Abstract
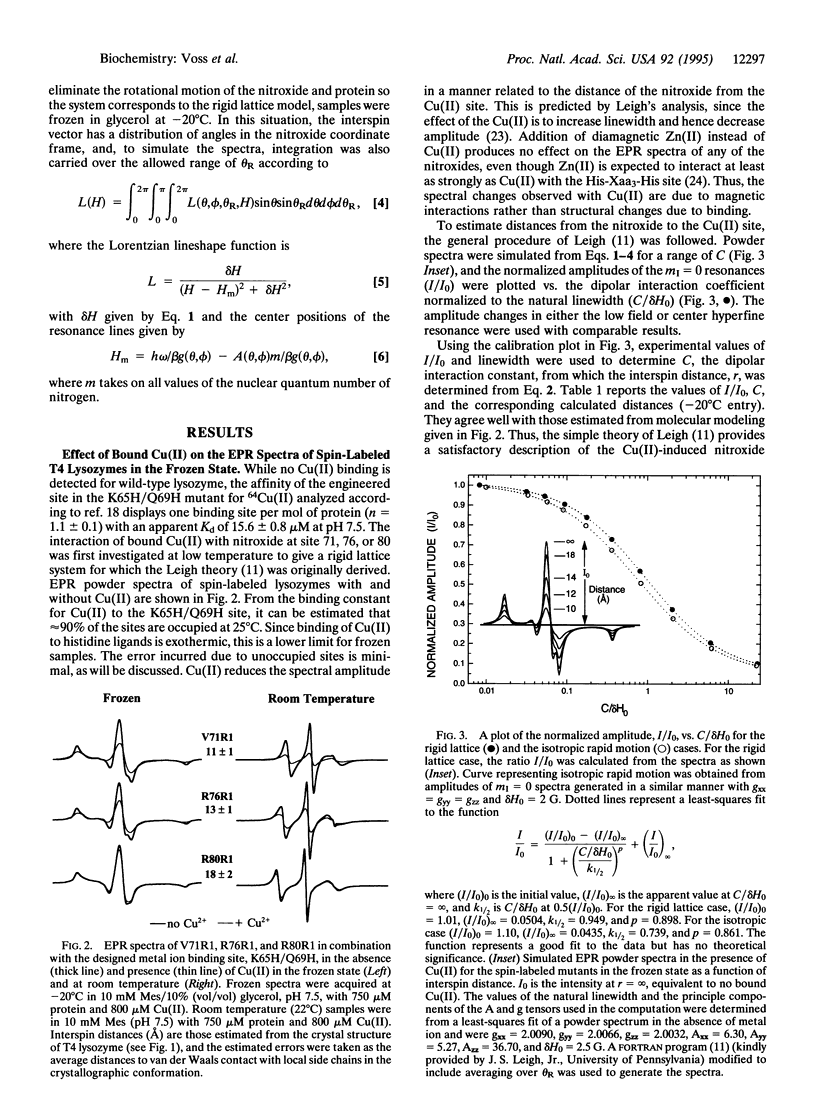
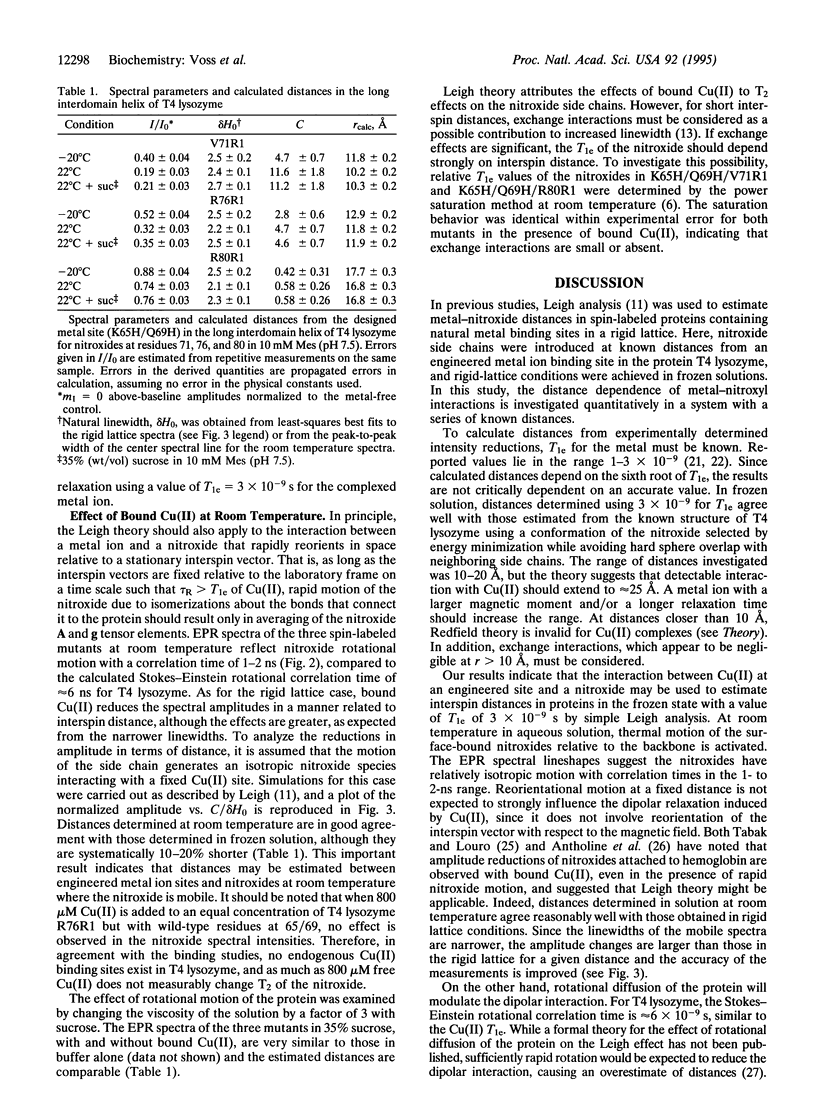
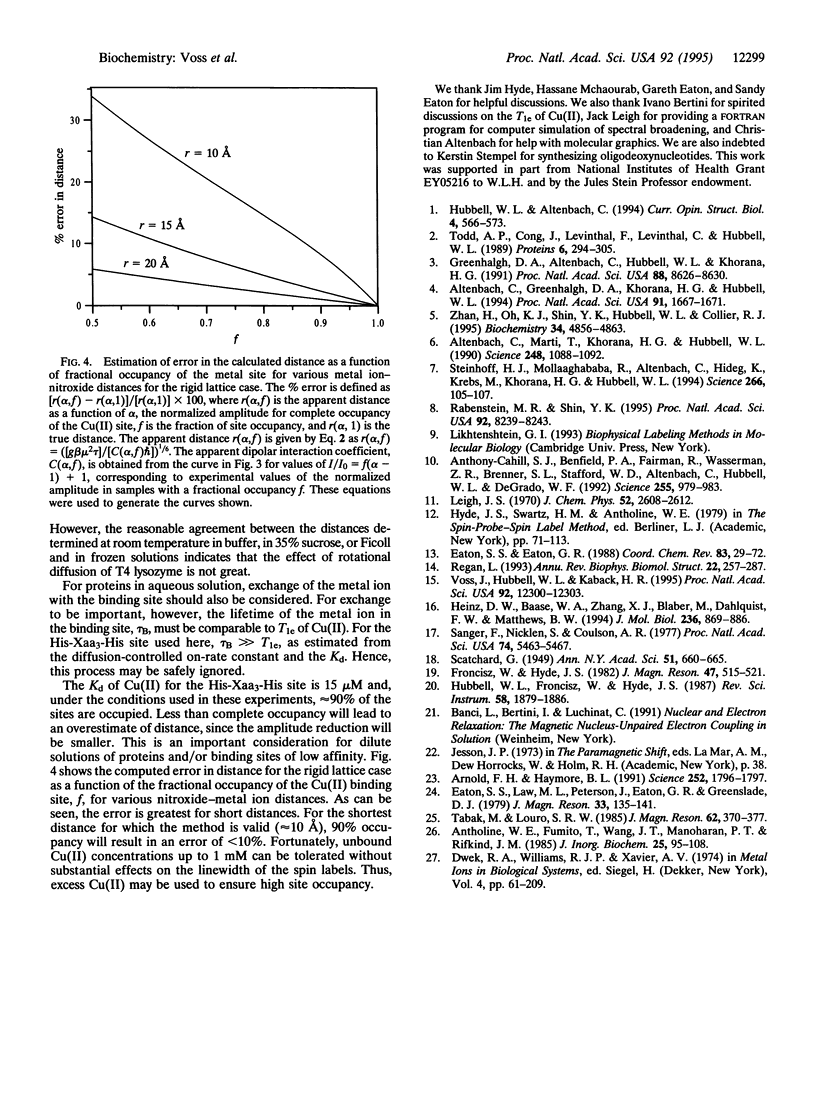
The use of molecular genetics to introduce both a metal ion binding site and a nitroxide spin label into the same protein opens the use of paramagnetic metalnitroxyl interactions to estimate intramolecular distances in a wide variety of proteins. In this report, a His-Xaa3-His metal ion binding motif was introduced at the N terminus of the long interdomain helix of T4 lysozyme (Lys-65 --> His/Gln-69 --> His) of three mutants, each containing a single nitroxide-labeled cysteine residue at position 71, 76, or 80. The results show that Cu(II)-induced relaxation effects on the nitroxide can be quantitatively analyzed in terms of interspin distance in the range of 10-25 A using Redfield theory, as first suggested by Leigh [Leigh, J.S. (1970) J. Chem. Phys. 52, 2608-2612]. Of particular interest is the observation that distances can be determined both under rigid lattice conditions in frozen solution and in the presence of motion of the spins at room temperature under physiological conditions. The method should be particularly attractive for investigating structure in membrane proteins that are difficult to crystallize. In the accompanying paper, the technique is applied to a polytopic membrane protein, lactose permease.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenbach C., Greenhalgh D. A., Khorana H. G., Hubbell W. L. A collision gradient method to determine the immersion depth of nitroxides in lipid bilayers: application to spin-labeled mutants of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1667–1671. doi: 10.1073/pnas.91.5.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altenbach C., Marti T., Khorana H. G., Hubbell W. L. Transmembrane protein structure: spin labeling of bacteriorhodopsin mutants. Science. 1990 Jun 1;248(4959):1088–1092. doi: 10.1126/science.2160734. [DOI] [PubMed] [Google Scholar]
- Antholine W. E., Taketa F., Wang J. T., Manoharan P. T., Rifkind J. M. Interaction between bound cupric ion and spin-labeled cysteine beta-93 in human and horse hemoglobins. J Inorg Biochem. 1985 Oct;25(2):95–108. doi: 10.1016/0162-0134(85)80018-0. [DOI] [PubMed] [Google Scholar]
- Anthony-Cahill S. J., Benfield P. A., Fairman R., Wasserman Z. R., Brenner S. L., Stafford W. F., 3rd, Altenbach C., Hubbell W. L., DeGrado W. F. Molecular characterization of helix-loop-helix peptides. Science. 1992 Feb 21;255(5047):979–983. doi: 10.1126/science.1312255. [DOI] [PubMed] [Google Scholar]
- Arnold F. H., Haymore B. L. Engineered metal-binding proteins: purification to protein folding. Science. 1991 Jun 28;252(5014):1796–1797. doi: 10.1126/science.1648261. [DOI] [PubMed] [Google Scholar]
- Greenhalgh D. A., Altenbach C., Hubbell W. L., Khorana H. G. Locations of Arg-82, Asp-85, and Asp-96 in helix C of bacteriorhodopsin relative to the aqueous boundaries. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8626–8630. doi: 10.1073/pnas.88.19.8626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz D. W., Baase W. A., Zhang X. J., Blaber M., Dahlquist F. W., Matthews B. W. Accommodation of amino acid insertions in an alpha-helix of T4 lysozyme. Structural and thermodynamic analysis. J Mol Biol. 1994 Feb 25;236(3):869–886. doi: 10.1006/jmbi.1994.1195. [DOI] [PubMed] [Google Scholar]
- Rabenstein M. D., Shin Y. K. Determination of the distance between two spin labels attached to a macromolecule. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8239–8243. doi: 10.1073/pnas.92.18.8239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan L. The design of metal-binding sites in proteins. Annu Rev Biophys Biomol Struct. 1993;22:257–287. doi: 10.1146/annurev.bb.22.060193.001353. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff H. J., Mollaaghababa R., Altenbach C., Hideg K., Krebs M., Khorana H. G., Hubbell W. L. Time-resolved detection of structural changes during the photocycle of spin-labeled bacteriorhodopsin. Science. 1994 Oct 7;266(5182):105–107. doi: 10.1126/science.7939627. [DOI] [PubMed] [Google Scholar]
- Todd A. P., Cong J., Levinthal F., Levinthal C., Hubbell W. L. Site-directed mutagenesis of colicin E1 provides specific attachment sites for spin labels whose spectra are sensitive to local conformation. Proteins. 1989;6(3):294–305. doi: 10.1002/prot.340060312. [DOI] [PubMed] [Google Scholar]
- Voss J., Hubbell W. L., Kaback H. R. Distance determination in proteins using designed metal ion binding sites and site-directed spin labeling: application to the lactose permease of Escherichia coli. Proc Natl Acad Sci U S A. 1995 Dec 19;92(26):12300–12303. doi: 10.1073/pnas.92.26.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan H., Oh K. J., Shin Y. K., Hubbell W. L., Collier R. J. Interaction of the isolated transmembrane domain of diphtheria toxin with membranes. Biochemistry. 1995 Apr 11;34(14):4856–4863. doi: 10.1021/bi00014a043. [DOI] [PubMed] [Google Scholar]




