Abstract
Objective
Aflatoxin is known to cross the placental barrier and exposures in utero could influence genomic programming, fetal growth and development, resulting in long term health effects. We aimed to determine aflatoxin exposure in Gambian women at two stages of pregnancy and during the rainy and dry seasons.
Methods
We examined aflatoxin exposure in pregnant Gambian women at early (<16 weeks) and later (16 weeks onward) stages of pregnancy and at different times of the year, during the rainy (June to October 2009) or dry (November to May 2010) season, using aflatoxin albumin adducts (AF-alb).
Results
Mean AF-alb was higher during the dry season than in the rainy season, in both early and later pregnancy although the difference was strongest in later pregnancy. There was a modest increase in AF-alb in later than early pregnancy (geometric mean 41.8 vs 34.5 pg/mg, P <.0.05), but this was restricted to the dry season when exposures were generally higher.
Conclusions
The study confirmed that Gambian pregnant women were exposed to aflatoxin throughout the pregnancy, with higher levels in the dry season. There was some evidence in the dry season that women in later pregnancy had higher AF-alb levels than those in earlier pregnancy. Further research on the effects of exposure to this potent mutagen and carcinogen throughout pregnancy, including the epigenetic modification of fetal gene expression and impact on pre- and post-natal growth and development, are merited.
Keywords: Aflatoxin, in utero exposure, seasonality
Introduction
Aflatoxins are naturally-occurring secondary fungal metabolites mostly produced by Aspergillus species. Aflatoxin B1 (AFB1) is the most common and has been classified as a Group 1 human carcinogen by the International Agency for Research on Cancer (IARC 2002). Numerous acute out-breaks of aflatoxin poisoning through the consumption of highly contaminated grain have been reported, of which one of the largest occurred in Kenya in 2004, resulting in 317 cases of hepatic failure and 125 deaths (Azziz-Baumgartner et al. 2005; Strosnider et al. 2006). Aflatoxin is associated with child growth faltering (Gong et al. 2002, 2004) and possibly immune function impairment (Turner et al. 2003; Jiang et al. 2005). Aflatoxin is a well-established and potent mutagen and teratogen as demonstrated in experimental models (Butler & Wigglesworth 1966; Qureshi et al. 1998; Marin et al. 2002).
Aflatoxin contaminates a large proportion of the world’s staple foods, including maize and groundnuts (Wild & Gong 2010) and an estimated 4.5 billion people are exposed worldwide (Williams et al. 2004). Fungal growth and aflatoxin production can occur both in the field and during storage under warm and humid conditions (Hell et al. 2000). Changes in temperature and precipitation can also influence contamination levels (Cotty & Jaime-Garcia 2007). Consequently, season has been identified as an important factor in determining aflatoxin exposure in West Africa (Hell et al. 2000; Wild et al. 2000; Cotty & Jaime-Garcia 2007). In The Gambia, aflatoxin levels are usually higher following harvest and a period of storage during the dry season from November to May than during the rainy season from June to October (Wild et al. 2000). The rainy season has also been called the ‘hungry’ season (vs. the dry ‘harvest’) because it is a period of intense physical activity but at a time when stores of staple foods are depleted (Thomson et al. 1966).
Exposure to aflatoxin has been consistently reported to be high in The Gambia (Allen et al. 1992; Turner et al. 2000; Wild et al. 2000). Maternal exposure is significantly associated with lower height and weight gain in infants during the first year of life (Turner et al. 2007). Such effects are consistent with aflatoxin crossing the placental barrier and being bio-activated in utero by hepatic cytochrome P450s (Li et al. 1997; Abdulrazzaq et al. 2002; Turner et al. 2007). However, it is unclear whether aflatoxin exposure changes during pregnancy, and how such a change may adversely affect the health of the mother and her child. This study aimed to understand aflatoxin exposure status during the early and later stages of pregnancy in rural Gambian women and explored possible interactions with seasonal influence on this relationship.
Methods
Study subjects
The study site was based in the West Kiang region of The Gambia, a rural subsistence farming community of savannah and farmland. Women aged 18–45 years old were invited to participate in the ‘Methyl Donors and Epigenetics’ (MDEG) study which followed a sub-cohort of women to study the impact of nutrition at time of conception on DNA methylation patterns in offspring. The MDEG study was embedded in an on-going trial of pre-natal and infant nutritional supplementation on infant immune development: the ‘ENID’ Trial (Early Nutrition and Immune Development; ISRCTN49285450). Full details of the ENID Trial are presented elsewhere (Moore et al. 2012).
Once enrolled, women were visited by a field assistant monthly to record the date of last menstrual period. Where a menses had been missed, pregnancy and date were confirmed by a urine pregnancy test, and for those who were positive in the urine test, a subsequent obstetric ultrasound test in the MRC Keneba clinic for date confirmation. Data on height, weight, village and self-reported ethnicity were also collected. A blood sample was collected before 16 weeks of pregnancy (defined as early pregnancy) and at 16–33 weeks of pregnancy (defined as “later pregnancy”). Blood samples were available from 134 women at early pregnancy, of whom 99 were followed up in the ENID study at later pregnancy and had blood samples available from this stage of pregnancy. Both sets of blood samples from these 99 women were used for this study to investigate aflatoxin exposure at different pregnancy stages and the impact of seasonality on exposure. For individual women there was a minimum of six weeks between the collection of the early and later pregnancy samples. The blood sampling fell either in the rainy (June to October) or dry (November to May) season.
Blood was collected in EDTA vacutainer tubes and centrifuged at 2,750 g for 10 minutes at 4 °C. The plasma was separated and stored as aliquots at −80 °C, before being shipped to the University of Leeds, UK for blood albumin and AF-alb biomarker analysis.
Ethical approval was obtained from the Gambia Government/MRC Gambia Joint Ethics Committee and written informed consent was obtained from each participating woman.
Blood albumin and AF-alb biomarker analysis
Plasma samples were analysed for AF-alb using a competitive ELISA, as previously described (Chapot and Wild 1991) with modification. In brief, plasma (150–250 μl) was digested overnight with 5 mg/ml pronase at 37 °C. The digested protein was then purified using C18 cartridges (Waters, Hertfordshire, UK) and analysed by competitive ELISA. Samples were analysed in triplicate over two separate days. Intra-assay coefficient of variation (CV) was < 15 % and inter-assay CV was ≤ 25 %. Three positive quality controls of known AF-alb levels and one negative control were analysed alongside each batch of samples. The AF-alb level is presented with reference to the amount of albumin in the sample from which the AF-alb was measured (i.e. pg AF-alb/mg albumin). The limit of detection for AF-alb was 0.6 pg/mg albumin.
An aliquot (20 μl) from each plasma sample was used to measure albumin levels using a commercial kit (Bio-quant, San Diego, CA, USA) based on the bromocresol green (BCG) method.
Statistical analysis
AF-alb was not normally distributed and was natural-log transformed prior to statistical analyses. Geometric means (GM) with 95% confidence intervals (CI) are presented for AF-alb levels. Differences in albumin and AF-alb levels by stage of pregnancy and by season of blood collection were tested using the Student’s t-test. The ANOVA model was used to test the interaction relationship between the stage of pregnancy and season of blood collection in relation to AF-alb or albumin levels. Spearman rank correlation analysis was used to assess the relationship between the AF-alb at the two stages of pregnancy. A P < 0.05 was considered statistically significant, and P <0.01 as highly significant. All data analyses were performed on Stata IC software (version 11, StataCorp, College Station, TX, USA).
Results
At the time the early pregnancy samples were collected, the average age of the women (mean ± SD) was 28.9 ± 6.5 years (range, 18–45 years). 70.3% of the women were of Mandinka ethnicity, 10.1% Fula and 19.6% of other or unknown ethnicity.
Early pregnancy samples were obtained from 47 women during the dry season and from 87 women during the rainy season. The mean gestation week of these women (mean ± SD) was 8 ± 4 weeks. Of the 99 women who were followed-up for AF-alb during later pregnancy, 47 provided samples during the dry season and 52 during the rainy season. Of the original 134 women who were recruited, 35 were not followed up as they had either moved away or opted out of the study. The mean gestation week at the second sampling was 27 ± 3 weeks (range 16–33 weeks). There was an average 19 ± 5-week (range 6–33 weeks) gap between the two collections.
Blood albumin and AF-alb biomarker
The blood albumin and AF-alb levels by season of blood collection and stage of pregnancy are summarized in Table 1. The blood albumin levels were significantly higher during the early stages of pregnancy compared to the later stage (mean ± SD: 37.2 ± 9.1 vs 48.1 ± 14.9 mg/ml, P<0.01), and there was no significant difference in albumin levels between the dry or rainy season.
Table 1.
Blood albumin (mg/ml) and AF-alb (pg/mg albumin) levels by season and stage of pregnancy
| Albumin Dry Season Mean ± SD |
Rainy Season Mean ± SD |
Total Mean ± SD |
|
|---|---|---|---|
| Early pregnancy | 50.2 ± 14.6 (N = 47) | 47.0 ± 15.0 (N = 87) | 48.1 ± 14.9 (N = 134) |
| Later pregnancy | 37.8 ± 8.7* (N = 47) | 36.6 ± 9.7* (N = 52) | 37.2 ± 9.1* (N = 99) |
|
| |||
| Total (Mean ± SD) | 44.0 ± 13.5 | 43.2 ± 14.1 | |
| AF-alb Dry Season GM (95% CI) |
Rainy Season GM (95% CI) |
Total GM (95% CI) |
|
|---|---|---|---|
| Early pregnancy | 40.6 (29.5, 55.8) (N = 47) | 31.6 (26.2, 38.1) (N = 87) | 34.5 (29.3, 40.7) (N = 134) |
| Later pregnancy | 68.7 (52.6, 89.7)* (N = 47) | 26.6 (21.9, 32.4)a (N = 52) | 41.8 (34.7, 50.3)b (N = 99) |
|
| |||
|
Total GM (95% CI) |
52.8 (42.8, 65.2) | 29.6 (25.8, 34.0)c | |
Significant difference between data at early and later stages of pregnancy, P < 0.05
Significant difference between AF-alb levels in dry and rainy seasons at the later stage of pregnancy, P < 0.01
Significant difference between total AF-alb levels between the early and later stages of pregnancy, P < 0.05
Significant difference between total AF-alb levels between dry and rainy seasons, P < 0.01
All samples tested had detectable AF-alb levels. AF-alb levels were not compared between villages due to the small number of subjects per village; age and ethnicity were not correlated with AF-alb levels.
In relation to season of blood collection, AF-alb was higher during the dry season than the rainy season (GM: 52.8 vs 29.6 pg/mg, P < 0.01), although when stratified by stage of pregnancy a statistically significant difference was only seen among the women in later pregnancy (GM: 68.7 vs 26.6 pg/mg, P < 0.01) (Table 1). The levels of AF-alb in relation to rainfall in each month of blood collection are shown in Figure 1.
Figure 1.
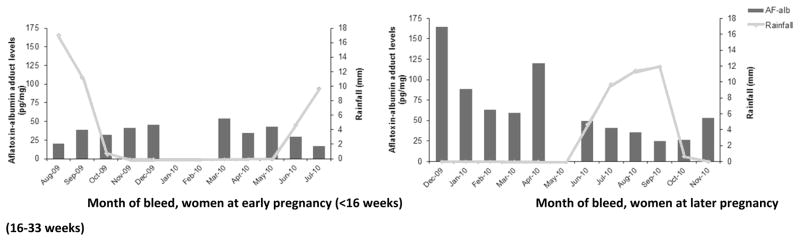
Geometric means of aflatoxin-albumin adduct presented by month of bleed with amount of rainfall per month collected during 2009–2010
AF-alb levels were higher during later than early pregnancy (GM: 41.8 pg/mg vs 34.5 pg/mg, P <0.05). Significant difference was limited to samples collected during the dry season (GM: 68.7 pg/mg at the later stage vs 40.8 pg/mg at the early stage, P <0.05).
The interaction between season of blood collection and stage of pregnancy in explaining AF-alb and albumin levels was further investigated using an ANOVA model (Table 2). For AF-alb levels, the overall model was statistically significant (F = 10.87, P < 0.01), with a significant interaction effect on AF-alb between pregnancy stage and season (P <0.01), and with a 2-fold difference between the exposure in later pregnancy in the dry season as compared to early or later pregnancy during the rainy season. No interaction was found between pregnancy stage and season on blood albumin.
Table 2.
ANOVA analysis indicating the interaction of stage of pregnancy and season of blood collection on AF-alb but not on Albumin
| Variables | Blood AF-alb F, p |
Blood Albumin F, p |
|---|---|---|
| Pregnancy stage (early vs later pregancy) | 2.14, 0.145 | 45.5, <0.01 |
| Season of blood collection (dry vs rainy season) | 24.41, < 0.01 | 1.55, 0.21 |
| Pregnancy stage * season of blood collection | 8.29, <0.01 | 0.33, 0.56 |
Correlation between a woman’s AF-alb level at early and later pregnancy was tested using Spearman’s correlation analysis. The rank order of AF-alb at early and later stage of pregnancy were positively correlated (Spearman’s coefficient: 0.231, P <0.05).
Discussion
This study investigated possible changes in aflatoxin exposure during pregnancy, confirming ubiquitous exposure in this population at similar levels to those previously reported in adult subjects (Wild & Turner 2002, Turner et al. 2007; Wild et al. 1990, 1992, 2000). The main determinant of a pregnant woman’s exposure is season (highest AF-alb occurring in the dry season), with limited evidence for an effect of stage of pregnancy (higher AF-alb in later stages). The current findings have important implications for in utero child health effects, as it has been established that AFB1 can cross the placenta (Partanen et al. 2010) and exposure to aflatoxin in utero has been previously linked to impaired child growth (Turner et al. 2007).
The diet in West Kiang typically consists of a staple (refined white rice, millet or maize) with a sauce. This sauce is often a groundnut sauce mixed with vegetables, but sauces can also be made with other ingredients such as green leaves or fish (Prentice et al. 1993). The most likely sources of aflatoxin exposure are contaminated groundnuts and, to a lesser extent (because of lower consumption), maize, which together form key components of the diet in The Gambia (Hudson et al. 1992). Groundnuts are usually harvested between September and November (Roberts et al. 1982; Turner et al. 2000); afterwards they are either sold or stored in conditions that make them further susceptible to insect damage and fungal growth.
In the dry season we did observe higher levels of aflatoxin exposure in women during later pregnancy than early pregnancy but not during the rainy season. There is a significant interaction between season of collection and pregnancy stage, which explains why the effect of late-stage pregnancy on AF-alb was seen only in the dry season. Overall, the stage of pregnancy is not strongly associated with AF-alb but season is.
The rainy season in the Gambia is a period of heavy farming activity and food shortages (Paul et al. 1979; Roberts et al. 1982). It is possible that severe food shortage in the rainy/hungry season has restricted the women from consuming more food to meet the increasing demand in later pregnancy, whilst in the dry season, when food is more abundant, the increased demand in later pregnancy can be satisfied. In the dry season, when groundnuts and maize are stored, aflatoxin levels rise. Thus aflatoxin exposure increases due to rising contamination and consumption during the same period.
Seasonal variation in the presence of the R249S mutation, a p53 249 codon G to T mutation characteristic of human hepatocellular carcinoma in high AFB1 populations, has been observed in Gambian children and adults, where the dry season served as a surrogate indicator of high aflatoxin exposure (Villar et al. 2011). R249S serum concentrations were higher during the dry season, which is a period of high AFB1 exposure, than in the rainy season in hepatitis B virus (HBV)- negative subjects.
It has been reported that CYP3A enzyme activity can increase during pregnancy (Tracy et al. 2005), and the CYP3A4 and CYP3A5 isoforms play an important role in the metabolism and conversion of AFB1 to the toxic AFB1-8,9-epoxide which binds DNA or albumin (Guengerich et al. 1998; Wojnowski et al. 2004). Such modifications to metabolism of AFB1 during later pregnancy may also contribute to the higher AF-alb levels observed in the present study in later compared to earlier pregnancy.
A key question is why there is no difference in AF-alb levels in early pregnancy by season. This may be partly explained by the significant dip in basal and resting metabolic rates in Gambian women during early pregnancy compared to non-pregnant women, before a steady increase in both rates as pregnancy progresses (Lawrence et al. 1984; Poppitt et al. 1993). A lower metabolic rate during early pregnancy suggests a potentially lower food intake during this period, minimising the effect of season on AF-alb levels.
Our data show that aflatoxin exposure for individual women was significantly correlated between early and later stages of pregnancy, suggesting that despite the effect of seasonality on levels of toxin intake, the level of contamination of household food stores is also relevant, although we did not directly measure aflatoxin levels in grains or groundnuts.
It is worth noting that albumin concentrations were lower in later than early pregnancy. This is consistent with previous observations in French and Canadian women, whereby albumin levels dropped as pregnancy progressed (Bacq et al. 1996; Walker et al. 1999), possibly due to the increase in blood volume in later pregnancy (Pirani et al. 1973; Gallery et al. 1979). However, as the AF-alb level is presented after adjustment for albumin concentration, a discrepancy in albumin concentrations should not have a significant influence on the aflatoxin exposure biomarker.
A critical ‘window of vulnerability’ exists in the early stages of fetal development, during which dietary exposures to aflatoxin, as in the case of other environment toxins, may affect the genomic programming of the foetus, including epigenetic changes that can subsequently modify gene expression (Gluckman et al. 2008). These changes can alter DNA methylation or histone modification patterns, which can affect mechanisms involved in growth, development and cell cycle regulation leading to an increased risk of cancer development. The changes introduced at this stage can be maintained throughout life and can adversely affect health in adulthood. However, aflatoxin exposure throughout pregnancy, including the later stages where the baby gains weight rapidly, may also affect fetal development and growth as demonstrated in an earlier study from The Gambia (Turner et al. 2007). Thus the timing of a child’s conception is likely to influence their exposure to aflatoxin in utero and possibly the subsequent adverse health effects.
In summary, the study confirmed exposure to aflatoxin throughout pregnancy among women in The Gambia. Levels were higher in the dry season and there was some evidence that in this season women in later pregnancy had higher AF-alb levels than those in earlier pregnancy. Further research on the effects of exposure to this potent mutagen and carcinogen throughout pregnancy, including the effects on fetal gene expression are merited, given the ubiquitous nature of this exposure in many sub-Saharan African populations.
Acknowledgments
We thank all the study participants as well as the field, laboratory and data office staff at MRC Keneba. YY Gong and CP Wild are supported by the National Institute of Environmental Health Sciences, USA. We would also like to acknowledge the finance contribution from IARC toward JM Castelino’s PhD study. MRC Keneba is supported by the UK Medical Research Council and the study within which this analysis was embedded was funded by the Wellome Trust.
References
- Abdulrazzaq YM, Osman N, Ibrahim A. Fetal exposure to aflatoxins in the United Arab Emirates. Annals of Tropical Paediatrics. 2002;22:3–9. doi: 10.1179/027249302125000094. [DOI] [PubMed] [Google Scholar]
- Allen SJ, Wild CP, Wheeler JG, et al. Aflatoxin exposure, malaria and hepatitis-B infection in rural Gambian children. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1992;86:426–430. doi: 10.1016/0035-9203(92)90253-9. [DOI] [PubMed] [Google Scholar]
- Azziz-Baumgartner E, Lindblade K, Gieseker K, et al. Case-control study of an acute aflatoxicosis outbreak, Kenya, 2004. Environmental Health Perspectives. 2005;113:1779–1783. doi: 10.1289/ehp.8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacq Y, Zarka O, Brechot JF, et al. Liver function tests in normal pregnancy: a prospective study of 103 pregnant women and 103 matched controls. Hepatology. 1996;23:1030–1034. doi: 10.1002/hep.510230514. [DOI] [PubMed] [Google Scholar]
- Butler WH, Wigglesworth J. The effects of aflatoxin B1 on the pregnant rat. British Journal of Experimental Pathology. 1966;47:242–247. [Google Scholar]
- Chapot B, Wild CP. ELISA for quantification of aflatoxin-albumin adducts and their application to human exposure assessment. In: Warhol M, van Velzen D, Bullock GR, editors. Techniques in Diagnostic Pathology. Vol. 2. CA: Academic Press, San Diego; 1991. pp. 135–155. [Google Scholar]
- Cotty PJ, Jaime-Garcia R. Influences of climate on aflatoxin producing fungi and aflatoxin contamination. International Journal of Food Microbiology. 2007;119:109–115. doi: 10.1016/j.ijfoodmicro.2007.07.060. [DOI] [PubMed] [Google Scholar]
- Gallery ED, Hunyor SN, Gyory AZ. Plasma volume contraction: a significant factor in both pregnancy-associated hypertension (pre-eclampsia) and chronic hypertension in pregnancy. Quarterly Journal of Medicine. 1979;48:593–602. [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of In Utero and Early-Life Conditions on Adult Health and Disease. New England Journal of Medicine. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong YY, Cardwell K, Hounsa A, et al. Dietary aflatoxin exposure and impaired growth in young children from Benin and Togo: cross sectional study. British Medical Journal. 2002;325:20–21. doi: 10.1136/bmj.325.7354.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong YY, Hounsa A, Egal S, et al. Postweaning exposure to aflatoxin results in impaired child growth: A longitudinal study in Benin, west Africa. Environmental Health Perspectives. 2004;112:1334–1338. doi: 10.1289/ehp.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich FP, Johnson WW, Shimada T, et al. Activation and detoxication of aflatoxin B1. Mutation Research. 1998;402:121–128. doi: 10.1016/s0027-5107(97)00289-3. [DOI] [PubMed] [Google Scholar]
- Hell K, Cardwell KF, Setamou M, Poehling HM. The influence of storage practices on aflatoxin contamination in maize in four agroecological zones of Benin, West Africa. Journal of Stored Products Research. 2000;36:365–382. doi: 10.1016/s0022-474x(99)00056-9. [DOI] [PubMed] [Google Scholar]
- Hudson GJ, Wild CP, Zarba A, Groopman JD. Aflatoxins isolated by immunoaffinity chromatography from foods consumed in the Gambia, West Africa. Natural Toxins. 1992;1:100–105. doi: 10.1002/nt.2620010208. [DOI] [PubMed] [Google Scholar]
- IARC . Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. IARC monographs on the evaluation of carcinogenic risks to humans/World Health Organization. International Agency for Research on Cancer. 2002;82:1–556. [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Jolly PE, Ellis WO, et al. Aflatoxin B1 albumin adduct levels and cellular immune status in Ghanaians. International Immunology. 2005;17:807–814. doi: 10.1093/intimm/dxh262. [DOI] [PubMed] [Google Scholar]
- Lawrence M, Lamb WH, Lawrence F, Whitehead RG. Maintenance energy cost of pregnancy in rural Gambian women and influence of dietary status. The Lancet. 1984;324:363–365. doi: 10.1016/s0140-6736(84)90538-5. [DOI] [PubMed] [Google Scholar]
- Li Y, Yokoi T, Katsuki M, et al. In vivo activation of aflatoxin B1 in C57BL/6N mice carrying a human fetus-specific CYP3A7 gene. Cancer Research. 1997;57:641–645. [PubMed] [Google Scholar]
- Marin DE, Taranu I, Bunaciu RP, et al. Changes in performance, blood parameters, humoral and cellular immune responses in weanling piglets exposed to low doses of aflatoxin. Journal of Animal Science. 2002;80:1250–1257. doi: 10.2527/2002.8051250x. [DOI] [PubMed] [Google Scholar]
- Moore SE, Fulford AJ, Darboe MK, et al. A randomized trial to investigate the effects of prenatal and infant nutritional supplementation on infant immune development in rural Gambia: the ENID trial: Early Nutrition and Immune Development. BMC pregnancy and childbirth. 2012;12:107. doi: 10.1186/1471-2393-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberne PM, Butler WH. Acute and Chronic Effects of Aflatoxin on the Liver of Domestic and Laboratory Animals: A Review. Cancer Research. 1969;29:236–250. [PubMed] [Google Scholar]
- Partanen HA, El-Nezami HS, Leppanen JM, et al. Aflatoxin B1 transfer and metabolism in human placenta. Toxicological Sciences. 2010;113:216–225. doi: 10.1093/toxsci/kfp257. [DOI] [PubMed] [Google Scholar]
- Paul AA, Muller EM, Whitehead RG. The quantitative effects of maternal dietary energy intake on pregnancy and lactation in rural Gambian women. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1979;73:686–692. doi: 10.1016/0035-9203(79)90022-1. [DOI] [PubMed] [Google Scholar]
- Pirani BB, Campbell DM, MacGillivray I. Plasma volume in normal first pregnancy. Journal of Obstetrics and Gynaecology of the British Commonwealth. 1973;80:884–887. doi: 10.1111/j.1471-0528.1973.tb02146.x. [DOI] [PubMed] [Google Scholar]
- Poppitt SD, Prentice AM, Jéquier E, et al. Evidence of energy sparing in Gambian women during pregnancy: a longitudinal study using whole-body calorimetry. The American Journal of Clinical Nutrition. 1993;57:353–364. doi: 10.1093/ajcn/57.3.353. [DOI] [PubMed] [Google Scholar]
- Prentice AM, Laskey M, Shaw J, et al. The calcium and phosphorus intakes of rural Gambian women during pregnancy and lactation. British Journal of Nutrition. 1993;69:885–885. doi: 10.1079/bjn19930088. [DOI] [PubMed] [Google Scholar]
- Qureshi MA, Brake J, Hamilton PB, et al. Dietary exposure of broiler breeders to aflatoxin results in immune dysfunction in progeny chicks. Poultry Science. 1998;77:812–819. doi: 10.1093/ps/77.6.812. [DOI] [PubMed] [Google Scholar]
- Roberts SB, Paul AA, Cole TJ, Whitehead RG. Seasonal changes in activity, birth weight and lactational performance in rural Gambian women. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1982;76:668–678. doi: 10.1016/0035-9203(82)90239-5. [DOI] [PubMed] [Google Scholar]
- Strosnider H, Azziz-Baumgartner E, Banziger M, et al. Workgroup Report: Public Health Strategies for Reducing Aflatoxin Exposure in Developing Countries. Environmental Health Perspectives. 2006;114:1898–1903. doi: 10.1289/ehp.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A, Billewicz W, Thompson B, McGregor I. Body weight changes during pregnancy and lactation in rural African (Gambian) women. BJOG: An International Journal of Obstetrics & Gynaecology. 1966;73:724–733. doi: 10.1111/j.1471-0528.1966.tb06075.x. [DOI] [PubMed] [Google Scholar]
- Tracy TS, Venkataramanan R, Glover DD, Caritis SN. Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A Activity) during pregnancy. American Journal of Obstetrics and Gynecology. 2005;192:633–639. doi: 10.1016/j.ajog.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Turner PC, Collinson AC, Cheung YB, et al. Aflatoxin exposure in utero causes growth faltering in Gambian infants. International Journal of Epidemiology. 2007;36:1119–1125. doi: 10.1093/ije/dym122. [DOI] [PubMed] [Google Scholar]
- Turner PC, Mendy M, Whittle H, et al. Hepatitis B infection and aflatoxin biomarker levels in Gambian children. Tropical Medicine & International Health. 2000;5:837–841. doi: 10.1046/j.1365-3156.2000.00664.x. [DOI] [PubMed] [Google Scholar]
- Turner PC, Moore SE, Hall AJ, et al. Modification of immune function through exposure to dietary aflatoxin in Gambian children. Environmental Health Perspectives. 2003;111:217–220. doi: 10.1289/ehp.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar S, Le Roux-Goglin E, Gouas DA, et al. Seasonal variation in TP53 R249S-mutated serum DNA with aflatoxin exposure and hepatitis B virus infection. Environmental Health Perspectives. 2011;119:1635. doi: 10.1289/ehp.1103539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MC, Smith GN, Perkins SL, et al. Changes in homocysteine levels during normal pregnancy. American Journal of Obstetrics and Gynecology. 1999;180:660–664. doi: 10.1016/s0002-9378(99)70269-3. [DOI] [PubMed] [Google Scholar]
- Wild CP, Gong YY. Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis. 2010;31:71–82. doi: 10.1093/carcin/bgp264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild CP, Yin F, Turner PC, et al. Environmental and genetic determinants of aflatoxin–albumin adducts in The Gambia. International Journal of Cancer. 2000;86:1–7. doi: 10.1002/(sici)1097-0215(20000401)86:1<1::aid-ijc1>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Williams JH, Phillips TD, Jolly PE, et al. Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. The American Journal of Clinical Nutrition. 2004;80:1106–1122. doi: 10.1093/ajcn/80.5.1106. [DOI] [PubMed] [Google Scholar]
- Wojnowski L, Turner PC, Pedersen B, et al. Increased levels of aflatoxin-albumin adducts are associated with CYP3A5 polymorphisms in The Gambia, West Africa. Pharmacogenetics and Genomics. 2004;14:691–700. doi: 10.1097/00008571-200410000-00007. [DOI] [PubMed] [Google Scholar]


