of (−)-epicatechin were less pronounced in increasing the growth of the C. coccoides–E. rectale group.
Interestingly, the growth of beneficial bacteria (Bifidobacterium and Lactobacillus) was relatively unaffected or favored by dietary polyphenols [122, 129]. Resveratrol, a potent antioxidant found in wine, favored the growth of Bifidobacterium and Lactobacillus [124] and abolished the expression of virulence factors of Proteus mirabilis for invasion of human urothelial cells [130]. Anthocyanins from berries have also been proven to inhibit the growth of pathogenic Staphylococcus, Salmonella, H. pylori and B. cereus [126, 127]. Phenolics, and flavonoids may also reduce the ability of L. rhamnosus to adhere to intestinal epithelial cells [131].
Polyphenols are widely found in plant products (fruits, vegetable, tea leaves). Humans were largely herbivorous for the first 4 million years or so of history. Polyphenol-tolerant and degrading gut microbes must have evolved to be commensal; polyphenols thus serve the function as a gate stopper for microbes (pathogens) that enter the gut occasionally.
STARVATION
Undernourishment in general leads to an abundance of enteric pathogens, such as Campylobacteraceae (35-fold more compared with healthy control subjects), Helicobacteraceae (12-fold) and Bacteroidaceae (4-fold) [132]. On the other hand, Enterobacteriaceae, Shewanellaceae, Thermotogaceae, Eubacteriaceae, Streptococcaceae, Methanosarcinaceae and Thermoprotei were reduced by half.
GENERAL CONCLUSION
Diet is clearly a major determining factor of the gut microbiome. The response of the gut microbiota to dietary impact is often rapid; alteration was observed within 24 hours. The enterotype is, however, determined by long-term dietary habits in terms of the proportion and type of carbohydrates, protein and fat. Few nonnutritive dietary components have been studied; polyphenols show potential as moderators of gut microbiome homeostasis. Nonnutritive dietary components should be the focus in future study of the dietary effects on the gut microbiome.
REFERENCES
- 1.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. 2005. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122: 107–118 [DOI] [PubMed] [Google Scholar]
- 2.Mazmanian SK, Round JL, Kasper DL. 2008. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 453: 620–625 [DOI] [PubMed] [Google Scholar]
- 3.Troy EB, Kasper DL. 2010. Beneficial effects of Bacteroides fragilis polysaccharides on the immune system. Front Biosci 15: 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.García-Lafuente A, Antolín M, Guarner F, Crespo E, Malagelada JR. 2001. Modulation of colonic barrier function by the composition of the commensal flora in the rat. Gut 48: 503–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mital BK, Garg SK. 1995. Anticarcinogenic, hypocholesterolemic, and antagonistic activities of Lactobacillus acidophilus. Crit Rev Microbiol 21: 175–214 [DOI] [PubMed] [Google Scholar]
- 6.Miyauchi E, Morita H, Tanabe S. 2009. Lactobacillus rhamnosus alleviates intestinal barrier dysfunction in part by increasing expression of zonula occludens-1 and myosin light-chain kinase in vivo. J Dairy Sci 92: 2400–2408 [DOI] [PubMed] [Google Scholar]
- 7.Irvine EJ, Marshall JK. 2000. Increased intestinal permeability precedes the onset of Crohn’s disease in a subject with familial risk. Gastroenterology 119: 1740–1744 [DOI] [PubMed] [Google Scholar]
- 8.Vorobjova T, Uibo O, Ojakivi I, Teesalu K, Panarina M, Heilman K, Uibo R. 2011. Lower expression of tight junction protein 1 gene and increased FOXP3 expression in the small bowel mucosa in coeliac disease and associated type 1 diabetes mellitus. Int Arch Allergy Immunol 156: 451–461 [DOI] [PubMed] [Google Scholar]
- 9.Deitch EA. 1990. Bacterial translocation of the gut flora. J Trauma 30:(Suppl): S184–S189 [DOI] [PubMed] [Google Scholar]
- 10.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. 2007a. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56: 1761–1772 [DOI] [PubMed] [Google Scholar]
- 11.Goldin BR, Swenson L, Dwyer J, Sexton M, Gorbach SL. 1980. Effect of diet and Lactobacillus acidophilus supplements on human fecal bacterial enzymes. J Natl Cancer Inst 64: 255–261 [DOI] [PubMed] [Google Scholar]
- 12.Huycke MM, Abrams V, Moore DR. 2002. Enterococcus faecalis produces extracellular superoxide and hydrogen peroxide that damages colonic epithelial cell DNA. Carcinogenesis 23: 529–536 [DOI] [PubMed] [Google Scholar]
- 13.Vesterlund S. 2009. Production on antimicrobial substances. In Handbook of probiotics and Prebiotics, 2nd ed. Lee YK, Salminen S (eds).Wiley, Hoboken, pp. 391–394. [Google Scholar]
- 14.Reid G, Burton J. 2002. Use of Lactobacillus to prevent infection by pathogenic bacteria. Microbes Infect 4: 319–324 [DOI] [PubMed] [Google Scholar]
- 15.Gibson GR, Wang X. 1994. Regulatory effects of bifidobacteria on the growth of other colonic bacteria. J Appl Bacteriol 77: 412–420 [DOI] [PubMed] [Google Scholar]
- 16.Louis P, Flint HJ. 2009. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett 294: 1–8 [DOI] [PubMed] [Google Scholar]
- 17.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. 2008. The role of butyrate on colonic function. Aliment Pharmacol Ther 27: 104–119 [DOI] [PubMed] [Google Scholar]
- 18.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. 2005. Diversity of the human intestinal microbial flora. Science 308: 1635–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller TL, Wolin MJ. 1986. Methanogens in human and animal intestinal tracts. Sys. Appl Microbiol 7: 223–229 [Google Scholar]
- 20.Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, Parameswaran P, Crowell MD, Wing R, Rittmann BE, Krajmalnik-Brown R. 2009. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA 106: 2365–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker A. 2007. Say hello to our little friends. Nat Rev Microbiol 5: 572–573 [DOI] [PubMed] [Google Scholar]
- 22.Fiedorek SC, Pumphrey CL, Casteel HB. 1990. Breath methane production in children with constipation and encopresis. J Pediatr Gastroenterol Nutr 10: 473–477 [DOI] [PubMed] [Google Scholar]
- 23.Weaver GA, Krause JA, Miller TL, Wolin MJ. 1986. Incidence of methanogenic bacteria in a sigmoidoscopy population: an association of methanogenic bacteria and diverticulosis. Gut 27: 698–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tap J, Mondot S, Levenez F, Pelletier E, Caron C, Furet JP, Ugarte E, Mun oz-Tamayo R, Paslier DLE, Nalin R,, Dore J, Leclerc M. 2009. Towards the human intestinal microbiota phylogenetic core. Environ Microbiol 11: 2574–2584 [DOI] [PubMed] [Google Scholar]
- 25.Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, Brown D, Stares MD, Scott P, Bergerat A Louis P, McIntosh F, Johnstone AM, Lobley GE, Parkhill J, Flint HJ. 2011. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J 5: 220–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Louis P, Young P, Holtrop G, Flint HJ. 2010. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA transferase gene. Environ Microbiol 12: 304–314 [DOI] [PubMed] [Google Scholar]
- 27.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Dore´ J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, Bork P, Ehrlich SD, Wang J. 2010. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464: 59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sørensen SJ, Hansen LH, Jakobsen M. 2010. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 5:(2): e9085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. 2010. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 32: 815–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fava F, Danese S. 2011. Intestinal microbiota in inflammatory bowel disease: Friend of foe? World J Gastroenterol 17: 557–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhodes JM. 2007. The role of Escherichia coli in inflammatory bowel disease. Gut 56: 610–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willing B, Halfvarson J, Dicksved J, Rosenquist M, Jarnerot G, Engstrand L, Tysk C, Jansson JK. 2009. Twin studies reveal specific imbalances in the mucosa-associated microbiota of patients with ileal Crohn’s disease. Inflamm Bowel Dis 15: 653–660 [DOI] [PubMed] [Google Scholar]
- 33.Lim M, Adams JD, Wilcox M, Finan P, Sagar P, Burke D. 2009. An assessment of bacterial dysbiosis in pouchitis using terminal restriction fragment length polymorphisms of 16S ribosomal DNA from pouch effluent microbiota. Dis Colon Rectum 52: 1492–1500 [DOI] [PubMed] [Google Scholar]
- 34.Zella GC, Hait EJ, Glavan T, Gevers D, Ward DV, Kitts CL, Korzenik JR. 2011. Distinct microbiome in pouchitis compared to healthy pouches in ulcerative colitis and familial adenomatous polyposis. Inflamm Bowel Dis 17: 1092–1100 [DOI] [PubMed] [Google Scholar]
- 35.Keighley MR, Arabi Y, Dimock F, Burdon DW, Allan RN, Alexander-Williams J. 1978. Influence of inflammatory bowel disease on intestinal microflora. Gut 19: 1099–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van de Merwe JP, Schröder AM, Wensinck F, Hazenberg MP. 1988. The obligate anaerobic faecal flora of patients with Crohn’s disease and their first-degree relatives. Scand J Gastroenterol 23: 1125–1131 [DOI] [PubMed] [Google Scholar]
- 37.Bulois P, Desreumaux P, Neut C, Darfeuille-Michaud A, Cortot A, Colombel JF. 1999. Infectious agents and Crohn’s disease. Clin Microbiol Infect 5: 601–604 [DOI] [PubMed] [Google Scholar]
- 38.De Palma G, Nadal I, Medina M, Donat E, Ribes-Koninckx C, Calabuig M, Sanz Y. 2010. Intestinal dysbiosis and reduced immunoglobulin-coated bacteria associated with coeliac disease in children. BMC Microbiol 10: 63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schippa S, Iebba V, Barbato M, Di Nardo G, Totino V, Checchi MP, Longhi C, Maiella G, Cucchiara S, Conte MP. 2010. A distinctive ‘microbial signature’ in celiac pediatric patients. BMC Microbiol 10: 175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaahtovuo J, Munukka E, Korkeama¨ki M, Luukkainen R, Toivanen P. 2008. Fecal microbiota in early rheumatoid arthritis. J Rheumatol 35: 1500–1505 [PubMed] [Google Scholar]
- 41.Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen ML, Bolte E, McTeague M, Sandler R, Wexler H, Marlowe EM, Collins MD, Lawson PA, Summanen P, Baysallar M, Tomzynski TJ, Read E, Johnson E, Rolfe R, Nasir P, Shah H, Haake DA, Manning P, Kaul A. 2002. Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis 35:(Suppl 1): S6–S16 [DOI] [PubMed] [Google Scholar]
- 42.Parracho HM, Bingham MO, Gibson GR, McCartney AL. 2005. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol 54: 987–991 [DOI] [PubMed] [Google Scholar]
- 43.Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, Youn E, Summanen PH, Granpeesheh D, Dixon D, Liu M, Molitoris DR, Green JA., 3rd2010. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 16: 444–453 [DOI] [PubMed] [Google Scholar]
- 44.Armougom F, Henry M, Vialettes B, Raccah D, Raoult D. 2009. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PLoS ONE 4: e7125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balamurugan R, George G, Kabeerdoss J, Hepsiba J, Chandragunasekaran AMS, Ramakrishna BS. 2010. Quantitative differences in intestinal Faecalibacterium prausnitzii in obese Indian children. Br J Nutr 103: 335–338 [DOI] [PubMed] [Google Scholar]
- 46.Duncan SH, Lobley GE, Holtrop G, Ince J, Johnstone AM, Louis P, Flint HJ. 2008. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes Lond 32: 1720–1724 [DOI] [PubMed] [Google Scholar]
- 47.Kalliomäki M, Collado MC, Salminen S, Isolauri E. 2008. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr 87: 534–538 [DOI] [PubMed] [Google Scholar]
- 48.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. 2005. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA 102: 11070–11075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Collado MC, Isolauri E, Laitinen K, Salminen S. 2008. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am J Clin Nutr 88: 894–899 [DOI] [PubMed] [Google Scholar]
- 50.Tiihonen K, Ouwehand AC, Rautonen N. 2010. Effect of overweight on gastrointestinal microbiology and immunology: correlation with blood biomarkers. Br J Nutr 103: 1070–1078 [DOI] [PubMed] [Google Scholar]
- 51.Onoue M, Kado S, Sakaitani Y, Uchida K, Morotomi M. 1997. Specific species of intestinal bacteria influence the induction of aberrant crypt foci by 1,2-dimethylhydrazine in rats. Cancer Lett 113: 179–186 [DOI] [PubMed] [Google Scholar]
- 52.Scanlan PD, Shanahan F, Clune Y, Collins JK, O’Sullivan GC, O’Riordan M, Holmes E, Wang Y, Marchesi JR. 2008. Culture independent analysis of the gut microbiota in colorectal cancer and polyposis. Environ Microbiol 10: 789–798 [DOI] [PubMed] [Google Scholar]
- 53.Sears CL, Pardoll DM. 2011. Perspective: alpha-bugs, their microbial partners, and the link to colon cancer. J Infect Dis 203: 306–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hirayama K, Rafter J. 2000. The role of probiotic bacteria in cancer prevention. Microbes Infect 2: 681–686 [DOI] [PubMed] [Google Scholar]
- 55.FAO/WHO Joint Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid bacteria. October 2001
- 56.Kalliomäki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. 2003. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet 361: 1869–1871 [DOI] [PubMed] [Google Scholar]
- 57.Kalliomäki M, Salminen S, Poussa T, Isolauri E. 2007. Probiotics during the first 7 years of life: a cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J Allergy Clin Immunol 119: 1019–1021 [DOI] [PubMed] [Google Scholar]
- 58.Round JL, Mazmanian SK. 2009. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 9: 313–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tamboli CP, Caucheteux C, Cortot A, Colombel JF, Desreumaux P. 2003. Probiotics in inflammatory bowel disease: a critical review. Best Pract Res Clin Gastroenterol 17: 805–820 [DOI] [PubMed] [Google Scholar]
- 60.Bixquert Jiménez M.2009. Treatment of irritable bowel syndrome with probiotics. An etiopathogenic approach at last? 101: 553-64. [DOI] [PubMed]
- 61.Hayatsu H, Hayatsu T. 1993. Suppressing effect of Lactobacillus casei administration on the urinary mutagenicity arising from ingestion of fried ground beef in the human. Cancer Lett 73: 173–179 [DOI] [PubMed] [Google Scholar]
- 62.Aso Y, Akazan H. 1992. Prophylactic effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer. BLP Study Group. Urol Int 49: 125–129 [DOI] [PubMed] [Google Scholar]
- 63.Aso Y, Akaza H, Kotake T, Tsukamoto T, Imai K, Naito S. 1995. Preventive effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer in a double-blind trial. The BLP Study Group. Eur Urol 27: 104–109 [DOI] [PubMed] [Google Scholar]
- 64.Spanhaak S, Havenaar R, Schaafsma G. 1998. The effect of consumption of milk fermented by Lactobacillus casei strain Shirota on the intestinal microflora and immune parameters in humans. Eur J Clin Nutr 52: 899–907 [DOI] [PubMed] [Google Scholar]
- 65.Fujisawa T, Shinohara K, Kishimoto Y, Terada A. 2006. Effect of miso soup containing natto on the composition and metabolic activity of human fecal flora. Microb Ecol Health Dis 18: 79–84 [Google Scholar]
- 66.Simon GL, Gorbach SL. 1984. Intestinal flora in health and disease. Gastroenterology 86: 174–193 [PubMed] [Google Scholar]
- 67.Dethlefsen L, Eckburg PB, Bik EM, Relman DA. 2006. Assembly of the human intestinal microbiota. Trends Ecol Evol 21: 517–523 [DOI] [PubMed] [Google Scholar]
- 68.Flint HJ. 2006. The significance of prokaryote diversity in the human gastrointestinal tract. In Prokaryotic Diversity: Mechanisms and Significance. Logan NA, Lappin-Scott HM, Oyston PCF (eds). Cambridge, UK: Cambridge University Press, pp. 65–90. SGM Symposium vol. 66.
- 69.Ley RE, Peterson DA, Gordon JI. 2006. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124: 837–848 [DOI] [PubMed] [Google Scholar]
- 70.Crittenden R, Playne M. 2009. Prebiotics. In Handbook of probiotics and Prebiotics, 2nd ed. Lee YK, Salminen S (eds).Wiley, Hoboken. pp. 535–581. [Google Scholar]
- 71.German JB, Freeman SL, Lebrilla CB, Mills DA. 2008. Human milk oligosaccharides: evolution, structures and bioselectivity as substrates for intestinal bacteria. Nestle Nutr Workshop Ser Pediatr Program 62: 205–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marcobal A, Sonnenburg JL. 2012. Human milk oligosaccharide consumption by intestinal microbiota. Clin Microbiol Infect 18:(Suppl 4): 12–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marcobal A, Barboza M, Sonnenburg ED, Pudlo N, Martens EC, Desai P, Lebrilla CB, Weimer BC, Mills DA, German JB, Sonnenburg JL. 2011. Bacteroides in the infant gut consume milk oligosaccharides via mucus-utilization pathways. Cell Host Microbe 10: 507–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hooper LV, Midtvedt T, Gordon JI. 2002. How host-microbial interactions shape the nutrient environemnt of the mammalian intestine. Annu Rev Nutr 22: 283–307 [DOI] [PubMed] [Google Scholar]
- 75.Ramnani P, Gaudier E, Bingham M, van Bruggen P, Tuohy KM, Gibson GR. 2010. Prebiotic effect of fruit and vegetable shots containing Jerusalem artichoke inulin: a human intervention study. Br J Nutr 104: 233–240 [DOI] [PubMed] [Google Scholar]
- 76.Ramirez-Farias C, Slezak K, Fuller Z, Duncan A, Holtrop G, Louis P. 2009. Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br J Nutr 101: 541–550 [DOI] [PubMed] [Google Scholar]
- 77.Davis LMG, Martı’nez I, Walter J, Goin C, Hutkins RW. 2011. Barcoded pyrosequencing reveals that consumption of galactooligosaccharides results in a highly specific bifidogenic response in humans. PLoS ONE 6: e25200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salminen S, Bouley MC, Boutron-Rualt MC, Cummings J, Frank A, Gibson G, Isolauri E, Moreau MC, Roberfroid M, Rowland I. 1998. Functional food science and gastrointestinal physiology and function. Br J Nutr 80:(Suppl 1): S147–S171 [DOI] [PubMed] [Google Scholar]
- 79.Adlerberth I, Ahrne S, Johansson ML, Molin G, Hanson LA, Wold AE. 1996. A manose-specific adherence mechanism in Lactobacillus plantarum conferring binding to the human colonic cell line HT-29. Appl Environ Microbiol 62: 2244–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ofek I, Beachey EH, Sharon N. 1978. Surface sugars of animal cells as determinants of recognition in bacterial adherence. Trends Biochem Sci 3: 159–160 [Google Scholar]
- 81.Yamamoto K, Miwa T, Taniguchi H, Nagano T, Shimamura K, Tanaka T, Kumagi H. 1996. Binding specificity of Lactobacillus to glycolopids. Biochem Biophys Res Commun 228: 148–152 [DOI] [PubMed] [Google Scholar]
- 82.Lee YK, Puong KY. 2002. Competition for adhesion between probiotics and human gastrointestinal pathogens in the presence of carbohydrate. Br J Nutr 88:(Suppl 1): S101–S108 [DOI] [PubMed] [Google Scholar]
- 83.Neeser JR, Granato D, Rouvet M, Servin A, Teneberg S, Karlsson KA. 2000. Lactobacillus johnsonii La1 share carbohydrate-binding specificities with several enteropathogenic bacteria. Glycobiol 10: 1193–1199 [DOI] [PubMed] [Google Scholar]
- 84.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD. 2011. Linking long-term dietary patterns with gut microbial enterotypes. Science 334: 105–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shinohara K, Ohashi Y, Kawasumi K, Terada A, Fujisawa T. 2010. Effect of apple intake on fecal microbiota and metabolites in humans. Anaerobe 16: 510–515 [DOI] [PubMed] [Google Scholar]
- 86.Lee YK, Low KY, Siah K, Drummond LM, Kok An Gwee KA. 2012. Kiwifruit (Actinidia deliciosa) changes intestinal microbial profile. Microb Ecol Health Dis 23: 18572–18576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Macfarlane GT, Gibson GR. 1997. Carbohydrate fermentation, energy transduction and gas metabolism in the human large intestine. In Gastrointestinal Microbiology, Vol. 1. Mackie RI, White BA (eds). Chapman and Hall, London. pp. 269–318. [Google Scholar]
- 88.McIntyre A, Gibson PR, Young GP. 1993. Butyrate production from dietary fiber and protection against large bowel cancer in a rat model. Gut 34: 386–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. 2002. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett 217: 133–139 [DOI] [PubMed] [Google Scholar]
- 90.Bindelle J, Pieper R, Montoya CA, Van Kessel AG, Leterme P. 2011. Nonstarch polysaccharide-degrading enzymes alter the microbial community and the fermentation patterns of barley cultivars and wheat products in an in vitro model of the porcine gastrointestinal tract. FEMS Microbiol Ecol 76: 553–563 [DOI] [PubMed] [Google Scholar]
- 91.Louis P, Scott KP, Duncan SH, Flint HJ. 2007. Understanding the effects of diet on bacterial metabolism in the large intestine. J Appl Microbiol 102: 1197–1208 [DOI] [PubMed] [Google Scholar]
- 92.Pieper R, Bindelle J, Rossnagel B, Van Kessel A, Pascal Leterme P. 2009. Effect of carbohydrate composition in barley and oat cultivars on microbial ecophysiology and proliferation of Salmonella enterica in an in vitro model of the porcine gastrointestinal tract. Appl Environ Microbiol 75: 7006–7016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Topping D. 2007. Cereal complex carbohydrates and their contribution to human health. J Cereal Sci 46: 220–229 [Google Scholar]
- 94.Duncan SH, Belenguer A, Holtrop G, Johnstone AM, Flint HJ, Lobley GE. 2007. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl Environ Microbiol 73: 1073–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abell GCJ, Cooke CM, Bennett CN, Conlon MA, McOrist AL. 2008. Phylotypes related to Ruminococcus bromii are abundant in the large bowel of humans and increase in response to a diet high in resistant starch. FEMS Microbiol Ecol 66: 505–515 [DOI] [PubMed] [Google Scholar]
- 96.Leitch ECM, Walker AW, Duncan SH, Holtrop G, Flint HJ. 2007. Selective colonization of insoluble substrates by human colonic bacteria. Environ Microbiol 72: 667–679 [DOI] [PubMed] [Google Scholar]
- 97.Benno Y, Endo K, Mizutani T, Namba Y, Komori T, Mitsuoka T. 1989. Comparison of fecal microflora of elderly persons in rural and urban areas of Japan. Appl Environ Microbiol 55: 1100–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Benno Y, Endo K, Miyoshi H, Okuda T, Koishi H, Mitsuoka T. 1989. Effect of rice fiber on human fecal microflora. Microbiol Immunol 33: 435–440 [DOI] [PubMed] [Google Scholar]
- 99.Finegold SM, Attebery HR, Sutter VL. 1974. Effect of diet on human fecal flora: comparison of Japanese and American diets. Am J Clin Nutr 27: 1456–1469 [DOI] [PubMed] [Google Scholar]
- 100.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. 2010. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA 107: 14691–14696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Martens EC, Lowe EC, Chiang H, Pudlo NA, Wu M, McNulty NP, Abbott DW, Henrissat B, Gilbert HJ, Bolam DN, Gordon JI. 2011. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol 9: e1001221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Neyrinck AM, Sam Possemiers S, Druart C, van de Wiele T, De Backer F, Cani PD, Larondelle Y, Delzenne NM. 2011. Prebiotic effects of wheat arabinoxylan related to the increase in bifidobacteria, roseburia and Bacteroides/Prevotella in diet-induced obese mice. PLoS ONE 6: e20944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bown RL, Gibson JA, Sladen GE, Hicks B, Dawson AM. 1974. Effects of lactulose and other laxatives on ileal and colonic pH as measured by a radiotelemetry device. Gut 15: 999–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, Gibson GR, Delzenne NM. 2007b. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 50: 2374–2383 [DOI] [PubMed] [Google Scholar]
- 105.Kankaanpaa PE, Salminen SJ, Isolauri E, Lee YK. 2001. The influence of polyunsaturated fatty acids on probiotic growth and adhesion. FEMS Microbiol let 194: 149-153. [DOI] [PubMed]
- 106.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. 1996. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity induced insulin resistance. Science 271: 665–668 [DOI] [PubMed] [Google Scholar]
- 107.Kahn BB, Flier JS. 2000. Obesity and insulin resistance. J Clin Invest 106: 473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK. 2005. Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes 54: 2939–2945 [DOI] [PubMed] [Google Scholar]
- 109.Wellen KE, Hotamisligil GS. 2005. Inflammation, stress, and diabetes. J Clin Invest 115: 1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr2003. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reddy BS, Weisburger JH, Wynder EL. 1975. Effects of high risk and low risk diets for colon carcinogenesis on fecal microflora and steroids in man. J Nutr 105: 878–884 [DOI] [PubMed] [Google Scholar]
- 112.Kabeerdoss J, Devi RS, Mary RR, Ramakrishna BS. 2012. Faecal microbiota composition in vegetarians: comparison with omnivores in a cohort of young women in southern India. Br J Nutr 108: 953–957 [DOI] [PubMed] [Google Scholar]
- 113.Bernalier A, Lelait M, Rochet V, Grivet JP, Gibson GR, Durand M. 1996. Acetogenesis from H2 and CO2 by methane and non-methane-producing human colonic bacterial communities. FEMS Microbiol Ecol 19: 193–202 [Google Scholar]
- 114.Simmering R, Pforte H, Jacobasch G, Blaut M. 2002. The growth of the flavonoid-degrading intestinal bacterium, Eubacterium ramulus, is stimulated by dietary flavonoids in vivo. FEMS Microbiol Ecol 40: 243–248 [DOI] [PubMed] [Google Scholar]
- 115.Devillard E, McIntosh FM, Duncan SH, Wallace RJ. 2007. Metabolism of linoleic acid by human gut bacteria: different routes for biosynthesis of conjugated linoleic acid. J Bacteriol 189: 2566–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hoskins LC. 1993. Mucin degradation in the human gastrointestinal tract and its significance to enteric microbial ecology. Eur J Gastroenterol Hepatol 5: 205–213 [Google Scholar]
- 117.Macfarlane GT, Gibson GR, Cummings JH. 1992. Comparison of fermentation reactions in different regions of the human colon. J Appl Bacteriol 72: 57–64 [DOI] [PubMed] [Google Scholar]
- 118.Cichewicz RH, Thorpe PA. 1996. The antimicrobial properties of chile peppers (Capsicum species) and their uses in Mayan medicine. J Ethnopharmacol 52: 61–70 [DOI] [PubMed] [Google Scholar]
- 119.Sivam GP, Lampe JW, Ulness B, Swanzy SR, Potter JD. 1997. Helicobacter pylori–in vitro susceptibility to garlic (Allium sativum) extract. Nutr Cancer 27: 118–121 [DOI] [PubMed] [Google Scholar]
- 120.Scalbert A, Williamson G. 2000. Dietary intake and bioavailability of polyphenols. J Nutr 130: 2073S–2085S [DOI] [PubMed] [Google Scholar]
- 121.Bazzocco S, Mattila I, Guyot S, Renard CM, Aura AM. 2008. Factors affecting the conversion of apple polyphenols to phenolic acids and fruit matrix to short-chain fatty acids by human faecal microbiota in vitro. Eur J Nutr 47: 442–452 [DOI] [PubMed] [Google Scholar]
- 122.Medina E, Garcia A, Romero C, de Castro A, Brenes M. 2009. Study of the anti-lactic acid bacteria compounds in table olives. Int J Food Sci Technol 7: 1286–1291 [Google Scholar]
- 123.Lee HC, Jenner AM, Low CS, Lee YK. 2006. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res Microbiol 157: 876–884 [DOI] [PubMed] [Google Scholar]
- 124.Larrosa M, Yanéz-Gascón MJ, Selma MV, González-Sarrías A, Toti S, Cerón JJ, Tomás-Barberán F, Dolara P, Espín JC. 2009. Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J Agric Food Chem 57: 2211–2220 [DOI] [PubMed] [Google Scholar]
- 125.Molan AL, Lila AA, Mawson J, De S. 2009. In vitro and in vivo evaluation of the prebiotic activity of water-soluble blueberry extracts. World J Microbiol Biotechnol 25: 1243–1249 [Google Scholar]
- 126.Nohynek LJ, Alakomi HL, Kahkonen MP, Heinonen M, Helander IM, Oksman-Caldentey KM, Puupponen-Pimiä RH. 2006. Berry phenolics: antimicrobial properties and mechanisms of action against severe human pathogens. Nutr Cancer 54: 18–32 [DOI] [PubMed] [Google Scholar]
- 127.Puupponen-Pimiä R, Nohynek L, Hartmann-Schmidlin S, Kähkönen M, Heinonen M, Määttä-Riihinen K, Oksman-Caldentey KM. 2005. Berry phenolics selectively inhibit the growth of intestinal pathogens. J Appl Microbiol 98: 991–1000 [DOI] [PubMed] [Google Scholar]
- 128.Vendrame S, Guglielmetti S, Riso P, Arioli S, Klimis-Zacas D, Porrini M. 2011. Six-week consumption of a wild blueberry powder drink increases bifidobacteria in the human gut. J Agric Food Chem 59: 12815–12820 [DOI] [PubMed] [Google Scholar]
- 129.Tzounis X, Vulevic J, Kuhnle GG, George T, Leonczak J, Gibson GR, Kwik-Uribe C, Spencer JP. 2008. Flavanol monomer-induced changes to the human faecal microflora. Br J Nutr 99: 782–792 [DOI] [PubMed] [Google Scholar]
- 130.Wang WB, Lai HC, Hsueh PR, Chiou RYY, Lin SB, Liaw SJ. 2006. Inhibition of swarming and virulence factor expression in Proteus mirabilis by resveratrol. J Med Microbiol 55: 1313–1321 [DOI] [PubMed] [Google Scholar]
- 131.Parkar SG, Stevenson DE, Skinner MA. 2008. The potential influence of fruit polyphenols on colonic microflora and human gut health. Int J Food Microbiol 124: 295–298 [DOI] [PubMed] [Google Scholar]
- 132.Gupta SS, Mohammed MH, Ghosh TS, Kanungo S, Nair GB, Mande SS. 2011. Metagenome of the gut of a malnourished child. Gut Pathog 3: 7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]


