Abstract
Thrombosis is characterized by congenital and acquired procatarxis. Lactic acid bacteria-fermented soybean milk products (FS-LAB) inhibit hepatic lipid accumulation and prevent atherosclerotic plaque formation. However, the therapeutic efficacy of FS-LAB against thrombosis has yet to be investigated. In this study, FS-LAB were administered subcutaneously into the tails of rats, with the subsequent intravenous administration of κ-carrageenan 12 hr after the initial injection. In general, administration of κ-carrageenan induces thrombosis. The length of the infarcted tail regions was significantly shorter in the rats administered a single-fold or double-fold concentration of the FS-LAB solution compared with the region in control rats. Therefore, FS-LAB exhibited significant antithrombotic effects. Our study is the first to characterize the properties of FS-LAB and, by testing their efficacy on an in vivo rat model of thrombosis, demonstrate the potency of their antithrombotic effect.
Keywords: thrombosis, fermented soybean milk products, lactic acid bacteria, rat tail, κ-carrageenan
The risk factors for thrombosis can be divided into either congenital or acquired procatarxis. Blood coagulation systems are controlled by a series of cascade reactions, which involve many coagulation factors [1, 2].
Because much progress has been made in our knowledge of thrombosis, it is expected that a more detailed mechanism of the disease will be elucidated. Therefore, it is valuable to create in vivo models of thrombosis to examine the mechanisms leading to its development. Bice et al. and Kod’ousek et al. reported that carrageen-induced gangrene of the tail, when analyzed histologically, is thrombosis [3, 4]. Based on these articles, we produced a carrageenan-induced thrombosis model in the rat tail [5]. In addition, we reported that the evaluation of nattokinase efficacy for the treatment of thrombosis was feasible using this in vivo rat model [6].
Few studies, if any, have been carried out to determine if there is an association between lactic acid bacteria (LAB) and thrombosis. The use of LAB for the treatment of diarrhea and candidiasis has been studied in detail at a clinical level [7,8,9]. LAB exert their effects on various disorders by enhancing immunity. In addition, the effects of LAB on hypertension and hyperlipemia have been investigated at a laboratory level [10, 11]. Therefore, we hypothesize that LAB can have an potential effect on thrombosis.
Fermented soybean milk products produced using LAB (FS-LAB) are products that are prepared from soybean milk that use LAB in the fermentation process. In the present study, we did not directly administer LAB. Instead, we used their products derived from fermented soybean milk, which inhibits hepatic lipid accumulation [12]. Moreover, fermented soybean milk can significantly decrease serum cholesterol and atherosclerotic plaque formation in the aorta [13].
Most previous evaluation methods that have tested the efficacy of antithrombotic agents have been performed in vitro, such as estimation of euglobulin activity by the standard fibrin plate method and assessment of the level of a decomposition product by serum fibrin/fibrinogen or tissue-type plasminogen activator (t-PA) antigen levels [14].
The aim of this study was to investigate the antithrombotic efficacy of FS-LAB. To examine the potential antithrombotic effects of FS-LAB, κ-carrageenan was administered intravenously in the tails of rats 12 hr after subcutaneous injection of FS-LAB, thereby inducing thrombus formation. The length of the infarcted tail region was then measured. The efficacy of FS-LAB in preventing prevent thrombus formation was evaluated by comparing the lengths of the infarcted tail regions in control rats with those in drug-administered rats.
FS-LAB were kindly provided by BIOGENOMICS Co., Ltd. (PS-B1, Nagasaki, Japan). PS-B1 was used as FS-LAB. PS-B1 is a fermented soybean milk product produced using LAB. The LAB are comprised of Lactobacillus genera (L. gasseri, L. acidophilus, L. paracasei, L. brevis, L. casei, L. delbrueckii subsp. bulgaricus, L delbrueckii subsp. delbrueckii) and Bifidobacterium genera (B. longum subsp. longum, B.bifidum, B. adolescentis). Fibrinogen from bovine plasma thrombin from bovine plasma, and κ-carrageenan were purchased from Wako Pure Chemical Industries (Osaka, Japan). All other materials and solvents were of analytical grade and were used without further purification.
The stock solution of FS-LAB contained citric acid and ethanol, which protect the product from decomposing. The tails of the rats became swollen when the FS-LAB stock solution was injected; therefore, κ-carrageenan could not be injected intravenously. To resolve this issue, we prepared a working stock of FS-LAB solution as follows. The stock FS-LAB solution was evaporated, and a 4-fold condensed FS-LAB solution was prepared by adding phosphate-buffered saline (PBS) to the FS-LAB residue after evaporation.
We prepared a 5 mg/ml fibrinogen solution by dissolving 100 mg fibrinogen from bovine plasma in 20 ml PBS (pH 7.4) and divided the mixture into 1 ml aliquots. Then, the 4-fold condensed FS-LAB and PBS were added into the fibrinogen aliquots to attain a total volume of 2 ml so that the concentration of each sample was 2×, 1×, 0.5×, 0.2×, 0.1× and 0× that of the FS-LAB liquid concentrate. Each vial was incubated for 30 min at 37°C. In addition, thrombin from bovine plasma was dissolved in physiological saline solution, and 5,000 units/ml of thrombin solution was prepared. Finally, 0.2 ml of the thrombin solution was added to the fibrinogen and FS-LAB solution.
Animal experiments were approved by the Experimental Animal Research Committee at Nagasaki International University (Japan). Female 6-week-old Wistar rats, weighing 180–200 g, were supplied by Kyudo Co., Ltd. (Tosu, Saga, Japan). The animal house was maintained at 22 ± 3°C with a relative humidity of 60% and a 12 h light–dark cycle (light period: 7:00 AM to 7:00 PM). All the animals were supplied with tap water and fed a normal pellet diet (CG-7™; CLEA Japan, Inc.) for at least 7 days before the experiments were performed.
The general procedures related to this rat model have been described previously [6]. Rats with tails that were longer than 13 cm were selected and anesthetized using diethyl ether for the experiments. A total of 0.5 ml of FS-LAB solution (1× or 2×) was injected hypodermically into the tail of each rat at 10 different points. Specifically, 0.05 ml of this solution was injected at 5 points on the right side of the tail and 5 points on the left side of the tail with a distance of 1 cm between each injection point [6]. At 12 hr after the FS-LAB solution was injected, κ-carrageenan was dissolved in saline and injected into the tail vein as follows. A site approximately 13 cm from the tip of the rat’s tail was ligated, and 1 mg/kg of body weight of κ-carrageenan was injected intravenously. After 10 min, the ligature was removed.
The lengths of the infarcted tail regions were measured at 6, 24 and 48 hr after the injection of κ-carrageenan. The rats in the control group were administered PBS and κ-carrageenan only. The dose–response relationship between FS-LAB and thrombogenesis was evaluated by intravenous injection of κ-carrageenan at a dose of 1 mg/kg of body weight.
The rats were categorized into 3 groups. Group 1 was the control group. The other 2 groups were injected with the FS-LAB solution; group 2 was administered the stock solution (1× FS-LAB), and group 3 was administered the concentrated solution (2× FS-LAB). The PBS-administered group contained 7 rats (group 1), the 1× FS-LAB-administered group contained 6 rats (group 2), and the 2× FS-LAB-administered group contained 7 rats (group 3).
Statistical differences between the control and FS-LAB-administered groups were analyzed using ANOVA, followed by a post hoc Tukey test. Probabilities of less than 5% (p<0.05) were considered statistically significant. All values are expressed as means ± standard deviation (SD).
Previously, the incidence of deep venous thrombosis in the Japanese population was low compared with incidences observed in Western populations. However, its manifestation has been increasing in Japan [15].
Establishing an in vivo method for evaluating the efficacy of new therapeutic drugs is extremely desirable. Previously, we reported that the efficacy of nattokinase against thrombosis could be readily assessed using an in vivo evaluation model [6]. The advantage of our method is that it enables easy evaluation of drug efficacy by comparing the lengths of the infarcted regions in drug-administered rat tails with lengths in control rat tails.
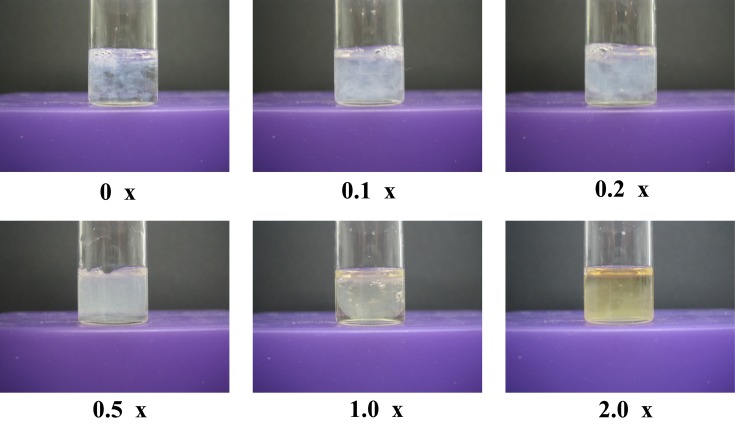
First, we performed an in vitro evaluation of the effects of FS-LAB. To achieve coagulation, a thrombin solution was added to a mixture of fibrinogen containing a varied concentration of FS-LAB solution. A fibrin clot was observed at all concentrations of FS-LAB solution. Therefore, FS-LAB do not have the ability to directly hinder the formation of a fibrin clot (Fig. 1). In addition, the appearance of each fibrin clot did not alter during the assessment time. Thrombolysis was not initiated because the absolute quantity of t-PA that was activated by FS-LAB was insufficient. Basically, the absolute quantity of t-PA in vitro is much less than that in the body. From these results, FS-LAB appear unable to directly prevent the production of a fibrin clot.
Fig. 1.
Photographs of fibrin clots 8 hr after mixing fibrinogen and thrombin.
The concentration of FS-LAB are indicate as 0 ×, 0.1 ×, 0.2 ×, 0.5 ×, 1.0 × and 2.0 ×.
Figure 2 shows the variation in the length of the infarcted tail region over the time. Intravenous injection of 1 mg/kg κ-carrageenan was performed 12 hr after the subcutaneous injection of 0.5 ml of FS-LAB (concentration of either 2× or 1×). After 6, 24 and 48 hr, the mean length of the infarcted tail regions was significantly shorter for the rats in the FS-LAB-administered groups compared with rats in the control group. The length of infarcted area in both the FS-LAB-administered groups was significantly decreased compared with the control group (p<0.05). However, there was no significant difference in length of the tail infarcted region between the rats administered the 2× FS-LAB solution and those administered the 1× FS-LAB solution, suggesting that the antithrombotic properties of FS-LAB are not dependent on the dose administered. The results of our study suggest that FS-LAB has significant effects against thrombosis. Figure 3 shows images of rat tails during development of an infarction at 24 hr after subcutaneous injection of κ-carrageenan. The mean length of the tail infarcted region in the FS-LAB-administered rats was shorter than that in the control rats. Typically, thrombosis occurs because of platelet aggregation or vasoconstriction, although platelet aggregation caused by cyclooxygenase inhibition has little or no effect on κ-carrageenan-induced thrombosis in rat tails [16]. Previously, it has been shown that serotonin (5-hydroxytryptamine-2 [5HT2]) receptor antagonists, such as ketanserin, can exhibit some therapeutic effects in serotonin activity-associated peripheral vasoconstrictive diseases [17]. The authors of this study observed an increase in the activity of exogenous serotonin, a decrease in the activity of serotonin-receptor antagonists, and serotonin depletion. However, administration of systemic or local vasodilating substances did not have any convincing effects on thrombosis. Therefore, the influence of vasoconstrictors and their dose-dependent effects (if applicable) must be investigated further [18]. However, Bekemeier and Hirschelmann reported that the unique inhibition of thromboxane synthesis by the administration of prostacyclin (PGI2) might reduce the occurrence of thrombosis in the tails of rats. Therefore, a thromboxane–PGI2 equilibrium appears to have a function in the induction of thrombosis [19]. However, the detailed mechanisms leading to thrombosis induction in rat tails remains to be elucidated.
Fig. 2.
Effect of FS-LAB on the mean length of the infarcted tail regions.
The horizontal axis shows the time elapsed after the κ-carrageenan injection; κ-carrageenan was injected intravenously 12 hr after subcutaneous injection of FS-LAB solution or PBS. Statistical analysis was performed using ANOVA with a post hoc Tukey test. Significant differences in the mean infarct length between 2× and 1× FS-LAB-administered groups are indicated by *p<0.05. The mean length of the infarcted regions of the FS-LAB-administered groups (2× and 1×) was compared with that of the PBS-administered group at 6, 24 and 48 hr. The PBS column represents the mean value ± SD of 7 measurements. The 1× FS-LAB column represents the mean ± SD of 6 measurements. The 2× FS-LAB column represents the mean value ± SD of 7 measurements.
Fig. 3.

Photographs of infarcted rat tails 24 hr after κ-carrageenan was injected for induction of thrombosis.
2×: rat tail after subcutaneous pre-injection with 2× FS-LAB 12 hr before κ-carrageenan injection. 1×: rat tail after subcutaneous pre-injection with 1× FS-LAB 12 hr before κ-carrageenan injection. PBS: rat tail after subcutaneous pre-injection with PBS 12 hr before κ-carrageenan injection.
In addition, the aforementioned reports describe different mechanisms underlying the thrombolytic effect of FS-LAB than those observed in our results. Therefore, a different model might be required to elucidate the mechanisms involved. Urano et al. reported that nattokinase deactivates plasminogen activator inhibitor, which causes activated plasminogen to be converted to plasmin. Consequently, the increased levels of plasmin dissolved the fibrin clot [20, 21]; a similar mechanism appears to be functioning for FS-LAB. Major plasminogen activators are mainly divided into t-PAs and urokinase-type plasminogen activators (u-PAs) [22, 23]. Nattokinase stimulates t-PA, and plasmin is produced from plasminogen [24]. Similarly, FS-LAB appears to stimulate t-PA. When activated by plasminogen activators such as t-PA, urokinase, and streptokinase, plasminogen is converted to plasmin [25, 26].
FS-LAB were injected into rat tails in this research. It is very difficult to administer FS-LAB into the human body because the safety of injecting FS-LAB is has not been established completely. However, our study suggests that FS-LAB have an effect on thrombosis.
The aim of the study was to investigate the efficacy of FS-LAB in preventing thrombosis. For this purpose, we administered FS-LAB in an experimental rat model of carrageenan-induced thrombosis in the rat tail. The length of the thrombotic or infarcted rattail regions was shorter in the FS-LAB-administered group than that in the control group. These results indicate that FS-LAB could inhibit thrombus formation in vivo. Thus far, no study has reported FS-LAB as a very effective inhibitor of thrombus formation. Our results indicate for the first time that FS-LAB have potential to prevent coagulation effects in thrombosis.
REFERENCES
- 1.Irani-Hakime N, Tamim H, Kreidy R, Almawi WY. 2000. The prevalence of factor V R506Q mutation-Leiden among apparently healthy Lebanese. Am J Hematol 65: 45–49 [DOI] [PubMed] [Google Scholar]
- 2.Helley D, Besmond C, Ducrocq R, da Silva F, Guillin MC, Bezeaud A, Elion J. 1997. Polymorphism in exon 10 of the human coagulation factor V gene in a population at risk for sickle cell disease. Hum Genet 100: 245–248 [DOI] [PubMed] [Google Scholar]
- 3.Bice DE, Gruwell DG, Salvaggio JE, Hoffmann EO. 1972. Suppression of primary immunization by carrageenan - a macrophage toxic agent. Immunol Commun 1: 615–625 [DOI] [PubMed] [Google Scholar]
- 4.Kod’ousek R, Jezdínský J, Krajcí D. 2007. Histological and ultrastructural changes of cardiomyocytes in experimental rats with tail thrombosis following subplantar application of carrageenin. Med Princ Pract 16: 360–366 [DOI] [PubMed] [Google Scholar]
- 5.Hagimori M, Kamiya S, Yamaguchi Y, Arakawa M. 2009. Improving frequency of thrombosis by altering blood flow in the carrageenan-induced rat tail thrombosis model. Pharmacol Res 60: 320–323 [DOI] [PubMed] [Google Scholar]
- 6.Kamiya S, Hagimori M, Ogasawara M, Arakawa M. 2010. In vivo evaluation method of the effect of nattokinase on carrageenan-induced tail thrombosis in a rat model. Acta Haematol 124: 218–224 [DOI] [PubMed] [Google Scholar]
- 7.Kerac M, Bunn J, Seal A, Thindwa M, Tomkins A, Sadler K, Bahwere P, Collins S. 2009. Probiotics and prebiotics for severe acute malnutrition (PRONUT study): a double-blind efficacy randomised controlled trial in Malawi. Lancet 374: 136–144 [DOI] [PubMed] [Google Scholar]
- 8.Lei V, Friis H, Michaelsen KF. 2006. Spontaneously fermented millet product as a natural probiotic treatment for diarrhoea in young children: an intervention study in Northern Ghana. Int J Food Microbiol 110: 246–253 [DOI] [PubMed] [Google Scholar]
- 9.Ehrström S, Daroczy K, Rylander E, Samuelsson C, Johannesson U, Anzén B, Påhlson C. 2010. Lactic acid bacteria colonization and clinical outcome after probiotic supplementation in conventionally treated bacterial vaginosis and vulvovaginal candidiasis. Microbes Infect 12: 691–699 [DOI] [PubMed] [Google Scholar]
- 10.Usinger L, Ibsen H, Linneberg A, Azizi M, Flambard B, Jensen LT. 2010. Human in vivo study of the renin-angiotensin-aldosterone system and the sympathetic activity after 8 weeks daily intake of fermented milk. Clin Physiol Funct Imaging 30: 162–168 [DOI] [PubMed] [Google Scholar]
- 11.Taranto MP, Perdigón G, Médici M, De Valdez GF. 2004. Animal model for in vivo in vivo evaluation of cholesterol reduction by lactic acid bacteria. Methods Mol Biol 268: 417–422 [DOI] [PubMed] [Google Scholar]
- 12.Kitawaki R, Nishimura Y, Takagi N, Iwasaki M, Tsuzuki K, Fukuda M. 2009. Effects of lactobacillus fermented soymilk and soy yogurt on hepatic lipid accumulation in rats fed a cholesterol-free diet. Biosci Biotechnol Biochem 73: 1484–1488 [DOI] [PubMed] [Google Scholar]
- 13.Tsai TY, Chu LH, Lee CL, Pan TM. 2009. Atherosclerosis-preventing activity of lactic acid bacteria-fermented milk-soymilk supplemented with Momordica charantia. J Agric Food Chem 57: 2065–2071 [DOI] [PubMed] [Google Scholar]
- 14.Sumi H, Hamada H, Nakanishi K, Hiratani H. 1990. Enhancement of the fibrinolytic activity in plasma by oral administration of nattokinase. Acta Haematol 84: 139–143 [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi T. 2008. Advancement of prophylaxis and therapy for venous thromboembolism. Rinsho Byori 56: 589–599 [PubMed] [Google Scholar]
- 16.Gervasi GB, Bartoli C, Carpita G. 1991. A new low molecular weight heparan sulphate antagonizes kappa-carrageenan-induced thrombosis in rats. Pharmacol Res 24: 59–63 [DOI] [PubMed] [Google Scholar]
- 17.Seibold JR, Jageneau AH. 1984. Treatment of Raynaud’s phenomenon with ketanserin, a selective antagonist of the serotonin2 (5-HT2) receptor. Arthritis Rheum 27: 139–146 [DOI] [PubMed] [Google Scholar]
- 18.Bekemeier H, Hirschelmann R. 1986. Influence of serotonin, serotonin antagonists, some vasoactive substances and temperature on carrageenin-induced tail thrombosis in rats and mice. Agents Actions 18: 581–585 [DOI] [PubMed] [Google Scholar]
- 19.Bekemeier H, Hirschelmann R. 1988. Role of eicosanoids in the kappa-carrageenin rat tail thrombosis. Biomed Biochim Acta 47: S260–S263 [PubMed] [Google Scholar]
- 20.Suzuki Y, Kondo K, Matsumoto Y, Zhao BQ, Otsuguro K, Maeda T, Tsukamoto Y, Urano T, Umemura K. 2003. Dietary supplementation of fermented soybean, natto, suppresses intimal thickening and modulates the lysis of mural thrombi after endothelial injury in rat femoral artery. Life Sci 73: 1289–1298 [DOI] [PubMed] [Google Scholar]
- 21.Urano T, Ihara H, Umemura K, Suzuki Y, Oike M, Akita S, Tsukamoto Y, Suzuki I, Takada A. 2001. The profibrinolytic enzyme subtilisin NAT purified from Bacillus subtilis cleaves and inactivates plasminogen activator inhibitor type 1. J Biol Chem 276: 24690–24696 [DOI] [PubMed] [Google Scholar]
- 22.Peng Y, Huang Q, Zhang RH, Zhang YZ. 2003. Purification and characterization of a fibrinolytic enzyme produced by Bacillus amyloliquefaciens DC-4 screened from douchi, a traditional Chinese soybean food. Comp Biochem Physiol B Biochem Mol Biol 134: 45–52 [DOI] [PubMed] [Google Scholar]
- 23.Yamashita J, Ogawa M, Yamashita S, Nakashima Y, Saishoji T, Nomura K, Inada K, Kawano I. 1993. Differential biological significance of tissue-type and urokinase-type plasminogen activator in human breast cancer. Br J Cancer 68: 524–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujita M, Hong K, Ito Y, Fujii R, Kariya K, Nishimuro S. 1995. Thrombolytic effect of nattokinase on a chemically induced thrombosis model in rat. Biol Pharm Bull 18: 1387–1391 [DOI] [PubMed] [Google Scholar]
- 25.Roldán-Olarte M, Jiménez-Díaz M, Miceli DC. 2005. Plasminogen detection in oocytes and plasminogen activator activities in the porcine oviduct during the estrous cycle. Zygote 13: 115–123 [DOI] [PubMed] [Google Scholar]
- 26.Collen D, Lijnen HR. 2005. Thrombolytic agents. Thromb Haemost 93: 627–630 [DOI] [PubMed] [Google Scholar]