Abstract
Bifidobacterium breve MCC-117 is able to significantly reduce the expression of inflammatory cytokines in porcine intestinal epithelial (PIE) cells and to improve IL-10 levels in CD4+CD25high Foxp3+ lymphocytes in response to heat-stable enterotoxigenic Escherichia coli (ETEC) pathogen-associated molecular patterns (PAMPs), while the immunoregulatory effect of B. adolescentis ATCC15705 was significantly lower than that observed for the MCC-117 strain. Considering the different capacities of the two bifidobacterium strains to activate toll-like receptor (TLR)-2 and their differential immunoregulatory activities in PIE and immune cells, we hypothesized that comparative studies with both strains could provide important information regarding the molecular mechanism(s) involved in the anti-inflammatory activity of bifidobacteria. In this work, we demonstrated that the anti-inflammatory effect of B. breve MCC-117 was achieved by a complex interaction of multiple negative regulators of TLRs as well as inhibition of multiple signaling pathways. We showed that B. breve MCC-117 reduced heat-stable ETEC PAMP-induced NF-κB, p38 MAPK and PI3 K activation and expression of pro-inflammatory cytokines in PIE cells. In addition, we demonstrated that B. breve MCC-117 may activate TLR2 synergistically and cooperatively with one or more other pattern recognition receptors (PRRs), and that interactions may result in a coordinated sum of signals that induce the upregulation of A20, Bcl-3, Tollip and SIGIRR. Upregulation of these negative regulators could have an important physiological impact on maintaining or reestablishing homeostatic TLR signals in PIE cells. Therefore, in the present study, we gained insight into the molecular mechanisms involved in the immunoregulatory effect of B. breve MCC-117.
Keywords: bifidobacteria, anti-inflammatory activity, porcine intestinal epithelial cells, Toll-like receptors negative regulators, Toll-like receptor 2
INTRODUCTION
In recent years, great advances have been made with regard to knowledge of the mechanism involved in probiotic effects, especially those related to the capacity to stimulate the immune system [1,2,3]. In this regard, it was reported that Toll-like receptor (TLR)-2 may play an important regulatory role in the recognition of bifidobacteria that possess an immunoinhibitory effect. Bifidobacteria have been shown to inhibit the production of tumor necrosis factor (TNF)-α and interleukin (IL)-6 induced by immunostimulatory lactobacilli in blood immune cells via interaction with TLR2 [4]. In addition, Hoarau et al. [5] demonstrated that the supernatant of Bifidobacterium breve C50 induces dendritic cell (DCs) maturation through TLR2, with a high level of IL-10 production. A study, using DCs from TLR2−/− mice reported that bifidobacteria induced much higher levels of IL-12 and lower IL-10 levels in DCs from TLR2−/− mice when compared with wild-type DCs [4].
Considering these observations, we isolated porcine TLR2 (pTLR2) cDNA from ileal Peyer’s patches (PPs) and, using a human cell line, developed a method for evaluating the immune responses to immunobiotic bifidobacteria by constructing a pTLR2-expressing transfectant (HEKpTLR2 cells) [6, 7]. We used the HEKpTLR2 immunoassay system to evaluate various bifidobacteria, belonging to different species with regard to the activation pattern of TLR2-overexpressing cells. As has been observed in studies in which bifidobacteria were evaluated using immune cells [8], we observed a strain-specific capacity of bifidobacteria to stimulate HEKpTLR2 cells. Of the strains tested, Bifidobacterium breve MCC-117 was the one that showed the highest nuclear factor κ B (NF-κB) activity in the HEKpTLR2 immunoassay system, while other strains of the same species were less effective or did not modify this parameter [9]. In addition, we evaluated mitogenic activities of the same strains of bifidobacteria in order to assess whether a correlation exists between the two methods for the selection of probiotics. Considering the results obtained using the two screening methods and the analysis of the correlation between the methods by a linear regression function and coefficient of determination, immunoregulatory bifidobacterium strains were classified in two groups: (i) strains with a high stimulatory capacity of HEKpTLR2 cells and high mitogenic activity, such as B. breve MCC-117, and (ii) strains with a low/moderate stimulatory capacity of HEKpTLR2 but with a high mitogenic activity, such as B. adolescentis ATCC15705 [9, unpublished results].
We also evaluated the anti-inflammatory effect of both strains by using co-cultures of intestinal epithelial cells (IECs) from pig (PIE cells) and immune cells from porcine PPs and heat-stable pathogen-associated molecular patterns (PAMPs) from enterotoxigenic Escherichia coli (ETEC) as an inflammatory challenge [9]. We demonstrated that B. breve MCC-117 treatment was able to significantly reduce the expression of inflammatory cytokines in PIE cells in response to heat-stable ETEC PAMPs. In addition, B. breve MCC-117 treatment was able to significantly reduce the levels of IFN-γ in both CD4+ and CD8+ lymphocytes and improved IL-10 levels in CD4+CD25high Foxp3+ lymphocytes [9]. On the other hand, the immunoregulatory effect of B. adolescentis ATCC15705 was significantly lower than that observed for the MCC-117 strain [9].
Considering the different capacity of B. breve MCC-117 and B. adolescentis ATCC15705 to activate TLR2 and their differential immunoregulatory activities in PIE and immune cells, we hypothesized that comparative studies with both strains could provide important information regarding the molecular mechanism(s) involved in the anti-inflammatory activity of bifidobacteria. Therefore, in the present study, we aimed to gain insight into the molecular mechanisms involved in the immunoregulatory effect of B. breve MCC-117 by studying pro-inflammatory cytokines production and mitogen-activated protein kinases (MAPK), phosphatidylinositol 3-kinase (PI3 K) and NF-κB pathways activation in PIE cells. Moreover, we evaluated role of TLR2 and TLR negative regulators in the regulation of the inflammatory response induced by B. breve MCC-117.
MATERIALS AND METHODS
Microorganisms
Bifidobacteria were provided by Morinaga Milk Industry Co., Ltd. (Zama, Japan). Bifidobacterium breve MCC-117 and Bifidobacterium adolescentis ATCC15705 were grown in Man-Rogosa-Sharpe broth and agar (Difco, Detroit, MI, USA) supplemented with 0.05% (w/v) cysteine (Sigma, Tokyo, Japan), and incubated at 37°C for 16 hr under anaerobic conditions (AnaeroGen; Oxoid, Basingstoke, UK). Cultures were then centrifuged at 1900 g for 10 min, and bifidobacteria were washed with phosphate-buffered saline (PBS) and resuspended in DMEM cell culture medium at the appropriate concentrations [9].
Enterotoxigenic Escherichia coli (ETEC) strain 987P was kindly provided by Dr M. Nakazawa, National Institute of Animal Health (Tsukuba, Japan). ETEC cells were plated into tryptic soy agar (Becton, Dickinson and Company) supplemented with 5% sheep blood (Nippon Biotest Laboratories Inc., Tokyo, Japan). After an overnight incubation at 37°C, a single colony was transferred to tryptic soy broth and grown for 24 hr at 37°C with shaking (200 rpm). After overnight incubation, the subcultures of bacteria that had been grown until the mid-log phase were centrifuged at 1900 g for 10 min at 4°C and washed with PBS, heat-treated at 65°C for 30 min and resuspended in DMEM cell culture medium as described previously [10]. Hereafter, we refer to this preparation as heat-stable ETEC pathogen-associated molecular patterns (PAMPs).
PIE cells
PIE cells, which are nontransformed intestinal cultured cells originally derived from intestinal epithelia isolated from an unsuckled neonatal swine [11], were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) (Invitrogen Corporation, Carlsbad, CA, USA) supplemented with 10% fetal calf serum (FCS), 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in an atmosphere of 5% CO2. PIE cells grow rapidly and are well adapted to culture conditions even without transformation or immortalization. However, the proliferative ability of PIE cells diminishes after 50 passages in culture. Therefore, we used PIE cells only between the 20th and 40th passages in these experiments.
Anti-inflammatory assay in PIE cells
PIE cells were seeded at 3 × 104 cells/12-well plate on type I collagen-coated plates (Iwaki, Tokyo, Japan) and cultured for 3 days. After changing the medium, bifidobacteria (5 × 107 cells/ml) were added for 48 hr, and the cytokine expression was analyzed. Similarly, in an ETEC challenge experiment, bifidobacteria (5 × 107 cells/ml) were added, and 48 hr later, each well was washed vigorously with medium at least three times to eliminate all stimulants; and then cells were stimulated with heat-stable ETEC PAMPs (equivalent to 5 × 107 cells/ml) for 12 hours. In a blocking experiment, unlabeled anti-human TLR2-mouse IgG (Clone #TL2.1, Cat. #309710, Biolegend, San Diego, CA, USA) and its isotype control antibody (MOPC-173, Cat. #400224) were used. Cultured PIE cells were incubated with the unlabeled anti-TLR2 or isotype control antibody for 12 hr before stimulation with bacteria.
Quantitative expression analysis by real-time PCR
We performed two-step real-time quantitative PCR to characterize the expression of mRNAs in PIE cells and immune cells. Total RNA was isolated from each PIE or immune cell sample using TRIzol reagent (Invitrogen). All cDNAs were synthesized using a Quantitect Reverse Transcription (RT) Kit (Qiagen, Tokyo, Japan) according to the manufacturer’s recommendations. Real-time quantitative PCR was carried out using an Applied Biosystems Real-time PCR System 7300 (Applied Biosystems, Warrington, United Kingdom) and the Platinum SYBR Green qPCR SuperMix-UDG (uracil-DNA glycosylase) with ROX (6-carboxyl-X-rhodamine) (Invitrogen). The primers for IL-6, IL-8, MCP-1, A20, SIGIRR, Tollip, Bcl-3 and IRAK-M used in this study were previously described [10, 12] (Table 1). The PCR cycling conditions were 5 min at 50°C, followed by 5 min at 95°C, and then 40 cycles of 15 sec at 95°C, 30 sec at 60°C, and 30 sec at 72°C. The reaction mixtures contained 2.5 μl of sample cDNA and 7.5 μl of master mix, which included the sense and antisense primers. Expression of β-actin in each sample was assessed, and the β-actin data were used as an internal control to normalize differences between samples and to calculate relative expression levels.
Table 1. Primer sequences used in this study.
| Sense primer | Antisense primer | |
| Porcine β-actin | CATCACCATCGGCAACGA | GCGTAGAGGTCCTTCCTGATGT |
| Porcine IL-6 | TGGATAAGCTGCAGTCACAG | ATTATCCGAATGGCCCTCAG |
| Porcine IL-8 | GCTCTCTGTGAGGCTGCAGTT | TTTATGCACTGGCATCGAAGTT |
| Porcine MCP-1 | ACAGAAGAGTCACCAGCAGCAA | GCCCGCGATGGTCTTG |
| Porcine SIGIRR | ATGTGAAGTGTCGGCTCAATGT | TTCATCTCCACCTCCCCATACT |
| Porcine Tollip | TACCGTGGGCCGTCTCA | CCGTAGTTCTTCGCCAACTTG |
| Porcine A20 | CCTCCCTGGAAAGCCAGAA | GTGCCACAAGCTTCCTCACTT |
| Porcine BCL-3 | CGACGCGGTGGACATTAAG | ACCATGCTAAGGCTGTTGTTTTC |
| Porcine MKP-1 | AACGAGGGTCAGGCTTTTCC | TCCCCAATGTGCTGAGTTCAG |
| Porcine IRAK-M | TGGAGCAGCCTTGAATCCTT | TGGATAACACGTTTGGGAATCTT |
Western blotting
PIE cells cultured (1.8 × 105 cells/dish) in 60-mm dishes were stimulated with bacteria as described previously. PIE cells were then washed and stimulated with ETEC for the indicated time period. The PIE cells were subsequently then washed three times with PBS and resuspended in 200 μl of CelLytic M Cell Lysis Reagent (Sigma) including protease and phosphates inhibitors (Complete Mini, PhosSTOP; Roche, Mannheim, Germany). Cells were transferred into Eppendorf tubes and boiled for 5 min at 95°C. Protein concentration was measured using a bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL, USA). Total protein samples (8 μg/sample) were loaded onto a 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Separated proteins were transferred electrophoretically to a nitrocellulose membrane. Phosphorylation of PI3 K, p38, Jun N-terminal protein kinase (JNK), and extracellular signal-regulated kinase (ERK) MAPK, and IκBα degradation were evaluated using anti-phosphated PI3 K, p38, anti-phosphated JNK, anti-phosphated ERK, and anti-IκB antibodies, respectively (Cell Signaling Technology, Beverly, MA, USA), according to the manufacturer’s instructions. The membranes were then stripped with Ten Minute Western Blot Re-Probe Kit (#JZ-008, Jacksun Easy Biotech, Inc., New York, USA) for the detection of each total protein. The optical density of each band was measured using Image J (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Statistical analyses were performed using the GLM and REG procedures available in the SAS computer program (SAS, 1994). Comparisons between mean values were carried out using one-way analysis of variance and Fisher’s least significant difference (LSD) test. For these analyses, P values of <0.05 were considered significant.
RESULTS
Immunoregulatory activity of B. breve MCC-117 in PIE cells
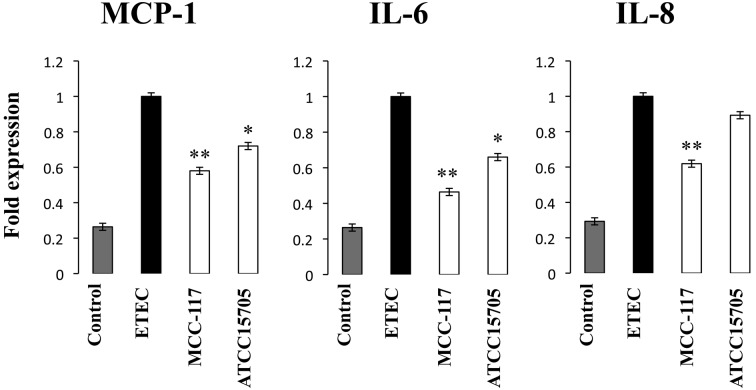
As we demonstrated before [10], challenge of PIE cells with heat-stable ETEC PAMPs significantly increased the levels of MCP-1, IL-6 and IL-8 mRNAs (Fig. 1). Pretreatment of PIE cells with both B. breve MCC-117 or B. adolescentis ATCC15705 significantly reduced the levels of MCP-1 and IL-6; however, the MCC-117 strain was more efficient than ATCC15705 in downregulating the levels of these cytokines. Only B. breve MCC-117 decreased the levels of IL-8 mRNAs in response to heat-stable ETEC PAMPs in PIE cells (Fig. 1).
Fig. 1.
Expression of cytokines in porcine intestinal epithelial (PIE) cells after stimulation with heat-stable Enterotoxigenic Escherichia coli (ETEC) pathogen-associated molecular patterns (PAMPs). PIE cells were pretreated with Bifidobacterium breve MCC-117 or Bifidobacterium adolescentis ATCC15705 for 48 hours and, stimulated with heat-stable ETEC PAMP and then the expression of MCP-1, IL-6 and IL-8 was studied at hour 12 post stimulation. The results represent four independent experiments. Significantly different from control: *p<0.05; **p<0.01.
Effect of B. breve MCC-117 on signaling pathways in PIE cells
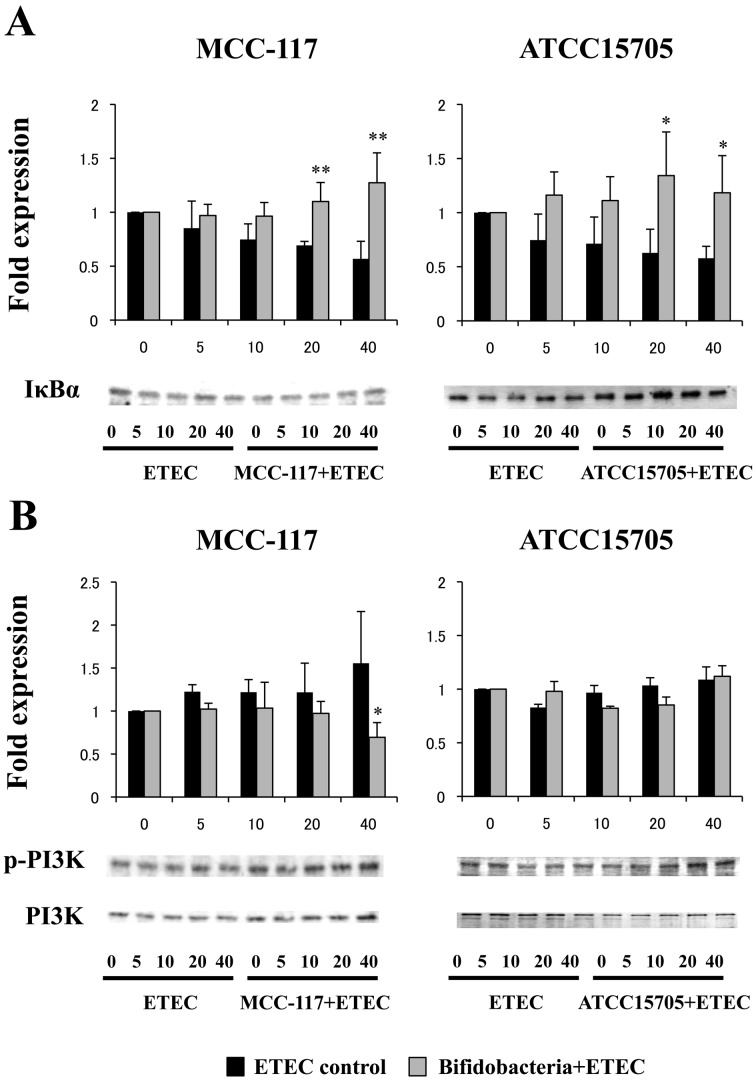
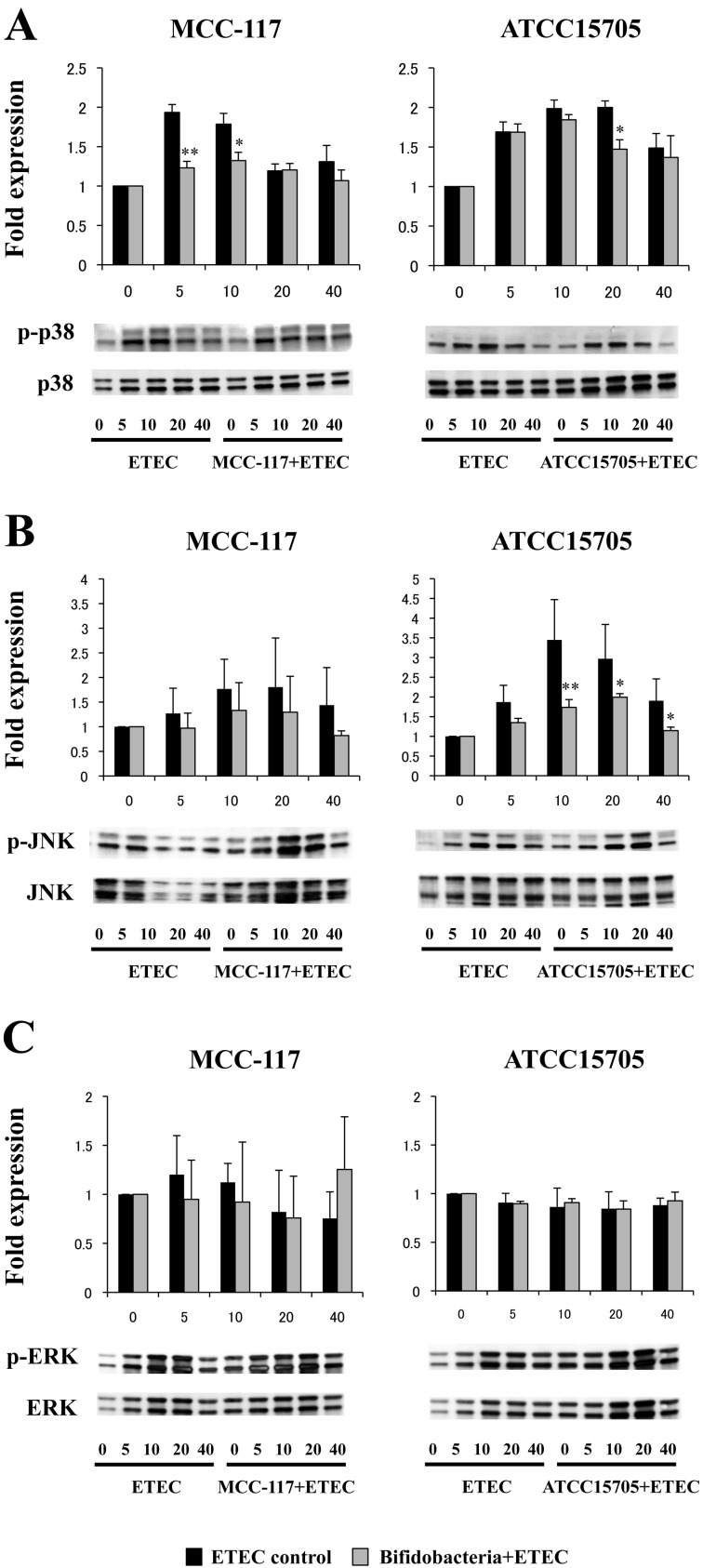
We next evaluated whether B. breve MCC-117 was able to attenuate heat-stable ETEC PAMP-mediated pro-inflammatory responses by modulating the NF-κB or PI3 K pathways (Fig. 2). Challenge of PIE cells with heat-stable ETEC PAMPs significantly reduced the levels of the counter-regulatory factor IκBα (Fig. 2A) and increased phosphorylation of PI3 K (Fig. 2B). PIE cells previously stimulated with B. breve MCC-117 or B. adolescentis ATCC15705 did not show a significant degradation of IκBα between 20 and 40 minutes after ETEC PAMP challenge, indicating an inhibitory effect on the NF-κB pathway for both strains (Fig. 2A). However, only B. breve MCC-117 was able to significantly reduce phosphorylation of PI3 K at 40 minutes after ETEC PAMP challenge (Fig. 2B). We also examined the relationship between MAPK activation and regulation of pro-inflammatory cytokines in PIE cells by B. breve MCC-117 (Fig. 3). PIE cells were stimulated with B. breve MCC-117, B. adolescentis ATCC15705 or control medium, and the activation profiles of p38, ERK and JNK were compared. Stimulation of PIE cells with heat-stable ETEC PAMPs significantly increased the phosphorylation of p38 (Fig. 3A) and JNK (Fig. 3B). On the other hand, no modifications in the phosphorylation of ERK were observed in PIE cells after heat-stable ETEC PAMP challenge (Fig. 3C). Phosphorylation of p38 in B. breve MCC-117-treated PIE was significantly reduced when compared with control cells after the challenge with ETEC PAMPs (Fig. 3A). B. breve MCC-117 was able to reduce p38 phosphorylation between minutes 5 and 10, while B. adolescentis ATCC15705 did not reduce the phosphorylation in a early time but a similar effect was observed only at minute 20 (Fig. 3A). In addition, PIE cells treated with B. breve MCC-117 showed a similar tendency of JNK phosphorylation to that observed in the control (Fig. 3B). On the other hand, reduced phosphorylation of JNK was observed in B. adolescentis ATCC15705-treated PIE cells between minutes 10 and 40 (Fig. 3B). The time course of ERK phosphorylation induced by heat-stable ETEC PAMPs in PIE cells treated with B. breve MCC-117 or B. adolescentis ATCC15705 showed a similar tendency to that observed in the control (Fig. 3C).
Fig. 2.
Western blot analysis of activation of NF-kB and IP3 K pathways in porcine intestinal epithelial (PIE) cells after challenge with heat-stable Enterotoxigenic Escherichia coli (ETEC) pathogen-associated molecular patterns (PAMPs). PIE cells were pretreated with Bifidobacterium breve MCC-117 or Bifidobacterium adolescentis ATCC15705 for 48 hours and, stimulated with heat-stable ETEC PAMPs. Levels of the counter-regulatory factor IκBα and phosphorylation of IP3 K were studied at the indicated times post stimulation. For the evaluation of IkBa degradation, the expressions were compared with the values obtained at time 0 that were set as 1. For the phosphorylation of PI3 K, the phosphorylated protein/total protein ratio was calculated, and the differences were compared with the values obtained at time 0 that were set as 1. Significantly different from the control at the same time point: *p<0.05; **p<0.01.
Fig. 3.
Western blot analysis of the activation of p38, JNK and ERK mitogen-activated protein kinases in porcine intestinal epithelial (PIE) cells after challenge heat-stable Enterotoxigenic Escherichia coli (ETEC) pathogen-associated molecular patterns (PAMPs). PIE cells were pretreated with Bifidobacterium breve MCC-117 or Bifidobacterium adolescentis ATCC15705 for 48 hours and, stimulated with heat-stable ETEC PAMPs. Phosphorylation of p38, JNK and ERK was studied at the indicated times post stimulation. For the phosphorylation of p38, JNK and ERK, each phosphorylated protein/total protein ratio was calculated, and the differences were compared with the values obtained at time 0 that were set as 1. Significantly different from the control at the same time point: *p<0.05; **p<0.01.
Effect of B. breve MCC-117 on negative regulators of the TLR signaling pathway in PIE cells
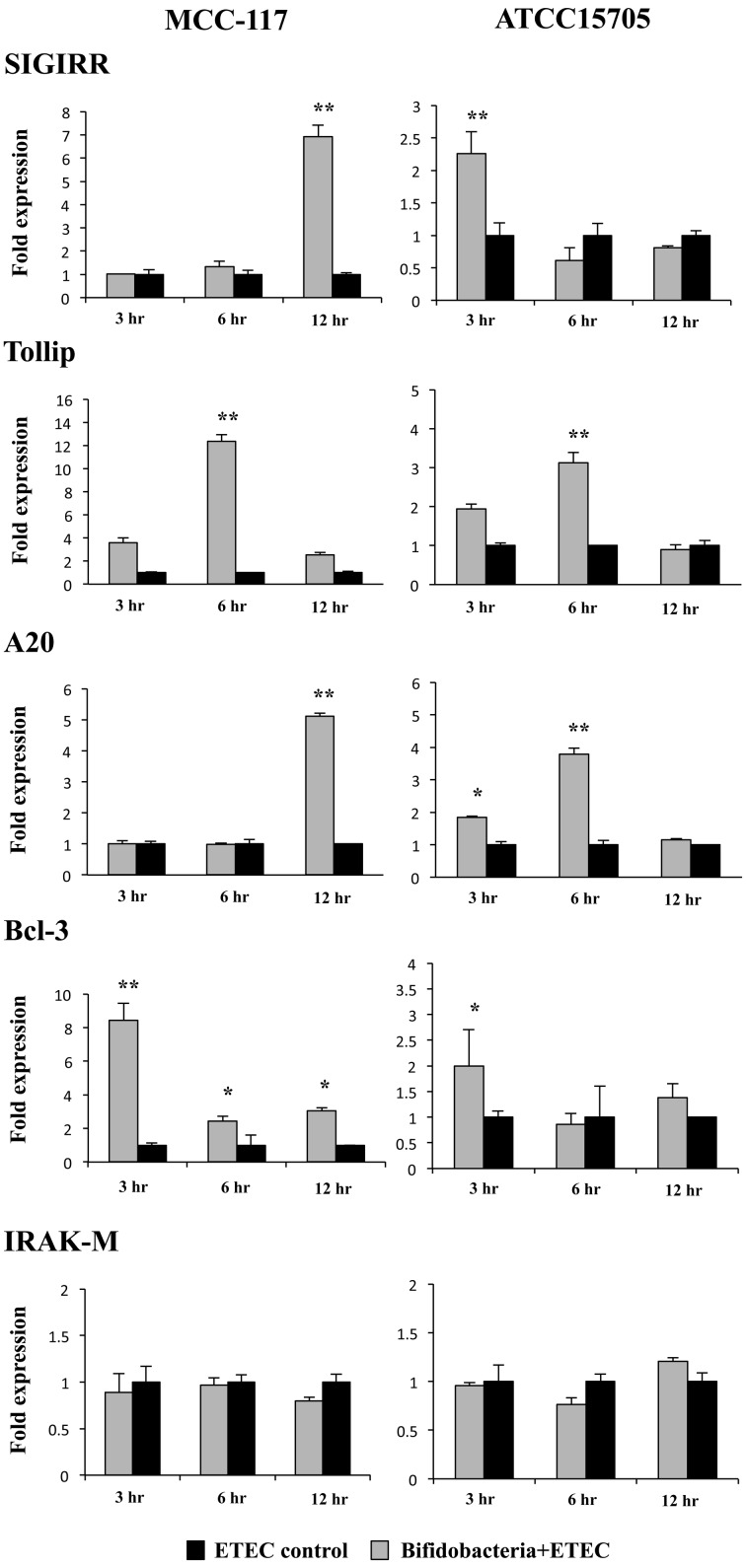
We studied the negative regulators that are known to mediate the TLR signaling pathway. For this reason, PIE cells were stimulated for 48 hours with B. breve MCC-117 or B. adolescentis ATCC15705, and the expression of single immunoglobulin IL-1-related receptor (SIGIRR), Toll interacting protein (Tollip), A20, B-cell lymphoma 3-encoded protein (Bcl-3) and interleukin-1 receptor-associated kinase M (IRAK-M) was determined by real-time PCR (Fig. 4). None of the treatments were able to significantly induce changes in the expression of IRAK-M (Fig. 4). On the other hand, both B. breve MCC-117 and B. adolescentis ATCC15705 treatments were capable of upregulating the expression of SIGIRR, Tollip, A20 and Bcl-3; however we observed several differences between the treatments when analyzing in the fold levels of upregulation as well as the time course (Fig. 4). Both strains upregulated the mRNA levels of Bcl-3 and Tollip at hours 3 and 6, respectively; however, the fold increases were significantly higher in PIE cells treated with B. breve MCC-117 (8.5-fold and 13-fold for Bcl-3 and Tollip, respectively) when compared with B. adolescentis ATCC15705 (2-fold and 3.1-fold for Bcl-3 and Tollip, respectively). In addition, expression of SIGIRR was upregulated early (hour 3) in B. adolescentis ATCC15705-treated PIE cells, while this TLR negative regulator was increased at hour 12 in MCC-117-treated cells. Moreover, SIGIRR was upregulated 2.3-fold and 7-fold in ATCC15705- and MCC-117-treated PIE cells, respectively (Fig. 4). Similarly, expression of A20 was upregulated at hour 6 in B. adolescentis ATCC15705-treated PIE cells, this while negative regulator was increased at hour 12 in MCC-117-treated cells. We also observed that A20 was upregulated 4-fold and 5-fold in ATCC15705- and MCC-117-treated PIE cells, respectively (Fig. 4).
Fig. 4.
Expression of toll-like receptor negative regulators in porcine intestinal epithelial (PIE) cells. PIE cells were pretreated with Bifidobacterium breve MCC-117 or Bifidobacterium adolescentis ATCC15705 for 48 hours and, stimulated with heat-stable ETEC PAMPs. Then, the expression of IRAK-M, SIGIRR, Bcl-3, Tollip and A20 negative regulators was studied at the indicated time points. The results represent four independent experiments. Significantly different from control at the same time point: *p<0.05; **p<0.01.
Role of TLR2 in the anti-inflammatory effect of B. breve MCC-117
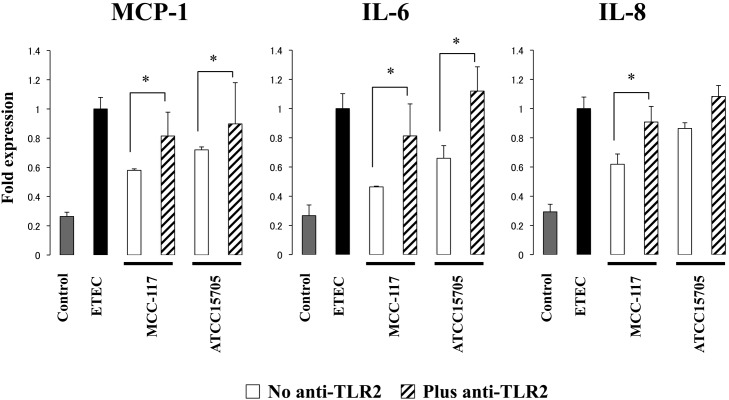
To study the role of TLR2 in the immunomodulatory effect of B. breve MCC-117 and B. adolescentis ATCC15705, we next performed comparative studies with anti-TLR2 blocking antibodies (Fig. 5). Use of anti-TLR2 antibodies abolished reductions in the IL-6, IL-8 and MCP-1 mRNA levels induced by B. breve MCC-117 and the reduction in MCP-1 achieved by B. adolescentis ATCC15705.
Fig. 5.
Role of TLR2 in the immunomodulatory effect of Bifidobacterium breve MCC-117 in porcine intestinal epithelial (PIE) cells. PIE cells were pretreated with Bifidobacterium breve MCC-117 or Bifidobacterium adolescentis ATCC15705 plus anti-TLR2 antibodies. Untreated adherent cells were used as controls. PIE cells were stimulated with heat-stable Enterotoxigenic Escherichia coli (ETEC) pathogen-associated molecular patterns (PAMPs), and then the expression of MCP-1, IL-6 and IL-8 was studied at hour 12 post stimulation. The results represent four independent experiments. Significantly different from the anti-TLR2 untreated group: *p<0.05.
DISCUSSION
Over the past decade, it has become clear that immunobiotics can interact with mucosal immune cells to modulate specific functions of the mucosal immune system [1, 2]. Increasing evidence also suggests that induction of epithelial signaling by immunobiotics can modulate barrier functions and, defensin production and regulate inflammatory signaling [3]. The most well-understood signaling mechanisms involve the innate pattern recognition receptors such as the TLRs. Several works have demonstrated that TLR2 is required for some probiotic lactobacilli to exert their immunomodulatory effects. Studies in a primary culture of IECs from conventional mice showed that immunostimulatory lactobacilli induce the release of cytokines by the cells via TLR2 [13]. In addition, lactobacilli are able to increase the expression of TLR2 in intestinal DCs and macrophages [12, 14]. Moreover, it was suggested that the strong induction of Th-polarizing DCs by lactobacillus strains is dependent on lipoteichoic acid interaction with TLR2 [4]. On the other hand, it has been demonstrated that TLR2 seems to play an important regulatory role in the recognition of lactobacilli that possess an immunoinhibitory effect. Recently, we demonstrated that stimulation of PIE cells with Lactobacillus jensenii TL2937 is able to downregulate the levels of IL-6, IL-8 and MCP-1 produced in response to heat-stable ETEC PAMPs or LPS challenges and that TLR2 is partially involved in this immunoregulatory effect of L. jensenii TL2937 in PIE cells [10]. More recently, we demonstrated that direct exposure of porcine antigen-presenting cells (APCs) from porcine PPs to L. jensenii TL2937 activated CD172a+ APCs and caused them to become phenotypically and functionally mature and to display tolerogenic properties, and that these effects were partially dependent of TLR2 activation [12].
These and other studies show that there has been a significant advance in the knowledge of the immunological mechanisms involved in the beneficial effects of lactobacilli. On the other hand, the mechanisms involved in the immunoregulatory activities of bifidobacteria have been less studied. Recent work has begun to elucidate the mechanisms as well as the effector molecules involved in the immunomodulatory effect of bifidobacteria [15,16,17]. It is believed that the beneficial effects of bifidobacteria require biological interactions with the intestinal epithelium. Therefore, in this work, we aimed to study the molecular mechanism and receptors involved in the anti-inflammatory effect of previously selected bifidobacterium strains. We demonstrated that B. breve MCC-117 significantly downregulates levels of IL-8, MCP-1 and IL-6 in PIE cells challenged with heat-stable ETEC PAMPs. Moreover, we showed that the MCC-117 strain is able to attenuate the pro-inflammatory response by modulating the NF-κB and p38 MAPK pathways. We have previously reported that L. jensenii TL2937 induces a similar anti-inflammatory effect on PIE cells by modulating the activation of both NF-κB and p38 MAPK pathways [10]. Moreover, another group reported that soluble factors of bifidobacteria can inhibit phosphorylation and subsequent degradation of NF-κB inhibitor IκBα along with phosphorylation of the AP-1 pathway component p38-MAPK, reducing CXCL8 expression in HT29-19A cells [15]. Therefore, inhibition of these pathways seems to be crucial in the anti-inflammatory activity of immunobiotics, and moreover, the study of the inhibition of NF-κB and p38 MAPK pathways in PIE cells could be used as biomarkers for the screening and selection of effective immunoregulatory strains as we have demonstrated recently [18].
The family of PI3 K enzymes is largely responsible for the phosphorylation of phosphatidylinositol lipids in response to cellular stimuli. PI3 K is a heterodimeric enzyme that consists of a regulatory (p85) and a catalytic (p110) subunit. Once activated, PI3 K regulates TLR signaling in both negative and positive ways. Direct evidence for the involvement of PI3 K in TLR-signaling was initially shown by Arbibe et al. [19], who demonstrated that site-directed mutagenesis of specific tyrosine residues within the cytosolic domain of TLR2 resulted in a loss in the ability of p85 to associate with TLR2 and abrogated the ability of TLR2 to induce NF-κB transcriptional activity. Furthermore, inhibition of PI3 K during TLR2 stimulation, through use of either Ly294002 or dominant-negative Akt has been shown to greatly reduce NF-κB activation [19]. Studies on PI3 K knock out mice, however, support the idea that PI3 K negatively regulates TLR activation, as signaling by TLR2, TLR4, TLR5, and TLR9 is elevated in p85a-deficient mice [20, 21]. So, it appears that PI3 K is an important TLR activation component that affects signaling in different ways depending on cell type and readout. In this work, we demonstrated that stimulation of PIE cells with heat-stable ETEC PAMPs activated the PI3 K pathway, indicating that PI3 K is involved in TLR4 signaling in PIE cells. In addition, we showed that B. breve MCC-117 treatment significantly inhibited the PI3 K pathway, and that this pathway was not affected when PIE cells were treated with the ATCC15705 strain. These results indicate that the PI3 K pathway would have an important role in the immunoregulatory effect of B. breve MCC-117.
To further dissect the mechanism(s) that underlies the anti-inflammatory effect of B. breve MCC-117, we assessed the effect of this strain on expression of negative regulators of TLRs in PIE cells. The expression levels of SIGIRR, Tollip, A20, Bcl-3 and IRAK-M were evaluated, and we found that SIGIRR, Tollip, A20, and Bcl-3 mRNA expression was upregulated in PIE cells stimulated with B. breve MCC-117. It was demonstrated that molecules such as Tollip, SIGIRR, zinc finger protein with ubiquitin-modifying activity A20 and peroxisome proliferator-activated receptor-γ (PPAR-γ) inhibit TLR signaling in human IECs [22]. TOLLIP ensures a state of nonresponsiveness in cultured enterocytes at re-exposure to LPS, due to downregulation of TLR surface expression and decreased phosphorylation of IRAK-1 [23]. A20 inhibits activation of NF-kB via inflammatory cytokine receptors, TLR and NOD2, by ubiquitin-editing activities. A20 suppresses TLR2-mediated production of IL-8 in enterocytes and induces hyporesponsiveness to repeated stimulation with LPS [24]. In addition, the Bcl-3 protein functions as an inhibitor of NF-κB activity and has been proposed to function in the process of LPS tolerance [25]. Thus, the induction of these four negative regulators by B. breve MCC-117 in PIE cells may be important for establishing tolerance against TLR4 activation. Moreover, our results indicate that the degree of upregulation of SIGIRR, Tollip, A20 and Bcl-3 would be also important for the anti-inflammatory effect of bifidobacteria, since we observed upregulation of the four negative regulators in B. adolescentis ATCC15705-treated PIE cells, but with significantly lower levels when compared with the MCC-117 strain.
In addition, we previously demonstrated that A20, Bcl-3 and Tollip mRNA levels can be upregulated by immunobiotics in a TLR2-dependent manner [10, 12]. In the present work, we showed that the three negative regulators of TLRs were upregulated by the MCC-117 and ATCC15705 strains, with B. breve MCC-117 being the most effective strain for the induction of Tollip and Bcl-3. Therefore, we also aimed to confirm if TLR2 was involved in the anti-inflammatory effect of the MCC-117 strain by using anti-TLR2 blocking antibodies. The results showed that the reduction in inflammatory cytokines and chemokines induced by bifidobacterium treatments were abolished in PIE cells treated with anti-TLR2 antibodies, indicating an important role of this PRR in the immunoregulatory effect of B. breve MCC-117.
Cell wall components of the lactobacilli can potentially signal through binding to TLR2. Apart from lipoproteins, TLR2 has been reported to bind the lipid chains anchoring lipoteichoic acid (LTA) in the cytoplasmic membrane of Gram-positive bacteria such as lactobacilli. Mutations of LTA in lactobacilli significantly modify their immunomodulatory properties. It has been shown, for example, that mutation of LTA in Lactobacillus plantarum NCIMB8826 induced a reduction in the secretion of proinflammatory cytokines by peripheral blood mononuclear cells (PBMCs) and monocytes when compared with the wild type strain [26]. Furthermore, the amount of IL-10 produced in co-culture of PBMCs with the mutant was significantly increased compared with the parent strain [26]. More recently, it was reported that deletion of the phosphoglycerol transferase gene that primes the synthesis of bacterial LTA in L. acidophilus NCK56 resulted in a strain lacking LTA. Interestingly, the mutant strain NCK2025 downregulated production of IL-12 and TNF-α in co-culture with human monocyte-derived DCs but also significantly enhanced IL-10 when compared with wild type strain [27]. Therefore, as suggested by Wells [28], the capacity of different species and strains to stimulate TLR2 signaling varies considerably, and that this may reflect differences in the expression level of certain lipoproteins, their release into the medium, the amount of LTA and its structure/modification and the effect of shielding factors such as exopolysacchrides. So, comparatively studying the immunoregulatory effects of cell wall components such as LTA from both B. breve MCC-117 and B. adolescentis ATCC15705 could further increase our knowledge of bifidobacteria effector molecules. This point is an interesting topic for future research.
In addition, we observed that B. breve MCC-117 increased expression of SIGIRR, a TLR negative regulator that is not induced by TLR2 activation in PIE cells [10]. This finding indicated that a PRR(s), other than TLR2, may mediate the anti-inflammatory effects of B. breve MCC-117. Moreover, we observed that the upregulation of SIGIRR induced by the MCC-117 strain was significantly higher than that induced by ATCC15705; indicating that MCC-117 would have a higher capacity to interact with the unknown PRR(s) when compared with the ATCC15705 strain. Identification of the unknown receptor or receptors is another interesting topic for future investigations.
In conclusion, we demonstrated in this work that the immunoregulatory effect of B. breve MCC-117 was achieved by complex interactions of multiple negative regulators of TLRs as well as by inhibition of multiple signaling pathways. We recently demonstrated that several strains with immunoregulatory capabilities used a common mechanism to induce tolerance in PIE cells [18]. Immunoregulatory strains are able to interact with TLR2, upregulate A20 expression in PIE cells and beneficially modulate the subsequent TLR4 activation by reducing the activation of MAPK and NF-kB pathways and the production of pro-inflammatory cytokines. In the present work, we confirmed these findings by demonstrating that B. breve MCC-117 is also able to activate TLR2 in PIE cells and induce tolerance through A20 upregulation. In addition, we extended our previous findings by showing for the first time that B. breve MCC-117 reduced heat-stable ETEC PAMP-induced expression of pro-inflammatory cytokines by modulating PI3 K activation.
The upregulation of negative regulators of TLRs by B. breve MCC-117 could have an important physiological impact on maintaining or reestablishing homeostatic TLR signals in PIE cells like other bifidobacterium strains previously evaluated in our laboratory such as B. longum BB536 and B. breve M-16V [18]. Moreover, considering that B. breve MCC-117 is able to significantly reduce the levels of IFN-γ in CD4+ lymphocytes and improve IL-10 levels in CD4+CD25high Foxp3+ lymphocytes co-cultured with PIE cells [9], we can speculate that B. longum BB536 and B. breve M-16V could have a similar effect in our in vitro co-culture system with PIE and immune cells from Peyer’s patches.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research (B)(2) (No. 21380164, 24380146) and Challenging Exploratory Research (No. 23658216) from the Japan Society for the Promotion of Science (JSPS), the Kieikai Research Foundation and the Japan Racing Association to Dr. H. Kitazawa. Dr. Julio Villena was supported by JSPS (Postdoctoral Fellowship for Foreign Researchers, Program No. 21-09335).
REFERENCES
- 1.Lebeer S, Vanderleyden J, De Keersmaecker SC. 2010. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol 8: 171–184 [DOI] [PubMed] [Google Scholar]
- 2.Bron PA, van Baarlen P, Kleerebezem M. 2011. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat Rev Microbiol 10: 66–78 [DOI] [PubMed] [Google Scholar]
- 3.Villena J, Aso H, Alvarez S, Kitazawa H. 2012. Porcine toll-like receptors and their crosstalk with immunobiotics: impact in the regulation of gut inflammatory immunity. In: Probiotics: Sources, Types and Health Benefits. NOVA Science publishers, pp. 53–83. [Google Scholar]
- 4.Zeuthen LH, Fink LN, Frokiaer H. 2008. Toll-like receptor 2 and nucleotide-binding oligomerization domain-2 play divergent roles in the recognition of gut-derived lactobacilli and bifidobacteria in dendritic cells. Immunology 124: 489–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoarau C, Lagaraine C, Martin L, Velge-Roussel F, Lebranchu Y. 2006. Supernatant of Bifidobacterium breve induces dendritic cell maturation, activation, and survival through a Toll-like receptor 2 pathway. J Allergy Clin Immunol 117: 696–702 [DOI] [PubMed] [Google Scholar]
- 6.Tohno M, Shimosato T, Kawai Y, Aso H, Ikegami S, Taketomo N, Saito T, Kitazawa H. 2007. Advanced molecular immunoassay system for immunobiotic lactic acid bacteria using a transfectant of toll-like receptor 2. Anim Sci J 78: 195–205 [Google Scholar]
- 7.Kitazawa H, Tohno M, Shimosato T, Saito T. 2008. Development of molecular immunoassay system for probiotics via toll-like receptors based on food immunology. Anim Sci J 79: 11–21 [Google Scholar]
- 8.López P, Gueimonde M, Margolles A, Suarez A. 2010. Distinct Bifidobacterium strains drive different immune responses in vitro. Int J Food Microbiol 138: 157–165 [DOI] [PubMed] [Google Scholar]
- 9.Fujie H, Villena J, Tohno M, Morie K, Simazu T, Aso H, Suda Y, Iwabuchi N, Xiao J, Iwatsuki K, Kawai Y, Saito T, Kitazawa H. 2011. Toll-like receptor-2 activating bifidobacteria strains differentially regulate inflammatory cytokines in porcine intestinal epithelial cell culture system: finding new anti-inflammatory immunobiotics. FEMS Immunol Med Microbiol 63: 129–139 [DOI] [PubMed] [Google Scholar]
- 10.Shimazu T, Villena J, Tohno M, Fujie H, Hosoya S, Shimosato T, Aso H, Suda Y, Kawai Y, Saito T, Makino S, Ikegami S, Itoh H, Kitazawa H. 2012. Immunobiotic Lactobacillus jensenii elicit anti-inflammatory activity in porcine intestinal epithelial cells by modulating negative regulators of the toll-like receptor signaling pathway. Infect Immun 80: 276–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moue M, Tohno M, Shimazu T, Kido T, Aso H, Saito T, Kitazawa H. 2008. Toll-like receptor 4 and cytokine expression involved in functional immune response in an originally established porcine intestinal epitheliocyte cell line. Biochim Biophys Acta 1780: 134–144 [DOI] [PubMed] [Google Scholar]
- 12.Villena J, Suzuki R, Fujie H, Chiba E, Takahashi T, Tomosada Y, Shimazu T, Aso H, Ohwada S, Suda Y, Ikegami S, Itoh H, Alvarez S, Saito T, Kitazawa H. 2012. Immunobiotic Lactobacillus jensenii modulates the Toll-like receptor 4-induced inflammatory response via negative regulation in porcine antigen-presenting cells. Clin Vaccine Immunol 19: 1038–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vinderola G, Matar C, Perdigón G. 2005. Role of the epithelial cells in the immune effects mediated by gram-positive probiotic bacteria. Involvement of Toll-like receptors. Clin Diagn Lab Immunol 12: 1075–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohamadzadeh M, Olson S, Kalina WV, Ruthel G, Demmin GL, Warfield KL, Bavari S, Klaenhammer TR. 2005. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc Natl Acad Sci USA 102: 2880–2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heuvelin E, Lebreton C, Grangette C, Pot B, Cerf-Bensussan N, Heyman M. 2009. Mechanisms involved in alleviation of intestinal inflammation by Bifidobacterium breve soluble factors. PLoS ONE 4: e5184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Mahony D, Murphy S, Boileau T, Park J, O’Brien J, Groeger D, Konieczna P, Ziegler M, Scully P, Shanahan F, Kiely B, O’Mahony L. 2010. Bifidobacterium animalis AHC7 protects against pathogen-induced NF-kB activation in vivo. BMC Immunol 11: 63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khokhlova EV, Smeianov VV, Efimov BA, Kafarskaia LI, Pavlova SI, Shkoporov AN. 2012. Anti-inflammatory properties of intestinal Bifidobacterium strains isolated from healthy infants. Microbiol Immunol 56: 27–39 [DOI] [PubMed] [Google Scholar]
- 18.Tomosada Y, Villena J, Murata K, Chiba E, Shimazu T, Aso H, Iwabuchi N, Xiao JZ, Saito T, Kitazawa H. 2013. Immunoregulatory effect of bifidobacteria strains in porcine intestinal epithelial cells through modulation of ubiquitin-editing enzyme A20 expression. PLoS ONE 8: e59259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arbibe L, Mira JP, Teusch N, Kline L, Guha M, Mackman N. 2000. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat Immunol 1: 533–540 [DOI] [PubMed] [Google Scholar]
- 20.Fukao T, Tanabe M, Terauchi Y, Ota T, Matsuda S, Asano T. 2002. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol 3: 875–881 [DOI] [PubMed] [Google Scholar]
- 21.Yu Y, Nagai S, Wu H, Neish AS, Koyasu S, Gewirtz AT. 2006. TLR5-mediated phosphoinositide 3-kinase activation negatively regulates flagellin-induced proinflammatory gene expression. J Immunol 176: 6194–6201 [DOI] [PubMed] [Google Scholar]
- 22.Shibolet O, Podolsky DK. 2007. TLRs in the Gut. IV. Negative regulation of Toll-like receptors and intestinal homeostasis: addition by subtraction. Am J Physiol Gastrointest Liver Physiol 292: G1469–G1473 [DOI] [PubMed] [Google Scholar]
- 23.Otte JM, Cario E, Podolsky DK. 2004. Mechanisms of cross hyporesponsiveness to Toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology 126: 1054–1070 [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Ouyang Y, Guner Y, Ford HR, Grishin AV. 2009. Ubiquitin-editing enzyme A20 promotes tolerance to lipopolysaccharide in enterocytes. J Immunol 183: 1384–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wessells J, Baer M, Young HA, Claudio E, Brown K, Siebenlist U, Johnson PF. 2004. BCL-3 and NF-kappa B p50 attenuate lipopolysaccharide-induced inflammatory responses in macrophages. J Biol Chem 279: 49995–50003 [DOI] [PubMed] [Google Scholar]
- 26.Grangette C, Nutten S, Palumbo E, Morath S, Hermann C, Dewulf J, Pot B, Hartung T, Hols P, Mercenier A.2005. Enhanced antiinflammatory capacity of a Lactobacillus plantarum mutant synthesizing modified teichoic acids. Proc Natl Acad Sci USA 102: 10321−10326. [DOI] [PMC free article] [PubMed]
- 27.Mohamadzadeh M, Pfeiler EA, Brown JB, Zadeh M, Gramarossa M, Managlia E, Bere P, Sarraj B, Khan MW, Pakanati KC. 2011. Regulation of induced colonic inflammation by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc Natl Acad Sci USA 108: 4623–4630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wells JM. 2011. Immunomodulatory mechanisms of lactobacilli. Microb Cell Fact 10: S17 [DOI] [PMC free article] [PubMed] [Google Scholar]