Abstract
Vascular smooth muscle cells (VSMCs) are derived from distinct embryonic origins. Vessels originating from differing smooth muscle cell populations have distinct vascular and pathological properties involving calcification, atherosclerosis, and structural defects such as aneurysm and coarctation. We hypothesized that domains within a single vessel, such as the aorta, vary in phenotype based on embryonic origin. Gene profiling and myographic analyses demonstrated that embryonic ascending and descending aortic domains exhibited distinct phenotypes. In vitro analyses demonstrated that VSMCs from each region were dissimilar in terms of cytoskeletal and migratory properties, and retention of different gene expression patterns. Using the same analysis, we found that these same two domains are indistinguishable in the adult vessel. Our data demonstrate that VSMCs from different embryonic origins are functionally distinct in the embryonic mouse, but converge to assume a common phenotype in the aorta of healthy adults. These findings have fundamental implications for aortic development, function and disease progression.
Keywords: Smooth muscle, neural crest, mesoderm, vasculature
1. Introduction
Heterogeneity of blood vessels is intrinsic and critical to cardiovascular function. Differences in vascular structure are reflected in the three layers comprising definitive arteries and veins (from lumen outward): the tunica intima, media, and adventitia. For example, regional variation in endothelial cells of the tunica intima regulates vessel permeability [1], while great variability in connective tissue elements has been noted along the entirety of the vascular tree [2, 3]. Significant heterogeneity in structure and function has been noted for the tunica media [4, 5], whose major cellular components are vascular smooth muscle cells (VSMCs) or pericytes. In the large conducting arteries of the cardiac outflow tract, this layer contains significant amounts of elastin contributing to the distensible properties that are essential for reacting to blood volume and pressure changes during the cardiac cycle [6]. Conversely, medium-sized muscular arteries (e.g. coronary and mesenteric vessels) contain less elastin but are enriched in VSMCs critical for regulation of vessel diameter and distribution of blood to specific tissues of the body. In addition to differences in their basic morphology, these vessels have distinct susceptibility to diseases often linked to the tunica media [2, 3, 7–17].
VSMC progenitors arise from distinct embryonic sources including splanchnic mesoderm [18], somitic mesoderm [19], neural crest (NC) [20, 21], mesothelia, [22] and others [23, 24]. Interestingly, differences in VSMC characteristics such as gene expression have been attributed to their embryonic origin [25]. Also, studies suggest that when vessels prone to atherosclerosis are placed in a vascular region that does not typically develop atherosclerosis, they retain their predisposition to disease [26]. Additionally, differentiation of VSMCs from embryonic stem cells through NC- or mesoderm-lineages provide evidence that VSMC characteristics are programmed based on embryonic origin [13]. These studies suggest that individual VSMC characteristics are strongly determined by embryonic origin. However, definitive evidence that embryonic origin dictates vascular phenotype has not been provided [23].
While different vessels arise from disticnt embryonic origins, the proximal aorta is particularly interesting because its VSMCs arise from two distinct embryonic origins: NC and somitic mesoderm [19, 21]. In the mouse, at 9.5 days post coitum (dpc), NC cells migrate from the dorsal neural tube and contribute to the pharyngeal arch arteries and aortic sac. At 12.5 dpc, NC facilitates a complex septation and remodeling of the outflow tract and pharyngeal arteries resulting in the pulmonary trunk and the aorta [27]. After outflow tract septation and remodeling, a separate population of NC cells invest the vessel and differentiate into VSMCs [20]. At 9.0 dpc, cells from lateral plate mesoderm begin to migrate around the ventral side of descending aorta [19]. By 9.5 dpc, cells from somitic mesoderm begin populating the dorsal side of the descending aorta [19]. At 11.5 dpc, the entire descending aorta is ensheathed by VSMCs derived from the somitic mesoderm. The border that forms between the NC-derived VSMCs of the ascending aortic arch (aAo) and mesoderm-derived VSMCs of the descending aorta (dAo) is maintained throughout development and into adulthood [20, 21]. This juxtaposition of VSMCs from different embryonic origins in the aorta affords the opportunity to test the hypothesis that embryonic origin of VSMCs relates to the vascular function of distinct regions within a single vessel.
Not only are the embryonic origins of these VSMC populations distinct, but the regions of the vasculature are differentially susceptible to vascular disease, namely atherosclerosis and calcification of the aorta. Transplantation studies in which an atherosclerotic-prone vessel was placed in a region of the vasculature that does not develop atherosclerosis demonstrated that the propensity to develop atherosclerosis is intrinsic to the vessel itself [26]. Furthermore, under ex vivo calcifying conditions, the ascending aorta calcified much more rapidly compared to the descending aorta [16]. Even if VSMCs were isolated from the ascending aorta, they retained this propensity to calcify, linking this condition to VSMC biology. These data support our hypothesis that VSMCs from different regions of the vasculature are intrinsically different and have the potential to contribute to disease.
Additionally, in human patients, the aortic border between the NC- and somitic mesoderm-derived VSMCs is the location of several cardiovascular defects: coarctation of the aortic arch, interrupted aortic arch type A, and aortic aneurism. Coarctation of the aorta is a narrowing of the aorta at the level of the ductus arteriosus [28]. Interrupted aortic arch type A is a complete discontinuation of the aortic lumen distal to the left subclavian artery. Aortic aneurisms are dilations of the aorta, which can occur in different regions of the aorta, but are typically found at borders between VSMCs of different embryonic origins [29]. Regardless of the defect, typical treatment involves surgical repair of the aorta. Understanding the biology of these different VSMC populations and their juxtaposition can inform the study and treatment of these human conditions.
Using gene profiling, myography, and cell biological strategies, we demonstrate that NC- and somitic mesoderm-derived VSMCs of the embryonic aorta are significantly different throughout development. Surprisingly, using the same modalities, we demonstrate that the two domains are indistinguishable in the normal, healthy adult. Thus, this unexpected result demonstrates that embryonic origin does not dictate adult phenotype in the specific case of the aorta and has major implications for our understanding of vascular development and disease.
2. Materials and Methods
2.1. Animals
Mice were from a mixed genetic background and maintained in accordance with protocols approved by the Vanderbilt University Institutional Animal Care and Use Committee (IACUC). Timed matings were performed to obtain embryos at specific stages of development. Noon on the day a vaginal plug was observed was considered day 0.5 dpc. The Tg(Wnt1-Cre)11Rth (called Wnt1-Cre) transgenic mouse line was used to lineage-map NC cells with Gt(ROSA)26Sortm1(EYFP)Cos (called R26RYFP). The Tg(H2-K1-tsA5811Kio) line (called Immorto) was used to conditionally immortalize isolated VSMCs. This line expresses a temperature sensitive SV-40 large T antigen in the presence of interferon gamma.
2.2. Microarray
For the microarray conducted on adult tissues, the ascending aorta (aAo), descending aorta (dAo), coronary artery, and mesenteric arteries were isolated from three individual 8 week-old female mice. Tissue was flash frozen prior to homogenization and RNA isolation. Samples were run on the Affymetrix mouse Gene 1.0 ST arrays.
The embryonic microarray was conducted using tissue from 13.5 dpc embryos. To obtain enough tissue without the need to amplify the RNA sample, two biological samples were pooled for each experimental sample. A total of three experimental samples were run on Affymetrix Mouse Exon/Gene (WT) arrays.
Microarray images were scanned with an Affymetrix high resolution GenePix 4000B scanner in the Vanderbilt Functional Shared Resource (http://www.thefgsr.com/). Raw .CEL files were subsequently uploaded into Partek Genomics Suite version 6.6 (Partek Incorporated, St. Louis, MO) and processed using Robust Multi-chip Average (RMA) normalization [30, 31].
Following RMA normalization, Partek analysis software was used to perform pairwise comparisons of average group values and one-way ANOVA for analysis of aAo, dAo, superior mesenteric artery, and coronary artery tissues. Only probes that resulted in a fold-change of at least 1.5 with a Benjamini and Hochberrg (B-H) multiple hypothesis corrected p value of less than 0.05 were considered significantly altered. All six possible individual pairwise comparisons were performed with an expectation of 1.5-fold difference between each comparison. Data were uploaded to the Gene Expression Omnibus (GEO) repository and complied with MIAME standards. The GEO accession numbers are: GSE50250 and GSE50251. To generate the heat map, hierarchical clustering was performed using UPGMA (unweighted pair-group method of average linkage) and Euclidian distance as the dissimilarity measure in Partek.
2.3. Real Time PCR
Quantitative reverse transcription PCR (qRT-PCR) was completed to validate the expression of genes identified from the microarray. RNA samples were harvested from the aAo and dAo of 13.5, 15.5, and 17.5 dpc embryos, 8 week-old adults and cell lines. In all cases, RNA was isolated using Qiagen RNeasy Mini Kits (74104). Ambion Turbo DNase (AM2238) was used to remove any DNA from the samples. Reverse transcription was done with the Invitrogen SuperScript III First-Strand Synthesis System (18080-051). qRT-PCR was performed using Promega Gotaq qPCR master Mix (A6001) on Bio-Rad CFX96 Detection System. Primers are displayed in Supplemental Table 1. All experiments were run in duplicate, and the relative amount of RNA was determined by comparison with Hypoxanthine guanine phosphoribosyl transferase (Hprt) mRNA. Student’s paired t-tests were used to determine statistical significance. N=5.
2.4. Myography
For fetal studies, pregnant females were anesthetized by intraperitoneal injection of 0.4 ml 2.5% Avertin (2,2,2-tribromoethanol in tert-amyl alcohol; Sigma-Aldrich, St Louis, MO), followed by isoflurane inhalation (Baxter, Deerfield, IL) to facilitate fetal anesthesia.
Anesthetized fetuses were submerged in ice-cold, deoxygenated (95% N2, 5% CO2) Krebs buffer. Krebs buffer was modified (109 mM NaCl, 34 mM NaHCO3, 4.7 mM KCl, 0.9 mM MgSO4, 1.0 mM KH2PO4, 11.1 mM dextrose, and 2.5 mM CaCl2) to maintain stable pH (7.30–7.35) and relative hypoxia in the vessel bath (dissolved oxygen content = 1.5–1.8%; measured pO2 = 38–45 Torr). Aortae were dissected from 15.5 dpc (n=8) and 19.5 dpc fetuses (n=10) and transferred to custom microvessel perfusion chambers (Instrumentation and Model Facility, The University of Vermont, Burlington, VT) filled with chilled, deoxygenated Krebs buffer, as described [32]. Adult aortae (n=8) were similarly isolated, then placed in chambers perfused with oxygenated Krebs (21% O2, 5% CO2, balance N2). Brachiocephalic, left carotid, and left subclavian arteries were tied off at their base; the ductus arteriosus was tied off in fetal tissues. The excised vessel preparation was positioned and secured on ~120 µm (fetal) to ~500 µm (adult) pipette tips.
Once mounted, the perfusion chamber was placed on an inverted microscope equipped with a video camera and an image-capture system (IonOptix, Milton, MA) to continuously record the intraluminal diameter of both the aAo and dAo simultaneously. Optical markers used to detect the lumen diameter were positioned at the narrowest point of the constricted vessel. Vessels were allowed to equilibrate at 37 °C for 30–40 minutes, then pressurized in a stepwise manner to 6 mm Hg (preterm), 20 mm Hg (term) or 80 mm Hg (adult). After equilibration, vessels were exposed to 50 mM KCl (×2) to ascertain vessel reactivity. Vasoconstrictive drugs and doses used for the subsequent myography experiments included: the thromboxane receptor agonist U-46619 (10−8 M; Cayman Chemical), phenylephrine (10−5 M, Sigma-Aldrich), endothelin-1 (ET-1; 10−7 M in acetic acid, Bachem). In some studies, the U-46619 preconstricted aorta was exposed to acetylcholine (10−5 M, Sigma-Aldrich) to assess endothelial-dependent vasodilatory responses. Aortae were also exposed to the NO-donor sodium nitroprusside (SNP; 10−5 M, Sigma-Aldrich) and the directacting vasodilator papaverine (10−4 M, Sigma-Aldrich). Drugs were dissolved in Krebs buffer or ethanol, unless otherwise specified. Final solvent concentration in the bath was ≤0.04%. Vessels were exposed to each agent until a stable baseline diameter was reached (typically 20–30 min). Drug concentrations refer to their final molar concentration in the bath. Differences between the aAo and dAo lumen diameter were evaluated by paired Student’s t-test.
2.5. VSMC isolation
Aortae from Immorto 8 week-old adults or 16.5 dpc embryos were dissected to separate the aAo from the dAo. To dissect the aAo, the aorta was cut away from the heart and the arch transected at the level of the left subclavian artery to prevent inclusion of tissue from the dAo (Supplemental Figure 1). YFP fluorescence in the Wnt1-Cre; R26RYFP NC lineage labeled mouse was also used to facilitate clean dissections of the aAo. The dAo was taken from the remaining tissue beginning just distal to the ductus arteriosus in embryos (the ligamentum arteriosum in adults) and continuing until the level of the diaphragm. Adult aortae were processed as previously described [33]. Embryonic aortae were processed by peeling the adventitia away from the vessel. Vessels were then cut open longitudinally and the endothelial cells gently scraped away. The remaining vessel was plated into a four well culture dish and cells migrated away from the explant over one week. The tissue was then discarded and the explanted cells were trypsinized and replated to start cell lines. Cells were cultured in DMEM, high glucose; 10% fetal calf serum, 1% penicillin/streptomycin. Cells were immortalized by changing the culture conditions to 33°C and adding 30 units/mL interferon gamma. Cells were removed from immortalizing conditions 48 hours prior to conducting experiments. Clonal cell lines were derived by dissociating aortic outgrowths with 0.25% trypsin, and plating cells at clonal cell densities. Isolated clones were selected with cloning rings and further propagated. Clonal lines of VSMCs were not cultured past 5 passages in order to ensure retention of smooth muscle characteristics. These cells maintained expression of characteristic VSMC markers smooth muscle actin (SMA) and smooth muscle myosin heavy chain (smMHC) both in the explant and as clonal cell lines (Supplemental Figure 2). Three clonal lines from each region of the aorta were studied for both the embryonic and adult time points.
2.6. Immunofluorescence and Imaging
Cells were fixed with 2% formaldehyde and 0.25% Triton-X in PBS. Antibodies used include: mouse anti- α smooth muscle actin (Sigma, 1:200), rabbit anti-smooth muscle myosin heavy chain (Biomedical Tech, 1:200), mouse anti-vinculin (Sigma, 1:200). Actin filaments were labeled with phalloidin 568nm (Invitrogen, 1:100), and DNA was labeled with DAPI (1:10,000). Imaging of immunofluorescence in isolated cells was performed on an Olympus FV_1000 inverted microscope in the Vanderbilt University Cell Imaging Shared Resource Core. Fluorescent images are z-projections generated using ImageJ software. Quantification of the VSMC migration assay was conducted using ImageJ software. Whole mount imaging of aortae was performed using a Leica M165 FC dissecting microscope.
For quantification of focal adhesion area, fixed VSMCs on glass coverslips were blocked in 5% goat serum and 1% bovine serum albumin for 1 hr at RT. Cells were stained with α-vinculin antibody to label focal adhesions for 1 hr at RT and with anti-mouse IgG 488nm (Invitrogen, 1:500) and DAPI for 45 minutes at RT. Using ImageJ, background fluorescence and diffuse perinuclear labeling were removed using the threshold function. Focal adhesions were selected and analyzed using the “Analyze Particle” function. Focal adhesions were measured for area in pixels for both embryonic and adult VSMC derived lines. These data were analyzed with the Mann-Whitney U test.
2.7. In vitro assays
Boyden chamber assays were completed with 8µm Millicell inserts. 40,000 cells were loaded into each well and four wells were assayed for each of three cell lines derived from the different regions of the aorta from both 16.5 dpc embryos and 8 week-old adults. After 24 hours, the filters were removed and cells on the underside of the filter were visualized by Giemsa staining. The number of cells per area were counted and then averaged. Student’s paired t-tests were used to determine statistical significance.
Scratch assays were performed in 4 well chamber slides. Cells were plated at confluence (5×105 cells/well). 24 hours later, sheets of confluent cells were scratched with a p20 pipette tip with equal force applied to each well. Width of the wound at time 0 and after 6 hours was determined and the average percent wound closure was calculated for each cell line. Four wells of each of three cell lines derived from the different regions of the aorta from both 16.5 dpc embryos and 8 week-old adults were quantified. Statistical significance was determined with a Student’s paired t-test.
3. Results
3.1. Ascending and descending domains of the embryonic aorta exhibit distinct patterns of gene expression
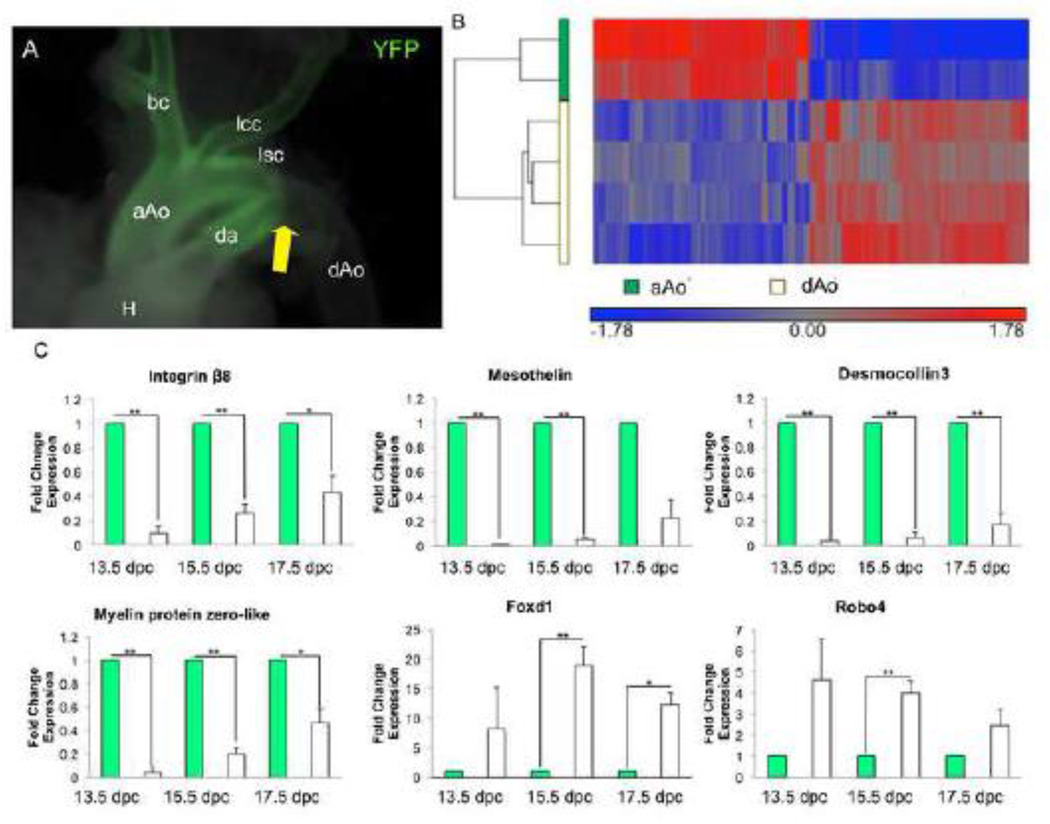
The ascending (aAo) and descending (dAo) domains of the aorta are derived from NC and somitic mesoderm respectively. The aAo and dAo of 13.5dpc embryos were subjected to microarray analyses to explore whether distinct components of a single vessel derived from diverse progenitor pools have varied patterns of gene expression. This stage was chosen because it is the first day after reorganization of the truncus arteriosus into the aortic arch and pulmonary trunk. Wnt-1-Cre; R26RYFP NC lineage labeled aortae were used to accurately dissect aortic regions containing NC and mesodermal derivatives within the developing aorta (Figure 1A). In comparing gene expression patterns of the aAo versus the dAo, we found a total of 1,475 significantly differentially hybridized probes. Significance was defined as expression of at least 1.5-fold difference and a B-H p value of <0.05. Of these 1,475 differentially hybridized probes, 459 genes were upregulated in the aAo and 597 genes were upregulated in the dAo (Figure 1B). Using the Database for Annotation, Visualization and Integrated Discovery (DAVID), we analyzed the gene ontology (GO) terms associated with each gene identified in the microarray (Table 1). Because we were interested in the smooth muscle characteristics of cell adhesion and cell migration, we focused on GO term categories related to those processes. One of the GO terms containing a number of genes highly expressed in the aAo was “cellular adhesion”. In the dAo, genes related to the GO terms “cell migration” and “cell motion” were highly expressed in comparison with the aAo. The genes that were most differentially expressed in these categories were the ones we proceeded to verify. qRT-PCR of selected gene products identified by microarray verified their differential expression in the aAo versus dAo at 13.5, 15.5, and 17.5 dpc (Figure 1C). Sustained differences in gene expression patterns across the timeframe measured suggest that these data reflect definitive phenotypes in these embryonic structures.
Figure 1.
Embryonic regions of the aorta have distinct gene expression profiles. A) Wnt1-Cre; R26RYFP lineage labeled aorta demonstrating the border between the aAo and dAo. White arrow points to the border between the NC- and somitic-derived VSMCs. B) Hierarchical clustering of 1,475 probes detected as significantly different (at least 1.5-fold, B-H p value < 0.05) between control aAo and dAo samples. Values shown are log base 2, and bright red, bright blue, and gray indicate the highest, lowest, and median normalized signal values, respectively. Vertical dendrograms represent the individual samples, of which there are two to four replicates for each sample type. C) qRT-PCR on RNA taken from aorta samples throughout development. Abbreviations: aAo, ascending aorta; bc, brachiocephalic artery; da, ductus arteriosus; dAo, descending aorta; H, heart; lcc, left common carotid; lsc, left subclavian. Error bars represent SEM. *, p≤0.05; **, p≤0.01
Table 1.
Example GO terms differentially regulated in different regions of embryonic aorta.
| Gene Ontology Category | aAo | dAo |
|---|---|---|
| Biological Process | Number of Genes | |
| G protein signaling | 10 | - |
| Ion transport | 39 | 39 |
| Cell adhesion | 35 | 80 |
| Ion homeostasis | 22 | 29 |
| Cell surface receptor linked signaling | - | 95 |
| Response to wounding | - | 48 |
| Cell migration | - | 30 |
| Cell motion | - | 41 |
| Immune response | - | 46 |
| Extracellular structure organization | - | 18 |
| Regulation of blood vessel size | - | 8 |
3.2. Cell lines derived from aAo and dAo retain phenotypic distinctions observed in vivo
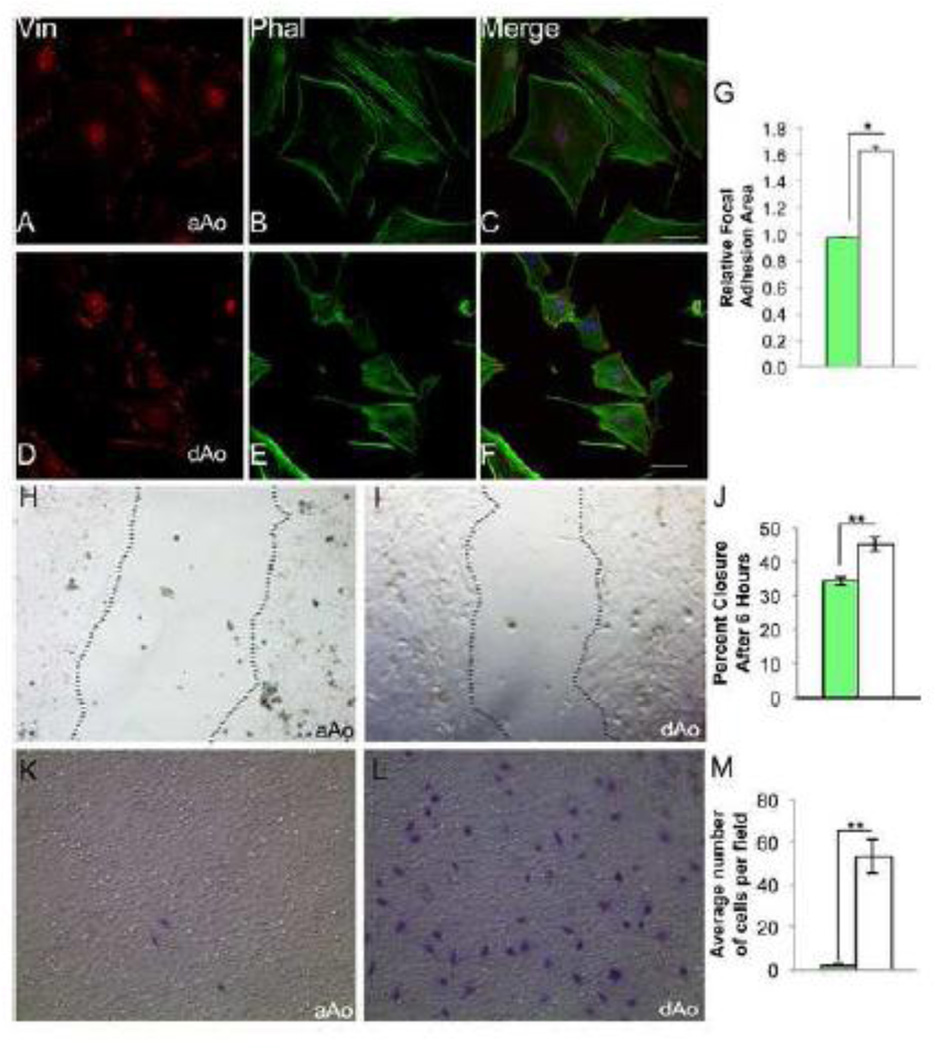
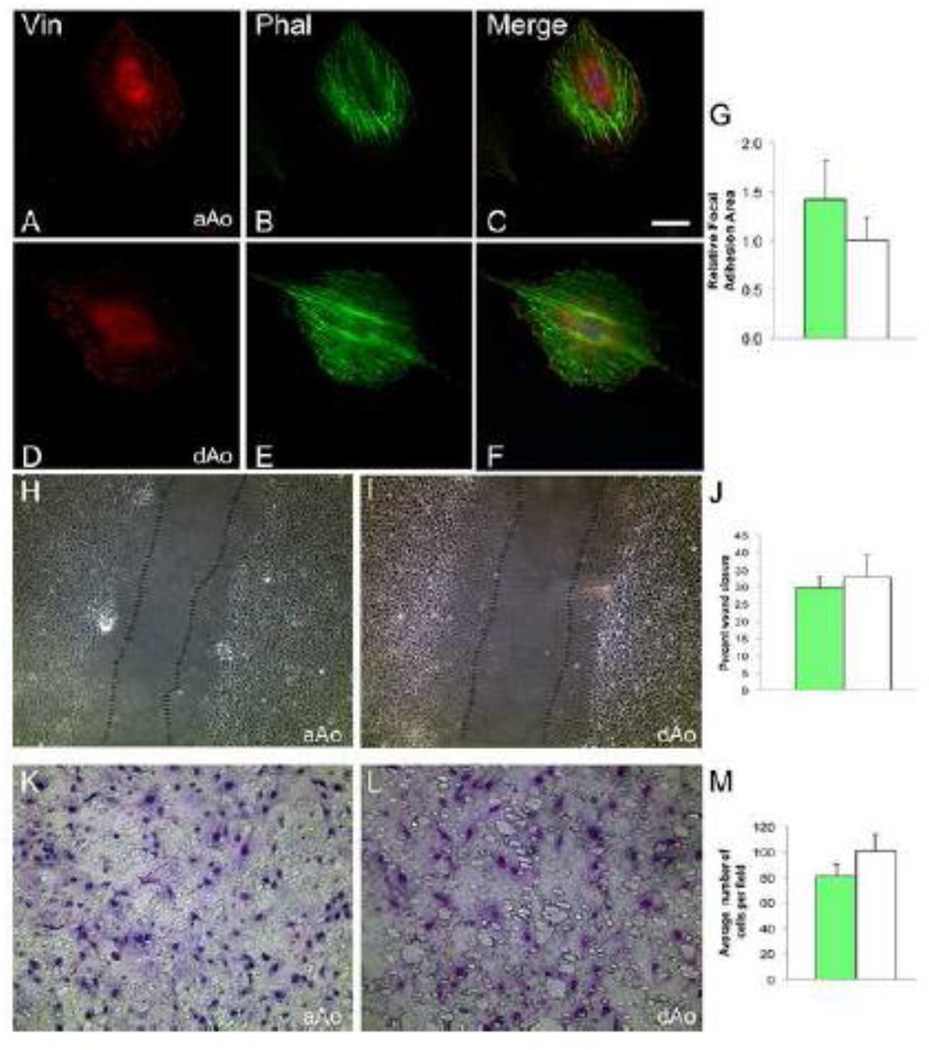
Microarray and gene expression studies suggested that developing aAo and dAo exhibit fundamental phenotypic differences in motility and contractility. We derived cells lines from distinct regions of the aorta of embryonic 16.5 dpc Immorto mice to assess these characteristics at the cellular level and determine whether gene expression variation observed in vivo was retained in VSMCs. Importantly, clonal cell lines derived from embryonic aAo and dAo maintained the differential expression of genes identified from the microarray of native tissue (Supplemental Figure 3). In observing these cells, immediate and consistent differences were observed between lines derived from embryonic aAo versus dAo domains. First, when VSMC cell lines derived from the two domains were stained with phalloidin to highlight filamentous actin and an antibody against vinculin to visualize focal adhesions, significant and reproducible variation in the distribution of actin filaments and focal adhesions was revealed (Figure 2). Embryonic aAo VMSCs were relatively larger (4.5 times greater area, p<0.05) and had many focal adhesions scattered throughout the cell whereas the embryonic dAo VSMCs were consistently smaller and had significantly larger focal adhesions (Figure 2 A, D, and G). The localization of actin filaments also differed between embryonic aAo versus dAo cell lines with more organized parallel filaments evident in cells from the dAo (Figure 2 B and E). Importantly, while variation was observed in these cells, antibodies against SMA and MHC (Supplemental Figure 2) confirmed that all derived cell lines maintained expression of markers of differentiated vascular smooth muscle.
Figure 2.
Localization of cytoskeletal elements and migratory characteristics differ between cells derived from embryonic aAo versus dAo. A–C) Embryonic aAo VSMCs labeled with anti-vinculin antibody and phalloidin. D–F) Cells derived from the embryonic dAo labeled with anti-vinculin antibodies and phalloidin. DNA labeled with DAPI. G) Quantification of relative focal adhesion area. H, I) Scratch assay using embryonic aAo and dAo VSMC lines, 6 hours after scratch was applied. J) Quantification of percent wound closure in embryonic VSMC lines. K, L) Boyden chamber assay on embryonic VSMCs t=24 hours. Cells stained with Giemsa demonstrate the number of cell that invaded the membrane. M) Quantification of number of cells migrated after 24 hours. Scale bars represent 50µm. Abbreviations: Vin, vinculin; Phal, phalloidin. Error bars represent SEM. *, p≤0.05; **, p≤0.01.
These differences in cytoskeletal organization together with the gene expression profiles observed in intact vessels and clonal cell lines suggested that the embryonic aAo and dAo cells may also vary in migratory abilities [34]. To test this hypothesis, scratch assays on confluent cell cultures were conducted to compare high-density cell migration in VSMCs from different embryonic origins. Six hours after injury, cell lines derived from embryonic dAo VSMCs moved at a significantly higher rate when compared to the migration of cell lines derived from embryonic aAo VSMCs (Figure 2 H–J). Next, single cell migratory properties were assessed using Boyden chamber assays. As predicted, and consistent with sheet movement analyses, embryonic dAo VSMC-derived cell lines were consistently much more migratory when compared to aAo VSMCs-derived cell lines (Figure 2 K–M).
3.3. Regions of the aorta have distinct contractile properties in the embryo
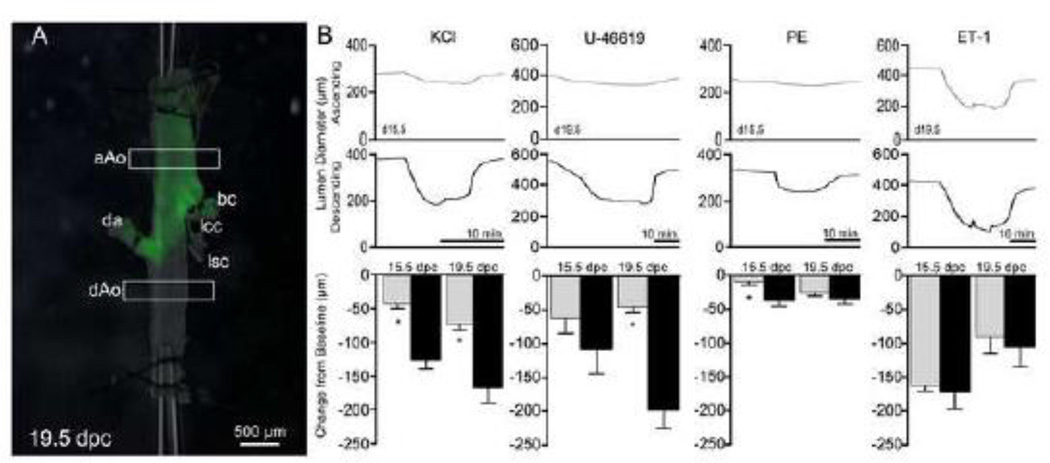
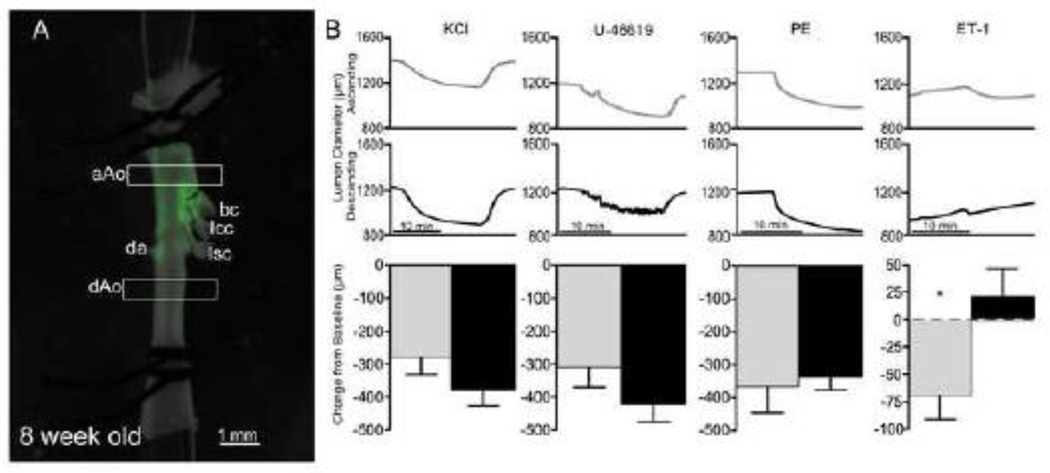
We next determined whether the observed phenotypic differences extended to contractile properties in these two domains derived from NC and mesodermal progenitors. To test the relative contractility of the vessel, aortae from 15.5 and 19.5 dpc embryos were cannulated and the change in lumen diameter in response to applied vasoconstrictors was measured (Figure 3). Importantly, this myographic approach allowed for the simultaneous analysis of both domains within the same vessel. With the addition of general (potassium chloride) and specific agonists (U46619 and phenylephrine), the embryonic dAo was significantly more contractile than the aAo counterpart at both gestational time points (Figure 3B). Taken together, these data demonstrate that subdomains of an individual artery seeded by progenitors of varying origin exhibit distinct phenotypes in the embryonic state and these differences were maintained in cell lines derived from the regions of interest.
Figure 3.
Region-specific contractile response of the embryonic aorta. A) Lineage labeled 19.5 dpc aorta mounted on cannula for experimental analysis. Boxes represent regions where lumen diameters are measured. B) Example traces from aAo (gray) and dAo (black) with the addition of different vasoconstrictors, PE (phenylephrine), ET-1 (endothelin-1). Abbreviations: aAo, ascending aorta; bc, brachiocephalic artery; da, ductus arteriosus; dAo, descending aorta; lcc, left common carotid; lsc, left subclavian. Error bars represent SEM. *, p≤0.05; **, p≤0.01.
3.4 Phenotypic convergence of aAo and dAo domains in the adult aorta
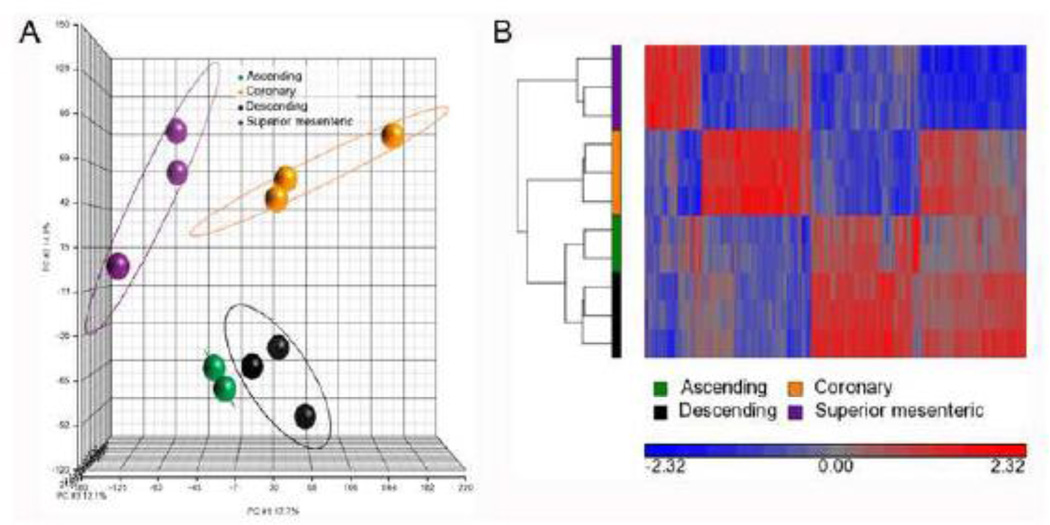
Given the phenotypic heterogeneity of ascending and descending domains of the embryonic aorta, microarray analysis was conducted on these same regions in the adult vessel to determine whether this divergence persisted. In turn, these results were compared to expression patterns observed for the superior mesenteric and coronary arteries whose myogenic components are derived from serosal and epicardial mesothelia respectively [18, 35, 36]. Interestingly, while significant variation was observed between the aorta, mesenteric, and coronary vessels, expression profiles of the aAo and dAo of the adult did not vary. In these analyses, 1,496 probes were differentially hybridized across these four vessels (Figure 4 A and B). Within this probe set, unique expression profiles for the superior mesenteric, coronary, and aorta as a whole were readily identified (Figure 4B). Surprisingly, of the 1,496 differentially hybridized probes, none were significantly differentially expressed between the ascending and descending aorta (Figure 4). Furthermore, the differentially expressed genes that were verified with qRT-PCR in embryonic vessels were either not expressed or expressed at very low levels in the adult aorta. While in the embryo the data suggested two distinct gene expression profiles in the aAo and dAo, the adult aorta has statistically uniform gene expression profiles.
Figure 4.
Adult vessels have different gene expression patterns, however the regions of the aorta have similar profiles. A) Principal components analysis of four different blood vessel tissues: ascending aorta (red), descending aorta (green), coronary artery (blue), and superior mesenteric artery (purple) is shown. The colors represent the different sample replicates, as indicated in the legend, and circles mark the ellipsoids (+/− standard a deviation of 2) for each group. The X-axis, Y-axis, and Z-axis components represent 17.7%, 14.8%, and 12.1%, respectively, of the total variability between experimental replicates. B) Hierarchical clustering of 1,496 probes detected as significantly different (at least 1.5-fold, B-H p value < 0.05) between ascending aorta, descending aorta, coronary, and superior mesenteric arteries. Values shown are log base 2, and bright red, bright blue, and gray indicate the highest, lowest, and median normalized signal values, respectively. Vertical dendrograms represent the individual samples, of which there are three replicates for each sample type (two replicates for ascending aortic tissue).
In light of this finding, we tested the other basic parameters that distinguish embryonic domains within the adult aorta. VSMC lines from the adult aAo and dAo were derived using the same methodologies employed on embryos. Interestingly, morphology and cytoskeletal characteristics of clonal VMSC-derived lines isolated from adult aAo and dAo domains were also remarkably similar (Figure 5 A–G). Additionally, migratory patterns of cell sheets after scratch injury and of single cells in Boyden chamber analyses from both adult domains were tested to probe phenotypic characteristics (Figure 5 H–M). In both settings, movement of cells derived from either domain was indistinguishable using quantitative analyses in contrast to those observed for their embryonic counterparts. Finally, analyses of vessel contractility were conducted on young adults using the same myographic modalities employed on embryonic counterparts. Here, we determined that the contractile properties of the aAo and dAo were remarkably similar with no significant difference in response to any of the antagonists cited above (Figure 6). The single exception to this pattern is endothelin-1, an endothelially-expressed gene product, which has potent non-region-specific effects in the fetal aorta, but only limited effects on the adult aAo and dAo. Application of vasodilators did not reveal any pattern of dilation in the different regions of the aorta in either the embryo or the adult (Supplemental Figure 4).
Figure 5.
VSMC lines from adult aAo and dAo have similar localization of cytoskeletal elements and migratory characteristics. A–C) Representative adult aAo VSMCs labeled with anti-vinculin antibody and phalloidin. D–F) Representative adult dAo VSMC lines labeled with anti-vinculin antibodies and phalloidin. DNA labeled with DAPI. G) Quantification of relative focal adhesion area. H, I) Scratch assay of adult aAo and dAo VSMC lines after 6 hours. J) Quantification of percent wound closure in adult VSMC cultures. K, L) Boyden chamber assay on adult VSMC lines t=24 hours. M) Quantification of number of cells migrated after 24 hours. Scale bars represent 50µm. Abbreviations: aAo, ascending aorta; dAo, descending aorta; Vin, vinculin; Phal, phalloidin. Error bars represent SEM. *, p≤0.05; **, p≤0.01.
Figure 6.
Regions of the adult aorta have similar contractile responses to vasoconstrictors. A) Lineage labeled 8 week old aorta on cannula. Boxes represent regions where lumen diameters are measured. B) Example traces from aAo (gray) and dAo (black) with the addition of different vasoconstrictors, PE (phenylephrine), ET-1 (endothelin-1). Abbreviations: aAo, ascending aorta; bc, brachiocephalic artery; da, ductus arteriosus; dAo, descending aorta; H, heart; lcc, left common carotid; lsc, left subclavian. Error bars represent SEM. *, p≤0.05.
5. Discussion
The data presented in this study conclusively demonstrate the unique characteristics of the embryonic regions of the aorta, which phenotypically converge in the adult. This is a novel finding that establishes a roadmap for future analyses to determine how this process of convergence occurs and what happens when the VMSCs of the aorta falter in patterning. Further investigation can now be done to examine the variables that influence convergence. One exciting manipulation would be to expose embryonic regions of the aorta to different flow conditions to change the biomechanical environment. It is possible that the change in blood pressure and flow that occurs at birth is the event that results in vascular convergence. These data firmly establish a platform on which a more complete understanding of vascular maturation can be built.
Additionally, these data may serve as a paradigm to explain how apparently homogeneous smooth muscle populations in the adult respond differently to injury or disease. For example, it is not uncommon for tissues in the adult to revert to embryonic characteristics when stressed. Similarly, cells of the nephron reactivate developmental programs to repair damage done to the kidney [37, 38]. In this context, reverting to a developmental program allows the cells of the tissue to repair and maintain function. The heart is another example of an organ that reactivates developmental characteristics to protect against stress [39, 40]. It has been hypothesized, however, that long term these developmental characteristics may lead to disease in the adult [40]. Therefore, one potential explanation for the difference in disease progression of these two regions of the aorta may be that, despite their similar adult characteristics, these domains revert to their divergent embryonic state to cope with stress. This reversion may, over time, result in the differential pattern of disease found in different regions of the aorta including atherosclerosis and calcification as well as aortic aneurism at the junction between regions [16, 26, 29]. We would predict that a metabolically stressed mouse model would exhibit the embryonic patterns we have described in the different regions of the aorta.
The plasticity of smooth muscle cells in culture has been well documented [41]. VSMCs are easily manipulated by the conditions under which they are cultured. Therefore, it is entirely possible that the in vitro experiments described above do not perfectly replicate the in vivo setting. Due to the sensitivity of these cells, it was critical that all clones were treated the same. Based on the stability of both VSMC markers and the differential expression of genes identified from the microarray, these data clearly indicate significant differences between the embryonic cell lines derived from diverse origins and the maintenance of these differences over the timeframe studied in vitro.
Our studies lay the groundwork to determine whether the fundamental differences in embryonic VSMCs play a role in vascular diseases of the adult. The data described here lay the groundwork for the determination embryonic patterns converge in the adult and the role this plays in the context of disease.
Supplementary Material
Highlights.
Embryonic aortic regions of diverse origin differ in physiology and expression.
Smooth muscle cells from these regions also diverge in migration and cell adhesion.
The same regions have convergent gene expression and physiology in adult aortae.
Smooth muscle cells from the adult aorta have comparable migration and adhesion.
Both embryonic origin and environment shape smooth muscle biology in development.
Acknowledgments
We would like to thank Alison LeGrone for her excellent mouse work and performing the RNA isolation on embryonic tissue.
Funding information:
AHA grant 12PRE10950005 to ERP
NIH grant R01 DK083234 to DMB
AHA grant in aid 11GRNT7690040 to PAL and DMB
NIH grants RO1 HL096967 and RO1 HL109199 to JR.
Abbreviations
- aAo
aortic arch
- dAo
descending aorta
- dpc
days post coitum
- ET-1
endothelin-1
- NC
neural crest
- PE
phenylephrine
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- SMA
smooth muscle actin
- SM-MHC
smooth muscle myosin heavy chain
- VSMCs
vascular smooth muscle cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: We have nothing to disclose.
Contributor Information
Elise R. Pfaltzgraff, Email: Elise.r.pfaltzgraff@vanderbilt.edu.
Elaine L. Shelton, Email: Elaine.l.shelton@vanderbilt.edu.
Cristi L. Galindo, Email: Cristi.l.galindo@vanderbilt.edu.
Brian L. Nelms, Email: bnelms@fisk.edu.
Christopher W. Hooper, Email: Christopher.w.hooper@vanderbilt.edu.
Stanley D. Poole, Email: Stan.poole@vanderbilt.edu.
Patricia A. Labosky, Email: Patricia.labosky@nih.gov.
David M. Bader, Email: David.bader@vanderbilt.edu.
Jeff Reese, Email: Jeff.reese@vanderbilt.edu.
References
- 1.Katora ME, Hollis TM. Regional variation in rat aortic endothelial surface morphology: relationship to regional aortic permeability. Exp Mol Pathol. 1976;24:23–34. doi: 10.1016/0014-4800(76)90054-x. [DOI] [PubMed] [Google Scholar]
- 2.Gadson PF, Jr, Rossignol C, McCoy J, Rosenquist TH. Expression of elastin, smooth muscle alpha-actin, and c-jun as a function of the embryonic lineage of vascular smooth muscle cells. In Vitro Cell Dev Biol Anim. 1993;29A:773–781. doi: 10.1007/BF02634344. [DOI] [PubMed] [Google Scholar]
- 3.Ruckman JL, Luvalle PA, Hill KE, Giro MG, Davidson JM. Phenotypic stability and variation in cells of the porcine aorta: collagen and elastin production. Matrix Biol. 1994;14:135–145. doi: 10.1016/0945-053x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 4.van Meurs-van Woezik H, Klein HW, Markus-Silvis L, Krediet P. Comparison of the growth of the tunica media of the ascending aorta, aortic isthmus and descending aorta in infants and children. J Anat. 1983;136:273–281. [PMC free article] [PubMed] [Google Scholar]
- 5.Stenmark KR, Mecham RP. Cellular and molecular mechanisms of pulmonary vascular remodeling. Annu Rev Physiol. 1997;59:89–144. doi: 10.1146/annurev.physiol.59.1.89. [DOI] [PubMed] [Google Scholar]
- 6.Fawcett DW, Bloom W, Raviola E. A textbook of histology. 12th ed. New York: Chapman & Hall; 1994. [Google Scholar]
- 7.Flaim SF, Ress RJ, Mest S. Regional variation in norepinephrine-stimulated calcium uptake in rabbit aorta. Pharmacology. 1985;30:1–11. doi: 10.1159/000138043. [DOI] [PubMed] [Google Scholar]
- 8.Sufka KJ, Stratton DB, Giordano J. Regional differences in serotonergic contractile sensitivity mediated by 5-HT2 receptors in rat aorta. Artery. 1990;18:47–53. [PubMed] [Google Scholar]
- 9.Thieszen SL, Dalton M, Gadson PF, Patterson E, Rosenquist TH. Embryonic lineage of vascular smooth muscle cells determines responses to collagen matrices and integrin receptor expression. Exp Cell Res. 1996;227:135–145. doi: 10.1006/excr.1996.0258. [DOI] [PubMed] [Google Scholar]
- 10.Gadson PF, Jr, Dalton ML, Patterson E, Svoboda DD, Hutchinson L, Schram D, et al. Differential response of mesoderm- and neural crest-derived smooth muscle to TGF-beta1: regulation of c-myb and alpha1 (I) procollagen genes. Exp Cell Res. 1997;230:169–180. doi: 10.1006/excr.1996.3398. [DOI] [PubMed] [Google Scholar]
- 11.Andersen MR, Stender S. Endothelial nitric oxide synthase activity in aorta of normocholesterolemic rabbits: regional variation and the effect of estrogen. Cardiovasc Res. 2000;47:192–199. doi: 10.1016/s0008-6363(00)00072-9. [DOI] [PubMed] [Google Scholar]
- 12.Ko YS, Coppen SR, Dupont E, Rothery S, Severs NJ. Regional differentiation of desmin, connexin43, and connexin45 expression patterns in rat aortic smooth muscle. Arterioscler Thromb Vasc Biol. 2001;21:355–364. doi: 10.1161/01.atv.21.3.355. [DOI] [PubMed] [Google Scholar]
- 13.Cheung C, Bernardo AS, Trotter MW, Pedersen RA, Sinha S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nat Biotechnol. 2012;30:165–173. doi: 10.1038/nbt.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trigueros-Motos L, Gonzalez-Granado JM, Cheung C, Fernandez P, Sanchez-Cabo F, Dopazo A, et al. Embryological-Origin-Dependent Differences in Hox Expression in Adult Aorta: Role in Regional Phenotypic Variability and Regulation of NF-kappaB Activity. Arterioscler Thromb Vasc Biol. 2013 doi: 10.1161/ATVBAHA.112.300539. [DOI] [PubMed] [Google Scholar]
- 15.Reslan OM, Yin Z, do Nascimento GR, Khalil RA. Subtype-Specific Estrogen Receptor-Mediated Vasodilator Activity in the Cephalic, Thoracic and Abdominal Vasculature of Female Rat. J Cardiovasc Pharmacol. 2013 doi: 10.1097/FJC.0b013e31828bc88a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leroux-Berger M, Queguiner I, Maciel TT, Ho A, Relaix F, Kempf H. Pathological calcification of adult vascular smooth muscle cells differs upon their crest or mesodermal embryonic origin. J Bone Miner Res. 2011 doi: 10.1002/jbmr.382. [DOI] [PubMed] [Google Scholar]
- 17.Subbiah MT, Bale LK, Dinh DM, Kottke BA, Deitemeyer D. Regional aortic differences in atherosclerosis-susceptibility: changes in prostaglandin biosynthesis and cholesterol accumulation in response to desoxycorticosterone (DOCA)-salt induced hypertension. Virchows Arch B Cell Pathol Incl Mol Pathol. 1981;37:309–315. doi: 10.1007/BF02892579. [DOI] [PubMed] [Google Scholar]
- 18.Wilm B, Ipenberg A, Hastie ND, Burch JB, Bader DM. The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development. 2005;132:5317–5328. doi: 10.1242/dev.02141. [DOI] [PubMed] [Google Scholar]
- 19.Wasteson P, Johansson BR, Jukkola T, Breuer S, Akyurek LM, Partanen J, et al. Developmental origin of smooth muscle cells in the descending aorta in mice. Development. 2008;135:1823–1832. doi: 10.1242/dev.020958. [DOI] [PubMed] [Google Scholar]
- 20.Le Lievre CS, Le Douarin NM. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. Journal of embryology and experimental morphology. 1975;34:125–154. [PubMed] [Google Scholar]
- 21.Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- 22.Que J, Wilm B, Hasegawa H, Wang F, Bader D, Hogan BL. Mesothelium contributes to vascular smooth muscle and mesenchyme during lung development. Proc Natl Acad Sci U S A. 2008;105:16626–16630. doi: 10.1073/pnas.0808649105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gittenberger-de Groot AC, DeRuiter MC, Bergwerff M, Poelmann RE. Smooth muscle cell origin and its relation to heterogeneity in development and disease. Arterioscler Thromb Vasc Biol. 1999;19:1589–1594. doi: 10.1161/01.atv.19.7.1589. [DOI] [PubMed] [Google Scholar]
- 24.Rinkevich Y, Mori T, Sahoo D, Xu PX, Bermingham JR, Jr, Weissman IL. Identification and prospective isolation of a mesothelial precursor lineage giving rise to smooth muscle cells and fibroblasts for mammalian internal organs, and their vasculature. Nat Cell Biol. 2012;14:1251–1260. doi: 10.1038/ncb2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Gu S, Al-Sabeq B, Wang S, He J, Tam A, et al. Origin-specific epigenetic program correlates with vascular bed-specific differences in Rgs5 expression. Faseb J. 2012;26:181–191. doi: 10.1096/fj.11-185454. [DOI] [PubMed] [Google Scholar]
- 26.Haimovici H, Maier N. Experimental canine atherosclerosis in autogenous abdominal aortic grafts implanted into the jugular vein. Atherosclerosis. 1971;13:375–384. doi: 10.1016/0021-9150(71)90080-3. [DOI] [PubMed] [Google Scholar]
- 27.Kirby ML, Gale TF, Stewart DE. Neural crest cells contribute to normal aorticopulmonary septation. Science. 1983;220:1059–1061. doi: 10.1126/science.6844926. [DOI] [PubMed] [Google Scholar]
- 28.Tanous D, Benson LN, Horlick EM. Coarctation of the aorta: evaluation and management. Curr Opin Cardiol. 2009;24:509–515. doi: 10.1097/HCO.0b013e328330cc22. [DOI] [PubMed] [Google Scholar]
- 29.Majesky MW, Dong XR, Hoglund VJ. Parsing aortic aneurysms: more surprises. Circ Res. 2011;108:528–530. doi: 10.1161/CIRCRESAHA.111.240861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 31.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reese J, O'Mara PW, Poole SD, Brown N, Tolentino C, Eckman DM, et al. Regulation of the fetal mouse ductus arteriosus is dependent on interaction of nitric oxide and COX enzymes in the ductal wall. Prostaglandins Other Lipid Mediat. 2009;88:89–96. doi: 10.1016/j.prostaglandins.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geisterfer AA, Peach MJ, Owens GK. Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ Res. 1988;62:749–756. doi: 10.1161/01.res.62.4.749. [DOI] [PubMed] [Google Scholar]
- 34.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dettman RW, Denetclaw W, Jr, Ordahl CP, Bristow J. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Dev Biol. 1998;193:169–181. doi: 10.1006/dbio.1997.8801. [DOI] [PubMed] [Google Scholar]
- 36.Landerholm TE, Dong XR, Lu J, Belaguli NS, Schwartz RJ, Majesky MW. A role for serum response factor in coronary smooth muscle differentiation from proepicardial cells. Development. 1999;126:2053–2062. doi: 10.1242/dev.126.10.2053. [DOI] [PubMed] [Google Scholar]
- 37.Jiang YS, Jiang T, Huang B, Chen PS, Ouyang J. Epithelial-mesenchymal transition of renal tubules: divergent processes of repairing in acute or chronic injury? Med Hypotheses. 2013;81:73–75. doi: 10.1016/j.mehy.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 38.Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A. 2010;107:4194–4199. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oka T, Xu J, Molkentin JD. Re-employment of developmental transcription factors in adult heart disease. Semin Cell Dev Biol. 2007;18:117–131. doi: 10.1016/j.semcdb.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajabi M, Kassiotis C, Razeghi P, Taegtmeyer H. Return to the fetal gene program protects the stressed heart: a strong hypothesis. Heart Fail Rev. 2007;12:331–343. doi: 10.1007/s10741-007-9034-1. [DOI] [PubMed] [Google Scholar]
- 41.Chamley-Campbell J, Campbell GR, Ross R. The smooth muscle cell in culture. Physiol Rev. 1979;59:1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.