Abstract
Salivary immunoglobulin A (IgA) is used as an immunity marker, as saliva can be easily collected, noninvasively with little stress. However, several saliva collection methods can be used. Our comparison between samples collected using different methods demonstrated that the salivary IgA secretion rate in samples collected using an aspiration method was significantly correlated with that in samples collected using a swab method. Moreover, the significant circadian variation in salivary IgA secretion rate in the aspirated saliva suggested that the aspiration method does not suppress salivary IgA secretion rate variability compared with the swab method. Therefore, the aspiration method should be considered as the preferable saliva collection method.
Keywords: Saliva collection, IgA, aspiration, swab
The mucosal immune system maintains human health by forming the first line of defense in the body. Secretory immunoglobulin A (SIgA) is an antibody that is mainly secreted from the mucosal tissue of the digestive tract, lungs, respiratory tract, etc. It is the major antibody in the mucosal secretion, and it plays an important role in the prevention of disease by inhibiting the invasion of pathogenic microorganisms, allergens, etc. into the mucosal tissue [1]. Therefore, evaluation of SIgA can be used to assess the risk of infection.
SIgA is found in exocrine secretions such as saliva, nasal secretion, bronchoalveolar lavage fluids, intestinal fluids, and breast milk. Salivary IgA is generally considered as an immunity or stress marker, because saliva can be easily collected, noninvasively with little stress. Many studies have demonstrated that salivary IgA concentrations decline with intensive exercise, psychological stress, or aging [2,3,4,5,6,7,8,9,10,11,12,13,14,15]. Moreover, a correlation has been reported between the decline in salivary IgA and the incidence of upper respiratory tract infection (URTI) [16,17,18,19,20,21,22,23,24]. Thus, enhancement or preservation of salivary IgA secretion is considered to contribute to better health and has been reported to be influenced by lactic acid bacteria and food factors [25,26,27,28,29].
Studies investigating the causes of salivary IgA reduction have used various saliva collection methods. However, the spitting method for collecting unstimulated saliva has been the most commonly used collection method in studies examining the relationship between salivary IgA and the incidence of URTI. Salivary IgA is known to exhibit intraindividual and interindividual variations that are influenced by multiple factors, such as saliva collection methods, circadian changes, and lifestyle [2,3,4,5,6,7,8,9,10,11,12,13,14,15, 30]. Thus, more consistent salivary IgA measurements should be obtained for proper evaluation of salivary IgA secretion. The spitting, swab, and aspiration methods are the main saliva collection methods used [31]. Unstimulated, slightly stimulated, and stimulated saliva is collected using the spitting, aspiration, and swab methods, respectively. Michishige et al. demonstrated that, among the 3 saliva collection methods, the spitting and aspiration methods showed a significant positive correlation of salivary IgA concentrations [32]. The association between salivary IgA and URTI risk has been exhibited not only through salivary IgA concentrations but also through salivary IgA secretion rate [16,17,18,19,20,21,22,23,24]. Therefore, in this study, we compared the salivary IgA secretion rate in saliva samples collected using aspiration and swab methods.
Healthy male and female individuals aged 20–30 years and those aged 65 or over were included in this study, with the following exclusion criteria: (1) smoking; (2) intense exercising; (3) blood test results, and blood pressure and pulse rate values significantly outside the normal range; (4) history of digestive system or immune system disease including pneumonia, cancer, inflammatory colitis, and rheumatoid arthritis; (5) periodontitis or oral cavity bleeding; (6) antibiotic administration within 2 weeks prior to the blood test; and (7) intake of drugs that could influence digestive system or immune system function such as antiflatulents, antidiarrhetics, drugs that promote digestive function, steroids, immunosuppressants, etc. In addition to the above listed criteria, individuals considered unsuitable for the study by the supervising doctor were excluded from the study. The study specifics were explained to the participants, and a written informed consent was obtained from each subject before the study was performed. Subsequently, the investigator interviewed and examined the candidate subjects, and blood biochemical and hematological tests were conducted. As a result, a total of 60 subjects were selected for the study, including 15 individuals from each of the 4 participant groups, which consisted of young men, young women, elderly men, and elderly women. This study was approved by the Ethics Committees of HUMA CORP, a contract research organization, and performed in accordance with the Helsinki Declaration.
Subjects were asked to avoid alcoholic beverages and to fast from 9:00 pm on the night prior to the day of saliva collection. Saliva was collected from 10:00 am to 12:00 am and from 2:00 pm to 4:00 pm. Subjects had an assigned meal between 8:30 am and 9:00 am, after which they brushed their teeth and rinsed their mouths with tap water. Subjects rested prior to saliva collection. At 9:55 am, the subject rinsed their oral cavities 3 times with tap water, and at 10:00 am, they were asked to swallow the saliva in their oral cavity and to immediately tilt their head slightly forward. The tip of the tracheal tube (TOP Corporation, Tokyo, Japan) was connected to a low-pressure aspiration pump (SEASTAR Corporation, Tokyo, Japan) and set lightly at each subject’s back teeth, and collection of saliva was initiated. Immediately after initiating saliva collection, the subject was asked to close his/her mouth, and saliva was collected for 5 min in an exclusive tube while carefully avoiding contact between the aspiration port of the tracheal tube and the subject’s cheek and tongue. Saliva collected was temporarily preserved on ice. This procedure was repeated for saliva collection at 10:15 am (with a 10:20 am saliva collection time) and at 10:35 am (corresponding to a 10:40 am saliva collection time). Each sample of collected saliva was also temporarily preserved on ice. For all 3 collections, the subject was asked to maintain a resting condition in a sitting position from 5 min before saliva collection, and until the completion of saliva collection.
Fifteen minutes after all 3 saliva samples were collected by the aspiration method, we also collected 3 samples by the swab method, using a noncovered cotton roll kit (Salivette®, Sarstedt, Nümbrecht, Germany). At 10:55 am, the subjects rinsed their oral cavities 3 times with tap water, and at 11:00 am, they were asked to swallow the saliva in their oral cavities. A piece of cotton roll was placed on each subject’s right back teeth, and another was placed on his/her left back teeth. During the 1 min of saliva collection, the subject was asked to masticate once per second at the beat of a metronome. The 2 cotton rolls containing the saliva were then immediately separately placed in 2 exclusive tubes for temporary preservation on ice. Then, 2 new pieces of cotton roll were similarly placed on the subject’s right and left back teeth, and the subject was also asked to masticate once per second at the beat of a metronome, for the 1 min duration of saliva collection. The 2 cotton rolls that contained the saliva were then immediately collected, respectively placed in the 2 exclusive tubes, and preserved on ice. This saliva collection procedure was repeated for a total of 5 times. Then, at 11:15 am (saliva collection time of 11:20 am) and 11:35 am (11:40 am saliva collection time), saliva was collected following the same procedure used at 10:55 am, and each sample was temporarily preserved on ice. For all 3 saliva collections conducted over a 1-hr period, the subject was asked to maintain a resting conditions in a sitting position from 5 min before collection until the completion of saliva collection. Subjects had an assigned meal at 12:30 pm and then brushed their teeth and rinsed their mouths with tap water. Then, saliva samples were collected at 2:00 pm, 2:20 pm and 2:40 pm by the aspiration method, and at 3:00 pm, 3:20 pm, and 3:40 pm by the swab method.
The weight of saliva collected every minute for 5 min using Salivette®cotton rolls was calculated as the difference in weight between the exclusive tube used for saliva collection, which held 10 pieces of saliva-containing cotton rolls, and the same tube empty. We assumed that the weight of collected saliva represented the volume of collected saliva. Subject recruitment, selection and saliva collection were carried out by Huma corp.
Subjects were included in the analysis if their collected samples achieved the volume required for salivary IgA concentration measurement, irrespective of the saliva collection method, collection frequency, and collection time point. Salivary IgA concentration was measured by enzyme immunoassay using a commercial kit (Salivary Secretory IgA Indirect Enzyme Immunoassay Kit; Salimetrics, State College, PA, USA). Salivary IgA secretion rate (μg/5 min) was calculated by multiplying the salivary IgA concentration (μg/ml) by the saliva secretion rate (ml/5 min). Comparison between salivary IgA secretion rate (μg/5 min) was conducted using one-way or multi-factor ANOVA, including the following factors: samplings, age, gender, sampling time point, and the number of sampling times. Correlation of corresponding salivary IgA secretion rate between samples collected by the aspiration method and swab method was analyzed using Pearson’s correlation coefficient. Statistical analyses were performed using SAS software (R9.1, SAS institute Japan, Tokyo, Japan). A two-tailed p-value <0.05 was considered significant for all tests.
We selected a total of 60 participants, who were equally divided between young men, young women, elderly men, and elderly women, such that 15 participants represented each group; however, an elderly woman withdrew from the study for personal reasons. We analyzed the samples of subjects who provided all 12 saliva samples using both the aspiration and swab methods. Accordingly, the subjects included in the analysis were categorized as: 9 young men, 8 young women, 9 elderly men and 7 elderly women. A summary of the salivary IgA secretion rate of these 33 subjects is presented in Table 1. The salivary IgA secretion rate was higher in the saliva samples collected by the swab method than in those collected by the aspiration method. This could be due to the effect of masticatory stimulation of the amount of saliva secretion (data not shown). Aufricht et al. reported that the salivary IgA concentration was inversely correlated with the salivary secretion rate in saliva collected by the spitting method [30]. By the swab method, the salivary IgA secretion rate of the first sample collected in the morning from elderly participants was significantly higher than those of the second and third samples collected. Additionally, the salivary IgA secretion rate of the first afternoon sample collected from elderly participants was significantly higher than that of the third sample collected. Although such differences were not detected in samples collected from young participants, the average values of the first samples were higher than those of the second and third samples, both in the morning and in the afternoon sample collections. On the other hand, by the aspiration method, only the first morning sample collected from elderly participants was significantly higher than that of the third samples; however, no other significant differences were observed between samples collected from young and elderly subjects.
Table 1. Salivary IgA secretion rate (μg/5 min).
| Saliva | Young (N = 17) |
Elderly (N = 16) |
|||
| Morning | Afternoon | Morning | Afternoon | ||
| Swab | 1st | 588 ± 357 | 591 ± 304 | 697 ± 242 | 650 ± 240 |
| 2nd | 480 ± 292 | 530 ± 297 | 524 ± 187 | 536 ± 163 | |
| 3nd | 503 ± 282 | 522 ± 293 | 492 ± 179 | 497 ± 141 | |
| Aspiration | 1st | 346 ± 199 | 293 ± 148 | 355 ± 215 | 396 ± 186 |
| 2nd | 263 ± 134 | 358 ± 239 | 264 ± 94 | 381 ± 153 | |
| 3nd | 277 ± 159 | 306 ± 157 | 236 ± 94 | 320 ± 147 | |
Values are means ± SD.
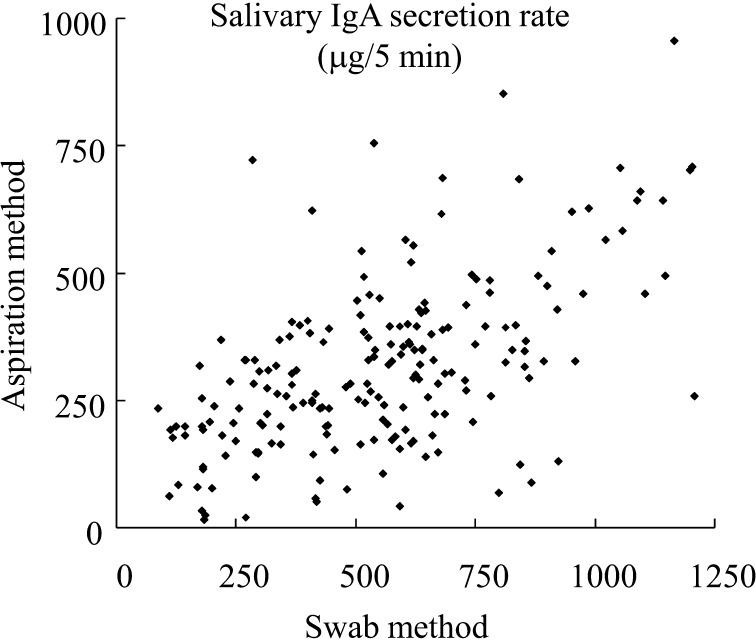
All 396 samples collected from the 33 subjects by the swab method and aspiration method were analyzed. Comparison analysis of corresponding salivary IgA secretion rate (μg/5 min) between samples collected by the aspiration method and swab method revealed a significant correlation (p<0.01) with a Pearson’s correlation coefficient of 0.573 (Fig. 1). Michishige et al. reported that the salivary IgA concentration was significantly correlated in samples collected by the aspiration and spitting methods [32]. Correlation between the salivary secretion rate in samples collected by aspiration and spitting methods is expected, since unstimulated and slightly stimulated saliva are collected using these methods. This observation and our results suggest that salivary IgA secretion rate in saliva samples collected by aspiration, swab, and spitting methods could be correlated.
Fig. 1.
Correlation of salivary IgA secretion rate in samples collected by swab and aspiration methods. Correlation was analyzed using Pearson’s correlation coefficient.
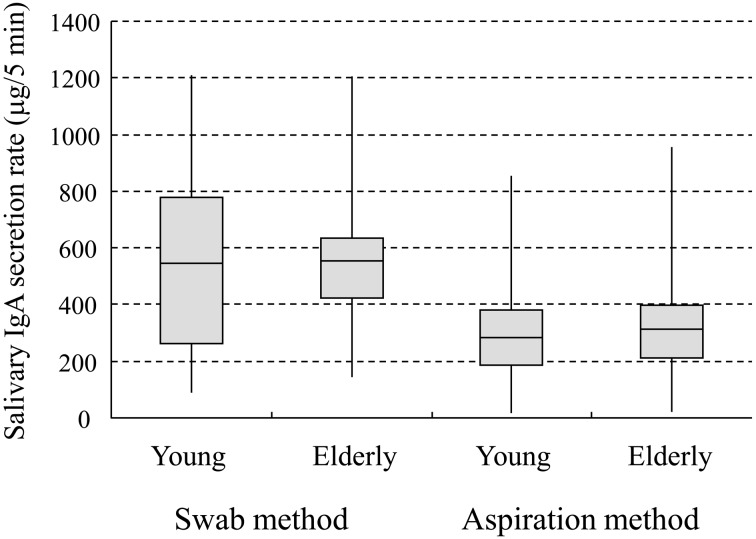
Table 2 demonstrates the statistical analysis results based on 4 factors: age, gender, sampling time and the number of samples collected. A significant difference was detected between sampling time points when using the aspiration method but not the swab method. It has been reported by Miletic et al. that differences between morning and afternoon could be caused by circadian changes in salivary IgA [3]. Therefore, we consider that the aspiration method allows the detection of such circadian-related differences. The swab method showed a highly significant difference according to the number of samples collected. This might indicate a wide variation in salivary IgA secretion rate for samples collected by the swab method; it might also be caused by a decrease in saliva secretion volume between the first and third saliva collections among the young subjects. Our results also confirmed that, among young subjects, IgA secretion rate of saliva samples collected using the low-pressure aspiration pump was more tightly distributed than those of samples collected using the swab method (Fig. 2).
Table 2. Factors influencing salivary IgA secretion according to the saliva collection method.
| Age | Gender | Sampling time point | Number of samples collected | |
| (Young/Elderly) | (Male/Female) | (Morning/Afternoon) | (1, 2, 3 times) | |
| p value | ||||
| Swab method | 0.4293 | 0.1484 | 0.8403 | 0.0081 |
| Aspiration Method | 0.4668 | 0.3628 | 0.0314 | 0.1069 |
p values were obtained using multi-factor ANOVA (factors: sampling method, age, gender, sampling time point and number of samples collected). Results show correlation of corresponding salivary IgA secretion rate between samples collected by the aspiration method and swab method.
Fig. 2.
Box-whisker plot of salivary IgA secretion rate
Distribution of salivary IgA secretion rate (µg/5 min) among the young and elderly participants, in samples collected by the swab and aspiration methods. The box-whisker plot displays the mean, quartiles, and the minimum and maximum values observed for each group. The plot elements and the statistics they represent are as follows: the length of the box represents the interquartile range (the distance between the 25th and 75th percentiles); the horizontal line in the box interior represents the mean; and the vertical lines issuing from the box extend to the minimum and maximum values of the analysis variable.
By the swab method, at least 1 out of 6 saliva samples could not be collected from 6 young men, 7 young women, 6 elderly men, and 7 elderly women. Meanwhile, saliva samples were not collected from 3 young men and 3 elderly women when using the aspiration method, and these participants were among those who were unable to provide saliva samples using the swab method as well. When using the swab method, and even if saliva secretion was stimulated by mastication, we were sometimes unable to collect saliva from cotton rolls due to a low saliva flow rate. We also could not accurately measure saliva flow when cotton rolls failed to absorb all the secreted saliva. These show that the aspiration method can be a highly effective method for saliva sample collection. Michishige et al. recommended using the aspiration method for saliva collection because some elderly people cannot spit well [32]. Moreover, Jones et al. reported that 69% of elderly people preferred the aspiration method to the spitting method [33]. Although the swab method makes it easy to obtain saliva samples, our results showed wide variation in salivary IgA secretion rate. In addition, there are people who cannot spit saliva well due to various kinds of diseases. On the other hand, the aspiration method requires an aspiration pump but has certain strong points, such as allowing control of the aspiration period and providing high precision in saliva collection from almost all kinds of subjects.
Michishige et al. also investigated whether the concentrations of each total protein, kallikrein activity, trypsin activity and trypsin-like protease in aspirated saliva were significantly positively correlated with those in spitted saliva [32]. As described above, one of the significant benefit of the aspiration method is accurate saliva collection. Considering our salivary IgA results, the aspiration method would also be the preferable saliva collection method for examination of the secretion rate for these measurements.
In conclusion, the aspiration method leads the collected salivary IgA secretion rate to correlate with that of the swab method with high precision. Moreover, this method would prevent the failure of saliva collection and be used to collect saliva from almost all kinds of subjects under fixed conditions, such as the collection period. The aspiration method could provide a good and effective method for evaluating salivary IgA secretion rate and predicting URTI.
Acknowledgments
We thank Mr. Hiroshi Okamatsu, Otsuka Pharmaceutical Co., Ltd., for considerable contribution to this study and Prof. Fumiko Michishige, School of Nursing, Kyoto Tachibana University, for providing instructions regarding saliva collection.
REFERENCES
- 1.McKay DM, Perdue MH. 1993. Intestinal epithelial function: the case for immunophysiological regulation. Cells and mediators (1). Dig Dis Sci 38: 1377–1387 [DOI] [PubMed] [Google Scholar]
- 2.Challacombe SJ, Percival RS, Marsh PD. 1995. Age-related changes in immunoglobulin isotypes in whole and parotid saliva and serum in healthy individuals. Oral Microbiol Immunol 10: 202–207 [DOI] [PubMed] [Google Scholar]
- 3.Miletic ID, Schiffman SS, Miletic VD, Sattely-Miller EA. 1996. Salivary IgA secretion rate in young and elderly persons. Physiol Behav 60: 243–248 [DOI] [PubMed] [Google Scholar]
- 4.Phillips AC, Carroll D, Evans P, Bosch JA, Clow A, Hucklebridge F, Der G. 2006. Stressful life events are associated with low secretion rates of immunoglobulin A in saliva in the middle aged and elderly. Brain Behav Immun 20: 191–197 [DOI] [PubMed] [Google Scholar]
- 5.Gallagher S, Phillips AC, Evans P, Der G, Hunt K, Carroll D. 2008. Caregiving is associated with low secretion rates of immunoglobulin A in saliva. Brain Behav Immun 22: 565–572 [DOI] [PubMed] [Google Scholar]
- 6.Jafarzadeh A, Sadeghi M, Karam GA, Vazirinejad R. 2010. Salivary IgA and IgE levels in healthy subjects: relation to age and gender. Braz Oral Res 24: 21–27 [DOI] [PubMed] [Google Scholar]
- 7.Jemmott JB, 3rd, Borysenko JZ, Borysenko M, McClelland DC, Chapman R, Meyer D, Benson H. 1983. Academic stress, power motivation, and decrease in secretion rate of salivary secretory immunoglobulin A. Lancet 1: 1400–1402 [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Koh D, Ng V, Lee CY, Chan G, Dong F, Goh SH, Anantharaman V, Chia SE. 2002. Self perceived work related stress and the relation with salivary IgA and lysozyme among emergency department nurses. Occup Environ Med 59: 836–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng V, Koh D, Mok B, Lim LP, Yang Y, Chia SE. 2004. Stressful life events of dental students and salivary immunoglobulin A. Int J Immunopathol Pharmacol 17: 49–56 [DOI] [PubMed] [Google Scholar]
- 10.Tsuboi H, Hamer M, Tanaka G, Takagi K, Kinae N, Steptoe A. 2008. Responses of ultra-weak chemiluminescence and secretory IgA in saliva to the induction of angry and depressive moods. Brain Behav Immun 22: 209–214 [DOI] [PubMed] [Google Scholar]
- 11.Pacque PF, Booth CK, Ball MJ, Dwyer DB. 2007. The effect of an ultra-endurance running race on mucosal and humoral immune function. J Sports Med Phys Fitness 47: 496–501 [PubMed] [Google Scholar]
- 12.Moir H, Butcher L, Jones KP, Hughes MG, Neale H, Jia H, Al-Ismaily Z, Webb R. 2008. AMPK inactivation in mononuclear cells: a potential intracellular mechanism for exercise-induced immunosuppression. Appl Physiol Nutr Metab 33: 75–85 [DOI] [PubMed] [Google Scholar]
- 13.Arroyo-Morales M, Olea N, Ruíz C, del Castilo Jde D, Martínez M, Lorenzo C, Díaz-Rodríguez L. 2009. Massage after exercise–responses of immunologic and endocrine markers: a randomized single-blind placebo-controlled study. J Strength Cond Res 23: 638–644 [DOI] [PubMed] [Google Scholar]
- 14.Khaustova SA, Shkurnikov MU, Tonevitsky AG. 2010. Short highly intense exercise causes changes in salivary concentrations of hydrocortisone and secretory IgA. Bull Exp Biol Med 149: 635–639 [DOI] [PubMed] [Google Scholar]
- 15.Tsai ML, Chou KM, Chang CK, Fang SH. 2011. Changes of mucosal immunity and antioxidation activity in elite male Taiwanese taekwondo athletes associated with intensive training and rapid weight loss. Br J Sports Med 45: 729–734 [DOI] [PubMed] [Google Scholar]
- 16.Isaacs D, Webster AD, Valman HB. 1984. Immunoglobulin levels and function in pre-school children with recurrent respiratory infections. Clin Exp Immunol 58: 335–340 [PMC free article] [PubMed] [Google Scholar]
- 17.Gleeson M, McDonald WA, Pyne DB, Cripps AW, Francis JL, Fricker PA, Clancy RL. 1999. Salivary IgA levels and infection risk in elite swimmers. Med Sci Sports Exerc 31: 67–73 [DOI] [PubMed] [Google Scholar]
- 18.Novas AM, Rowbottom DG, Jenkins DG. 2003. Tennis, incidence of URTI and salivary IgA. Int J Sports Med 24: 223–229 [DOI] [PubMed] [Google Scholar]
- 19.Fahlman MM, Engels HJ. 2005. Mucosal IgA and URTI in American college football players: a year longitudinal study. Med Sci Sports Exerc 37: 374–380 [DOI] [PubMed] [Google Scholar]
- 20.Nieman DC, Henson DA, Dumke CL, Lind RH, Shooter LR, Gross SJ. 2006. Relationship between salivary IgA secretion and upper respiratory tract infection following a 160-km race. J Sports Med Phys Fitness 46: 158–162 [PubMed] [Google Scholar]
- 21.Hall H, Fahlman MM, Engels HJ. 2007. Echinacea purpurea and mucosal immunity. Int J Sports Med 28: 792–797 [DOI] [PubMed] [Google Scholar]
- 22.Neville V, Gleeson M, Folland JP. 2008. Salivary IgA as a risk factor for upper respiratory infections in elite professional athletes. Med Sci Sports Exerc 40: 1228–1236 [DOI] [PubMed] [Google Scholar]
- 23.Cunniffe B, Griffiths H, Proctor W, Davies B, Baker JS, Jones KP. 2011. Mucosal immunity and illness incidence in elite rugby union players across a season. Med Sci Sports Exerc 43: 388–397 [DOI] [PubMed] [Google Scholar]
- 24.Gleeson M, Bishop NC, Oliveira M, Tauler P. 2011. Daily probiotic’s (Lactobacillus casei Shirota) reduction of infection incidence in athletes. Int J Sport Nutr Exerc Metab 21: 55–64 [DOI] [PubMed] [Google Scholar]
- 25.Kotani Y, Shinkai S, Okamatsu H, Toba M, Ogawa K, Yoshida H, Fukaya T, Fujiwara Y, Chaves PH, Kakumoto K, Kohda N. 2010. Oral intake of Lactobacillus pentosus strain b240 accelerates salivary immunoglobulin A secretion in the elderly: a randomized, placebo-controlled, double-blind trial. Immun Ageing 7: 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tormo Carnicer R, Infante Piña D, Roselló Mayans E, Bartolomé Comas R. 2006. Intake of fermented milk containing Lactobacillus casei DN-114 001 and its effect on gut flora. An Pediatr (Barc) 65: 448–453 [DOI] [PubMed] [Google Scholar]
- 27.Lara-Villoslada F, Sierra S, Boza J, Xaus J, Olivares M. 2007. Beneficial effects of consumption of a dairy product containing two probiotic strains, Lactobacillus coryniformis CECT5711 and Lactobacillus gasseri CECT5714 in healthy children. Nutr Hosp 22: 496–502 [PubMed] [Google Scholar]
- 28.Mero A, Miikkulainen H, Riski J, Pakkanen R, Aalto J, Takala T. 2002. Effects of bovine colostrum supplementation on serum IGF-I, IgG, hormone, and saliva IgA during training. J Appl Physiol 93: 732–739 [DOI] [PubMed] [Google Scholar]
- 29.Crooks CV, Wall CR, Cross ML, Rutherfurd-Markwick KJ. 2006. The effect of bovine colostrum supplementation on salivary IgA in distance runners. Int J Sport Nutr Exerc Metab 16: 47–64 [DOI] [PubMed] [Google Scholar]
- 30.Aufricht C, Tenner W, Salzer HR, Khoss AE, Wurst E, Herkner K. 1992. Salivary IgA concentration is influenced by the saliva collection method. Eur J Clin Chem Clin Biochem 30: 81–83 [PubMed] [Google Scholar]
- 31.Navazesh M. 1993. Methods for collecting saliva. Ann N Y Acad Sci 694: 72–77 [DOI] [PubMed] [Google Scholar]
- 32.Michishige F, Kanno K, Yoshinaga S, Hinode D, Takehisa Y, Yasuoka S. 2006. Effect of saliva collection method on the concentration of protein components in saliva. J Med Invest 53: 140–146 [DOI] [PubMed] [Google Scholar]
- 33.Jones JM, Watkins CA, Hand JS, Warren JJ, Cowen HJ. 2000. Comparison of three salivary flow rate assessment methods in an elderly population. Community Dent Oral Epidemiol 28: 177–184 [DOI] [PubMed] [Google Scholar]