Abstract
This study aimed to examine the mechanism for differential effects of low- (LPPS) and high-phosphorus (HPPS) potato starches and high-amylose cornstarch (HACS) on rat cecal fermentation, the n-butyrate proportion in particular. In ileorectostomized rats, the in vivo resistant starch (RS) contents were determined to be 66% (LPPS), 66% (HPPS) and 36% (HACS), but the carbohydrate/nitrogen (C/N) ratios of the ileal digesta were comparable among the respective starch diets. In intact rats fed diets including similar amounts of RS, the cecal n-butyrate proportions in the LPPS- and HPPS-fed rats were equally higher than in the HACS-fed rats. The cecal starch contents were fivefold greater in the LPPS- and HPPS-fed rats than in the HACS-fed rats. The results suggest that potato starches and HACS are not equivalent n-butyrate producers in the rat cecum and that the slower fermentation rate of potato starches relative to HACS might be responsible for the higher n-butyrate proportion.
Keywords: potato starch, high-amylose cornstarch, cecal fermentation, butyrate
INTRODUCTION
Short-chain fatty acids (SCFA, mainly acetate, propionate and n-butyrate) are produced during bacterial fermentation in the large intestine. Among these, n-butyrate (butyrate) in particular has attracted much attention because it is the major energy substrate for colonocytes and may play an essential role in maintenance of the colonic mucosa [1, 2, 3].
Compared with non-starchy polysaccharides, starches have been shown to yield high proportions of butyrate by in vitro fermentation in human fecal inocula [4]. With respect to substrate supply to the large bowel microflora, resistant starch (RS) may be a good answer. RS is defined as the sum of starch and degradation products of starch that reach the large intestine [5]. Previous studies with rat experiments showed that both of raw potato starch (PS) and high-amylose cornstarch (HACS) had a major impact on the cecal production of butyrate [6, 7, 8]. At the same time, however, these studies consistently reported that PS unlike HACS enhanced the molar proportion of butyrate in the total SCFA even when the adaptation period of PS ingestion and dietary dosage of PS differed. However, the reason for this potential difference in butyrate production between the two starches is still unclear.
In vitro RS assays repeatedly confirmed that PS is much more resistant to α-amylase digestion than HACS [9, 10], but in vivo data is relatively scarce except for data from human subjects with ileorectostomy [11]. The difference in molar proportion of cecal butyrate between rats fed PS and HACS may be due to the different amounts of starch entering the large intestine when the rats consume the same amount of the respective starches. Also, starches from different sources have been suggested to affect the digestibility of protein in the diet differently [12, 13], implicating the possibility that the ratio of starch to nitrogen in the ileal digesta as a fermentation substrate in the large intestine may differ between in rats fed the diets including PS and HACS. Previous studies clearly indicated that the ratio of carbohydrate to nitrogen (C/N ratio) of fermentation substrate profoundly affected the production of butyrate in vitro [14] and in vivo [15, 16]. Accordingly, these findings may partly explain the difference in molar proportion of cecal butyrate between rats fed PS and HACS.
The aim of this study was to examine whether there is an intrinsic difference in the molar proportion of butyrate between PS and HACS. For this purpose, in vivo RS contents of PS and HACS in the small intestine were directly measured using ileorectostomized rats. The ratio of starch to nitrogen of the ileal digesta was also measured in the ileorectostomized rats. Then, we evaluated the cecal fermentation profile of PS and HACS in intact rats under the condition in which the in vivo RS contents were similarly set up in the diets. In the present study, we used two special kinds of PS with different phosphorus contents, since it has been suggested that besides the C/N ratio, phosphorus and sulfur are other important factors that act as fermentation substrates to increase cecal butyrate production [17].
MATERIALS AND METHODS
Materials
Normal potato starch (PS) was provided by Matsutani Chemical Co., Ltd. (Osaka, Japan). Additionally, two other kinds of PS, derived from different cultivars (Benimaru and Hokkaikogane) with different phosphorus contents, were provided by Jinno Potato Starch Factory Co., Ltd. (Obihiro, Japan). The phosphorus contents, as determined by the molybdovanadate method [18], were 550 mg/kg (normal PS), 419 mg/kg (Benimaru) and 708 mg/kg (Hokkaikogane), respectively. The latter two were referred to as low-phosphorus (LPPS) and high-phosphorus potato starches (HPPS). HACS was obtained from the National Starch and Chemical Company (Sydney, NSW, Australia), and its amylose content was determined to be 68% by an iodine-colorimetric method [19]. The phosphorus content of HACS was 159 mg/kg. The RS contents of LPPS, HPPS and HACS in vitro, as determined by the method of McClearly and Monaghan [10], were 75% (LPPS), 76% (HPPS) and 46% (HACS), respectively.
Care of animals
Male Wistar rats were purchased at 5 and 8 week-old from Shizuoka Laboratory Animal Center (Hamamatsu, Japan). They were housed in individual screen-bottomed stainless steel cages in a room with controlled temperature (23 ± 2°C) and lighting (light on from 8:00 to 20:00). Rats were fed a control diet for at least 5 days for adaptation. The diet was formulated from 250 g/kg casein, 652.25 g/kg sucrose (except in the preliminary experiment in which normal cornstarch (CS) was used as a carbohydrate source), and 50 g/kg corn oil [15]. The remainder of the diet consisted of vitamins and minerals (AIN-76 [20]). Body weight and food intake were recorded every morning before replenishing the diet. The study was approved by the Animal Use Committee of Shizuoka University, and the animals were maintained in accordance with the guidelines of Shizuoka University for the care and use of laboratory animals.
Preliminary study
Twenty-four 8-week-old Wistar rats were used in this study. Rats weighing 185–193 g were divided into 3 groups of 8 rats after acclimation and were allowed free access to the control diet or a diet either containing 200 g HACS or 200 g normal PS/kg. Supplementation of HACS and PS was performed by replacement of an equal amount of CS in the control diet. After feeding the respective diets for 15 days, rats were euthanized by decapitation under anesthesia with diethyl ether, the cecum was excised, and the contents were removed and weighed. The cecal contents were homogenized and then used for measurements of pH and organic acids.
Comparison of starch digestibility between LPPS, HPPS and HACS in ileorectostomized rats (experiment 1)
Five-week-old Wistar rats were used in this study. After overnight fasting, 24 rats weighing 104–120 g were subjected to an ileorectostomy in which the terminal ileum was connected to the rectum according to the method of Lambert [21], with some modifications [22]. To shorten the recovery period, we did not dissect the cecum and the colon, but the ileocecal valve was ligatured (closed), and then the colonic terminal was anastomosed to the stoma in the abdominal wall to allow the cecal and colonic contents to be excreted naturally. The surgery was performed for 2 consecutive days (12 rats/day were operated on). Postoperatively, the rats were not allowed food and water for the first 24 hr and then were fed the control diet for 14 days. The rats received a daily intramuscular injection of antibiotics [22] at surgery and for 5 days thereafter. The rats lost about 13 g of body weight during the immediate postoperative recovery period. However, they then gained weight, and constant growth rates (3–4 g of body weight gain/d) were achieved 4–5 days after surgery. Rats weighing 121–149 g were divided into four groups (six rats per each) and were freely fed one of the diets containing 200 g of CS, LPPS, HPPS or HACS per kilogram of the diet for 7 days. Thus there were four groups: CS, LPPS, HPPS and HACS (initial body weights 132 ± 5, 132 ± 5, 133 ± 6, and 132 ± 5 g, respectively). Supplementation of each starch was performed by replacement of an equal amount of sucrose in the control diet. Feces (ileal excreta) were collected for the last 3 days of the experimental period, freeze-dried, and stored at -40°C. After the digestibility study was performed, to measure the small intestinal transit time in ileorectostomized rats, all rats further consumed the respective diets at 0800–0900 and 1900–2000 hr for 5 days. After adaptation to meal feeding, rats weighing 165–190 g were fed 3 g of the respective diets including 5% carmine (a water-insoluble and unabsorbable dye) at 1900 hr. Their feces were monitored every 30 min for the first appearance of the red dye.
Comparison of cecal fermentation between LPPS, HPPS and HACS in intact rats (experiment 2)
Eight-week-old Wistar rats were used in this study. Thirty-two rats weighing 185–193 g were divided into 4 groups of 8 rats after acclimation and were allowed free access to diets either containing 200 g CS, 109 g LPPS, 106 g HPPS, or 200 g HACS per kilogram. Thus there were four groups: 20-CS, 10-LPPS, 10-HPPS and 20-HACS. In vivo RS contents were similarly set up at 72 g/kg diet among the 10-LPPS, 10-HPPS and 20-HACS diets according to the findings obtained in experiment 1. Supplementation of CS, LPPS, HPPS and HACS was performed by replacement of an equal amount of sucrose in the control diet. After feeding the respective diets for 15 days, rats were euthanized by decapitation under anesthesia with diethyl ether, the cecum was excised, and the contents were removed and weighed. The respective cecal contents were mixed well, and then divided into several portions. These were used for measurements of pH, organic acids, starch, and bacteria numbers.
Cecal pH and organic acids
A portion of the homogenate was diluted with the same weight of distilled water, and then cecal pH was measured with a compact pH meter (Model C-1, Horiba, Tokyo, Japan). Cecal organic acids were measured by the internal standard method using an HPLC (LC-10A, Shimadzu, Kyoto, Japan) equipped with a Shim-pack SCR-102H column (8 mm i.d. × 30 cm long, Shimadzu) and a conductibity detector (CDD-6A, Shimadzu) [15].
Flow cytometry analysis of cecal bacteria numbers
Population and viability of bacteria were analyzed by flow cytometry according to Ben-Amor et al. [23]. In brief, a portion (≈100 mg) of cecal contents was suspended in 1 mL anaerobic PBS containing 1 mM dithiothreitol and 0.01% (w/v) Tween20 and homogenized by vortexing for 3 min. After centrifugation at 700 × g for 1 min, the supernatant was carefully recovered and centrifuged at 6,000 × g for 3 min. The pellet was washed twice, resuspended in anaerobic PBS and then serially diluted. Thereafter, the diluted samples were incubated for 15 min at room temperature in anaerobic PBS supplemented with 104 particles/ml fluorospheres (Flow-Check Fluorospheres, Beckman Coulter, Tokyo, Japan), 1 mg/ml propidium iodide (Wako Pure Chemical Industries, Osaka, Japan), and 5 nM SYTO-BC (Molecular Probes, Eugene, OR, USA). Samples were analyzed by flow cytometry (Epics XL, Beckman Coulter). The cells were discriminated between SYTO BC-stained variable cells, propidium iodide-stained dead cells, and double-stained injured cells.
General procedures
Dietary and fecal nitrogen content was determined using the Kjeldahl method [24], and the conversion factor of 6.25 was used when calculating the protein content. Fecal and cecal starch contents were determined using a commercially available kit (Total Starch assay kit, Megazyme, Ireland) with a modification that involved preheating the samples in dimethyl sulfoxide at 100°C for 30 min [25].
Statistical analyses
Data were basically analyzed by one-way ANOVA. Significant differences among means were identified using the Tukey-Kramer test. When variances were not homogenous by the Bartlett test, data were logarithmically transformed. When variances were not homogenous even after logarithmic transformation, the data were presented as medians with range and then analyzed by Kruskal-Wallis ANOVA followed by the Kolmogorov-Smirnov 2-sample test. The statistical calculations were carried out using the StatView 5.0 computer software (SAS Institute), and a 5% level of probability was considered significant for all analyses. Regression analyses were performed using the Stat Cel 2 program (Tokyo Shoseki).
RESULTS
Preliminary experiment
Food intake and body weight gain did not differ among the groups. The weight of cecal contents and the cecal pH were higher and lower in the HACS and PS groups than those in the control group. The cecal pool sizes of various organic acids were equally greater in the HACS and PS groups than in the control group, but the molar proportion of butyrate in the PS group was significantly higher than in the HACS group (Table 1).
Table 1. Food intake, body weight gain, and cecal variables in intact rats fed the respective diets for 15 days (preliminary experiment).
| Control | HACS | PS | |
| Food intake, g/15 days | 242 ± 3 | 214 ± 3 | 213 ± 4 |
| Body weight gain, g/15 days | 50 ± 1 | 44 ± 3 | 48 ± 3 |
| Cecum | |||
| Contents, g | 1.8 ± 0.1a | 4.4 ± 0.3b | 5.6 ± 0.5b |
| pH | 7.7 ± 0.1a | 6.1 ± 0.1b | 6.3 ± 0.3b |
| Organic acid, μmol/cecum | |||
| Acetate | 104.3 (80.2–132.0) | 590.9 (328.3–783.6)* | 637.0 (165.3–1427)* |
| Propionate | 31.0 (3.2–36.0) | 272.7 (83.6–417.8)* | 193.6 (52.5–432.0)* |
| n-Butyrate | 9.9 (7.9–11.9) | 82.5 (43.2–146.4)* | 127.1 (115.1–171.5)* |
| Succinate | 1.4 (0.4–2.0) | 166.8 (102.4–216.4)* | 109.9 (2.7–244.2)* |
| Lactate | 0.0 (0.0–0.8) | 0.0 (0.0–509.5) | 129.1 (0.0–625.9)* |
| Formate | 0.0 (0.0–0.0) | 115.2 (0.0–248.1)* | 169.2 (2.1–397.9)* |
| Total organic acid | 141.2 ± 12.5a | 1180 ± 182.3b | 1349 ± 373.0b |
| Molar proportion of butyrate in SCFA1, % | 7.4 ± 0.7a | 10.0 ± 1.1a | 18.0 ± 4.4b |
Data are expressed as the mean ± SE or median (range), n=8. Values in a row not sharing a common superscript letter are significantly different (p<0.05) when analyzed by the Tukey-Kramer test. When variances were not homogenous, data were analyzed by Kruskal-Wallis one-way ANOVA followed by the Kolmogorov-Smirnov two-sample test. *p<0.05 vs. control. 1 Sum of acetate, propionate and n-butyrate.
Starch digestibility of LPPS, HPPS and HACS in ileorectostomized rats (experiment 1)
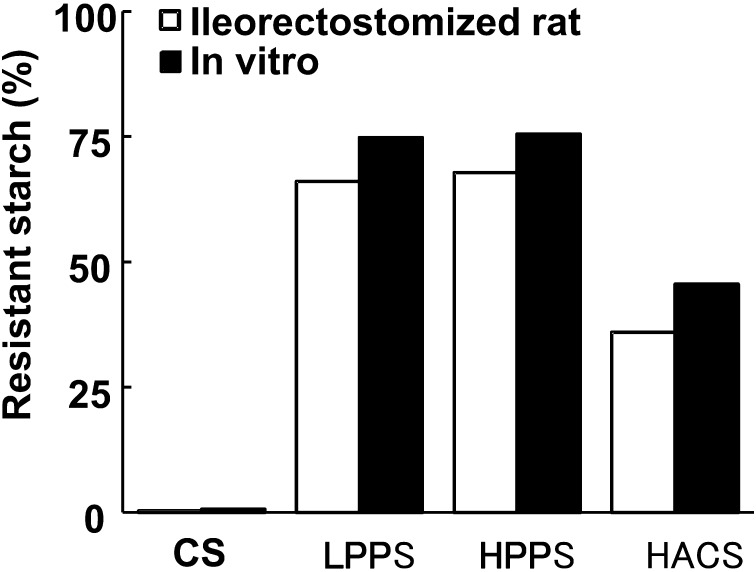
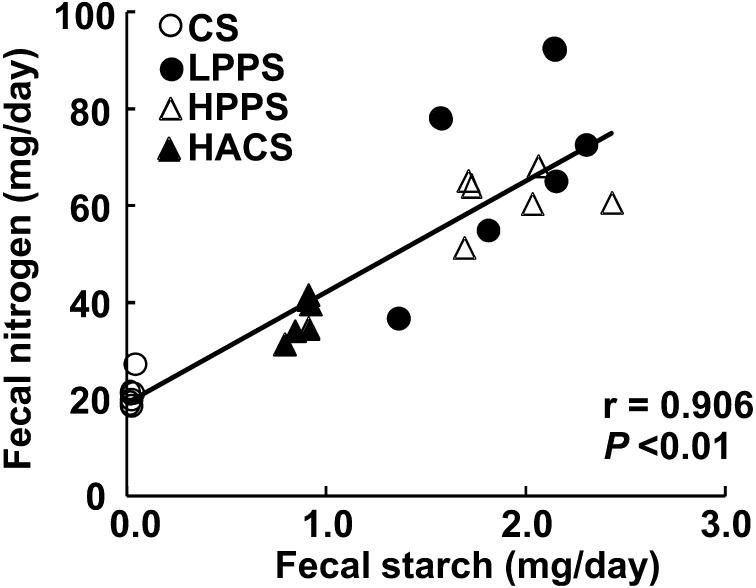
Total food intake and body weight gain for 7 days were the same among the dietary groups (Table 2)). Starch and nitrogen intakes were calculated from dietary intakes for the last 3 days of the experimental period, and significant differences were observed only between the CS and HPPS groups. Fecal excreta dry matter for the last 3 days was highest in the LPPS and HPPS groups, lowest in the CS group and intermediate in the HACS group. Starch excretion was significantly higher in the LPPS, HPPS and HACS groups than in the CS group, and the differences between the LPPS and HPPS groups versus the HACS groups were also significant. This translates to corresponding ileal digestibility of 99.7, 34.4, 35.5, and 63.6% for CS, LPPS, HPPS and HACS, respectively. Accordingly, the RS contents measured in the ileorectostomized rats were lower than in those determined in vitro by 12% (LPPS and HPPS) and 20% (HACS), respectively (Fig. 1). However, there was a significant correlation between in vitro and in vivo determinations (r= 0.998, p=0.002). Essentially similar trends were observed in nitrogen excretion and protein digestibility. The ratios of fecal starch to nitrogen were equally higher in the LPPS, HPPS and HACS groups than in the CS group, and a significant and positive correlation was observed between the excretion of fecal starch and nitrogen (Fig. 2). To verify the homogeneity of the intestinal movement after surgery, the small intestinal transit time was measured. The first appearance of red dye in the feces was observed within 3.0 – 4.0 hr in all groups, and no differences were observed among the groups.
Table 2. Food intake, body weight gain, fecal variables, starch and protein digestibility in ileorectostomized rats fed the respective diets for 7 days (experiment 1).
| CS | LPPS | HPPS | HACS | |
| Food intake, g/7 days | 95 (87–102) | 107 (70–119) | 109 (74–126) | 100 (96–104) |
| Body weight gain, g/7 days | 30 ± 2 | 25 ± 4 | 28 ± 5 | 30 ± 2 |
| Starch intake1, g/3 days | 7.13 ± 0.37a | 8.52 ± 0.57ab | 8.55 ± 0.30b | 7.35 ± 0.21ab |
| Nitrogen intake1, g/3 days | 1.41 ± 0.07a | 1.70 ± 0.11ab | 1.72 ± 0.06b | 1.52 ± 0.05ab |
| Fecal excretion2 | ||||
| Fecal dry matter, g/3 days | 1.17 ± 0.07a | 7.92 ± 0.64c | 8.02 ± 0.47c | 4.46 ± 0.11b |
| Fecal starch, g/3 days | 0.02 (0.02–0.05) | 5.91 (4.08–6.90)*, # | 5.64 (5.07–7.29)*, # | 2.73 (2.30–2.76)* |
| Starch digestibility, % | 99.7 (99.5–99.7) | 34.4 (30.0–38.1)*, # | 35.5 (23.5–37.4)*, # | 63.6 (63.1–65.2)* |
| Fecal nitrogen, mg/3 days | 61 (55–81) | 206 (109–276)*, # | 187 (153–203)*, # | 111 (93–124)* |
| Protein digestibility, % | 95.5 (94.4–96.2) | 89.2 (84.5–91.3)*, # | 89.3 (87.5–90.6)*, # | 93.0 (92.2–93.4)* |
| Fecal starch/nitrogen, (mg/mg) | 0.3 ± 0.1a | 29.8 ± 2.7b | 31.8 ± 2.1b | 24.1 ± 0.7b |
Data are expressed as the mean ± SE or median (range), n=6. Values in a row not sharing a common superscript letter are significantly different (p<0.05) when analyzed by the Tukey-Kramer test. When variances were not homogenous, data were analyzed by Kruskal-Wallis one-way ANOVA followed by the Kolmogorov-Smirnov two-sample test. *p<0.05 vs. CS; #p<0.05 vs. HACS. 1 Calculated from the food intake for the last 3 days of the experimental period. Starch intake was calculated from the dietary composition taking account of the moisture content of each diet (9.6 – 11.9%). Nitrogen intake was determined on the basis of Kjeldahl analyses of the respective diets. 2 Feces were collected for the last 3 days of the experimental period.
Fig. 1.
In vitro and in vivo comparisons of resistant starch values of cornstarch (CS), low-phosphorus potato starch (LPPS), high-phosphorus potato starch (HPPS) and high-amylose cornstarch (HACS).
Fig. 2.
Correlation between fecal excretion of starch and nitrogen in ileorectostomized rats fed the respective diets.
Cecal fermentation of LPPS, HPPS and HACS in intact rats (experiment 2)
Total food intake and body weight gain for 15 days were comparable among the dietary groups (Table 3). The weight of the cecal contents and the cecal pH were higher and lower in the 10-LPPS, 10-HPPS and 20-HACS groups than those in the 20-CS group. The cecal pool sizes of acetate, propionate and butyrate were greater in the 10-LPPS, 10-HPPS and 20-HACS groups than those in the 20-CS group. The cecal pool size of propionate in the 20-HACS group was also significantly different from those in the 10-LPPS and 10-HPPS groups. Cecal succinate was greater only in the HACS group compared with the other groups, while cecal lactate was detected only in a few samples of the respective groups. Substantial amounts of formate were detected in a few samples of the 10-LPPS, 10-HPPS and 20-HACS groups. The cecal pool sizes of total organic acids were highest in the HACS group, lowest in the 20-CS group and intermediate in the LPPS and HPPS groups. The cecal starch contents in the 10-LPPS, 10-HPPS and 20-HACS groups were greater than in the 20-CS groups, and the level in the 20-HACS group was significantly different from those in the 10-LPPS and 10-HPPS groups. The molar proportions of butyrate in the total SCFA (acetate, propionate and butyrate) were equally higher in the LPPS and HPPS groups than in the HACS group. Total bacterial counts in the cecum were equally greater in the 10-LPPS, 10-HPPS and 20-HACS groups than in the 20-CS group. The percentage of viable cells was significantly lower in the 20-CS group than in the 10-LPPS and 20-HACS groups, whereas that of dead cells was lower in the 20-HACS group than in the other groups.
Table 3. Food intake, body weight gain, and cecal variables in intact rats fed the respective diets for 15 days (experiment 2).
| 20-CS | 10-LPPS | 10-HPPS | 20-HACS | |
| Food intake, g/15 days | 242 ± 8 | 228 ± 6 | 235 ± 6 | 223 ± 5 |
| Body weight gain, g/15 days | 61 ± 3 | 56 ± 2 | 53 ± 3 | 53 ± 3 |
| Cecum | ||||
| Contents, g | 1.9 ± 0.1a | 5.0 ± 0.4b | 4.7 ± 0.3b | 5.1 ± 0.4b |
| pH | 7.7 (7.5–8.0) | 7.0 (6.6–7.5)*, # | 6.7 (6.0–7.3)* | 6.1 (5.5–6.7)* |
| Organic acid, μmol/cecum | ||||
| Acetate | 90.4 ± 7.3a | 220.8 ± 14.2b | 226.4 ± 25.1b | 307.0 ± 54.5b |
| Propionate | 31.5 ± 2.3a | 99.4 ± 15.4b | 103.5 ± 16.2b | 209.2 ± 43.9c |
| n-Butyrate | 0.0 (0.0–8.5) | 49.0 (0.0–87.2)* | 48.2 (29.1–127.7)* | 51.9 (0.0–114.4)* |
| Succinate | 2.5 (0.0–6.2) | 8.6 (0.0–155.0) | 63.3 (4.3–364.4) | 225.4 (52.5–787.6)* |
| Lactate | 0.0 (0.0–0.6) | 0.0 (0.0–10.8) | 0.0 (0.0–33.6) | 0.0 (0.0–220.3) |
| Formate | 0.5 (0.0–1.0) | 35.4 (0.0–100.6) | 21.0 (0.0–69.4) | 7.7 (0.0–121.4) |
| Total organic acid | 127.7 ± 10.4a | 438.2 ± 39.3b | 514.3 ± 68.4b | 905.8 ± 120.4c |
| Molar proportion of n-butyrate in SCFA1, % | 1.9 ± 0.9a | 12.1 ± 2.3c | 15.5 ± 1.9c | 6.8 ± 2.0b |
| Starch, mg/cecum | 7.4 ± 1.6a | 608.8 ± 62.1c | 530.3 ± 49.0c | 107.3 ± 10.5b |
| Bacteria, log counts/g | 10.8 ± 0.0a | 11.3 ± 0.1b | 11.2 ± 0.1b | 11.4 ± 0.1b |
| Viable cells, % | 49.1 ± 4.6a | 62.2 ± 7.0b | 59.0 ± 5.2ab | 72.4 ± 5.5b |
| Injured cells, % | 13.8 ± 2.7b | 5.8 ± 0.4a | 7.3 ± 0.8ab | 9.6 ± 2.0ab |
| Dead cells, % | 37.1 ± 4.9b | 32.0 ± 7.2b | 33.7 ± 5.2b | 18.0 ± 4.2a |
Data are expressed as the mean ± SE or median (range), n=8. Values in a row not sharing a common superscript letter are significantly different (p<0.05) when analyzed by the Tukey-Kramer test. When variances were not homogenous, data were analyzed by Kruskal-Wallis one-way ANOVA followed by the Kolmogorov-Smirnov two-sample test. *p<0.05 vs. 20-CS; #p<0.05 vs. HACS. 1 Sum of acetate, propionate and n-butyrate.
DISCUSSION
Our preliminary study produced results consistent with the previous studies [6, 8] in which the molar proportion of butyrate in the total SCFA was significantly higher in a PS-fed group than in a HACS-fed group at the same dietary levels of the respective starches. There are a wide variety of PS sources that differ in their characteristics, including granule structure and amylopectin and phosphorus contents [26]. In the subsequent experiments, therefore, we specified the variety of PS as low- (LPPS) and high-phosphorus (HPPS) contents.
The results with ileorectostomized rats showed that the small intestinal digestibility of LPPS (34.4%) and HPPS (35.5%) were equally lower than that of HACS (63.6%), but that those of LPPS and HPPS were virtually the same (in turn, the same RS values in vivo). This is unexpected because it is established that the esterified phosphate at the carbon-3 position of glucose moiety in the starch molecule contributes to the inhibition of hydrolysis by α-amylase in vitro [27]. One explanation for this might be raised from the observations of Mineo et al. showing that the esterified phosphate in the starch molecule could be cleaved by alkaline phosphatase in the rat small intestine [28]. Thus, PS digestibility in vivo does not depend on the phosphorus contents, but other possible factors such as crystallinity and size of the granule might be related. Fecal nitrogen excretion in ileorectostomized rats fed the LPPS and HPPS diets was significantly greater than in those fed the HACS diet. However, the fecal nitrogen excretion was positively and significantly correlated with the fecal starch excretion among the dietary groups, and as a result, there were no significant differences in the ratios of fecal starch to nitrogen between LPPS, HPPS and HACS.
The results with intact rats showed that all of the starches tested (LPPS, HPPS and HACS) had a major impact on cecal fermentation. However, the molar proportions of butyrate in rats fed LPPS and HPPS were equally and significantly higher than that in the rats fed HACS even when the amount of starch entering the cecum and the ratio of starch to nitrogen in the ileal digesta were theoretically equal among the diets. This simply means that PS and HACS are not equivalent butyrate producers in the rat cecum and that the phosphorus content in the starch granule is not related to the fermentation pattern of PS. In the present study, however, we did not measure the phosphorus content in the ileal digesta, and we cannot totally deny the possibility that the different fermentation profile between HACS and PSs might be due to the different phosphorus contents among starches. One interesting feature of this study is the fact that the cecal amounts of starch were equally greater in the LPPS and HPPS diets than in the HACS diet, suggesting that PS (LPPS, HPPS) and HACS were utilized at different fermentation rates. Martin et al. showed that unlike HACS, PS was slowly fermented in vitro with pig cecal microflora [29]. The reason why the fermentation rate of PS is relatively slow is not clear, but one explanation could be the difference in the granule sizes of PS and HACS [26]. The large granule size of PS suggests the possibility that the surface area of the granule for bacterial attachment is limited compared with HACS, which has a much smaller granule size (by five- to tenfold).
The principal substrates for cecal bacteria are carbohydrates and nitrogen, and this balance (C/N) affects fermentative metabolism and ATP formation by bacteria [14, 30]. When rapidly fermentable carbohydrates (HACS in this case) are supplied to the cecum in an excessive quantity relative to nitrogen, ATP production by substrate-level phosphorylation in the glycolytic pathway is accelerated, and this leads to an exhaustion of NAD+. Naturally, the pathways of dehydrogenase activities in the formation of lactate or succinate via pyruvate become active to regenerate NAD+ [30]. However, when carbohydrate fermentation is relatively slow (LPPS and HPPS in this case), pyruvate is further metabolized to acetate or butyrate [31]. In fact, besides SCFA, a considerable amount of succinate was detected in rats fed the HACS diet, presumably indicating that nitrogen supply was insufficient in the HACS group [15]. At present, however, it cannot be excluded that different compositions of microbiota might have been involved in the different fermentation profiles of PS and HACS.
In conclusion, the present study showed that there is an intrinsic difference in the molar proportion of butyrate between PS and HACS when they are used as fermentation substrates. The principal factor for the difference could be the slower fermentation rate of PS relative to HACS.
REFERENCES
- 1.Topping DL, Clifton P. 2001. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev 81: 1031–1064 [DOI] [PubMed] [Google Scholar]
- 2.Sakata T, Yajima T. 1984. Influence of short chain fatty acids on the epithelial cell division of digestive tract. Q J Exp Physiol 69: 639–648 [DOI] [PubMed] [Google Scholar]
- 3.Kripke SA, Fox AD, Berman JM, Settle RG, Rombeau JL. 1989. Stimulation of intestinal mucosal growth with intracolonic infusion of short-chain fatty acids. J Parent Enter Nutr 13: 109–116 [DOI] [PubMed] [Google Scholar]
- 4.Englyst HN, Hay S, Macfarlane GT. 1987. Polysaccharide breakdown by mixed populations of human faecal bacteria. FEMS Microbiol Ecol 45: 163–171 [Google Scholar]
- 5.Asp NG. 1992. Resistant Starch. Proceedings for the 2nd plenary meeting of EURESTA. Eur J Clin Nutr 46: 1–6 [PubMed] [Google Scholar]
- 6.Martin LJ, Dumon HJ, Lecannu G, Champ MM. 2000. Potato and high-amylose maize starches are not equivalent producers of butyrate for the colonic mucosa. Br J Nutr 84: 689–696 [PubMed] [Google Scholar]
- 7.Ferguson LR, Tasman-Jones C, Englyst H, Harris PJ. 2000. Comparative effects of three resistant starch preparations on transit time and short-chain fatty acid production in rats. Nutr Cancer 36: 230–237 [DOI] [PubMed] [Google Scholar]
- 8.Henningsson AM, Margareta E, Nyman GL, Björck IM. 2003. Influences of dietary adaptation and source of resistant starch on short-chain fatty acids in the hindgut of rats. Br J Nutr 89: 319–328 [DOI] [PubMed] [Google Scholar]
- 9.Goñi I, Garcia-Diz L, Manas E, Saura-Calixto F. 1996. Analysis of resistant starch:a method for foods and food products. Food Chem 56: 445–449 [Google Scholar]
- 10.McCleary BV, Monaghan DA. 2002. Measurement of resistant starch. J AOAC Int 85: 665–675 [PubMed] [Google Scholar]
- 11.Englyst HN, Cummings JH. 1987. Digestion of polysaccharides of potato in the small intestine of man. Am J Clin Nutr 45: 423–431 [DOI] [PubMed] [Google Scholar]
- 12.Beames RM, Eggum BO. 1981. The effect of type and level of protein, fibre and starch on nitrogen excretion patterns in rats. Br J Nutr 46: 301–313 [DOI] [PubMed] [Google Scholar]
- 13.Nageswara Rao C, Narasinga Rao BS. 1978. Influence of starches from different sources on protein utilization in rats. Br J Nutr 40: 1–8 [DOI] [PubMed] [Google Scholar]
- 14.MacFarlane GT, Macfarlane S. 1993. Factors affecting fermentation reactions in the large bowel. Proc Nutr Soc 52: 367–373 [DOI] [PubMed] [Google Scholar]
- 15.Morita T, Kasaoka S, Oh-hashi A, Ikai M, Numasaki Y, Kiriyama S. 1998. Resistant proteins alter cecal short-chain fatty acid profiles in rats fed high amylose cornstarch. J Nutr 128: 1156–1164 [DOI] [PubMed] [Google Scholar]
- 16.Morita T, Kasaoka S, Hase K, Kiriyama S. 1999. Oligo-L-methionine and resistant protein promote cecal butyrate production in rats fed resistant starch and fructooligosaccharide. J Nutr 129: 1333–1339 [DOI] [PubMed] [Google Scholar]
- 17.Kiriyama S. 2005. A personal account of the dawn and later development of luminacoid research in Japan. J Integr Study Diet Habbits. 16: 104–107 [Google Scholar]
- 18.Karim AA, Toon LC, Lee VP, Ong WY, Fazilah A, Noda T. 2007. Effects of phosphorus contents on the gelatinization and retrogradation of potato starch. J Food Sci 72: C132–C138 [DOI] [PubMed] [Google Scholar]
- 19.Knutson CA. 1986. A simplified colorimetric procedure for determination of amylose in maize starches. Cereal Chem 63: 89–92 [Google Scholar]
- 20.American Institute of Nutrition. 1977. Report of the American Institute of Nurtition ad hoc Committee on standards for nutritional studies. J Nutr 107: 1340–1348 [DOI] [PubMed] [Google Scholar]
- 21.Lambert R. 1965. In Surgery of the digestive System in the Rat. Thomas CC ed., Springfield, IL, pp 415–433. [Google Scholar]
- 22.Morita T, Kasaoka S, Kiriyama S, Brown IL, Topping DL. 2005. Comparative effects of acetylated and unmodified high-amylose maize starch in rats. Starch/Stärke 57: 246–253 [Google Scholar]
- 23.Ben-Amor K, Heilig H, Smidt H, Vaughan EE, Abee T, de Vos WM. 2005. Genetic diversity of viable, injured, and dead fecal bacteria assessed by fluorescence-activated cell sorting and 16S rRNA gene analysis. Appl Environ Microbiol 71: 4679–4689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller L, Houghton JA. 1945. J Biol Chem 159: 373–380 [Google Scholar]
- 25.Muir JG, Birkett A, Brown I, Jones G, O’Dea K. 1995. Food processing and maize variety affects amounts of starch escaping digestion in the small intestine. Am J Clin Nutr 61: 82–89 [DOI] [PubMed] [Google Scholar]
- 26.Gallant DJ, Bouchet B, Buléon A, Pérez S. 1992. Physical characteristics of starch granules and susceptibility to enzymatic degradation. Eur J Clin Nutr 46: S3–S16 [PubMed] [Google Scholar]
- 27.Takeda Y, Hizukuri S, Ozono Y, Suetake M. 1983. Actions of porcine pancreatic and Bacillus subtilis alpha-amylases and Aspergillus niger glucoamylase on phosphorylated (1–4)-alpha-D-glucan. Biochim Biophys Acta 749: 302–311 [DOI] [PubMed] [Google Scholar]
- 28.Mineo H, Morikawa N, Ohmi S, Ishida K, Machida A, Kanazawa T, Chiji H, Fukushima M, Noda T. 2010. Ingestion of potato starch containing esterified phosphorus increases alkaline phosphatase activity in the small intestine in rats. Nutr Res 30: 341–347 [DOI] [PubMed] [Google Scholar]
- 29.Martin LMJ, Dumon HJW, Champ MMJ. 1998. Production of short-chain fatty acids from resistant starch in a pig model. J Sci Food Agric 77: 71–80 [Google Scholar]
- 30.Macfarlane S, Macfarlane GT. 2003. Regulation of short-chain fatty acid production. Proc Nutr Soc 62: 67–72 [DOI] [PubMed] [Google Scholar]
- 31.Ito H, Takemura N, Sonoyama K, Kawagishi H, Topping DL, Conlon MA, Morita T. 2011. Degree of polymerization of inulin-type fructans differentially affects number of lactic acid bacteria, intestinal immune functions, and immunoglobulin A secretion in the rat cecum. J Agric Food Chem 59: 5771–5778 [DOI] [PubMed] [Google Scholar]