Abstract
Lactobacillus delbrueckii strains were assessed for their degradation patterns of various carbohydrates with specific reference to inulin-type fructans in comparison with those of Lactobacillus paracasei strains. Firstly, growth curves on glucose, fructose, sucrose and inulin-type fructans with increasing degrees of fructose polymerization (i.e., 1-kestose, fructo-oligosaccharides and inulin) of the strains were compared. L. paracasei DSM 20020 grew well on all these sugars, while the growth rates of the 4 L. delbrueckii strains were markedly higher on the fructans with a greater degree of polymerization than on fructose and sucrose. Secondly, sugar compositions of spent cultures of the strains of L. delbrueckii and L. paracasei grown in mMRS containing either the fructans or inulin were determined by thin layer chromatography, in which the spent cultures of L. paracasei DSM 20020 showed spots of short fructose and sucrose fractions, whereas those of the L. delbrueckii strains did not show such spots at all. These results suggest that, unlike the L. paracasei strains, the L. delbrueckii strains do not degrade the inulin-type fructans extracellularly, but transport the fructans capable of greater polymerization preferentially into their cells to be degraded intracellularly for their growth.
Keywords: inulin, fructans, fructose, Lactobacillus delbrueckii, Lactobacillus paracasei
INTRODUCTION
The ingestion of fermented food products or dietary supplements containing probiotic species of Lactobacillus and Bifidobacterium has been reported to promote gastrointestinal (GI) health in hosts by increasing the population of these bacteria in the GI tract [1, 2]. However, the beneficial effects of these bacteria may be transient due to colonization resistance by intestinal microbes, which restricts the ability of probiotic bacteria to become well established in the intestinal environment [3, 4]. A way to overcome this limitation is to include prebiotics in the host diet. A prebiotic can be defined as a nondigestible food ingredient that beneficially affects the host by selectively stimulating the growth of one or a limited number of bacteria in the colon, thus improving host health [5].
Inulin-type fructans are among the prebiotic substances that have been reported to selectively stimulate the growth and activity of certain strains of Lactobacillus and Bifidobacterium. Inulin-type fructans are linear D-fructose polymers linked by β (2-1)-glycosidic bonds, with a terminal glucose moiety that is linked by an α (1-2)-glycosidic bond, as in sucrose. Inulin and fructo-oligosaccharide (FOS) are well known as common inulin-type fructans [6]. The degree of polymerization (DP) of FOS varies between 3 and 5, a mixture of 1-kestose (DP 3), nystose (DP 4) and 1F-fructofuranosyl nystose (DP 5), whereas that of inulin can be 60 or even more [7]. The β (2-1) linkages of these fructans prevent their digestion in the upper part of the human gastrointestinal tract and are responsible for their reduced caloric value and dietary fiber-like effects [8].
These prebiotics stimulate the specific growth of bifidobacteria, which leads to the so-called bifidogenic effect [8, 9]. Moreover, it has been demonstrated that certain Lactobacillus strains, including strains of L. paracasei and L. delbrueckii, are able to grow on these prebiotics [10, 11, 12, 13, 14, 15, 16]. As for the degradation mechanism of inulin-type fructans, it has been reported that L. paracasei 1195 has an extracellular enzyme that is cell wall associated and responsible for the degradation of large fractions of FOS and inulin, leading to the extracellular accumulation of short fractions that can be taken up into the bacterial cell for further intracellular hydrolysis [17, 18]. In contrast, despite the wide use of the fructans as functional food ingredients and their well-studied prebiotic activity, very little is known about the relationship between the chain length of the fructans and the ability of L. delbrueckii to ferment them. We thus compared growth and degradation patterns among strains of L. delbrueckii and L. paracasei on various sugars including FOS and inulin.
MATERIALS AND METHODS
Bacterial strains used
The bacterial strains used in this study were as follow: L. delbrueckii TU-1, L. delbrueckii JCM 1002T, L. delbrueckii JCM 1012T, L. delbrueckii JCM 1248T, L. delbrueckii JCM 15610T, L. paracasei JCM 8130T, L. paracasei JCM 1171T and L. paracasei DSM 20020 (Table 1). All strains were stored at −80°C in de Man-Rogosa-Sharpe (MRS) broth (Oxoid, Basingstoke, United Kingdom) until use.
Table 1. Bacterial strains used in this study.
| Strain | Origin | Source |
| Lactobacillus paracasei JCM1171T | Pasteurized milk | JCM |
| Lactobacillus paracasei JCM8130T | Milk products | JCM |
| Lactobacillus paracasei DSM 20020 | Saliva of a child | DSMZ |
| Lactobacillus delbrueckii TU-1 | Commercial yogurt | This study |
| Lactobacillus delbrueckii JCM 1002T | Bulgarian yogurt | JCM |
| Lactobacillus delbrueckii JCM 1012T | Sour grain mash | JCM |
| Lactobacillus delbrueckii JCM 1248T | Emmental (Swiss) cheese | JCM |
It should be noted that L. delbrueckii TU-1 was isolated in our laboratory from a commercial yogurt. Briefly, approximately 10 g of the commercial yogurt was homogenized in saline, and 10-fold serial dilutions were prepared. Dilutions up to 10−8 of the initial suspension were plated on MRS agar plates. Plates were incubated anaerobically at 37°C for at least 48 hr. Colonies were subcultured and identified by Gram staining. The identification of yogurt strains was performed by PCRs with the species of L. delbrueckii specific primers as described by Tilsala et al. [19]. The primers used for identification of L. delbrueckii were as follows: Del I (5′-ACGGATGGATGGAGAGCAG-3′) and Del II (5′-GCAAGTTTGTTCTTTCGAACTC-3′). The amplification profile was as follows: 94°C for 30 sec, 62°C for 30 sec and 72°C for 15 sec, which was repeated for 30 cycles. A pre-incubation step at 94°C for 15 sec was also included. The PCR assay confirmed that TU-1 was L. delbrueckii.
Media and substrates used
Modified MRS medium [20], without glucose and supplemented with 0.5 g/l L-cysteine hydrochloride (Wako Pure Chemical Industries, Osaka, Japan), hereafter referred to as mMRS medium, was used as the basal fermentation medium throughout this study. The pH of the medium was adjusted to 6.5 before sterilization (121°C for 15 min).
Fructose, sucrose, 1-kestose (DP 3), FOS (DP 3−5), Fuji FFSC inulin, Fuji FF inulin or inulin was added to mMRS as the sole carbon source (2%, wt/vol). In all cases, these sugars were sterilized through membrane filtration using Millex® Syringe Filter Units (pore size, 0.45 μm; Merck Millipore, Darmstadt, Germany) and added aseptically to the sterile mMRS medium.
Fructose, sucrose, 1-kestose, FOS and inulin were purchased from Wako; Fuji FFSC inulin and Fuji FF inulin were purchased from Fuji Nihon Seito Corporation (Japan). Fuji FFSC inulin, a commercial powder made with sugar as a raw material, contains inulin (86.8%, wt/wt), another glucide (6.6%, wt/wt) and water (6.6%, vol/wt). The average DP of the Fuji FFSC inulin chains is reported to vary between 3 and 20, with an average of 8. Fuji FF is also a commercial powder made with sugar as a raw material according to information supplied by the company. The powder contains inulin (92.8%, wt/wt), another glucide (3.3%, wt/wt) and water (3.8%, vol/wt). The DP of the Fuji FF inulin chains is reported to vary between 3 and 30, with an average of 16 according to information supplied by the company. The DP of inulin (Wako) is reported to vary between 3 and 60 according to information supplied by the company. It should be noted that the polymerization range of inulin (Wako) is greater than those of Fuji FFSC inulin and Fuji FF inulin.
Fermentation experiments
To compare the growth patterns of L. delbrueckii and L. paracasei after fermenting different sugars, 4 L. delbrueckii strains and 3 L. paracasei strains were propagated twice in MRS broth anaerobically, and cultures obtained after 12 hr of growth anaerobically at 37°C were centrifuged (at 4,000 rpm for 15 min). After centrifugation, the bacterial pellet was washed once with phosphate-buffered saline (PBS, 0.8% NaCl, 0.02% KH2PO4, 0.115% Na2HPO4 [pH 7.4]) and re-suspended in PBS until its optical density at 660 nm (OD660) reached 0.4, followed by spotting of 50 µL of this suspension on 5 mL of MRS or mMRS containing fructose, sucrose, 1-kestose, FOS, Fuji FFSC inulin, Fuji FF inulin or inulin (2%, wt/vol at final concentration). Incubations of the media were performed anaerobically at 37°C in an anaerobic jar (Mitsubishi Gas Chemical, Japan) for up to 48 hr, during which their OD660 and pH were measured at 6, 12, 24, and 48 hr after incubation, respectively.
Sugar degradation analysis by thin layer chromatography (TLC)
To investigate the degradation patterns of the sugars added to the media, sugar compositions of the spent cultures after 12 hr and 24 hr of incubation were determined by thin layer chromatography (TLC) (Merck, silica gel 60 plate). Briefly, the spent cultures (approx. 2 µL each) of L. paracasei JCM 8130T, L. paracasei DSM 20020, L. delbrueckii TU-1 and L. delbrueckii JCM 1002T grown in mMRS containing 2% FOS for 12 hr and 24 hr were spotted along with solutions of fructose, sucrose, 1-kestose and FOS standard (2%, wt/vol) onto different lanes of a TLC plate. The plate was developed three times in ethyl acetate/acetic acid/ethanol/water (12:3:3:2) solvent. The spent cultures of L. paracasei JCM 8130T, L. paracasei DSM 20020, L. delbrueckii TU-1 and L. delbrueckii JCM 1002T grown in mMRS containing 2% Fuji FF inulin or inulin after 12 hr and 24 hr of incubation were also treated the same way. The plates were developed in 1-butanol/2-propanol/ethanol/water (3:2:3:4) solvent. Spots were visualized by spraying the plates with p-anisaldehyde (contains acetic acid, H2SO4) ethanol solution (Tokyo Chemical Industry, Tokyo, Japan) and heating them at 160°C for several minutes.
RESULTS
Fermentation experiments
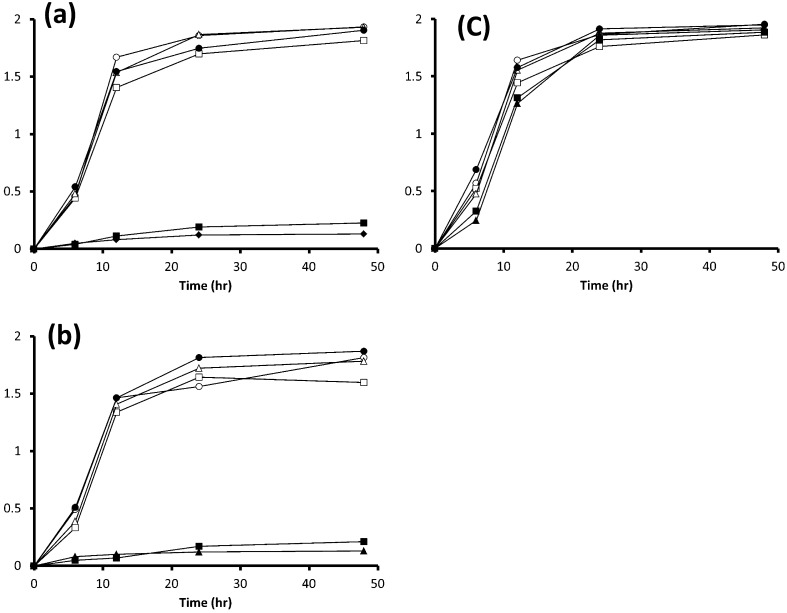
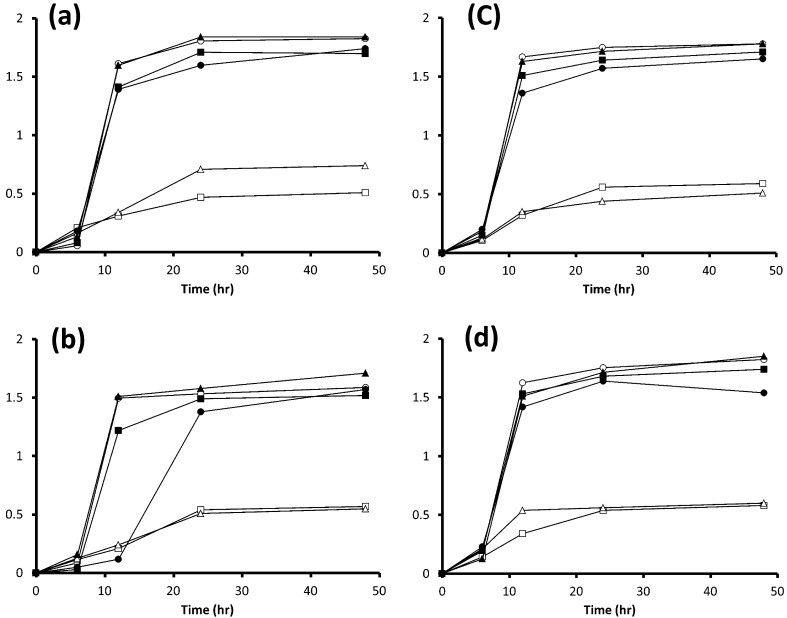
It should be noted that none of the strains used in the present study grew in mMRS (data not shown). The 3 strains of L. paracasei on glucose, fructose, sucrose, 1-kestose (data not shown) or FOS grew well and showed a similar growth pattern (Fig. 1) and pH in their spent cultures after 48 hr of incubation (Table 2). L. paracasei JCM 1171T and L. paracasei JCM 8130T did not grow on Fuji FFSC inulin (data not shown), Fuji FF inulin or inulin (Fig. 1a, 1b), while L. paracasei DSM 20020 grew well on them (Fig. 1c), and the acidification profiles during fermentation of glucose, fructose, sucrose, 1-kestose, FOS and inulin were comparable among them (Table 2). By contrast, the 4 L. delbrueckii strains did not grow well on fructose or sucrose but did grow well on glucose, 1-kestose (data not shown), FOS, Fuji FFSC inulin (data not shown), Fuji FF inulin and inulin (Fig. 2). It should be noted that the growth of 4 L. delbrueckii strains on inulin was apparently greater than that on any other sugars at the end of the incubation.
Fig. 1.
Growth curves in MRS medium or mMRS medium with a 2% (wt/vol) concentration of glucose, fructose, 1-kestose, FOS, Fuji FF inulin or inulin for 3 strains of L. paracasei. (a) L. paracasei JCM 8130T, (b) L. paracasei JCM 1171T, and (c) L. paracasei DSM 20020. ○, OD660 on glucose; □, OD660 on fructose; Δ, OD660 on sucrose; ●, OD660 on FOS; ■, OD660 on Fuji FF inulin; ▲, OD660 on inulin. Each experiment was performed in triplicate.
Table 2. Changes in pH after performing incubation of the media.
| Glucose | Fructose | Sucrose | 1-Kestose | FOS | Fuji FFSC inulin | Fuji FF inulin | Inulin | |
| Lactobacillus paracasei JCM1171T | 3.89 | 3.96 | 3.91 | 3.99 | 4.01 | 5.88* | 5.96* | 5.84* |
| Lactobacillus paracasei JCM8130T | 3.94 | 3.88 | 4.02 | 4.05 | 4.05 | 5.91* | 5.78* | 5.81* |
| Lactobacillus paracasei DSM 20020 | 4.02 | 3.98 | 3.89 | 4.05 | 4.11 | 4.09 | 4.14 | 4.12 |
| Lactobacillus delbrueckii TU-1 | 3.91 | 3.96 | 3.95 | 4.07 | 4.12 | 4.27 | 4.08 | 4.89 |
| Lactobacillus delbrueckii JCM 1002T | 3.87 | 4.01 | 3.91 | 4.15 | 4.21 | 4.25 | 4.15 | 5.14 |
| Lactobacillus delbrueckii JCM 1012T | 3.88 | 4.05 | 3.91 | 4.21 | 4.22 | 4.29 | 4.21 | 4.87 |
| Lactobacillus delbrueckii JCM 1248T | 3.97 | 4.06 | 4.01 | 4.12 | 4.15 | 4.11 | 4.25 | 4.98 |
pH levels were measured after 48 hr incubation in MRS or mMRS containing fructose, sucrose, 1-kestose, FOS, Fuji FFSC inulin, Fuji FF inulin, or inulin. Values presented are averages of 3 replicates. *, No growth was seen.
Fig. 2.
Growth curves in MRS medium or mMRS medium with a 2% (wt/vol) concentration of glucose, fructose, 1-kestose, FOS, Fuji FF inulin or inulin by 4 strains of L. delbrueckii. (a) L. delbrueckii TU-1, (b) L. delbrueckii JCM 1002T, (c) L. delbrueckii JCM 1012T and (d) L. delbrueckii JCM 1248T. ○, OD660 on glucose; □, OD660 on fructose; Δ, OD660 on sucrose; ●, OD660 on FOS; ■, OD660 on Fuji FF inulin; ▲, OD660 inulin. Each experiment was performed in triplicate.
Moreover, the decrease in pH after 48 hr of the 4 strains grown on inulin was smaller than that on glucose, even though their growth patterns on both sugars were comparable (Table 2). On the other hand, the pH levels after 48 hr of growth on fructose and sucrose were almost the same as that on glucose, even though their growth levels on fructose and sucrose were lower than that on glucose after 48 hr of incubation. Meanwhile, pH after 24 hr of growth on fructose was slightly higher than that on glucose (data not shown).
Sugar degradation analysis by thin layer chromatography (TLC)
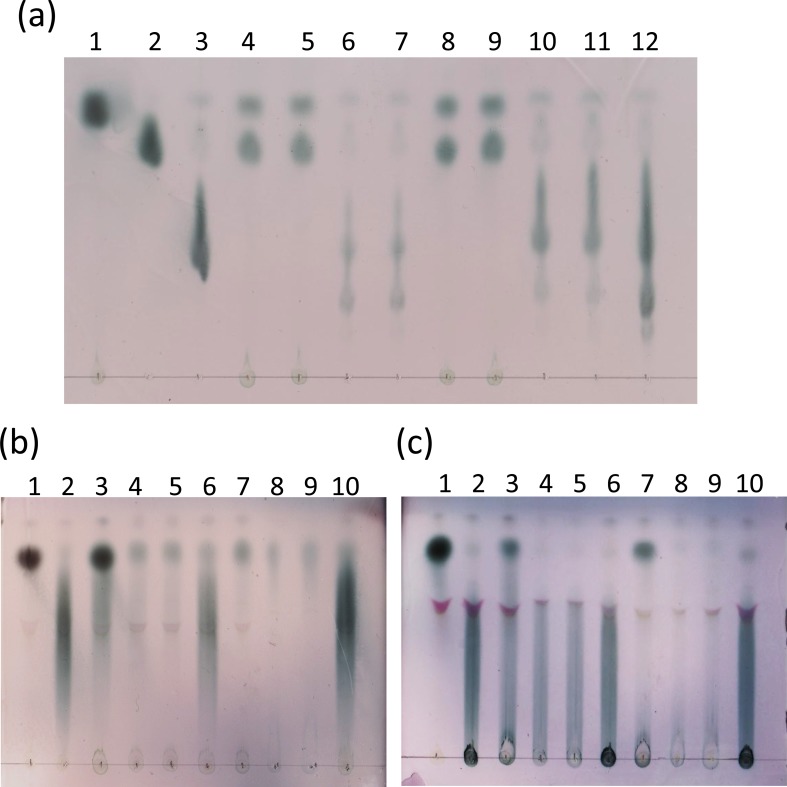
In mMRS with sugars added but not inoculated with any strains, each sugar was developed on the TLC plate in accordance with its molecular weight: a dark oval-shaped fructose spot was seen in the upper part of the plate (Fig. 3a, lane 1); a slightly elongated dark sucrose spot was seen under the fructose spot (Fig. 3a, lane 2); a further elongated dark 1-kestose spot was seen under the sucrose spot (Fig. 3a, lane 3); and a markedly extended FOS spot, a mixture of 1-kestose, nystose (DP 4), and 1F-fructofuranosyl nystose (DP 5), was seen under the sucrose spot, covering a much wider area than the 1-kestose spot (Fig. 3a, lane 12). The spent cultures of L. paracasei JCM 8130T and L. paracasei DSM 20020 grown in mMRS containing 2% FOS did not show any FOS spots after 12 hr and 24 hr of incubation but did show fructose and sucrose spots (Fig. 3a, lanes 4, 5, 8 and 9). In contrast, the spent cultures of L. delbrueckii TU-1 and L. delbrueckii JCM 1002T grown in mMRS containing 2% FOS showed a FOS spot after 12 hr of incubation but its darkness intensity was less than the reference FOS spot (Fig. 3a, lanes 6 and 7). Moreover, the lower part of the spot disappeared after 24 hr of incubation (Fig. 3a, lanes 10 and 11).
Fig. 3.
Thin layer chromatography analysis of sugar compositions of the spent cultures of the strains of L. paracasei and L. delbrueckii in mMRS containing FOS (a), Fuji FF inulin (b), or inulin (c) after 12 hr and 24 hr of incubation.
Strains were grown and harvested as follows: (a) lane 1, fructose (2%, wt/vol); lane 2, sucrose (2%, wt/vol); lane 3, 1-kestose (2%, wt/vol); lane 4, L. paracasei JCM 8130T (after 12 hr of incubation); lane 5, L. paracasei DSM 20020 (after 12 hr of incubation); lane 6, L. delbrueckii TU-1 (after 12 hr of incubation); lane 7, L. delbrueckii JCM 1002 T (after 12 hr of incubation); lane 8, L. paracasei JCM 8130T (after 24 hr of incubation); lane 9, L. paracasei DSM 20020 (after 24 hr of incubation); lane 10, L. delbrueckii TU-1 (after 24 hr of incubation); lane 11, L. delbrueckii JCM 1002 T (after 24 hr of incubation); lane 12, FOS (2%, wt/vol). (b) and (c) lane 1, fructose (2%, wt/vol); lane 2, L. paracasei JCM 8130T (after 12 hr of incubation); lane 3, L. paracasei DSM 20020 (after 12 hr of incubation); lane 4, L. delbrueckii TU-1 (after 12 hr of incubation); lane 5, L. delbrueckii JCM 1002 T (after 12 hr of incubation); lane 6, L. paracasei JCM 8130T (after 24 hr of incubation); lane 7, L. paracasei DSM 20020 (after 24 hr of incubation); lane 8, L. delbrueckii TU-1 (after 24 hr of incubation); lane 9, L. delbrueckii JCM 1002 T (after 24 hr of incubation); lane 10, Fuji FF inulin or inulin (2%, wt/vol).
In mMRS with sugars added but not inoculated with any strains, a dark oval-shaped fructose spot was seen in the upper part of the plate (Fig. 3b, lane 1), and a markedly extended Fuji FF inulin spot, for which the DP is reported to vary between 3 and 30, with an average of 16 according to information supplied by the company, was seen over a wide area (Fig. 3b, lane 10). The spent cultures of L. paracasei JCM 8130T inoculated in mMRS containing 2% Fuji FF inulin showed an intact Fuji FF inulin spot after 12 hr and 24 hr of incubation (Fig. 3b, lanes 2 and 6). The spent cultures of L. paracasei DSM 20020 grown in mMRS containing 2% Fuji FF inulin did not show the lower part of the Fuji FF inulin spot after 12 hr of incubation but did show the upper part of the spot with increased darkness intensity (Fig. 3b, lane 3), but only the upper part of the spot was seen after 24 hr of incubation (Fig. 3b, lane 7). In contrast, the spent cultures of L. delbrueckii TU-1 and L. delbrueckii JCM 1002T grown in mMRS containing 2% Fuji FF inulin did not show the lower part of the Fuji FF inulin spot after 12 hr of incubation (Fig. 3b, lanes 4 and 5). Moreover, the darkness intensity decreased further after 24 hr of incubation (Fig. 3b, lanes 8 and 9).
In mMRS with sugars added but not inoculated with any strains, a dark oval-shaped fructose spot was seen in the upper part of the plate (Fig. 3c, lane 1). A markedly extended spot covering a wide area and a dark oval-shaped non-migrating inulin spot, for which the DP is reported to vary between 3 and 60 according to information supplied by the company, was seen under the fructose spot (Fig. 3c, lane 10). The spent cultures of L. paracasei JCM 8130T inoculated in mMRS containing 2% inulin showed an intact inulin spot after 12 hr and 24 hr of incubation (Fig. 3c, lanes 2 and 6). The spent cultures of L. paracasei DSM 20020 grown in mMRS containing 2% inulin after 12 hr showed an inulin spot with decreased darkness intensity and a dark oval-shaped spot in the upper part of the plate (Fig. 3c, lane 3). Its darkness intensity, except for the upper part of the spot, decreased further after 24 hr of incubation (Fig. 3c, lane 7). In contrast, the spent cultures of L. delbrueckii TU-1 and L. delbrueckii JCM 1002T grown in mMRS containing 2% inulin showed an inulin spot after 12 hr of incubation but its darkness intensity (Fig. 3c, lanes 4 and 5) was weaker than the reference inulin spot (Fig. 3c, lane 10). Its darkness intensity decreased further after 24 hr of incubation (Fig. 3c, lanes 8 and 9).
DISCUSSION
Our sugar degradation analysis by TLC clearly indicated that L. paracasei DSM 20020 was capable of degrading the highly polymerized inulin into fructose and sucrose, whereas L. paracasei JCM 8130T failed to do so. It has been reported elsewhere [17, 18] that L. paracasei strains showed an extracellular β-fructosidase, which hydrolyzes inulin-type fructans to release free fructose, and thus they were capable of degrading inulin-type fructans into extracellular accumulations of free molecules of fructose and sucrose that can be subsequently transported into the cells to be metabolized. In contrast, cultures of L. delbrueckii TU-1 and L. delbrueckii JCM 1002T did not show such extracellular accumulations of fructose and sucrose during fermentation of inulin-type fructans. One of the explanations for this may be that, like the L. paracasei strains, the 2 L. delbrueckii strains degraded the inulin into fructose and sucrose but incorporated these sugars into the cells much more quickly than the L. paracasei strains. However, this was unlikely because the growth rates of the 2 L. delbrueckii strains were significantly slower on fructose and sucrose than on glucose in our fermentation experiments, suggesting that they were not efficient at utilizing these sugars. Moreover, our in silico BLAST search failed to identify any genes encoding extracellular inulinase homologues in the known genome of L. delbrueckii, suggesting that the strains do not degrade inulin-type fructans extracellularly. This in turn suggests that the L. delbrueckii strains have a transporting system in which larger molecules of inulin-type fructans are more preferably taken up into the cells. In this regard, some strains of Bifidobacterium spp. are known to be capable of taking up oligofructose (DP 2 to 8) into the cells through a transport system for it [21, 22, 23] and L. plantarum WCFS1 is capable of taking up 1-kestose (DP 3) and nystose (DP 4) into the cells through a sucrose transport system [24]. However, the ability to take up inulin of higher DP into cells has not been reported in any bacteria to our knowledge. As for the ability to take up a macromolecule into bacterial cells, Sphingomonas sp. strain A1 was found to incorporate alginate into the cells through an ABC (ATP-binding cassette) transporter system [25]. It will be necessary to investigate how L. delbrueckii strains utilize inulin-type fructans with a high DP with specific reference to any transporter system specialized for high DP fructans.
In our fermentation experiments, the 3 L. paracasei strains on glucose, fructose, sucrose, 1-kestose and FOS showed similar growth patterns. L. paracasei DSM 20020 grew well even on fructans with a greater DP (i.e., Fuji FFSC inulin, Fuji FF inulin, and inulin), while L. paracasei JCM 1171T and L. paracasei JCM 8130T failed to do so. Meanwhile, the growth rates of the 4 L. delbrueckii strains were significantly higher on the fructans with a greater DP than on fructose and sucrose, i.e., the fructans with a greater DP > fructose and sucrose. These results suggested that the 4 L. delbrueckii strains have a preferentially metabolize fructans with a greater DP. It has been reported for many Bifidobacterium species that their growth rates are higher on oligofructose than on fructose [26, 27]. However, Bifidobacterium or other species of Lactobacillus that preferentially metabolize fructans with a greater DP have not been reported.
The acidification profiles of L. paracasei DSM 20020 during fermentation of glucose and inulin were comparable with each other. In contrast, pH after 48 hr of growth of the 4 L. delbrueckii strains on fructose and sucrose were almost the same as that on glucose, even though their growth levels on fructose and sucrose were lower than that on glucose after the 48 hr incubation. Meanwhile, the pH levels after 24 hr of growth on fructose were slightly higher than that on glucose. These results suggested that the growth of the 4 L. delbrueckii strains on fructose was simply worse than that on glucose. On the other hand, the acid production of the 4 L. delbrueckii strains during fermentation of inulin was less than that on glucose, although their levels of growth were comparable on both sugars. This result suggested that the inulin degradation mechanisms of the 4 L. delbrueckii strains were different from that of L. paracasei DSM20020. With respect to metabolism of inulin-type fructans by L. paracasei, it has been reported that lactic acid is the major metabolic end product of L. paracasei subsp. paracasei 8700:2 but significant amounts of other metabolic end products such as acetic acid, formic acid and ethanol are also produced during fermentation of inulin-type fructans [17]. Thus, a possible explanation for this result is that the 4 L. delbrueckii strains took up inulin into the cells and degraded it intracellularly, which lead to a type of hetero-fermentation, producing ethanol as well as a relatively small amount of organic acids, resulting in a relatively higher pH of the spent media. Analysis of metabolites of the L. delbrueckii strains during fermentation with inulin will thus be necessary to evaluate this possibility.
Regarding the probiotic role of L. delbrueckii, it has been reported that these bacteria are responsible for enhanced host immune response [28] and have antioxidative activity [29]. Therefore, L. delbrueckii promotes GI health in humans and other animals in the GI tract. However, some authors have reported that L. delbrueckii is rarely found among the gut microbiota after ingestion due to its reduced capability to survive the restrictive conditions found during GI digestion [30,31,32]. Other researchers have provided evidence indicating that some L. delbrueckii strains can survive the GI transit but that the number of bacteria decreases considerably in that case [33]. If any of the probiotic strains of L. delbrueckii have a special transporting system for higher DP inulin-type fructans, it will be possible to use such fructans as prebiotic supplements that stimulate growth selectively in the host’s digestive tract because absorption of fructans is difficult in the host and fructans are degraded by other members of the gut microbiota. On the other hand, L. paracasei DSM 20020 degrades inulin to fructose extracellularly, which is subsequently utilized not only by the L. paracasei strain itself but also by other concomitant intestinal microbes. Further studies using in vitro gut simulation models and in vivo trials are needed to evaluate this possibility.
Acknowledgments
This work was supported by Special Coordination Funds for Promoting Science and Technology, Creation of Innovation Centers for Advanced Interdisciplinary Research Areas (Innovative Bioproduction Kobe), Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
REFERENCES
- 1.Fuller R, Gibson GR. 1997. Modification of the intestinal microflora using probiotics and prebiotics. Scand J Gastroenterol Suppl 222: 28–31 [DOI] [PubMed] [Google Scholar]
- 2.Rolfe RD. 2000. The role of probiotic cultures in the control of gastrointestinal health. J Nutr 130: 396S–402S [DOI] [PubMed] [Google Scholar]
- 3.Bouhnik Y, Pochart P, Marteau P, Arlet G, Goderel I, Rambaud JC. 1992. Fecal recovery in humans of viable Bifidobacterium sp. Ingested in fermented milk. Gastroenterology 102: 875–878 [DOI] [PubMed] [Google Scholar]
- 4.Goldin BR, Gorbach SL, Saxelin M, Barakat S, Gualtiere L, Salminen S. 1992. Survival of Lactobacillus species (strain GG) in human gastrointestinal tract. Dig Dis Sci 37: 121–128 [DOI] [PubMed] [Google Scholar]
- 5.Gibson GR, Roberfroid MB. 1995. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 125: 1401–1412 [DOI] [PubMed] [Google Scholar]
- 6.Gibson GR, Probert H, Van Loo J, Rastall RA, Roberfroid M. 2004. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev 17: 259–275 [DOI] [PubMed] [Google Scholar]
- 7.Roberfroid MB. 1993. Dietary fiber, inulin and oligofructose: a review comparing their physiological effects. Crit Rev Food Sci Nutr 33: 103–148 [DOI] [PubMed] [Google Scholar]
- 8.Roberfroid MB, Van Loo JAE, Gibson GR. 1998. The bifidogenic nature of chicory inulin and its hydrolysis products. J Nutr 128: 11–19 [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Gibson GR. 1993. Effects of the in vitro fermentation of oligofructose and inulin by bacteria growing in the human large intestine. J Appl Bacteriol 75: 373–380 [DOI] [PubMed] [Google Scholar]
- 10.Kaplan H, Hutkins RW. 2000. Fermentation of fructooligosaccharides by lactic acid bacteria and bifidobacteria. Appl Environ Microbiol 66: 2682–2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Müller M, Lier D. 1994. Fermentation of fructans by epiphytic lactic acid bacteria. J Appl Bacteriol 76: 406–411 [DOI] [PubMed] [Google Scholar]
- 12.Müller M, Seyfarth W. 1997. Purification and substrate specificity of an extracellular fructanhydrolase from Lactobacillus paracasei ssp. paracasei P 4134. New Phytol 136: 89–96 [Google Scholar]
- 13.Müller M, Steller J. 1995. Comparative studies of the degradation of grass fructan and inulin by strains of Lactobacillus paracasei subsp. paracasei and Lactobacillus plantarum. J Appl Bacteriol 78: 229–236 [Google Scholar]
- 14.Winters AL, Merry RJ, Műller M, Davies DR, Pahlow G, Műller T. 1998. Degradation of fructans by epiphytic and inoculant lactic acid bacteria during ensilage of grass. J Appl Microbiol 84: 304–312 [Google Scholar]
- 15.Kunová G, Rada V, Lisová I, Ročková S, Vlková E. 2011. In vitro fermentability of prebiotic oligosaccharides by lactobacilli. Czech J Food Sci 29: S49–S54 [Google Scholar]
- 16.Rurangwa E, Laranja JL, Van Houdt R, Delaedt Y, Geraylou Z, Van de Wiele T, Van Loo J, Van Craeyveld V, Courtin CM, Delcour JA, Ollevier F. 2009. Selected nondigestible carbohydrates and prebiotics support the growth of probiotic fish bacteria mono-cultures in vitro. J Appl Microbiol 106: 932–940 [DOI] [PubMed] [Google Scholar]
- 17.Makras L, Van Acker G, De Vuyst L. 2005. Lactobacillus paracasei subsp. paracasei 8700:2 degrades inulin-type fructans exhibiting different degrees of polymerization. Appl Environ Microbiol 71: 6531–6537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goh YJ, Lee JH, Hutkins RW. 2007. Functional analysis of the fructooligosaccharide utilization operon in Lactobacillus paracasei 1195. Appl Environ Microbiol 73: 5716–5724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tilsala-Timisjärvi A, Alatossava T. 1997. Development of oligonucleotide primers for the 16S-23S rRNA intergenic sequences for identifying different dairy and probiotic lactic acid bacteria by PCR. Int J Food Microbiol 35: 49–56 [DOI] [PubMed] [Google Scholar]
- 20.De Man J, Rogosa M, Sharpe M. 1960. A medium for the cultivation of lactobacilli. J Appl Bacteriol 23: 130–135 [Google Scholar]
- 21.Van der Meulen R, Makras L, Verbrugghe K, Adriany T, De Vuyst L. 2006. In vitro kinetic analysis of oligofructose consumption by Bacteroides and Bifidobacterium spp. indicates different degradation mechanisms. Appl Environ Microbiol 72: 1006–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falony G, Vlachou A, Verbrugghe K, De Vuyst L. 2006. Cross-feeding between Bifidobacterium longum BB536 and acetate-converting, butyrate producing colon bacteria during growth on oligofructose. Appl Environ Microbiol 72: 7835–7841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schell MA, Karmirantzou M, Snel B, Vilanova D, Berger B, Pessi G, Zwahlen MC, Desiere F, Bork P, Delley M, Pridmore RD, Arigoni F. 2002. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc Natl Acad Sci USA 99: 14422–14427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saulnier DMA, Molenaar D, de Vos WM, Gibson GR, Kolida S. 2007. Identification of prebiotic fructooligosaccharide metabolism in Lactobacillus plantarum WCFS1 through microarrays. Appl Environ Microbiol 73: 1753–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Momma K, Okamoto M, Mishima Y, Mori S, Hashimoto W, Murata K. 2000. A novel bacterial ATP-binding cassette (ABC) transporter system that allows uptake of macromolecules. J Bacteriol 182: 3998–4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van der Meulen R, Avonts L, De Vuyst L. 2004. Short fractions of oligofructose are preferentially metabolized by Bifidobacterium animalis DN-173 010. Appl Environ Microbiol 70: 1923–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossi M, Corradini C, Amaretti A, Nicolini M, Pompei A, Zanoni S, Matteuzzi D. 2005. Fermentation of fructooligosaccharides and inulin by bifidobacteria: a comparative study in pure and fecal cultures. Appl Environ Microbiol 71: 6150–6158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perdigón G, Vintini E, Alvarez S, Medina M, Medici M. 1999. Study of the possible mechanisms involved in the mucosal immune system activation by lactic acid bacteria. J Dairy Sci 82: 1108–1114 [DOI] [PubMed] [Google Scholar]
- 29.Saide JA, Gilliland SE. 2005. Antioxidative activity of lactobacilli measured by oxygen radical absorbance capacity. J Dairy Sci 88: 1352–1357 [DOI] [PubMed] [Google Scholar]
- 30.Tannock GW. 2003. The intestinal microflora. In Gut flora: nutrition, immunity and health, Fuller R, Perdigón G (eds), Backwell Press, Oxford, pp. 1–23. [Google Scholar]
- 31.del Campo R, Bravo D, Cantón R, Ruiz-Garbajosa P, García-Albiach R, Montesi-Libois A, Yuste FJ, Abraira V, Baquero F.2005. Scarce evidence of yogurt lactic acid bacteria in human feces after daily yogurt consumption by healthy volunteers. Appl Environ Microbiol 71: 547–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.García-Hernández J, Moreno Y, Chuan C, Hern’andez M. 2012. In vivo study of the survival of Lactobacillus delbruecki subsp. bulgaricus CECT 4005T and Streptococcus thermophilus CECT 801 by DVC-FISH after consumption of fermented milk. J Food Sci 77: 593–597 [DOI] [PubMed] [Google Scholar]
- 33.Mater DDG, Bretigny L, Firmesse O, Flores MJ, Mogenet A, Bresson JL, Corthier G. 2005. Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus survive gastrointestinal transit of healthy volunteers consuming yogurt. FEMS Microbiol Lett 250: 185–187 [DOI] [PubMed] [Google Scholar]