Abstract
Objectives
To assess whether a dementia care coordination intervention delays time to transition from home and reduces unmet needs in elders with memory disorders.
Design
18-month randomized controlled trial of 303 community-living elders. Setting: 28 postal code areas of Baltimore, MD.
Participants
Age 70+, with a cognitive disorder, community-living, English-speaking, and having a study partner available.
Intervention
18-month care coordination intervention to systematically identify and address dementia-related care needs through individualized care planning; referral and linkage to services; provision of dementia education and skill building strategies; and care monitoring by an interdisciplinary team.
Measurements
Primary outcomes were time to transfer from home and total percent of unmet care needs at 18 months.
Results
Intervention participants had a significant delay in time to all-cause transition from home and the adjusted hazard of leaving the home was decreased by 37% (HR = 0.63, 95% CI 0.42 to 0.94) compared to control participants. While there was no significant group difference in reduction of total percent of unmet needs from baseline to 18 months, the intervention group had significant reductions in the proportion of unmet needs in safety and legal/advance care domains relative to controls. Intervention participants had a significant improvement in self-reported quality of life (QOL) relative to control participants. No group differences were found in proxy-rated QOL, neuropsychiatric symptoms, or depression.
Conclusions
A home-based dementia care coordination intervention delivered by non-clinical community workers trained and overseen by geriatric clinicians led to delays in transition from home, reduced unmet needs, and improved self-reported QOL.
Keywords: Dementia, community-based, care coordination, memory disorders, intervention
OBJECTIVES
Alzheimer’s disease and related dementias affect 5.4 million Americans with 80% receiving care in the community by 15 million unpaid informal caregivers (CGs) (1). Dementia is associated with long term care placement, high health care costs, general medical complications (e.g., urinary tract infections, falls), functional dependency, serious behavioral problems, mortality, and reduced quality of life (QOL) (2-6).
Due to service system fragmentation and poor coordination, many dementia-related care needs are undetected, underevaluated, and unmet (7-11), contributing to excesses in poor outcomes and higher care costs. Practice recommendations support coordinated, comprehensive approaches that integrate evidence-supported strategies to maximize effectiveness in dementia management (6,12-14). However, dementia care is rarely delivered as a comprehensive, evidence-based set of services that link medical care with community-based supportive care (15-16). For instance, primary care, the hub of care for most dementia patients, faces significant time and resource challenges (16), making it difficult to respond to the complex and multidimensional care needs of both “patients” and “caregivers” or to evaluate and address non-medical supportive care needs.
Patient and family centric care models tailored to dementia that coordinate health and community care represent a promising mechanism to address the multiple and ongoing needs of this growing population, but are understudied. Five systematic reviews (17-21) and two meta-analyses (22,23) of efficacy of care coordination in dementia reveal there is a paucity of rigorous well-controlled trials, making it difficult to draw conclusions of the true impact of these approaches on most key outcomes. Most (70%) trials have been fair to poor quality, had substantial weaknesses in study design elements (e.g., non-masked assessment), small sample sizes, and/or lacked sufficiently detailed intervention protocols or characterization of the intervention. Of the few high quality trials conducted (22-35), there is evidence to support modest to moderate effects on improving care quality (27,29,34,35), patient QOL (26-29), reduction of neuropsychiatric symptoms (NPS) (27), and reduction of CG burden, unmet needs, and depression (25,26,27,29,31,35). Delaying or avoiding transition from the home is an especially salient outcome for individuals, as well as for health care reform cost containment efforts. However, with few exceptions (24,28), the beneficial impacts of these models on time to transition have either been untested (29,34,35) or elusive (26,27,30,32,33).
Building on best practice principles and prior studies, we tested the effect of a comprehensive, home-based care coordination intervention, Maximizing Independence (MIND) at Home, on delaying transition from the home and reducing unmet care needs in community-residing elders with memory disorders. We hypothesized that intervention participants would remain in their homes significantly longer and have fewer unmet care needs at 18 months compared to control participants. Secondarily, we evaluated intervention efficacy on participant QOL, NPS, and depression. The trial methods and intervention protocol were designed to enhance the potential for implementation in community-level service contexts. MIND assumed a ‘real world’ approach by including heterogeneous participants (including persons with mild cognitive impairment); implementation of an intervention protocol that was comprehensive yet not complex; and utilizing non-clinical community workers as frontline coordinators to maximize the potential future workforce capable of implementing MIND.
METHODS
This was an 18-month prospective, single-blind, parallel group randomized pilot trial design comparing the MIND care coordination intervention to augmented usual care in a cohort of 303 elders age 70+ with cognitive disorders (265 with dementia, 38 with mild cognitive impairment) living at home in Baltimore, MD (clinicaltrials.gov; NCT01283750). This study was approved by the Johns Hopkins Medicine Institutional Review Board. Oral consent was obtained from participants (i.e., persons with cognitive disorder) during a telephone screen. Written consent was obtained from the participants and their study partners (i.e. a reliable family member or friend who knew the participant well) at the initial in-home assessment. For participants too impaired to provide consent, proxy consent was obtained from a legally authorized representative using the Maryland Health Care Decisions Act as a guide, with assent obtained from the participant.
Study Recruitment
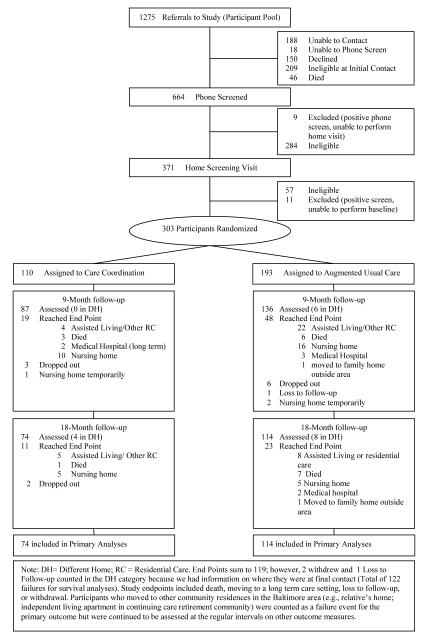
Figure 1 shows the flow of individuals through the study. Community-residing individuals with memory disorders were recruited from July 2008 to May 2010 in Baltimore, MD. Participants were identified through multiple approaches including referrals from 16 service organizations whose staff were trained in dementia case-finding, (b) letters from service providers to their clients, and (c) general community outreach activities.
Figure 1.
Flowchart of study participation
Eligible participants were age 70+, English-speaking, community-residing in the northwest Baltimore area (i.e., 28 postal-codes), had a reliable study partner available who was willing to participate in all study visits, met Diagnostic and Statistical Manual, Fourth Edition, Text Revision (DSM-IV-TR) criteria for dementia or Cognitive Disorder Not Otherwise Specified (COG DO NOS) (36), and had >1 unmet care needs on the Johns Hopkins Dementia Care Needs Assessment (JHDCNA) (10,37). Individuals in a crisis situation (i.e., signs of abuse, neglect, risk of danger to self or others) were excluded.
Eligibility was determined in two stages. First, a phone screen was administered to screen for cognitive impairment using the Telephone Interview for Cognitive Status (TICS) (11items, score range 0- 41, lower scores indicated more impairment) (38), and the Short Informant Questionnaire for Cognitive Disorders in the Elderly (IQCODE) (16 items, range 16-80, higher scores indicated more impairment) (39), administered to the study partner. Positive screens (e.g., TICS <31 and IQCODE >52 cut-offs [38,39]) then received an in-home screening assessment by a clinician (i.e., registered nurse [RN] or geriatric psychiatrist) to confirm DSM-IV-TR eligibility criteria and complete the JHDCNA. Data collected included family and medical history, medications, medical and psychiatric diagnoses and treatments, a brief neurological and mental status examination, any available clinical or lab reports, information on function, behavior, cognition, physical health, formal and informal support networks, service use, and a visual inspection of the home environment. Eligible participants then received a baseline (BL) visit to perform quantitative assessments of QOL, activities of daily living (ADLs), NPS, and depression. Study partners took part in each data collection visit.
Randomization
Participants were randomized by the PI within 48 hours of the BL visit to intervention or augmented usual care group (1:2 allocation), using a custom Excel program which generated a random number from a uniform distribution. Stratified urn randomization was used to encourage balance on our stratification variable (lived with/without a CG). The stratification variable was based on the rationale that participants living with CGs may have more support in daily functioning and the initiation and implementation of recommended care strategies, and have caregivers who are more aware of their daily living needs (40), all of which may be associated with transition from home.
Intervention Conditions
Augmented usual care (control) participants, study partners, and primary care physician (PCP) received the written results of the JHDCNA following the BL visit, including recommendations for each identified unmet need. They also received a brief resource guide developed for the study that provided program and contact information for eleven local and national aging service organizations.
Intervention participants, their study partners, and PCP received the written JHDCNA results and18 months of care coordination by an interdisciplinary care team comprised of non-clinical community workers (Coordinators) linked to a RN and a geriatric psychiatrist. The manualized care coordination protocol consisted of four key components: identification of needs and individualized care planning based on the JHDCNA to address unmet needs and to match the priorities and preferences of the patient and family; provision of dementia education and skill building strategies; coordination, referral and linkage to services; and care monitoring. Care components are individually tailored to current unmet needs and updated based on emergent needs of participants and CGs. After randomization, coordinators reviewed the JHDCNA assessment, conducted an in-home visit with the participant and study partner to review and prioritize needs, and developed the care plan. The study partner and/or participant, when appropriate, then implemented the plan with guidance from the coordinator. A menu of care options/strategies was available for each unmet need item and consisted of referral and linkage to resources/services; CG memory disorder education and skill building; informal counseling, problem-solving (Table 1). All recommended resource referrals were selected from those available locally. The protocol pre-specified two in-home visits (initial visit and 18 month visit), and monthly contacts to maintain engagement with the care team. Otherwise, the type and frequency of coordinator involvement with the participant and family was individualized over the 18 months and driven by need-level, care plan, and family preference. Needs were monitored over time and new strategies were implemented when necessary. Emergent needs were identified by the coordinators and incorporated into care plans. When appropriate, coordinators took a direct role to ensure follow-through with recommended strategies/care options (e.g., reminders of appointments, attending outpatient visits or nursing home rehabilitation meetings, pricing medical equipment or services, assisting with service program applications, providing educational material, and modeling management techniques).
Table 1.
JHDCNA Domains and Care Option/Strategy Examples
| Memory Care Needs Domains of Participants |
No. Items |
Abbreviated Care Option/Strategy Examples* |
|---|---|---|
| A. Evaluation / Diagnosis | 5 | In-depth review by DCC/DCS; Referral to PCP or specialist physician for dementia evaluation and workup; neurologic evaluation, substance abuse referral. |
| B. Treatment of Cognitive Symptoms |
2 | Evaluate whether a medication might be indicated and refer to PCP or Geriatrician or physician specializing in memory disorders for discussion/evaluation |
| C. Treatment of Neuropsychiatry Symptoms |
5 | In-depth review and characterization of concerning symptoms by DCC; Assessment of potential causes (e.g. UTI, constipation, pain); Refer to PCP or Geriatrician or physician specializing in memory disorders for discussion/evaluation of possible medication indications. |
| D. Behavior Management | 3 | In-depth review and characterization of concerning symptoms by DCC; provide instruction on specific behavior management/caregiver skills counseling; Assessment of potential causes (e.g. UTI, constipation, pain); Refer to Alzheimer’s Association |
| E. Medication Management | 4 | Initial review of medications by DCS; Request PCP or prescribing physician to evaluate polypharmacy or regimen adjustment; Assist in coordination of multiple prescribing physicians/pharmacies. |
| F. Medication Administration | 3 | Create medication administration routine that promotes compliance; Coordinate second party supervision or medication administration; Recommend specific devices or reminder tools |
| G. General Medical/Health Care |
8 | Referral to PCP, medical specialist or geriatric care manager; Recommend family and PCP consider hospice care |
| H. Allied Health Specialist Care |
4 | Referral to PCP. Recommend referral by PCP to PT, OT, SLP, home health care agency. |
| I. Safety | 7 | Identify possible environmental hazards (e.g. scatter rugs, out of date food, fall risks, fire risks, wander risks, guns/power tools) and make a plan to address each. Referral to driving evaluation program; home safety evaluation. Recommend asking PCP for PT, OT referral. |
| J. Assistance with Daily Activities |
10 | Arrange for informal or formal assistance for needed service. Provide caregiver skills counseling |
| K. Meaningful Activities | 6 | Evaluate and develop a list of activities that would match preferences, personality, and lifestyle and help caregiver implement. Provide caregiver skills counseling for help with creating a daily routine structure; Refer to friendly visitor programs, senior center, adult day, transportation service, etc. |
| L. Legal Issues / Advance Care Planning |
5 | Recommend patient and family engage in end-of-life care discussions with PCP and family members; Referral to eldercare attorney, or state attorney office about POA, will, advance directives |
| M. Assistance with Health Insurance |
5 | Review current medical needs, medications and referral to SHIP (Senior Health Insurance Program), CMS, US Veterans Affairs, AARP, ect. |
| N. Patient Education | 1 | Refer to PCP for discussion of illness. Refer to Alzheimer’s Association support group |
| O. Caregiver Availability | 3 | Identify and arrange for someone to take responsibility for intermittent phone checks, in-person visits, supervision. |
| P. Other Patient Needs | – | Dependent on needs listed |
|
Memory Care Need
Domains of Caregivers |
No.
Items |
Abbreviated Care Option/Strategy Examples * |
| Q. Caregiver Education | 3 | Educate CG about dementia course and impact; provide written learning material; inform of educational events or local resources (health fairs, clinicians, senior centers, day care/home care services, support groups); instruct and counsel CG on care management issues (behavioral issues, ADLs, communication, family conflicts, planning, safety) |
| R. Resource Referrals | 5 | Refer to local or national chapter of Alzheimer’s Association; eldercare attorney (e.g. estate planning, will, power of attorney, advanced directives); Maryland Dept. of Aging or local agency; private geriatric care management services; Adult protective services. |
| S. Caregiver Mental Health Care | 4 | Proactively monitor CG stress levels; provide informal counseling, help with coping skills, and emotional support; Refer to licensed mental health professional; Arrange and plan regular respite care periods |
| T. Caregiver General Medical/ Health Care | 3 | Referral to PCP; specialist physician; other health care professional (e.g., dentist, optometrist, PT) |
| U. Other Caregiver Needs | – | Dependent on needs listed |
Listed recommended interventions are not exhaustive. Actual recommendations based on the individual’s specific need within a category.
Note: Each need item was assessment as being either “fully met” (i.e. need is being addressed and potential benefits of available interventions have been achieved to the extent possible for the individual) or “unmet” (i.e. (1) it has not been addressed and potentially beneficial interventions are available, or (2) it has been or is being addressed but potential benefits of available interventions have not yet been achieved).
The three coordinators (2 FTE bachelors-prepared with Marketing or Psychology degrees, and 0.5 FTE with social work masters degree) were employees of two community-based social service agencies hired explicitly for the study and located at the agencies based on a priori design. None had prior formal training or certifications in geriatric case management or dementia care. Coordinators were trained over a 1 month period. This structured training was provided by the study’s clinical investigators and colleagues from a range of disciplines (e.g., geriatric psychiatry, geriatric medicine, nursing, social work) affiliated with the Bayview Memory Center. It included didactic and interactive sessions on dementia care and management, community resource identification, family engagement, rapport, and CG skill building, the JHDCNA, the Dementia Care Management System (DCMS) clinical tracking software, human subjects research principles, and HIPAA; JHDCNA home-visit needs assessment observations; clinical care observations (i.e., inpatient, outpatient, and long-term care); and proficiency assessments. The geriatric psychiatrist and RN provided direct support and clinical guidance to coordinators, led weekly in-person 2-hour meetings to review recommendations, cases, and protocol adherence, and were accessible by cell phone and email. Coordinators used a customized web-based application, the DCMS, specifically designed for MIND. The DCMS provided decision support and secure information sharing across the care team. It was used to track care plans, clinical progress, service and provider referrals, and service use. Built-in query and reporting capabilities enabled tracking of protocol fidelity and self-monitoring of the implementation process. Fidelity was ensured through the initial coordinator training; (2) observation of the coordinators by the RN or geriatric psychiatrist during the first several independent field visits; (3) weekly in-person care team meetings; and (4) monitoring of the Coordinators’ use and data entries into the DCMS clinical tracking software.
Measures
Participant characteristics assessed included demographics (age, sex, self-identified race, education), living arrangement (residing with a caregiver or not; years living at residence), medications, medical diagnoses, use of 22 formal services (e.g., home health care, homemaker, nutrition), health care use in the past year (hospitalizations, emergency department visits), Mini-Mental State Exam (MMSE) (41), and Psychogeriatric Dependency Rating Scale-Behavior (PGDRS-B) (42) to assess functional status.
Time to transfer out of the home
Time to transfer out of the home was collected through study partner report by masked evaluators at 4.5 (telephone), 9 (in-home), 14.5 (telephone), and 18-months (in-home). In cases of permanent transfer from home, the date, destination, and primary reason for relocation were recorded. For temporary transfers (e.g., in-patient hospital, rehabilitation facility), the location was recorded and evaluators followed up at the next scheduled interval to determine the participant’s location. For death of the participant, the date, location, and cause of death were recorded. If death occurred outside of the home, evaluators recorded the date the participant left the home, the destination(s) and duration of stay in each destination prior to death. Extended surveillance by unmasked evaluators was conducted at 4.5 month intervals post-intervention for all participants until 12/1/2011. Time was expressed in days from enrollment to time censor or event (i.e., all-cause permanent transfer or death).
Unmet care needs
The JHDCNA is a multidimensional, manualized tool used to identify 19 common care need categories for participants (71 items) and CGs (15 items) (Table 1) (10,37). JHDCNA was developed by a multidisciplinary group of clinical dementia experts through an iterative process based on best practices, suggesting face and content validity, and our prior studies have suggested convergent and discriminant validity (10,43). Need items have standardized descriptions and definitions, listings of indicators of needs, and a linked menu of potential care strategies/options to address each need. Evaluators document needs and assess each as being either “fully met” or “unmet” (definition in Table 1). Total percent of unmet care needs based on the JHDCNA ([# of unmet need items/ # need items assessed]*100), was determined at the initial in-home screening visit and at 18-months. The proportion of unmet items in six pre-specified need categories (Evaluation and Treatment of Memory Symptoms; Neuropsychiatric Symptom Management; Home and Personal Safety; General, Specialist and Allied Health Care; Daily and Meaningful Activities; Legal Issues/Advanced Care Planning) was also evaluated for treatment group differences. An unmasked RN rated the JHDCNA at the 18-month visit.
Secondary outcome measures
Secondary outcome measures were assessed at BL, 9, and 18-months by masked evaluators. These included the Quality of Life in AD, which was administered to participants (QOL-AD-participant) and study partners (QOL-AD-proxy) (44); the Alzheimer’s Disease Rated Quality of Life-40 item (ADRQL-40) scale, an informant rated disease-specific QOL instrument (45); the Neuropsychiatric Inventory-Q (NPI-Q), an informant rated questionnaire for NPS (46); and the Cornell Scale for Depression in Dementia (CSDD), a depression inventory for persons with dementia (47).
Analyses
Simple inferential statistics (i.e., Pearson chi-square and T-tests) were used to assess group differences at BL. Kaplan-Meier survival curves were constructed for each group. All models (except the Kaplan-Meier survival curves) were adjusted for living without a CG or with a CG variable (stratification variable). All participants were included in the outcome analysis as randomized, using the intention-to-treat approach. A Cox proportional hazards model was used to assess between-group survival differences. Linear mixed-effects regression models were used to estimate the effect of the intervention on the change in each continuous outcome measure relative to control using intention-to-treat. Terms for living with a CG, treatment group, time, and group x time interaction were included in the model. For total percent unmet needs from baseline to 18 months, groups were also modeled independently to assess the effect of time since the augmented usual care group also received the needs assessment and recommendations for intervention to reduce identified unmet needs. A generalized linear mixed-effects model (with binomial response distribution, logit link, and a random intercept for subjects) was used to model the six pre-specified JHDCNA domains using the same set of fixed effects. The estimate of interest for these models was the difference in slopes between the augmented usual care and intervention groups from 0 to 18 months on the response variables, and was calculated using an estimate statement in SAS 9.2. Tests were considered significant at an alpha level of .05.
RESULTS
Participant Characteristics
Baseline characteristics of participants and CGs are in Table 2. Participants were an average age of 84; mostly (64%) female; and racially diverse (29% non-White). Intervention (n=110) and augmented usual care (n=193) groups were balanced on BL participant and CG characteristics, except that intervention participants were taking more medications compared to control participants. Of those with dementia (n=265), 49% were in the mild stage, 37% moderate, and 14% severe (48).
Table 2.
Baseline Characteristics of Participants with a Memory Disorder
| Characteristic | Augmented Care Group (n=193) |
Intervention Group (n=110) |
Total | X 2 | t | p-value |
|---|---|---|---|---|---|---|
| Primary Participant Characteristics | ||||||
| Age, mean (SD), y | 83.9 (5.9) | 84.0 (5.8) | 83.9 (5.9) | −0.202 c | 0.840 | |
| Female, No. (%) | 120 (62.2) | 73 (66.4) | 193 (63.7) | 0.531 a | 0.466 | |
| Black/African American or Other Race, No. (%) |
55 (28.5) | 32 (29.1%) | 87 (28.7) | 0.012 a | 0.913 | |
| Education, mean (SD), y | 13.2 (3.9) | 13.0 (3.1) | 13.2 (3.6) | 0.430 e | 0.668 | |
| Living with Caregiver, No. (%) |
131 (67.9) | 80 (72.7) | 211 (69.6) | 0.780 a | 0.377 | |
| Time living at residence, means (SD), y |
22.0 (18.3) | 19.4 (18.2) | 21.1 (18.3) | 1.196 c | 0.233 | |
| Dementia, No. (%) | 166 (86.0) | 99 (90) | 265 (87.5) | 1.017 a | 0.313 | |
| Prescribed medication | ||||||
| Cholinesterase Inhibitors | 91 (47.2) | 45 (40.9) | 136 (44.9) | 1.103 a | 0.294 | |
| Memantine | 57 (29.5) | 29 (26.4) | 86 (28.4) | 0.346 a | 0.556 | |
| Antidepressants | 59 (30.6) | 38 (34.5) | 97 (32.0) | 0.509 a | 0.476 | |
| Antipsychotics | 15 (7.8) | A7 (6.4) V | 22 (7.3) | 0.206 a | 0.650 | |
| No. routine medications taking, mean (SD) |
6.1 (2.9) | 6.9 (3.4) | 6.4 (3.1) | −2.281 c | 0.023 | |
|
Cardiovascular disease,
No.(%) * |
154 (79.8) | 96 (87.3) | 250 (82.5) | 2.716 a | 0.099 | |
| Pulmonary disease, No(%) ± | 12 (6.2) | 7(6.4) | 19 (6.3) | 0.003 a | 0.960 | |
| Endocrine disease, No(%) ¥ | 104 (53.9) | 66 (60.0) | 170 (56.1) | 1.064 a | 0.302 | |
| ≥ 1 hospitalization in past year, No. (%) |
67 (34.7) | 37 (33.6) | 104 (34.3) | 0.036 a | 0.849 | |
| ≥ 1 ED visit in past year, No.(%) |
99 (51.6) | 50 (45.5) | 149 (49.3) | 1.044 a | 0.307 | |
| No. formal services/programs used, mean (SD) |
3.2 (1.7) | 3.2 (1.7) | 3.2 (1.7) | −0.111f | 0.912 | |
| MMSE, mean (SD) † | 19.2 (7.7) | 19.0 (7.9) | 19.1 (7.8) | 0.234 e | 0.815 | |
| NPI-Q, mean (SD) ‡ | 7.1 (6.2) | 7.2 (5.7) | 7.2 (6.0) | −0.101 e | 0.920 | |
| CSDD, mean (SD) ‡ | 6.1 (4.6) | 6.5 (4.8) | 6.2 (4.7) | −0.570 i | 0.569 | |
| PGDRS-B (mean, SD) ‡ | 9.5 (8.0) | 10.3 (7.8) | 9.8 (7.9) | −0.833 d | 0.406 | |
| Total % unmet JHDCNA needs, mean (SD) |
10.2 (6.5) | 9.8 (5.3) | 10.1 (6.1) | 0.553 c | 0.580 | |
| Caregiver Characteristics | ||||||
|
Augmented
Care Group (n=183) |
Intervention
Group (n=106) |
Total | X 2 | t | p-value | |
| Age, mean (SD), y | 67.3 (12.9) | 65.7 (13.9) | 66.7 (13.3) | 0.972 h | 0.332 | |
| Female, No. (%) | 136 (74.3) | 80 (75.5) | 216 (74.7) | 0.047 a | 0.828 | |
| Relationship | 1.226 b | 0.542 | ||||
| Spouse (%) | 83 (45.4) | 41 (38.7) | 124 (42.9) | |||
| Child (%) | 85 (46.4) | 55 (51.9) | 140 (48.4) | |||
| Other person (%) | 15 (8.2) | 10 (9.4) | 25 (8.7) | |||
| Time as caregiver for participant, mean (SD), mths |
38.4 (33.6) | 37.1 (30.5) | 37.9 (32.5) | 0.339 g | 0.735 | |
| Providing care to another, No. (%) |
41 (22.5) | 29 (27.9) | 70 (24.5) | 1.028 a | 0.311 | |
| Employed, No. (%) | 79 (43.4) | 57 (54.3) | 136 (47.4) | 3.161 a | 0.075 | |
Abbreviations: MMSE, Mini-Mental State Examination; NPI-Q, Neuropsychiatric Inventory-Questionnaire; CSDD, Cornell Scale for Depression in Dementia; PGDRS-B, Psychogeriatric Dependency Rating Scale; JHDCNA, Johns Hopkins Dementia Care Needs Assessment.
Cardiovascular disease includes hypertension, congestive heart failure, coronary artery disease, arrhythmia, valvular disease, aortic anueurysm, peripheral vascular disease, atrial fibrilliation
Pulmonary disease category includes chronic obstructive pulmonary disorder and asthma
Endocrine disease includes adrenal insufficiency, diabetes mellitus, hyperthyroidism, hypothyroidism, hyperlipidemia, hyperparathryoidism
Higher scores are better.
Higher scores are worse.
df = 1.
df =2.
df =301.
df=300.
df=298.
df=290.
df =286.
df=284.
df=281.
Frequency of MIND coordinator contacts regarding intervention participants by primary contact person is in Table 3. Descriptive data are provided for the full sample, and then for those who completed the 18 month visit in order to show the amount of effort required to care for participants over the entire program duration. Overall, coordinators made an average of 2 contacts (mean 1.8, SD 24.1) per month for 18 months (excluding left messages) (Table 3), mostly with the study partner. The RN or geriatric psychiatrist was present for all initial and 18-month in-home assessments and accompanied coordinators on at least 7 individual interim home visits over course of the trial, though their direct involvement with participants was not captured systematically.
Table 3.
Frequency of MIND care coordinator contacts regarding participants by primary contact and contact type*
| Phone | In-Person | Email/Mail/Fax | All Contact Types | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Primary Contact | Mean (SD) | Mdn | Range | Mean (SD) | Mdn | Range | Mean (SD) | Mdn | Range | Mean (SD) | Mdn | Range |
| Full sample † | ||||||||||||
| Participant | 5.1 (7.4) | 2 | 0 - 52 | 3.2 (2.2) | 3 | 1 - 15 | 0.7 (4.4) | 0 | 0 - 45 | 9.0 (9.8) | 6 | 1 - 61 |
| Study partner | 24.5 (14.9) | 23 | 0 – 66 | 0.9 (1.2) | 0.5 | 0 - 5 | 3.0 (9.1) | 0 | 0 - 63 | 28.3 (19.2) | 25 | 0 - 99 |
| Other Family a | 2.6 (7.7) | 0 | 0 – 58 | 0.1 (0.4) | 0 | 0 - 2 | 0.1 (1.1) | 0 | 0 - 11 | 2.9 (8.8) | 0 | 0 - 71 |
| Services Provider | 0.1 (0.3) | 0 | 0 - 2 | 0 | 0 | 0 | 0.01 (0.1) | 0 | 0 - 1 | 0.1 (0.4) | 0 | 0 - 3 |
| Health Provider b | 0.5 (1.3) | 0 | 0 - 7 | 0 | 0 | 0 | 0.1 (0.4) | 0 | 0 - 2 | 0.6 (1.4) | 0 | 0 - 7 |
| MIND clinician c | 0.3 (0.7) | 0 | 0 - 3 | 0.8 (1.3) | 0 | 0 - 7 | 0.1 (0.4) | 0 | 0 - 2 | 1.2 (1.7) | 1 | 0 - 8 |
| All contacts | 33.1 (19.7) | 30 | 1 - 145 | 5 (2.3) | 5 | 1 - 21 | 4.0 (12.3) | 1 | 0 - 89 | 42.1 (28.2) | 38 | 2 - 211 |
| All contacts | 23.1 (15.2) | 21 | 1 - 117 | 5 (2.3) | 5 | 1 - 21 | 4.0 (12.3) | 1 | 0 - 89 | 32.1 (24.1) | 27.5 | 2 - 183 |
| (excl. left messages) |
||||||||||||
| 18 month | ||||||||||||
| sample ‡ | ||||||||||||
| Participant | 5.8 (7.9) | 3.5 | 0 - 52 | 3.5 (2.3) | 3 | 1 - 16 | 0.9 (5.3) | 0 | 0 - 45 | 10.2 (10.7) | 7 | 1 - 61 |
| Study partner | 27.1 (14.9) | 26 | 0 - 63 | 1.1 (1.3) | 1 | 0 - 5 | 3.1 (8.3) | 1 | 0 - 48 | 31.3 | 28 | 0 - 89 |
| Other Family a | 2.9 (8.7) | 0 | 0 - 58 | 0.1 (0.5) | 0 | 0 - 2 | 0.2 (1.3) | 0 | 0 - 11 | 3.2 (10.1) | 0 | 0 - 71 |
| Services Provider | 0.1 (0.4) | 0 | 0 - 2 | 0 | 0 | 0 | 0.01 (0.1) | 0 | 0 - 1 | 0.1 (0.4) | 0 | 0 - 3 |
| Health Provider b | 0.5 (1.4) | 0 | 0 - 7 | 0 | 0 | 0 | 0.1 (0.4) | 0 | 0 - 2 | 0.7 (1.7) | 0 | 0 - 7 |
| MIND clinician c | 0.3 (0.7) | 0 | 0 - 3 | 0.9 (1.3) | 0 | 0 - 7 | 0.1 (0.4) | 0 | 0 - 2 | 1.3 (1.7) | 1 | 0 - 8 |
| All contacts | 36.6 (19.5) | 33.5 | 3 - 145 | 5.5 (3.1) | 5 | 1- 21 | 4.6 (12.8) | 1 | 0 - 89 | 46.7 (28.1) | 41.5 | 5 - 211 |
| All contacts | 25.6 (16.0) | 22 | 2-117 | 5.5 (3.1) | 5 | 1- 21 | 4.6 (12.8) | 1 | 0 - 89 | 35.7 (24.6) | 30 | 5 - 183 |
| (excl. left messages) |
||||||||||||
Abbreviations: Mdn, Median;
Lists contacts by primary contact person but other persons (e.g. patients, study partner, services provider, health provider, MIND clinician) may have been involved in the contact interaction. For example, a contact with the study partner listed as the primary contact may have also included another family member or a health care provider.
Includes family members, family friends, Powers of Attorney, or Health Care Agents involved in participant’s care.
Includes primary care physicians and specialty physicians, allied health professionals (OT, PT, Speech Therapists), social workers
May underestimate all contacts between care coordinators and a clinical member of the MIND care team regarding specific cases as it only represents contacts that took place outside of regularly scheduled weekly case review meetings.
Includes all randomized intervention participants (n=110)
Only includes intervention participants completed the 18 month study visit (n=76)
Effect of Intervention on Leaving the Home
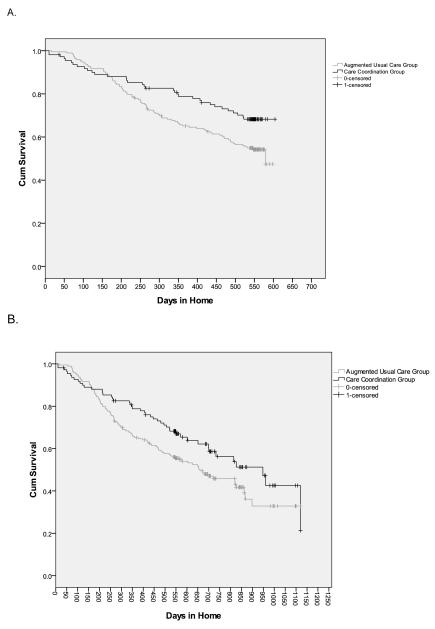
Survival curves comparing the intervention and the control groups are presented in Figure 2. At 18 months, intervention participants were less likely to permanently leave their home or die compared to controls (30.9% vs. 45.6%; χ2 = 6.28, 1 df, p=0.012) and remained in their home significantly longer (mean 496 days [SE 17.6, 95% CI 462 to 531] vs. 445 days [SE 13.6, 95% CI 418 to 471], Log rank X2 = 5.38, 1 df, p=0.020, mean difference=51 days) (Figure 2A). After adjustment for CG living in home, the hazard of leaving home was decreased by 37% (HR = 0.63, 95% CI 0.42 to 0.94, Wald test = 5.1, 1 df, p=0.022) compared to controls. Over the extended follow-up (median 26 months), the benefits were sustained (Figure 2B); intervention participants continued to be less likely to transition from home (41.8% vs. 53.4%) and remained in their home significantly longer than control participants (median 948 days [SE 113, 95%CI 727 to 1169] vs. 660 [SE 83.9, 95%CI 496 to 824]; Log Rank X2 = 4.1, 1 df, p=0.043), for a difference in median survival times of 288 days. The hazard of leaving the home was decreased by 30% (HR: 0.70, 95%CI 0.49-0.90, Wald test = 4.0, 1 df, p =0.046), after adjustment for CG living in home.
Figure 2.
Kaplan-Meier Survival graphs at 18 months (A) and for extended follow-up after intervention cessation [median 26 months followup,range,19-41 months] (B). A.
Effect of Intervention on Unmet Care Needs
There was no statistically significant group difference in reduction of total percent of unmet needs from baseline to 18 months (Table 4); however the percent of unmet needs decreased in both control and intervention groups when modeled independently (Control Group: −2.76, 95% CI −3.81 to −1.72, t = −5.21, 192 df, p <.001; Intervention Group: −4.33, 95% CI −5.35 to −3.31, t= −8.42, 109 df, p <.001). The intervention participants had a significantly greater reduction in proportion of unmet needs in Safety and Legal/Advance Care Planning domains relative to control participants (Table 4).
Table 4.
Outcomes at Baseline and 18 months *
| Estimated Mean (SE) | |||||
|---|---|---|---|---|---|
| Instrument | Augmented Usual Care (n=193) |
Intervention (n=110) |
Δ in Augmented Usual Care – Δ in Intervention from BL to 18 months (95%CI) |
Statistic (df) |
P- value |
| JHDCNA ‡ | |||||
|
Total Percent
Unmet Care Needs | |||||
| Baseline (n=303) | 10.5 (0.5) | 10.2 (0.6) | -- | ||
| 18 month (n=179) | 7.7 (0.4) | 5.9 (0.5) | −1.51 (−3.05 to 0.03) | t(301) = −1.9 |
0.054 |
| JHDCNA Domains ‡ | |||||
|
Evaluation and Treatment of Memory Symptoms a | |||||
| Baseline (n=303) | 7.6 (0.9) | 8.2 (1.2) | -- | ||
| 18 month (n=178) | 3.6 (0.7) | 2.7 (0.8) | −0.40 (−1.16 to 0.36), Point estimate = −1.56 ± |
t(175) = −1.0 |
0.299 |
| Neuropsychiatry Symptom Management b | |||||
| Baseline (n=303) | 4.5 (0.6) | 4.9 (0.9) | -- | ||
| 18 month (n=178) | 4.4 (0.8) | 3.0 (0.8) | −0.48 (−1.16 to 0.20), Point estimate = − 1.81 ± |
t(175)= −1.4 |
0.165 |
|
Home and Personal
Safety c | |||||
| Baseline (n=303) | 20.1 (1.0) | 20.4 (1.3) | |||
| 18 month(n=178) | 15.3 (1.1) | 11.0 (1.2) | −0.40 (−0.73 to − 0.06), Point estimate =− 4.62± |
t(175) = −2.3 |
0.021 |
| General, Specialist, and Allied Health Care d | |||||
| Baseline (n=303) | 10.6 (0.7) | 10.1 (0.9) | -- | ||
| 18 month (n=179) | 8.7 (0.8) | 7.6 (1.0) | −0.08 (−0.47 to 0.31) Point estimate = − 0.50± |
t(176) = −0.4 |
0.682 |
| Daily and Meaningful Activities e | |||||
| Baseline (n=303) | 7.5 (0.6) | 5.4 (0.7) | -- | ||
| 18 month (n=178) | 4.0 (0.6) | 2.9 (0.6) | 0.03 (−0.48 to 0.53) Point estimate = 1.03 ± |
t(175) = 0.1 |
0.911 |
| Legal Issues/Advanced Care Planning f | |||||
| Baseline (n=303) | 15.3 (2.1) | 17.9 (3.0) | -- | ||
| 18 month (n=179) | 9.9 (1.7) | 6.4 (1.6) | −0.66 (−1.20 to − 0.13) Point estimate = − 6.10 ± |
t(176) = −2.5 |
0.015 |
| QOL-AD-Self † | |||||
| Baseline (n=267) | 36.8 (0.5) | 37.2 (0.7) | -- | ||
| 9 month (n=189) | 36.7 (0.6) | 37.7 (0.8) | -- | ||
| 18 month (n=141) | 35.9 (0.6) | 38.2 (0.8) | 1.91 (0.22 to 3.59) | t(327) = 2.22 |
0.027 |
| ADRQL-40 † | |||||
| Baseline (n=302) | 92.8 (0.6) | 93.0 (0.7) | -- | ||
| 9 month (n=217) | 92.9 (0.6) | 92.7 (0.8) | -- | ||
| 18 month (n=177) | 91.1 (0.8) | 91.8 (1.0) | 0.51 (−1.23 to 2.24) | t(387) = 0.6 |
0.568 |
| QOL-AD-Informant † | |||||
| Baseline (n=289) | 31.3 (0.5) | 32.2 (0.6) | |||
| 9 month (n=216) | 33.1 (0.5) | 32.2 (0.6) | |||
| 18 month (n=173) | 31.7 (0.6) | 32.2 (0.7) | −0.42 (−1.96 to 1.12) | t(387) = −0.5 |
0.592 |
| CSDD ‡ | |||||
| Baseline (n=283) | 6.1 (0.37) | 6.5 (0.48) | -- | ||
| 9 month (n=211) | 6.2 (0.41) | 5.9 (0.53) | -- | ||
| 18 month (n=164) | 6.4 (0.46) | 6.9 (0.58) | 0.07 (--1.33 to 1.46) | t(350) = 0.1 |
0.925 |
| NPI-Q-Severity ‡ | |||||
| Baseline (n=300) | 6.9 (0.44) | 6.9 (0.58) | -- | ||
| 9 month (n=221) | 6.8 (0.49) | 6.2 (0.62) | -- | ||
| 18 month (n=176) | 6.9 (0.52) | 7.8 (0.65) | 0.85 (−0.55 to 2.25) | t(386) = 1.2 |
0.233 |
Abbreviations: JHDCNA, Johns Hopkins Dementia Care Needs Assessment; QOL-AD-Self, Quality of Life in Alzheimer’s Disease-Self Report; ADRQL-40, Alzheimer’s Disease Related Quality of Life scale-40 item; QOL-AD-Informant, Quality of life in Alzheimer’s Disease-Informant rated; CSDD, Cornell Scale for Depression in Dementia; NPI-Q, Neuropsychiatric Inventory-Questionnaire.
Intention-to-treat, mixed effects linear regression models adjusted for living with a caregiver.
Higher scores are worse.
Higher scores are better.
Estimates (with 95% CI) are expressed on the log odds scale and reflect the difference in change from 0 to 18 months between augmented usual care and intervention groups for the unmet needs domains. A point estimate is provided for the difference in percent change between augmented usual care and intervention groups and is calculated from the back transformed least squares estimates of the means at 0 and 18 months.
Includes items from JHDCNA Domains A and B, % unmet
Includes items from JHDCNA Domains C and D, % unmet
Includes items from JHDCNA Domains F and I, % unmet
Includes items from JHDCNA Domains G and H, % unmet
Includes items from JHDCNA Domains J and K, % unmet
Includes items from JHDCNA Domains L, % unmet
Secondary Outcomes
Self-reported QOL on the QOL-AD scale was significantly improved in the intervention group relative to the control group at 18 months (Table 4). The intervention did not impact informant-rated QOL (ADRQL-40; QOL-AD-Informant), NPS (NPI-Q), or participant depression (CSDD) (Table 4).
CONCLUSIONS
This initial study evaluated the efficacy of a home-based dementia care coordination model, MIND at Home, to delay transition from home and unmet needs and to improve quality of life in persons with memory disorders. The intervention was designed to be deliverable through community-based aging service providers or services networks and link community-based care with a medical team. Comparable to prior care coordination trials (24,27,29,32,33,35), this study tested a basic intervention approach involving tailored, dual-focused (patient and CG) needs assessments, care planning, and monitoring; an intervention duration of ≤ 2 years (18 months); a caseload of 44 families per coordinator; and a ‘usual care’ control condition. Our model is primarily differentiated from others through its intervention team composition (non-clinical community workers and mental health clinicians) and delivery model (implementation through community-based agencies that may not have explicit affiliations with integrated health systems or service networks); focus on a heterogeneous dementia population with diverse needs; and the content of the intervention itself (assessment on a comprehensive set of needs derived from decades of clinical expertise in geriatric psychiatry and dementia care, practice recommendations, and prior research). Thus, MIND offers a novel community-level model to address the needs of a heterogeneous memory-impaired population; link community, medical, and family resources; and expand the potential future dementia care workforce by frontline program staff without prior clinical experience.
Over 18 months, intervention participants had an estimated mean delay to transition out of their home of 51 days, with a median delay of 288 days over an extended follow-up period (median 26 months) compared to control participants. The greater ability to age-in-place in the context of improved self-reported QOL suggests the effect was not achieved at the expense of participant wellbeing. Transition to other care settings is sometimes necessary and appropriate for safety and patient and caregiver wellbeing. Level of care assessment and placement decision support was provided as part of the protocol. Figure 2A suggests that in the first 170 days of follow-up, the intervention group may have been leaving their homes at a rate faster than the control group (although non-significant). Later the curves diverge in favor of the intervention group. We surmise that this represents a subgroup of intervention participants who were not safe to remain at home and who were appropriately identified by the care coordinators and supported in transition decisions out of the home. Consistent with two prior trials (24,28), it appears that multicomponent supportive dementia care programs can improve the ability to age-in-place; this is the first study to our knowledge that has demonstrated a significant impact on time to leaving the home when the intervention duration is less than 24 months.
Like previous trials showing improved adherence to care guidelines (27,29,34,35), intervention participants experienced a significant reduction in proportions of unmet needs in Safety and Legal/Advanced Care Planning domains relative to control participants. MIND participants experienced a reduction in total percent of unmet care needs relative to controls (net between group difference = −1.5) that was not statistically significant, but that was likely clinically meaningful and represented a nearly 50% reduction in unmet needs from baseline to 18 months (10.2% to 5.9%). Both intervention and control groups, when modeled independently, had a significant decrease in total percent of unmet needs from baseline to 18 months. This is likely because the control group received a low-grade intervention consisting of an individualized written report on unmet care needs and recommendations for interventions. Thus, the effect of the MIND intervention was likely underestimated compared to a real world scenario in which patients and CGs do not receive an in-home assessment and recommendations.
A prior study of a collaborative model for dementia carried out in primary care reduced neuropsychiatric (NPI) symptoms but not depressive symptoms (CSDD) (27). In contrast, we did not find any significant group differences on NPS or depressive symptoms. It may be that NPS were lower in our study with less room for improvement. Also, the Callahan study (27) was carried out by nurse managers in collaboration with treating physicians in university-affiliated primary care clinics, suggesting that high levels of collaboration between nurse managers and physicians may have led to greater benefits and that the targeted population had access to high quality dementia care. In our study, we enrolled a community-based population with no specific connection to specialty or university-affiliated clinics.
Cost is a major factor in the uptake of interventions like MIND. While this study did not involve a prospective cost evaluation, post-hoc estimates suggest that the total annual cost per participant/caregiver dyad based on a caseload of 75 is $1,143 (based on 2009 values), which includes salary, fringe, travel, supplies, and overhead for all intervention team members (i.e., coordinator, psychiatrist, RN). The estimated annual cost per case is $738 if only considering coordinators (average salary of $40,000 plus fringe, travel, supplies and overhead). However since coordinator time per contact with participants was not measured and care team staff were involved in some research-related activities (e.g. consenting, study logistics/implementation meetings), these are preliminary estimates. Given the positive effect on being able to stay at home versus costly nursing home or assisted living placements, the findings imply a potential cost savings; although a prospective economic evaluation is necessary.
Delivery of MIND was designed to encourage flexibility, individualization, and efficiency. Coordinators averaged about 2 contacts per month, mostly with the study partner, showing fidelity to the pre-specified contact frequency. Contact frequency was quite variable (Table 3), however, and likely reflects the heterogeneity of the sample (e.g. cognitive status, need-levels), and differences in caregiver availability, and family preferences. Most (72%) contacts were phone-based, which implies benefits can be achieved in a potentially cost efficient manner. In fact, phone-based multicomponent dementia interventions designed for cost efficiency is an active and promising area of investigation (49). However, in the context of the MIND model and the observed effect on aging-in-place and significant reductions in unmet care needs related to safety, we believe the incorporation of prespecified and discretionary in-person home visits was essential, as this afforded the opportunity to visually identify a wide range of home and personal safety needs (e.g., fall risk, medication use adherence, wander risk) as well as the physical condition of participants and study partners.
Several limitations should be noted. Generalizability is limited because of the study sample, which was not a probability sample and represented an urban catchment area. Though carried out on the frontlines by non-clinical community workers, this approach is labor intensive, interdisciplinary, and requires support from mental health clinicians. We believe this is a strength, but it may present cost and implementation limitations. Also, coordinator services as tested in MIND do not currently qualify for Medicare/Medicaid reimbursement. However, Medicare and other insurance programs are currently examining use of care coordination models for quality improvement and efficiency (50). Other possible financing streams may include embedding MIND into Medicaid Waiver practice protocols and private pay programs. Due to project budget limitations, the 18-month unmet need data (JHDCNA) was collected by a non-blinded RN. However since intervention participants remained at home longer than controls, this may not have been an issue. Further, the exact mechanisms of action are not known. Given the diversity of the sample and their needs, we believe the intervention’s strength was the full package, rather than individual components. Finally, while MIND produced clinically and potentially financially important impacts on time to transfer from home, it had relatively modest effects on reducing unmet care needs and improving QOL, and did not impact other clinically relevant outcomes such as neuropsychiatric symptoms or participant depression, both of which predict institutionalization. Thus, the impact may be boosted by integrating discrete evidence-based approaches to address these specific areas.
This initial RCT demonstrates that a person-centered, home-based dementia care coordination intervention systematically delivered by community-based non-clinical coordinators supervised by geriatric clinicians is feasible, low-risk (no intervention-related adverse events), can delay transition out of the home , reduce unmet care needs, and improve self-reported QOL. This approach is responsive to the National Alzheimer’s Project Act (Public Law 111-375) and has the potential to reshape the current dementia care delivery paradigm by linking, in a novel and cost efficient way, medical and community based care services. Future research is warranted to replicate these promising preliminary results, assess cost offset potential, define which patient groups may benefit most (moderators), how benefits are achieved (mechanisms of action), to evaluate the model for sustainability and replicability in practice settings, and to extend the impact of MIND to include other important clinical outcomes.
Acknowledgments
Source of Funding: The project donors were: The Hoffberger Family Fund; LeRoy Hoffberger; The Harry and Jeannette Weinberg Foundation; Rosenberg Foundation; Hirschhorn Foundation; Stulman Charitable Foundation; Meyerhoff Foundation; Marc and Leonor Blum; Baltimore County Department of Aging; Blum Family; Lowell Glazer; Greif Family Fund; Marvin Schapiro Family Foundation; Lois and Phillip Macht; Eliasberg Family Foundation; Richard and Rosalee Davison; Alison & Arnold Richman; Moser Family Philanthropic Fund; Richard Lansburgh; Anonymous; and other supporting contributions Support was also provided by the National Institute of Mental Health/ National Institute on Aging (K01 MH085142). We wish express our sincere gratitude to Mr. LeRoy Hoffberger who served as the community champion for this project, to Drs. Ann Morrison, Shari Handel, and Adam Rosenblatt, and to The Associated: Jewish Community Federation of Baltimore, Jewish Community Services, Levindale Hebrew Geriatric Center, and the Alzheimer’s Association of Baltimore.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Under an agreement between DEMeasure and Drs. Black and Rabins, Dr. Black and Dr. Rabins are entitled to a share of income received by DEMeasure from sales of the Alzheimer’s Disease Related Quality of Life questionnaire and scale used in the study described in this article. Drs. Black and Rabins have ownership interests in DEMeasure. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies. Dr. Lyketsos has grant support from NIMH, NIA, Associated Jewish Federation of Baltimore, Weinberg Foundation, Forest, Glaxo-Smith-Kline, Eisai, Pfizer, Astra-Zeneca, Lilly, Ortho-McNeil, Bristol-Myers, Novartis, National Football League, Elan, Functional Neuromodulation Inc. Janssen. He is a consultant/Advisor for Astra-Zeneca, Glaxo-Smith Kline, Eisai, Novartis, Forest, Supernus, Adlyfe, Takeda, Wyeth, Lundbeck, Merz, Lilly, Pfizer, Genetech, Elan, NFL Players Association, NFL Benefits office, Avanir, Zinfandel, BMS. He has received honorarium or travel support from Pfizer, Forest, Glaxo-Smith Kline, Health Monitor. The remaining authors have no relevant conflicts or financial interests to disclose.
PREVIOUS PRESENTATION OF ABSTRACT: A limited amount of data presented in this manuscript was presented in a poster presentation and press briefing at the Alzheimer’s Association International Conference in Vancouver, BC, 2012. Reference: Samus Q, Johnston D, Black B, et al. Efficacy of a multidimensional home-based care coordination intervention for elders with memory disorders: The Maximizing Independence at Home trial. Alzheimer’s Association International Conference. Press release and poster presentation, Vancouver, BC,2012.
Work Cited
- 1.Alzheimer’s Association 2012 Alzheimer’s Disease Facts and Figures. Alzheimer’s & Dementia. 2012;8(2):131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Black B, Rabins P, German P. Predictors of nursing home placement among elderly public housing residents. The Gerontologist. 1999;39(5):559–568. doi: 10.1093/geront/39.5.559. [DOI] [PubMed] [Google Scholar]
- 3.Miller EA, Weissert WG. Predicting Elderly People’s Risk for Nursing Home Placement, Hospitalization, Functional Impairment, and Mortality: A Synthesis. Med Care Res Rev. 2000;57(3):259–297. doi: 10.1177/107755870005700301. [DOI] [PubMed] [Google Scholar]
- 4.Hebert R, Dubois M-F, Wolfson C, Chambers L, Cohen C. Factors Associated With Long-term Institutionalization of Older People With Dementia: Data From the Canadian Study of Health and Aging. J Gerontol A Biol Sci Med Sci. 2001;56(11):M693–699. doi: 10.1093/gerona/56.11.m693. [DOI] [PubMed] [Google Scholar]
- 5.Zhu CW, Scarmeas N, Torgan R, et al. Longitudinal study of effects of patient characteristics on direct costs in Alzheimer disease. Neurology. 2006;67(6):998–1005. doi: 10.1212/01.wnl.0000230160.13272.1b. [DOI] [PubMed] [Google Scholar]
- 6.Lyketsos C, Colenda C, Beck C, et al. Task Force of American Association for Geriatric Psychiatry Position statement of the American Association for Geriatric Psychiatry regarding principles of care for patients with dementia resulting from Alzheimer disease. Am J Geriatr Psychiatry. 2006;14(7):561–572. doi: 10.1097/01.JGP.0000221334.65330.55. [DOI] [PubMed] [Google Scholar]
- 7.Callahan CM, Hendrie HC, Tierney WM. Documentation and Evaluation of Cognitive Impairment in Elderly Primary Care Patients. Annals Of Internal Medicine. 1995;122(6):422–429. doi: 10.7326/0003-4819-122-6-199503150-00004. [DOI] [PubMed] [Google Scholar]
- 8.Gaugler JE, Kane RL, Kane RA, Newcomer R. Unmet Care Needs and Key Outcomes in Dementia. Journal of the American Geriatrics Society. 2005;53(12):2098–2105. doi: 10.1111/j.1532-5415.2005.00495.x. [DOI] [PubMed] [Google Scholar]
- 9.Miranda-Castillo C, Wood B, Galboda K, Ooman S, Olojugba C, Orrel M. Unmet needs, quality of life, and support networks of people with dementia living at home. Health Qual Life Outcomes. 2010;12(8):132. doi: 10.1186/1477-7525-8-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Black BS, Johnston D, Rabins PV, Morrison A, Lyketsos CG, Samus QM. Unmet needs of community-residing persons with dementia and their informal caregivers: Findings from the MIND at Home Study. Journal of the American Geriatrics Society. doi: 10.1111/jgs.12549. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnston D, Samus Q, Morrison A, et al. Identification of community-residing individuals with dementia and their unmet needs for care. Int J Geriatric Psychiatry. 2011;26(3):292–298. doi: 10.1002/gps.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Academy of Neurology Detection, diagnosis and management of dementia. 2001 < http://www.aan.com/professionals/practice/pdfs/dementia_guideline.pdf>.
- 13.Rabins P, Lyketsos C, Steele C. Practical Dementia Care. 2nd Edition Oxford University Press; New York: 2006. [Google Scholar]
- 14.National Collaborating Centre for Mental Health. Social Care Institute for Excellence [Accessed 1/20/2013];Dementia. The NICE-SCIE Guideline on supporting people with dementia and their carers in health and social care. 2011 http://www.nice.org.uk/nicemedia/live/10998/30320/30320.pdf. 2013.
- 15.Samus QM. The USA: US System for Dementia Care. In: de Waal H, O’Brien J, Ames D, Lyketsos C, editors. Designing and Implementing Successful Dementia Care Services. John Wiley & Sons, Ltd; West Sussex: 2013. [Google Scholar]
- 16.Callahan CM, Boustani M, Sachs GA, Hendrie HC. Integrating Care for Older Adults with Cognitive Impairment. Curr Alzheimer Res. 2009;6(4):368–374. doi: 10.2174/156720509788929228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brodaty H, Green A, Koschera A. Meta-analysis of psychosocial interventions for caregivers of people with dementia. J Am Geriatr Soc. 2003;51:657–664. doi: 10.1034/j.1600-0579.2003.00210.x. [DOI] [PubMed] [Google Scholar]
- 18.Pimouguet C, Lavaud T, Dartigues J, Helmer C. Dementia case management effectiveness on health care costs and resource utilization: a systematic review of randomized controlled trials. J Nutr Health Aging. 2010;14:669–676. doi: 10.1007/s12603-010-0314-4. [DOI] [PubMed] [Google Scholar]
- 19.Somme D, Trouve H, Dramé M, Gagnon D, Couturier Y, Saint-Jean O. Analysis of case management programs for patients with dementia: a systematic review. Alzheimers Dement. 2012;8:426–436. doi: 10.1016/j.jalz.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Knapp M, Iemmi V, Romeo R. Dementia care costs and outcomes: a systematic review. Int J Geriatr Psychiatry. 2012 doi: 10.1002/gps.3864. Online. ISSN 0885-6230 DOI: 10.1002/gps.3864o. [DOI] [PubMed] [Google Scholar]
- 21.Hickam DH, Weiss JW, Guise J-M, Buckley D, Motu’apuaka M, Graham E, Wasson N, Saha S. Comparative Effectiveness Review No. 99. Agency for Healthcare Research and Quality; Rockville, MD: Jan, 2013. Outpatient Case Management for Adults With Medical Illness and Complex Care Needs. (Prepared by the Oregon Evidencebased Practice Center under Contract No. 290-2007-10057-I.) AHRQ Publication No.13- EHC031-EF. www.effectivehealthcare.ahrq.gov/reports/final.cfm. [PubMed] [Google Scholar]
- 22.Spijker A, Vernooij-Dassen M, Vasse E, et al. Effectiveness of nonpharmacological interventions in delaying the institutionalization of patients with dementia: a meta-analysis. J Am Geriatr Soc. 2008;56:1116–1128. doi: 10.1111/j.1532-5415.2008.01705.x. [DOI] [PubMed] [Google Scholar]
- 23.Tam-Tham H, Cepoiu-Martin C, Ronksley PE, et al. Dementia case management and risk of long-term care placement: a systematic review and meta-analysis. Int J Geriatr Psychiatry. 2012 doi: 10.1002/gps.3906. Published online in Wiley Online Library ( wileyonlinelibrary.com) DOI: 10.1002/gps.3906. [DOI] [PubMed] [Google Scholar]
- 24.Eloniemi-Sulkava U, Notkola I-L, Hentinen M, et al. Effects of supporting communityliving demented patients and their caregivers: A randomized trial. J Am Geriatr Soc. 2001;49(10):1282–7. doi: 10.1046/j.1532-5415.2001.49255.x. PMID: 11890485. [DOI] [PubMed] [Google Scholar]
- 25.Mittelman M, Roth D, Coon D, et al. Sustained benefit of supportive intervention for depressive symptoms in Alzheimer’s caregivers. Am J Psychiatry. 2004;161:850–6. doi: 10.1176/appi.ajp.161.5.850. PMID: 15121650. [DOI] [PubMed] [Google Scholar]
- 26.Belle SH, Burgio L, Burns R, et al. Enhancing the Quality of Life of Dementia Caregivers from Different Ethnic or Racial Groups: A Randomized, Controlled Trial. Annals Of Internal Medicine. 2006;145(10):727–738. doi: 10.7326/0003-4819-145-10-200611210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Callahan C, Boustani M, Unverzagt F, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. Journal of the American Medical Association. 2006;295(18):2148–2157. doi: 10.1001/jama.295.18.2148. [DOI] [PubMed] [Google Scholar]
- 28.Mittelman MS, Haley WE, Clay OJ, Roth DL. Improving caregiver well-being delays nursing home placement of patients with Alzheimer disease. Neurology. 2006;67(9):1592–1599. doi: 10.1212/01.wnl.0000242727.81172.91. [DOI] [PubMed] [Google Scholar]
- 29.Vickrey B, Mittman B, Connor K, et al. The Effect of a Disease Management Intervention on Quality and Outcomes of Dementia Care: A Randomized, Controlled Trial. Ann Intern Med. 2006;145(10):713–726. doi: 10.7326/0003-4819-145-10-200611210-00004. [DOI] [PubMed] [Google Scholar]
- 30.Brodaty H, Mittelman M, Gibson L, et al. The effects of counseling spouse caregivers of people with Alzheimer disease taking donepezil and of country of residence on rates of admission to nursing homes and mortality. Am J Geriatr Psychiatry. 2009;17(9):734–43. doi: 10.1097/jgp.0b013e3181a65187. PMID: 19705519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mittelman MS, Brodaty H, Wallen AS, et al. A Three-country randomized controlled trial of a psychosocial intervention for caregivers combined with pharmacological treatment for patients with Alzheimer disease: Effects on caregiver depression. Am J Geriatr Psychiatry. 2008;16(11):893–904. doi: 10.1097/JGP.0b013e3181898095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eloniemi-Sulkava U, Saarenheimo M, Laakkonen M, et al. Family care as collaboration: effectiveness of a multicomponent support program for elderly couples with dementia. Randomized controlled intervention study. J Am Geriatr Soc. 2009;57(12):2200–8. doi: 10.1111/j.1532-5415.2009.02564.x. PMID: 20121986. [DOI] [PubMed] [Google Scholar]
- 33.Fortinsky RH, Kulldorff M, Kleppinger A, Kenyon-Pesce L. Dementia care consultation for family caregivers: Collaborative model linking an Alzheimer’s association chapter with primary care physicians. Aging & Mental Health. 2009;13(2):162–170. doi: 10.1080/13607860902746160. [DOI] [PubMed] [Google Scholar]
- 34.Ganz D, Koretz B, Bail J, et al. Nurse practicitioner comanagement for patients in an academic geriatric practice. American Journal of Managed Care. 2010;16(12):e343–355. [PMC free article] [PubMed] [Google Scholar]
- 35.Bass DM, Judge KS, Snow AL, Wilson NL, Morgan R, Looman WJ, McCarthy CA, Maslow K, Moye JA, Randazzo R, Garcia-Maldonado M, Elbein R, Odenheimer G, Kunik ME. Caregiver outcomes of Partners in Dementia Care: Effect of a care coordination program for Veterans with dementia and their family members and friends. Journal of the American Geriatric Society. 2013;61:1377–1386. doi: 10.1111/jgs.12362. [DOI] [PubMed] [Google Scholar]
- 36.American Psychological Association . Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision. 4th ed American Psychiatric Press, Inc; Washington, DC: 2000. [Google Scholar]
- 37.Black B, Johnston D, Handel S, et al. Manual for the Johns Hopkins Dementia Care Needs Assessment (JHDCNA) Baltimore, MD: 2008. [Google Scholar]
- 38.Brandt J, Spencer M, Folstein M. The Telephone Interview for Cognitive Status. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1988;1:111–117. [Google Scholar]
- 39.Jorm A. The informant questionnaire on cognitive decline in the elderly (IQCODE): a review. International Psychogeriatrics. 2004;16:275–293. doi: 10.1017/s1041610204000390. [DOI] [PubMed] [Google Scholar]
- 40.Clark PA, Bass DM, Looman WJ, McCarthy CA, Eckert S. Outcomes for patients with dementia from the Cleveland Alzheimer’s Managed Care Demonstration. Aging and Mental Health. 2004;8(1):40–51. doi: 10.1080/13607860310001613329. [DOI] [PubMed] [Google Scholar]
- 41.Folstein M, Folstein S, McHugh P. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 42.Wilkinson I, Graham-White J. Psychogeriatric dependency rating scales (PGDRS): a method of assessment for use by nurses. Br J Psychiatry. 1980;137:558–565. doi: 10.1192/bjp.137.6.558. [DOI] [PubMed] [Google Scholar]
- 43.Black B, Johnston D, Morrison A, Rabins P, Lyketsos C, Samus Q. Quality of life of community-residing persons with dementia based on self-rated and caregiver-rated measures. Quality of Life Research. 2012;21:1379–1389. doi: 10.1007/s11136-011-0044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Logsdon R, Gibbons L, McCurry S, Teri L. Quality of life in Alzheimer’s disease: patient and caregiver reports. Journal of Mental Health and Aging. 1999;5:21–32. [Google Scholar]
- 45.Kasper J, Black B, Shore A, Rabins PV. Evaluation of the validity and reliability of the Alzheimer Disease-related Quality of Life instrument. Alzheimer Disease and Associated Disorders. 2009;23(3):275–284. doi: 10.1097/WAD.0b013e31819b02bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaufer D, Cummings J, Ketchel P, et al. Validation of the NPI-Q, a Brief clinical form of the Neuropsychiatric Inventory. The Journal of Neuropsychiatry and Clinical Neurosciences. 2000;12(2):233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 47.Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell scale for depression in dementia. Biological Psychiatry. 1988;23(3):271. doi: 10.1016/0006-3223(88)90038-8. [DOI] [PubMed] [Google Scholar]
- 48.Perneczky R, Wagenpfeil S, Komossa K, Grimmer T, Diehl J, Kurz A. Mapping scores onto stages: mini-mental state examination and clinical dementia rating. Am J Geriatr Psychiatry. 2006;14(2):139–44. doi: 10.1097/01.JGP.0000192478.82189.a8. [DOI] [PubMed] [Google Scholar]
- 49.Tremont G, Davis J, Papandonatos GD, Grover C, Ott BR, Fortinsky RH, Gozalo P, Bishop DS. A telephone intervention for dementia caregivers:Background, design, and baseline characteristics. Contemporary Clinical Trials. 2013;36:338–347. doi: 10.1016/j.cct.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.US Department of Health Human Services [Accessed 6/1/2013];New Affordable Care Act support to improve care coordination for nearly 200,000 people with Medicare. 2011 http://www.hhs.gov/news/press/2011pres/06/20110606a.html.