Abstract
Study Design
Cross-sectional study.
Objective
The purpose of this study was to determine if a sustained fatiguing contraction of the dorsiflexor muscles alters the dynamic position sense (proprioception) and the associated central nervous system processing time of information from the ankle.
Background
Ankle injury has been hypothesized to be related to altered proprioception as a consequence of fatiguing exercise. Previous reports assessing proprioception include tests of motor performance (balance and limb repositioning) or tests of a joint under static conditions. This study used a novel experimental approach to test the effects of exercise on the somatosensory system of the ankle.
Methods and Measures
Nineteen healthy subjects were tested on their ability to extend the metacarpophalangeal joint of their left index finger when their left ankle was passively plantar flexed (0°–40°, 10 velocities) through a predetermined target angle (20°). Testing occurred before and after a fatiguing contraction of the dorsiflexor muscles.
Results
Subjects accurately indicated the ankle target angle up to ankle velocities of 70°/s (300 ms) both before and after the sustained fatiguing contraction. At velocities above 70°/s all subjects could no longer scale to accurately indicate the target angle with the index finger and consequently overshot the target. The central nervous system processing time was estimated to be approximately 85 milliseconds before and after the sustained contraction.
Conclusions
These results indicate that a sustained activity of the dorsiflexion muscles of the ankle minimally affects dynamic position sense and the ability to process dynamic position sense information. Understanding the impact of exercise on sensory system processing will be integral to establishing the scientific basis for rehabilitation programs that purport to train proprioception.
Keywords: ankle injuries, fatigue, movement sequence, proprioception
Approximately 5 to 10 million ankle injuries occur annually in the United States and over 5% of all athletic injuries occur at the ankle joint.3 The ankle is most frequently affected because of its structure and weight-bearing function.3 Ankle sprains during athletic events are often attributed to altered somatosensory perception (joint proprioception), especially at the end of exercise when the individual is fatigued.11,13,15,27,34 Kinesthetic information from the ankle joint is obtained through articular mechanoreceptors, cutaneous afferents, and muscle receptors (Golgi tendon organs and muscle spindle afferents). The muscle spindles are recognized as being the most important source of proprioceptive information.17
Strenuous exercise and associated muscle fatigue has been purported to distort joint proprioception during postural control.4,6,19,28,30,31 Furthermore, exercise leading to muscle fatigue alters proprioception by influencing the afferent spindle receptors in the muscles.25 Sustained muscle contraction is also associated with widespread changes in the central nervous system (CNS) at various spinal and supraspinal levels.16
The influence of exercise on proprioception has primarily been examined by methods that assess the ability of the individual to reposition the joint or by detecting the threshold to a slow movement.4–6,20,24,28,35,36 However, assessing joint position sense statically appears removed from the dynamic nature of most movements leading to injury. Moreover, when proprioception is assessed with active repositioning of the limb, it is uncertain whether altered position sense is from the effect of exercise on the motor system or the somatosensory system. An equally important question is whether one’s ability to process proprioceptive information is altered after sustained muscle activity. To our knowledge, no previous report has evaluated the ability to process somatosensory information following a defined bout of sustained muscle activity while using a dynamic position sense test.
Dynamic position sense is defined as the knowledge of the angular position and velocity of the moving joint obtained through various somatosensory receptors.37 The dynamic position sense of the ankle joint was evaluated by having the subjects extend the metacarpophalangeal (MCP) joint of their index finger (sequential movement) when the ankle was passively rotated through a predetermined target angle at various random angular velocities. To achieve temporal and spatial accuracy of performance, the nervous system must determine the angular position of the joint as well as the velocity at which the ankle is being rotated.9
For example, at slow velocities of movement the CNS can rely on position information and wait until the ankle is at the appropriate target angle. However, at faster velocities of movement, the CNS must process the early velocity information and predict when the ankle will arrive at the target. Eventually, at some peak threshold velocity, the CNS will not have available time to process the faster velocity information, which should lead to consistent overshoot errors. The time associated with this peak threshold velocity provides information about CNS processing.
Within this experimental paradigm the central processing time of the nervous system can be estimated.7,9 The CNS processing time is the time needed by the CNS to process kinematic information (velocity) obtained through proprioceptive input to coordinate the sequential upper extremity movement.9 By subtracting the peak threshold time for accurate dynamic position sense of the ankle from the simple reaction time, the CNS processing time can be estimated.7,9
Accordingly, the purposes of this study were (1) to examine if dynamic position sense during passive ankle motion is altered after a sustained contraction, and (2) to determine if CNS processing is altered by the fatiguing exercise. We hypothesized that the proprioceptive sensory systems and the associated CNS processing times are minimally influenced by sustained exercise of the dorsiflexor muscles when tested under these isolated dynamic conditions.
METHODS
A convenience sample of 19 healthy subjects (6 males, 13 females; mean age ± SD, 25.7 ± 5.6 years; mean height ± SD, 1.8 ± 0.1 m; mean body mass ± SD, 75 ± 8.2 kg) participated in this study. Subjects signed informed consent forms after being provided with a brief description of the study. The exclusion criteria for this study (determined by verbal report) included any previous history of ankle joint trauma, neurological deficits, significant degenerative joint diseases, rheumatologic diseases, and restricted joint mobility. The Human Subjects Institutional Review Board at The University of Iowa approved this research.
Experimental Task
An isokinetic dynamometer (Kincom 125 E Plus; Chattecx Corporation, Chattanooga, TN) was used to passively rotate the ankle through the defined range from 10° of dorsiflexion to 30° of plantar flexion (40° range) at 10 random velocities ranging from 10°/s to 90°/s. The predetermined target angle was set at 10° of plantar flexion (midway through the range). The motor task of the subject was to indicate the position of the target angle during passive ankle plantar flexion at various velocities of rotation by extending the MCP joint of the index finger. Each subject was seated upright in the isokinetic machine, with the knee flexed to 70° and the ankle strapped comfortably to the footplate using Velcro straps across the dorsal surface of the forefoot. A potentiometer affixed to the index finger recorded MCP extension. The subjects’ pronated forearm rested on an adjustable table by their side, with the palm on a wooden board and the index finger in an elastic loop connected to the lever of the potentiometer. The ankle was returned to the initial dorsiflexed position at a constant velocity of 10°/s for all trials.
Experimental Procedure
Before starting the experimental trials, the subjects were provided with 20 practice trials at constant velocities of 20°/s and 70°/s. Repetitions 1 through 5 ensured that each subject was familiarized with the task through the provision of online visual and verbal feedback. Visual feedback of passive ankle motion, position of target angle, and active MCP extension were given through a computer that was interfaced with the dynamometer and potentiometer (Figure 1). Subjects could see where the target angle was on the computer screen in reference to ankle displacement and thus coordinate their MCP extension accordingly. Verbal feedback consisted of 1 of 3 cues—“early,” “good,” or “late”—provided by the experimenters after the subjects completed each trial. This gave the subjects additional information other than what they perceived with visual feedback. During repetitions 6 through 10, each subject received visual and verbal feedback after each trial. As such, they were provided with knowledge of results after they completed the task. For the last 10 practice trials, the subjects received only verbal feedback and no visual feedback after each trial.
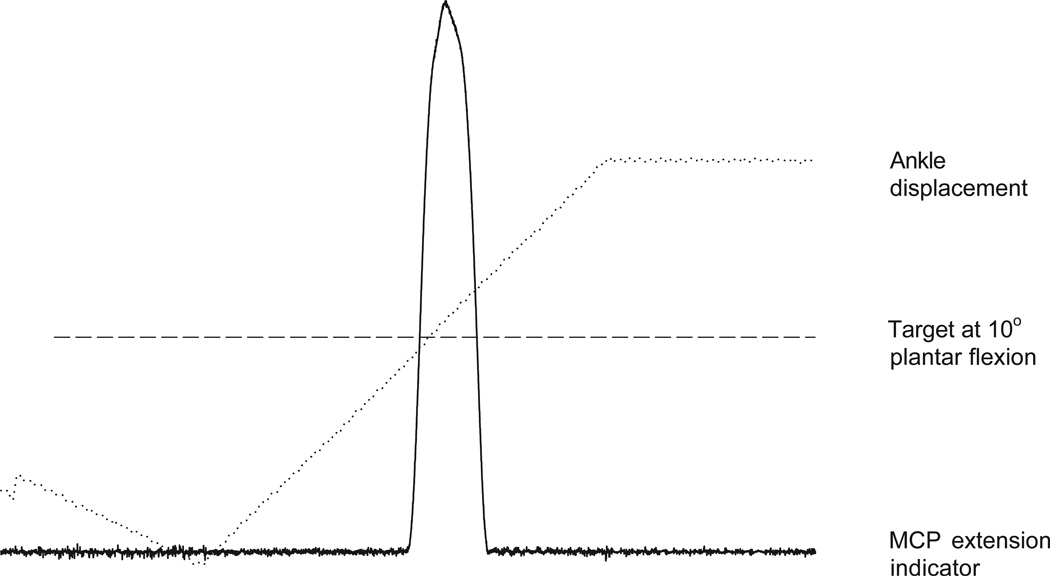
Figure 1.
Representative example of a single subject showing ankle displacement of 40°, target angle midway through the range, and metacarpophalangeal (MCP) extension indicator. Notice that this subject was slightly early in indicating the target location.
Twenty additional trials for the pre-exercise dynamic position sense testing followed the practice trials. These 20 trials were delivered at 10 random velocities of ankle rotation (2 trials at each velocity). No visual or verbal feedback or knowledge of results was provided. Blinders and a noise-reducing headset were provided to eliminate vision and sound. The subjects had to rely on ankle proprioception only to perform the task.
After the experimental trials, a subset of 10 subjects (mean ± SD age, 24.8 ± 4.5 years) was tested to estimate their reaction times. The reaction time was the time it took the subject to trigger index finger MCP extension in response to the start of passive ankle movement. The reaction time measure has been consistently faster than the time required to trigger at the target angle,8,33 indicating that subjects strive to process the sensory information when the goal is to be accurate with a sequential movement (extend the index finger).
After completing the target trials, each subject performed a maximal voluntary isometric contraction of the dorsiflexors at a neutral ankle position, while still seated in the same position. The force produced during the maximal voluntary isometric contraction was recorded through a force transducer. After maximal torque was determined, 50% of maximal torque was calculated and displayed visually to the subjects via an oscilloscope. As a standardized bout of exercise, subjects were instructed to maintain 50% isometric torque for as long as possible. Termination of the exercise occurred when subjects could no longer maintain 50% of maximal torque for approximately 3 seconds. Subjects maintained the isometric contraction for an average ± SD of 185 ± 70 seconds. The postexercise dynamic position sense testing occurred immediately after the completion of the exercise, using the same methods as the pre-exercise testing.
Signal Processing
A 12-bit resolution A–D converter was used to digitally sample all signals (ankle displacement, ankle velocity, and finger potentiometer). All data were sampled at a rate of 1000 Hz. Data were collected using Datapac 2000 software (RUN Technologies, Laguna Hills, CA). The dependent variables were time of indication (MCP extension), time to target, constant error, and absolute error. The time of indication (milliseconds) was calculated as the time from the beginning of ankle downward displacement (plantar flexion) until the beginning of MCP extension. MCP extension was standardized across subjects to indicate when the position of the joint was 5 SD above the position of the joint at baseline. The time to the target (milliseconds) was calculated as the time from the start of ankle plantar flexion to the time the target angle was reached. Ratios for time of indication and time to target were also calculated. Spatial error estimates were calculated using constant and absolute error measurements. Constant error was the average ankle angle (2 trials per velocity) at the time of indication. In constant error estimates, the overshoot angle and undershoot angle may cancel out each other because positive and negative angles are included when averaging. Absolute error, however, was the measure of the overall accuracy of performance because the absolute value of the angle error was averaged.
Statistical Analysis
Statistical analysis was performed using SAS for Windows, Version 7 (SAS Institute, Cary, NC). Repeated measures analysis of variance was used to test for significant differences in the time of indication, constant error, and absolute error measures between the pre-exercise and postexercise conditions. After testing for significant interaction, main effects and simple effects analyses were carried out. Results of all analyses were considered significant at P≤.05. Reliability of the time of indication and time to target was established between repeated trials at each velocity. There were no significant systematic differences between repeated trials for the time of indication (P = .21) and time to target (P = .32). The Pearson correlations were 0.90 and 0.99 for repeated measures for time of indication and time to target, respectively. Intraclass correlations (ICC3,1) were 0.87 and 0.95 for repeated measures for time of indication and time to target, respectively.
RESULTS
Data acquired from a representative trial is shown in Figure 1. The plantar flexion angle trace depicts the change in ankle displacement imposed by the isokinetic dynamometer as the ankle was passively rotated from 10° of dorsiflexion to 30° of plantar flexion (40° range) at a velocity of 20°/s. The horizontal dotted line represents the target angle. The bottom trace depicts the extension of the MCP joint of the index finger. Note that the subject was early in triggering for the target angle in this example.
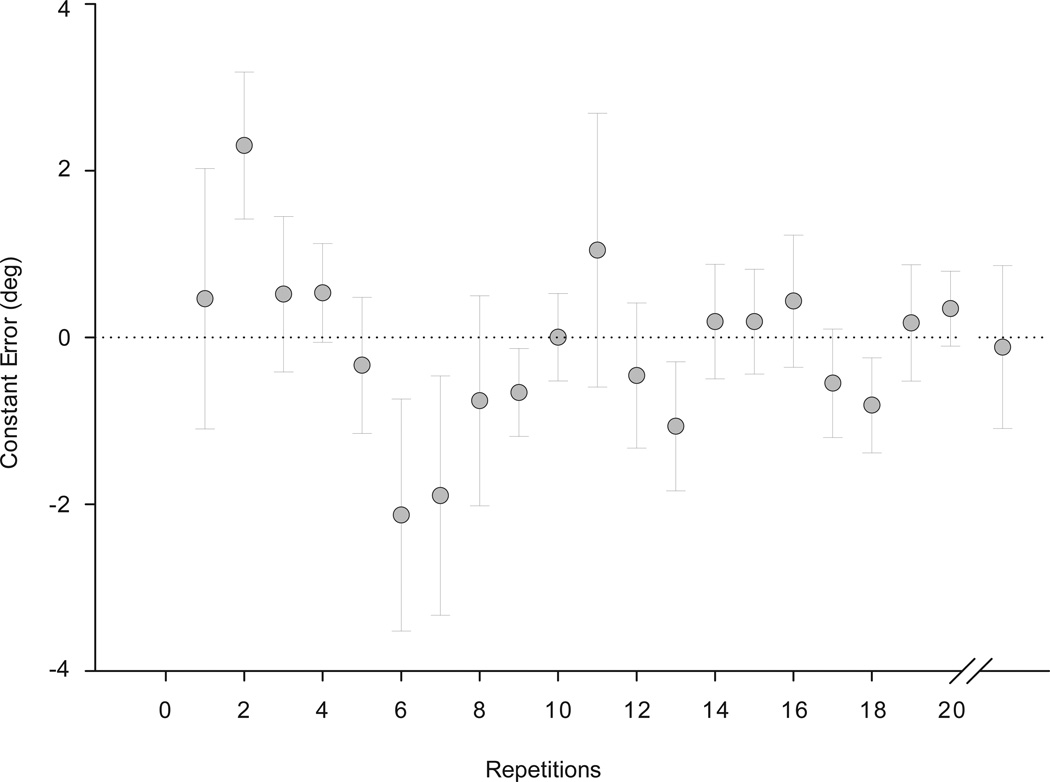
At the end of the practice trials at 20°/s velocity, the constant error decreased to less than 1°, demonstrating that the subjects understood the task (Figure 2). Even as the subjects progressed to the random velocities, the 20°/s trial (shown after repetition 20 in Figure 2) constant error was still within 1°. This verified that the subjects understood the task and had developed a level of competency with the task.
Figure 2.
Mean constant error values of the 19 subjects during the practice trials at a constant velocity of 20°/s. Data points are means of all subjects and error bars are standard errors. Repetitions 1, 6, and 11 were conversion zones for change in feedback. The last data point on the graph is the mean (standard error) of the target trials at the random velocity of 20°/s.
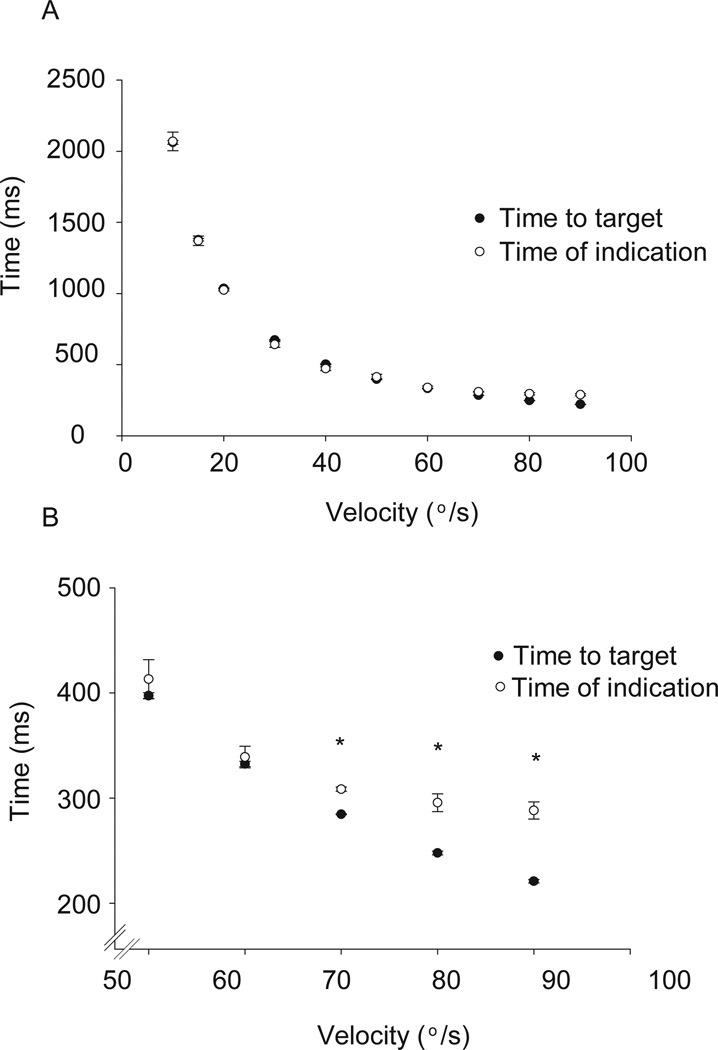
The postexercise time to target and time of indication are plotted as a function of velocity of ankle rotation (Figure 3). Both pre-exercise and postexercise conditions showed a strong agreement between the time that the ankle took to reach the target and the time that the subject indicated with the index finger (time of indication) when the ankle was in the target position. A comparison of the times of indication across ankle angular velocities revealed that subjects significantly decreased (P<.05) the times from velocities of 10°/s to 60°/s both before and after the fatigue protocol, which supported that the subjects scaled their response (decreased their time of indication to the target angle) as the velocity increased. However, at 70°/s and faster, subjects were unable to scale the response (decrease their time of indication) and therefore consistently overshot the target (Figure 3B). There was no significant difference in the time of indication for velocities of 70°/s, 80°/s, and 90°/s (P = .25).
Figure 3.
Temporal accuracy of performance of the postexercise condition. The time to target (closed circles) and time of indication (open circles) are plotted as a function of velocity of ankle rotation. Figure 3A shows all 10 velocities and 3B is the magnification of only faster velocities. Data points are means of all subjects while error bars are standard errors. Because the pre-exercise and postexercise conditions displayed similar trends, only the postexercise condition is shown here. Notice the time of indication (open circles) does not change with the time to target (closed circles) (3B). *Times of indication at 70°/s, 80°/s, and 90°/s are not different, but all are less than times of indication for 50°/s and 60°/s (P<.05).
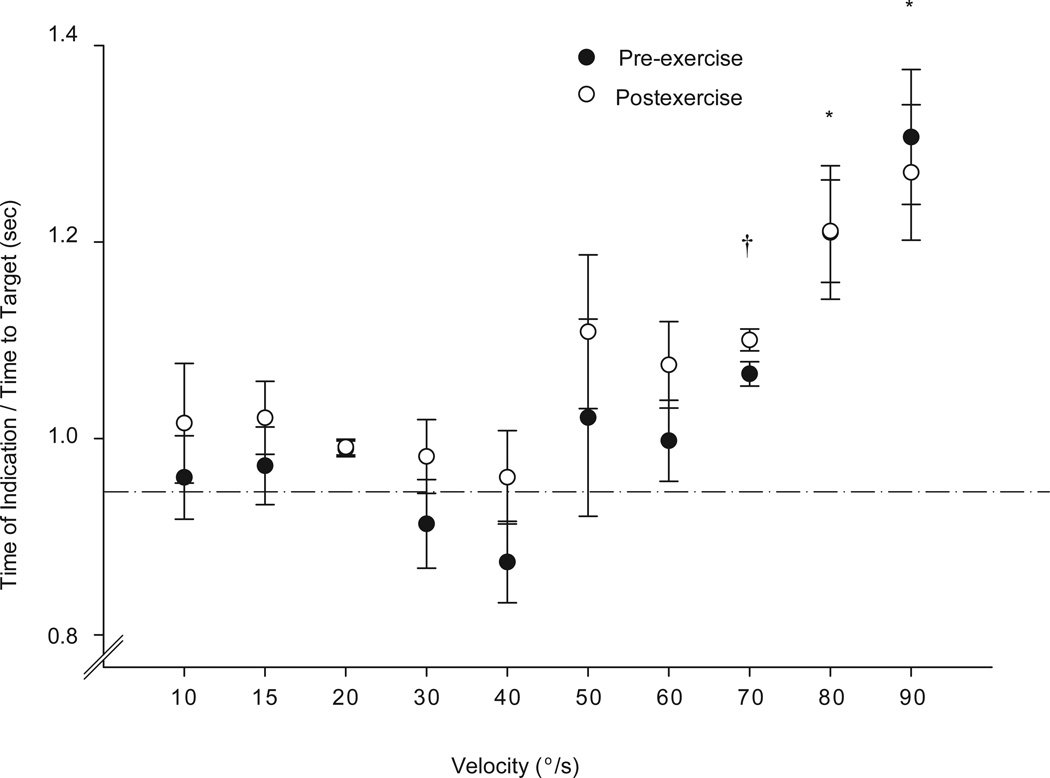
A direct comparison between the pre-exercise and postexercise conditions is presented by taking the ratio (time of indication/time to target) for the pre-exercise and postexercise conditions (Figure 4). A ratio of 1 indicates accuracy in temporal performance of the task. A 2-way repeated-measures ANOVA, with session and velocity as the 2 factors, was performed to compare the ratios before and after the exercise sessions. There was no significant interaction, indicating that the ratios were similar during the preexercise and postexercise conditions (P = .24). Main effects analysis indicated that there was no statistical difference between the pre-exercise and postexercise sessions for the ratios (P = .32). However, at the slower velocities, the ratios were closer to 1 in both pre-exercise and postexercise conditions, but progressively increased (P<.0001) at velocities higher than 70°/s (Figure 4). Post hoc contrasts indicated that ratios at 70°/s were significantly greater (P<.05) than ratios from 10°/s to 40°/s, and ratios at 80°/s and 90°/s were significantly greater than ratios from 10°/s to 70°/s (P<.05). This is consistent with the lower time of indication times observed with increasing velocity presented in Figure 3B.
Figure 4.
The ratios (time of indication/time to target) for the pre-exercise and postexercise conditions are shown as a function of velocity. A ratio of 1 indicates accuracy of performance of the task. Data points are means of all subjects while error bars are standard errors. There were no differences between the pre-exercise and postexercise conditions. *Ratios at 80°/s and 90°/s were significantly greater than ratios from 10°/s to 70°/s (P<.05).†Ratios at 70°/s were significantly (P<.05) greater than ratios from 10°/s to 40°/s.
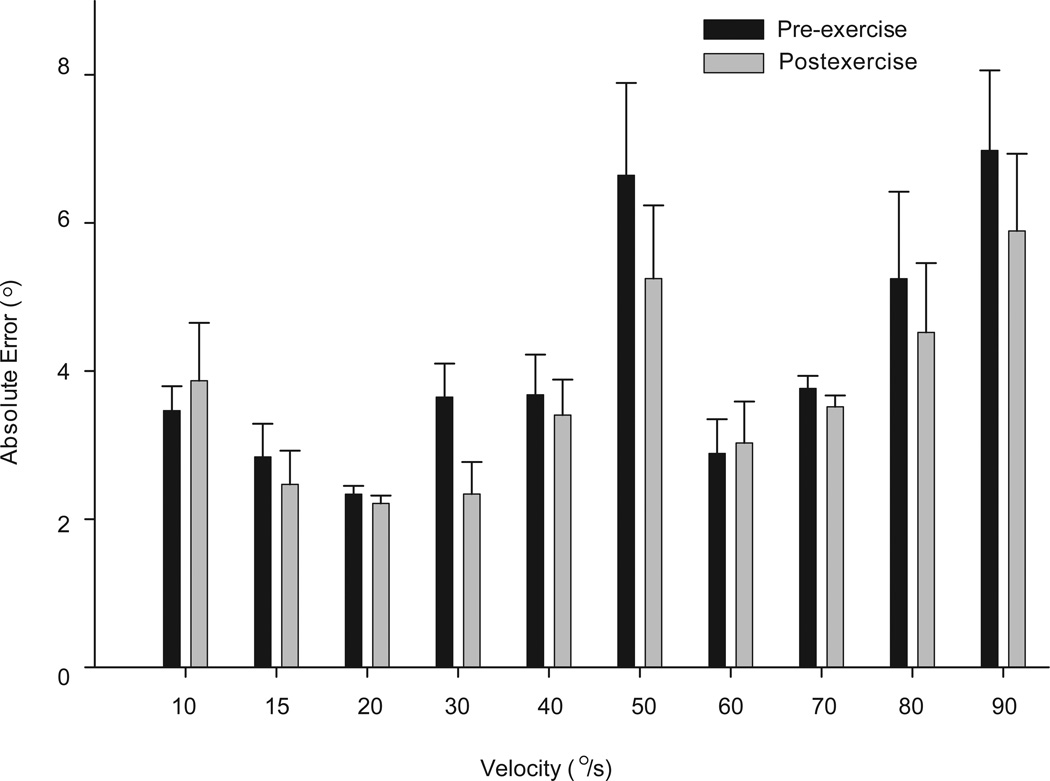
A 2-way repeated-measures ANOVA on the absolute errors of performance during the target trials showed that as the velocity of ankle plantar flexion increased, the absolute error increased for both exercise conditions (P<.001); but there was no significant interaction indicating that the error response was consistent between the pre-exercise and postexercise conditions (P = .07) (Figure 5). A power analysis indicated that this study was adequately powered (.82) based on an expected 15% difference in mean errors before versus after exercise.
Figure 5.
Absolute errors of performance of the target trials of the pre-exercise and postexercise conditions are compared. There was no significant exercise effect (P = .07). The greatest overall error occurred at 50°/s and 90°/s (P<.05). Bar graphs are means of all subjects while error bars are standard errors.
On average (±SD), the time of indication was approximately 300 ± 15 milliseconds at the higher velocities as the subjects attempted to interpret the early ankle movement to predict when the ankle target angle would be reached (Figure 3B). However, when a subset of the same subjects (n = 10) was asked to extend their index finger at the start of ankle movement (simple reaction time), rather than trying to interpret angular information to indicate the target angle, they could respond much more quickly (mean ± SD, 215 ± 35 milliseconds; P<.05). Accordingly, the difference between the reaction time (215 milliseconds) and the time necessary to process the ankle position in order to indicate when the target was reached (300 milliseconds) is the estimated time necessary for the CNS to process the proprioceptive information (mean ± SD, 85 ± 22 milliseconds). This was the minimum amount of time needed by the CNS to estimate the rate of ankle rotation, set the triggering angle, and send commands to extend the index finger.
DISCUSSION
In this study, a proprioceptive task was used to assess dynamic position sense during passive ankle motion after a bout of sustained muscle activity of the ankle dorsiflexors. To perform the task accurately, the CNS has to take into account both joint position and velocity of the ankle during joint rotation.7,9,10 Using time of indication as the dependent variable to assess ankle dynamic position sense helped to isolate the role of the somatosensory system without involving the motor component.
As shown by the low constant error at the end of the practice trials, this task was easy to learn. During the subsequent target trials, in both pre-exercise and postexercise conditions, the subjects were able to adjust their time of indication according to the velocity of joint rotation more so at the slower velocities. At the faster velocities (70°/s, 80°/s, and 90°/s) the ability to scale the response was diminished for both the pre-exercise and postexercise conditions (Figure 3B). This was also reflected in the ratios for the time of indication and time to target at these various velocities (Figure 4).
No significant change in absolute errors was seen after exercise. The ability to centrally process somatosensory information at the ankle appeared unchanged after exercise. If the central processing time had been delayed after a bout of exercise, the subject would then need a longer amount of time to perform the MCP extension using proprioceptive information from the ankle joint. Hence we would anticipate that the subjects would stop scaling their MCP extension responses at an earlier velocity (60°/s or earlier). Both before and after exercise, the CNS required approximately 85 milliseconds of central processing time to perform this task accurately.
Among all the peripheral somatosensory receptors, the muscle spindles are the most sensitive in detecting changes in muscle length and velocity.8,18 They are the most important contributors of afferent somatosensory information regarding ankle dynamic position sense.8 In this study, the muscle spindles of the ankle dorsiflexors would play an important role in ankle kinesthesia and have the potential to increase their sensitivity by increasing central drive to the gamma system. The gains of other afferent receptors, like the Golgi tendon receptors, Pacinian corpuscles, or Ruffinni nerve endings, are not modulated by central commands.17 No significant difference between the pre-exercise and postexercise conditions in this study suggests that the short bout of exercise (3 to 6 minutes) did not alter muscle spindle sensitivity.
The finding that sustained isometric exercise minimally affected dynamic position sense is inconsistent with some previous studies. Roberts et al30 reported that a moderate bout of cycling exercise led to impaired knee joint proprioception, as tested using threshold to detection of joint motion. Bartlett et al2 reported static joint position sense of the knee improved after a bout of warming-up exercise. Bouet and Gahery5 showed that the accuracy of active knee joint repositioning improves with moderate muscular exercise. Several plausible explanations may contribute to the discrepancy between the results of this study and previous reports. First, the previous studies examined static joint position sense and not dynamic position sense to assess joint proprioception. Second, previous reports included the motor system during active repositioning of the limb while testing proprioception, which may reflect a motor impairment attributed to the exercise rather than a sensory deficit. The use of a sequential upper extremity movement task, triggered via dynamic position sense from a lower extremity joint (ankle), is a novel approach to capture how someone uses proprioceptive information rather than merely recognizing the position of a joint.
Due to the frequent occurrence of ankle injuries in athletic activities, there is a growing need to prevent such injuries through education and rehabilitation programs.22 The current trend is to utilize balance exercises of increasing challenge to improve proprioception at the ankle joint. These exercises usually involve bipedal and unipedal balance tasks on different types of surfaces (firm floors, foams of various thicknesses, etc), and use of exercise bands, trampolines, and balance boards.12,14 There is no question that such exercises assist with balance control by improving motor performance, but there is no definite evidence that they specifically improve ankle proprioception.1 This may be because most of these studies utilize a motor task to evaluate the results of sensory training.21,29,32 Improvement in performance of balance tasks in standing with training could mean that there is an improvement in muscle strength and increased coordination between various sensory systems (vestibular and visual); however, it may not necessarily indicate enhanced joint proprioception. A future challenge would be to investigate if a longterm proprioceptive exercise, prescribed by physical therapists and trainers, would cause changes in the speed at which proprioceptive information is processed centrally.
It is important to note that this study examined the effects of localized muscle activity and therefore cannot address the effects of systemic cardiovascular exercise on the ability to process proprioceptive information. This point is important because central fatigue may be one of the primary contributors to injury in athletics. However, the period of recovery during the testing in this study is similar to brief resting periods seen in athletic competition. Accordingly, future studies addressing the effects of longterm aerobic exercise on central processing of proprioceptive information are warranted.
CONCLUSION
Our results suggest that a sustained activation of the dorsiflexor muscles minimally influences dynamic position sense and the ability to process dynamic position sense information from the ankle. The use of a sequential upper extremity task triggered via dynamic position sense of the ankle is a novel approach to examine the sensory component of the nervous system. Understanding the impact of exercise on sensory system processing will be integral to establishing the scientific basis for rehabilitation programs that purport to train proprioception.
Acknowledgments
Dr Shields is supported by NIH R01HD39445. The Human Subjects Institutional Review Board at The University of Iowa approved this research.
REFERENCES
- 1.Ashton-Miller JA, Wojtys EM, Huston LJ, Fry-Welch D. Can proprioception really be improved by exercises? Knee Surg Sports Traumatol Arthrosc. 2001;9:128–136. doi: 10.1007/s001670100208. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett MJ, Warren PJ. Effect of warming up on knee proprioception before sporting activity. Br J Sports Med. 2002;36:132–134. doi: 10.1136/bjsm.36.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birrer RB, Fani-Salek MH, Totten VY, Herman LM, Politi V. Managing ankle injuries in the emergency department. J Emerg Med. 1999;17:651–660. doi: 10.1016/s0736-4679(99)00060-8. [DOI] [PubMed] [Google Scholar]
- 4.Bjorklund M, Crenshaw AG, Djupsjobacka M, Johansson H. Position sense acuity is diminished following repetitive low-intensity work to fatigue in a simulated occupational setting: a critical comment. Eur J Appl Physiol. 2003;88:485–486. doi: 10.1007/s00421-002-0753-7. [DOI] [PubMed] [Google Scholar]
- 5.Bouet V, Gahery Y. Muscular exercise improves knee position sense in humans. Neurosci Lett. 2000;289:143–146. doi: 10.1016/s0304-3940(00)01297-0. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter JE, Blasier RB, Pellizzon GG. The effects of muscle fatigue on shoulder joint position sense. Am J Sports Med. 1998;26:262–265. doi: 10.1177/03635465980260021701. [DOI] [PubMed] [Google Scholar]
- 7.Cordo P, Bevan L, Gurfinkel V, Carlton L, Carlton M, Kerr G. Proprioceptive coordination of discrete movement sequences: mechanism and generality. Can J Physiol Pharmacol. 1995;73:305–315. doi: 10.1139/y95-041. [DOI] [PubMed] [Google Scholar]
- 8.Cordo P, Carlton L, Bevan L, Carlton M, Kerr GK. Proprioceptive coordination of movement sequences: role of velocity and position information. J Neurophysiol. 1994;71:1848–1861. doi: 10.1152/jn.1994.71.5.1848. [DOI] [PubMed] [Google Scholar]
- 9.Cordo P, Gurfinkel VS, Bevan L, Kerr GK. Proprioceptive consequences of tendon vibration during movement. J Neurophysiol. 1995;74:1675–1688. doi: 10.1152/jn.1995.74.4.1675. [DOI] [PubMed] [Google Scholar]
- 10.Cordo PJ. Kinesthetic coordination of a movement sequence in humans. Neurosci Lett. 1988;92:40–45. doi: 10.1016/0304-3940(88)90739-2. [DOI] [PubMed] [Google Scholar]
- 11.Davey PR, Thorpe RD, Williams C. Fatigue decreases skilled tennis performance. J Sports Sci. 2002;20:311–318. doi: 10.1080/026404102753576080. [DOI] [PubMed] [Google Scholar]
- 12.Eils E, Rosenbaum D. A multi-station proprioceptive exercise program in patients with ankle instability. Med Sci Sports Exerc. 2001;33:1991–1998. doi: 10.1097/00005768-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Fahlstrom M, Bjornstig U, Lorentzon R. Acute Achilles tendon rupture in badminton players. Am J Sports Med. 1998;26:467–470. doi: 10.1177/03635465980260032201. [DOI] [PubMed] [Google Scholar]
- 14.Freeman MA. Co-ordination exercises in the treatment of functional instability of the foot. Physiotherapy. 1965;51:393–395. [PubMed] [Google Scholar]
- 15.Gabbett TJ. Incidence of injury in amateur rugby league sevens. Br J Sports Med. 2002;36:23–26. doi: 10.1136/bjsm.36.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 17.Gandevia SC, Burke D. Does the nervous system depend on kinesthetic information to control natural limb movements? Behav Brain Res. 1992;15:614–632. [Google Scholar]
- 18.Gandevia SC, McCloskey DI. Joint sense, muscle sense, and their combination as position sense, measured at the distal interphalangeal joint of the middle finger. J Physiol. 1976;260:387–407. doi: 10.1113/jphysiol.1976.sp011521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gribble PA, Hertel J. Effect of lower-extremity muscle fatigue on postural control. Arch Phys Med Rehabil. 2004;85:589–592. doi: 10.1016/j.apmr.2003.06.031. [DOI] [PubMed] [Google Scholar]
- 20.Gurney B, Milani J, Pedersen M. Role of fatigue on proprioception of the ankle. J Exerc Physiol [online serial] 2000;3 [Google Scholar]
- 21.Hoffman M, Payne VG. The effects of proprioceptive ankle disk training on healthy subjects. J Orthop Sports Phys Ther. 1995;21:90–93. doi: 10.2519/jospt.1995.21.2.90. [DOI] [PubMed] [Google Scholar]
- 22.Holmer P, Sondergaard L, Konradsen L, Nielsen PT, Jorgensen LN. Epidemiology of sprains in the lateral ankle and foot. Foot Ankle Int. 1994;15:72–74. doi: 10.1177/107110079401500204. [DOI] [PubMed] [Google Scholar]
- 23.Horak FB, Hlavacka F. Somatosensory loss increases vestibulospinal sensitivity. J Neurophysiol. 2001;86:575–585. doi: 10.1152/jn.2001.86.2.575. [DOI] [PubMed] [Google Scholar]
- 24.Lattanzio PJ, Petrella RJ. Knee proprioception: a review of mechanisms, measurements, and implications of muscular fatigue. Orthopedics. 1998;21:463–470. doi: 10.3928/0147-7447-19980401-19. discussion 470–461. [DOI] [PubMed] [Google Scholar]
- 25.Lee HM, Liau JJ, Cheng CK, Tan CM, Shih JT. Evaluation of shoulder proprioception following muscle fatigue. Clin Biomech (Bristol, Avon) 2003;18:843–847. doi: 10.1016/s0268-0033(03)00151-7. [DOI] [PubMed] [Google Scholar]
- 26.Ljubisavljevic M, Anastasijevic R. Fusimotor system in muscle fatigue. J Peripher Nerv Syst. 1996;1:83–96. [PubMed] [Google Scholar]
- 27.Mohr M, Krustrup P, Bangsbo J. Match performance of high-standard soccer players with special reference to development of fatigue. J Sports Sci. 2003;21:519–528. doi: 10.1080/0264041031000071182. [DOI] [PubMed] [Google Scholar]
- 28.Pedersen J, Lonn J, Hellstrom F, Djupsjobacka M, Johansson H. Localized muscle fatigue decreases the acuity of the movement sense in the human shoulder. Med Sci Sports Exerc. 1999;31:1047–1052. doi: 10.1097/00005768-199907000-00019. [DOI] [PubMed] [Google Scholar]
- 29.Refshauge KM, Taylor JL, McCloskey DI, Gianoutsos M, Mathews P, Fitzpatrick RC. Movement detection at the human big toe. J Physiol. 1998;513(Pt 1):307–314. doi: 10.1111/j.1469-7793.1998.307by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts D, Ageberg E, Andersson G, Friden T. Effects of short-term cycling on knee joint proprioception in healthy young persons. Am J Sports Med. 2003;31:990–994. doi: 10.1177/03635465030310064001. [DOI] [PubMed] [Google Scholar]
- 31.Sharpe MH, Miles TS. Position sense at the elbow after fatiguing contractions. Exp Brain Res. 1993;94:179–182. doi: 10.1007/BF00230480. [DOI] [PubMed] [Google Scholar]
- 32.Sheth P, Yu B, Laskowski ER, An KN. Ankle disk training influences reaction times of selected muscles in a simulated ankle sprain. Am J Sports Med. 1997;25:538–543. doi: 10.1177/036354659702500418. [DOI] [PubMed] [Google Scholar]
- 33.Shields RK, Madhavan S, Cole KR, et al. Proprioceptive coordination of movement sequences in humans. Clin Neurophysiol. 2005;116:87–92. doi: 10.1016/j.clinph.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 34.Smith AM, Stuart MJ, Wiese-Bjornstal DM, Gunnon C. Predictors of injury in ice hockey players. A multivariate, multidisciplinary approach. Am J Sports Med. 1997;25:500–507. doi: 10.1177/036354659702500413. [DOI] [PubMed] [Google Scholar]
- 35.Sterner RL, Pincivero DM, Lephart SM. The effects of muscular fatigue on shoulder proprioception. Clin J Sport Med. 1998;8:96–101. doi: 10.1097/00042752-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Thelen DG, Brockmiller C, Ashton-Miller JA, Schultz AB, Alexander NB. Thresholds for sensing foot dorsiand plantarflexion during upright stance: effects of age and velocity. J Gerontol A Biol Sci Med Sci. 1998;53:M33–M38. doi: 10.1093/gerona/53a.1.m33. [DOI] [PubMed] [Google Scholar]
- 37.Verschueren SM, Brumagne S, Swinnen SP, Cordo PJ. The effect of aging on dynamic position sense at the ankle. Behav Brain Res. 2002;136:593–603. doi: 10.1016/s0166-4328(02)00224-3. [DOI] [PubMed] [Google Scholar]