Abstract
Psychopaths commit a disproportionate amount of violent crime, and this places a substantial economic and emotional burden on society. Elucidation of the neural correlates of psychopathy may lead to improved management and treatment of the condition. Although some methodological issues remain, the neuroimaging literature is generally converging on a set of brain regions and circuits that are consistently implicated in the condition: the orbitofrontal cortex, amygdala, and the anterior and posterior cingulate and adjacent (para)limbic structures. We discuss these findings in the context of extant theories of psychopathy and highlight the potential legal and policy implications of this body of work.
Psychopathy: the personality disorder
Psychopathy is a construct characterized by symptoms of emotional detachment and a propensity for disinhibited, impulsive behavior combined with a general callousness and lack of insight for the impact such behavior has on others [1]. Psychopathy is among the most important psychological constructs in legal settings, having strong predictive utility for recidivism (the tendency to re-offend), institutional adjustment and treatment outcomes [2,3]. Indeed, within 1 year of release, psychopaths are approximately three times more likely to recidivate than non-psychopaths, and four times more likely to violently recidivate [4]. Furthermore, this pattern is alarmingly stable and persistent. Juveniles with identifiable psychopathic traits demonstrate similar recidivism rates [5], and traditional therapeutic intervention strategies with adult psychopaths have often proven to be ineffective or even counterproductive [6–8].
The symptoms of psychopathy, defined by 200 years of clinical and forensic work, are well established. Psychometric analyses of these symptoms suggest theoretically relevant subcomponents representing features such as affective deficits and impulsive antisocial tendencies (Box 1). Forensic research indicates that the impulsive facets of psychopathy are particularly important in predicting criminal activity [3], and clinicians maintain that the affective deficits are fundamental to the construct, distinguishing psychopathy from other etiological paths to criminality [9]. An understanding of the nature of psychopathy has become an increasingly urgent need among a diverse set of researchers owing to the mental health, social, legal and philosophical implications stemming from research suggesting that psychopathy has a neural basis [10,11] with strong genetic heritability [12].
Box 1. (Sub)types of psychopathy.
There is a long history of dividing the symptoms of psychopathy into subtypes or classifying different types of psychopaths. Some of the more prominent divisions are described below.
Primary versus secondary psychopathy
Karpman suggested that primary psychopathy is the consequence of an intrinsic idiopathic deficit and that secondary psychopathy is the result of indirect factors (i.e. trauma exposure) [84,85]. The distinction has evolved somewhat and it is often suggested that primary psychopaths are characterized by lower anxiety and poverty of emotional expression, and tend to commit crimes that are fundamentally instrumental in nature; conversely, secondary psychopaths are more anxious, show more emotional volatility, and commit more impulsive, reactionary crimes [86]. The symptoms are more or less the same for primary versus secondary psychopathy, with the groups only differing in anxiety.
Successful and unsuccessful
Criminal activity, although a common correlate of psychopathy, is not a necessary component for its definition [1,9]. The putative ‘successful’ (or adaptive or non-criminal) psychopath, while possessing the core personality traits associated with psychopathy, either refrains from traditional criminal activity or possesses resources that allows him/her to avoid conviction [87]. It is unclear whether these subtypes are primarily distinguished by fundamental differences in the brain (or genetics) or by protective environmental factors such as socioeconomic status, IQ, education and parenting. It is also unclear whether ‘successful’ is a useful term [88], because psychopathic traits, at least at the clinical level, are associated with impairment in multiple domains of life, including interpersonal problems at home, work and school and with extended family, and general impairments in moral sensibility. Investigations that make use of this characterization often rely on self-reported criminal convictions as a distinguishing factor; however, this would not account for the full range of impairments associated with psychopathy, nor would it account for patterns of antisocial deviance not resulting in criminal conviction.
Measuring psychopathy and its subfactors
Several tools have been used to operationalize psychopathy in experimental settings, but Hare’s Psychopathy Checklist-Revised (PCL-R) [16,17] is the most widely validated and most popular. The PCL-R was developed for use in forensic settings, comprises 20 items (Table 1), each scored on a two-point scale, and has a recommended cutoff score of 30 (out of 40) for designation of psychopathy in forensic or clinical settings. Unfortunately, neuroimaging studies often use different group cutoffs, raising concerns about comparability across samples.
Several other tools have been developed for use in non-incarcerated samples, such as Hare’s Self-Report Psychopathy Scale [89], Levenson’s Self-Report Psychopathy Scale [90] and the Psychopathic Personality Inventory [91], each of which has particular strengths and weaknesses [92]. The psychopathic deviate scale of the Minnesota Multiphasic Personality Inventory (MMPI) [93] has also been used; however, it does not correlate well with PCL-R scores [89]. Owing in part to the modest correlations between expert rater devices such as the PCL-R and these self-report measures, considerable debate exists regarding whether these devices measure the same construct. Thus, readers of this literature should pay careful attention to the assessment procedure used.
Nevertheless, factor analyses of these measures typically support at least two underlying subcomponents of psychopathy, most commonly corresponding to affective deficits and impulsive/antisocial traits [94,95]. More complex, alternative solutions have been suggested [17,96]; even so, each of these alternative solutions has components that distinguish impulsive and antisocial behavior from emotional deficits. It is important to recognize that a DSM-IV diagnosis of antisocial personality disorder (ASPD) is most strongly related to the impulsive/antisocial factor of the PCL-R, and ASPD has been highly criticized as being synonymous with criminality, all but ignoring the affective traits that most consider central to the construct of psychopathy [15]. Thus, ASPD and psychopathy as assessed by the PCL-R are not the same constructs and have little overlap. It is crucial that readers of the literature consider the assessment procedure for psychopathy when reviewing and interpreting results.
Psychopathy is considered a personality disorder. It had been specifically included in the Diagnostic and Statistical Manual of Mental Disorders [13] until it was conceptually consolidated in version three and subsequent editions with antisocial personality disorder (ASPD) [14]; however, the ASPD designation, which the framers of the DSM-IV hoped would capture the essential components of psychopathy, has been criticized for its over-emphasis on behavioral outcomes (such as criminality) and under-emphasis of the core personality features such as affective deficits [15]. Thus, psychopathy and ASPD are not the same condition. The most widely used and validated instrument for the assessment of psychopathy in clinical, research and forensic contexts is Hare’s Psychopathy Checklist-Revised (PCL-R) [16,17]. The Hare PCL-R comprises the complete set of traits classically associated with psychopathy that contribute to considerable disturbances in character, behavior and maladaptive social functioning, which are remarkably stable [18]. Thus, psychopathy is consistent with the widely accepted mental illness definition of a personality disorder, especially when recognized in its full clinical manifestation.
An alternative view asserts that psychopathic traits may be characterized as conferring a competitive advantage in some circumstances [19]. For instance, an individual who is cunning and manipulative and maintains a general disregard for the wellbeing of others might do very well for him/herself in a variety of contexts ranging from basic competition for resources to politics and business. Indeed, executive boardrooms are reportedly rife with these characteristics [20]. Research focusing on such traits outside the traditional criminal or forensic context has sometimes referred to this brand of psychopath as adaptive, subclinical, non-criminal or successful [21]. Speculative notions about the value of such traits for survival and/or reproductive advantages are intuitively plausible. For example, during stressful times in our evolutionary history, a physiological make-up that is relatively immune to stress and anxiety may prevent the development of post-traumatic stress disorder. Likewise, a biology that craves novelty and a changing environment might have encouraged migration and a wider search for resources.
History and literature have provided us with examples seemingly consistent with psychopathy across cultures, yet the full clinical manifestation of psychopathy occurs in less than 1% of the general population [17]. Selective promotion of these traits may be limited because those recognized for these traits have consistently been ostracized, shunned and punished within social systems that rely on cooperation to thrive [19], a stigma pervasive across cultures [22]. The discussion of what should appropriately be called a mental health disorder is a familiar one among other psychiatric designations that probably occur on a continuum. It is important to recognize that, similar to other psychiatric conditions, moderate expressions of these traits may be advantageous in certain contexts; however, in its full clinical manifestation, psychopathy represents a maladaptive condition promoting severe functional deficits including impaired passive avoidance learning [23] and aversive conditioning [24], combined with the significant consequences of impulsive risk-taking and probable disadvantages for social integration. A great deal of variation exists in subclinical manifestations of psychopathic traits, so careful consideration should be given to research in which subjects do not meet full criteria for the disorder. Without explicit consideration of this variability, subtle differences in experimental outcomes are likely to contribute to inconsistencies and confusion. Despite some variability in such methodological issues, a general consistency has begun to emerge in the outcomes of neuroimaging investigations of psychopathy. Recent reports indicate dysfunction in limbic or paralimbic brain regions associated with the integration of emotional information into higher-order cognitive processes. The current review highlights the most recent of this literature and applies this to existing models of psychopathy.
Neurobiological models of psychopathy
Determination of the physiological correlates of psychopathy has been a concern of empirical research since at least the 1950s, when it was recognized that psychopaths fail to show appropriate autonomic responses to aversive stimuli [25]. Over the years, many models of psychopathy have been suggested, which we summarize below.
A common thread among models of psychopathy has emphasized abnormalities in the integration of emotional response into behavior, essentially recognizing aversive situations and acting to avoid them. This is a prominent feature of Lykken’s low fear hypothesis [26], which suggests that psychopaths (specifically primary psychopaths; Box 1) have a subdued fear response, something that ordinarily promotes avoidance of dangerous or embarrassing situations in healthy individuals. Likewise, using Gray’s two-factor reinforcement sensitivity theory [27], Fowles suggested that psychopaths have a weak behavioral inhibition system, leaving a relatively unconstrained behavioral activation system to run amok, promoting impulsive behavior [28]. Damasio’s somatic marker hypothesis [29] has also been invoked to suggest that psychopaths are inadequate in their ability to utilize somatic emotional cues for the purposes of anticipating punishment.
Modern neuroimaging investigations have contributed to the development of two prominent neurobiological theories of psychopathy put forth by Blair [10] and Kiehl [11]. These models share a number of attributes but also have some important differences. Both theories implicate components of the limbic system, a network of brain regions supporting the utilization of emotional information in behavioral regulation. Blair’s model has primarily emphasized dysfunction in the amygdala leading to the development of psychopathy. The amygdala is integral in forming associations between environmental cues and affective states and the activation of basic threat circuits. More recently, Blair emphasized the role of both the amygdala and the ventromedial prefrontal cortex in ongoing monitoring of behavior against established reinforcement expectancies [30]. This expansion of the model accounts for distinguishable forms of antisocial deviance observed in psychopathy.
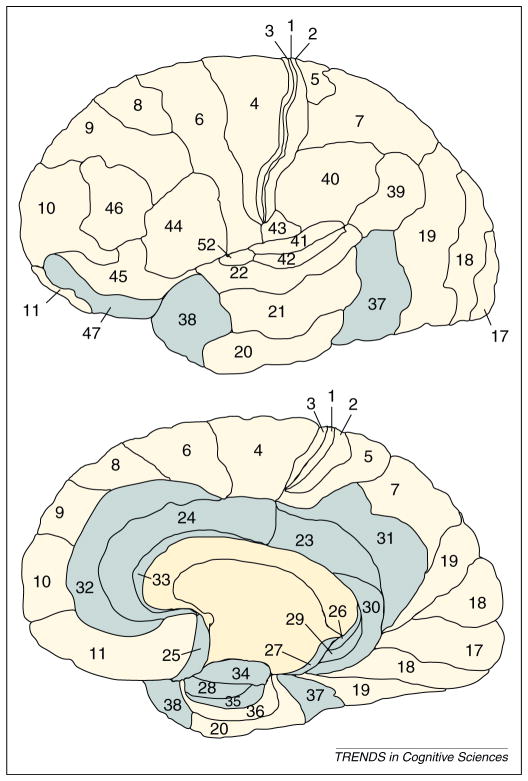
Kiehl’s paralimbic dysfunction model [11], relative to Blair’s model, describes more widely distributed abnormalities throughout the brains of psychopaths. This model is grounded in the study of cytoarchitectonics [31,32], which has identified closely related regions of the brain based on similarities in neuronal type, structure and density. The anterior superior temporal gyrus (temporal pole), anterior cingulate, posterior cingulate, orbitofrontal cortex, insula and parahippocampal regions are intimately connected to primary limbic regions including the amygdala, septal region and substantia innominata. The paralimbic system refers to primary limbic structures and this extended network of brain regions that provide a transition from subcortical structures to higher neocortical regions (Figure 1). Kiehl’s model accounts for evidence that psychopaths present with abnormalities in areas beyond primary limbic structures such as the anterior and posterior cingulate cortex, the temporal pole, insula and parahippocampal gyrus [11,33].
Figure 1.
A cytoarchitectonic map of the human brain [31]. This map divides regions of the brain according to similarity in the types and density of neurons. For example, primary visual (17), auditory (41), and motor (4) regions have similar neuronal organization. Prefrontal and parietal cortex are also similar in structure. Paralimbic regions include the amygdala (34), orbital frontal cortex (25, 47), anterior (32, 33, 24) and posterior cingulate (23, 26, 29, 30, 31), temporal pole (38), parahippocampal area (27, 28, 35, 37) and insula (not depicted).
A third model deserves attention here, although it may be better described as a cognitive model of psychopathy. Newman’s response modulation hypothesis suggests that apparent deficits in the processing of emotional information by psychopaths may be attributable to problems in switching attention to stimuli with emotional salience or peripheral stimuli in general [34]. While the attention of healthy individuals is occupied automatically and involuntarily by stimuli that are relevant to species safety and survival, some evidence suggests that psychopaths have deficiencies in this allocation of attention, which adversely impacts the integration of this information into ongoing behavioral modification. Primary support for this notion comes in the form of diminished performance in passive avoidance learning tasks [35], diminished interference in a primary task due to peripherally presented distractors [36] and abnormal modification of startle reflexes in psychopaths [37]. If their capacity to attend to emotional information is compromised, this may help to explain a fundamental reason behind the apparent failure of psychopaths to integrate emotional information into higher cognitive function. Future neuroimaging research in this area is much needed because functional anatomical units supporting different modes of attention are poorly understood.
Recent neuroimaging and psychopathy
Escalating attention to psychopathy has been accompanied by a meteoric rise in neuroimaging research on this topic, partly because of rapidly improving technology and methodological advances. This trend reveals a pressing need for frequent review and synthesis of this information, along with careful consideration of methodological variation.
Recent reviews indicate that the most common structural and functional abnormalities observed in the brains of psychopaths are distributed in frontal and temporal cortical regions [38–40]. Particularly relevant are core limbic structures of the medial temporal lobe, such as the hippocampus and amygdala, along with specific portions of the frontal lobe including the ventromedial/orbitofrontal and frontopolar regions. Other functional units that are commonly implicated in psychopathy are the superior temporal cortex, cingulate cortex, striatum and insula. The most recent data generally support these previous findings. Furthermore, recent research has begun to identify with greater specificity the subtypes and facets of psychopathy distinctly related to these neural abnormalities. Here we address the most recent data attributing differences in psychopaths to three broad functional units: the amygdala, prefrontal cortex and extended paralimbic structures. We discuss these findings in the context of available models of psychopathy and conclude that both Blair’s and Kiehl’s models continue to be supported, yet there remains a need for greater clarity in accounting for specific features of psychopathy (Box 1), which may be addressed through careful attention to certain methodological details outlined below (Box 2).
Box 2. Methodological concerns.
Inherent in the rapid growth of both neuroimaging and psychopathy research is a great deal of variability in methodological practices, which can drastically influence outcomes and adversely affect coherence between studies. We summarize below a list of methodological issues that require attention. For a more detailed review, see Koenigs et al.[97].
Assessment tools
Several measures have been developed to assess psychopathy, ranging from structured interviews designed to be carried out in forensic settings by trained experts to self-report measures designed to be implemented in normal-range community samples. Debate persists over the consistency of the construct across measures. Readers of the literature must pay attention to the psychopathy assessment procedure used because the different measures may not agree.
Cutoffs
Some assessment tools have recommended cutoffs for designating psychopathy; for instance, the PCL-R recommends a cutoff of 30 for incarcerated samples. These thresholds are often not adhered to in attempts to increase the number of purported psychopaths in a particular sample. This raises concerns regarding computation of effect sizes and comparisons across studies.
Factors and subtypes
The construct of psychopathy may be divided into several relevant subcomponents including, but not limited to, comparisons of successful and unsuccessful psychopaths, primary and secondary psychopaths, and factor elements such as affective traits and antisociality. Careful attention should be given to the precise comparisons being used in reports of a given effect.
Supression effects
Factor 1 (affective) and factor 2 (impulsive/antisocial) elements of psychopathy are correlated with one another; however, evidence suggests that these factors may have opposing effects on some physiological outcomes. Failure to account for these components separately may, in some cases, dilute valuable effects of interest.
Categorical and dimensional analysis
Psychopathy may be viewed as existing on a continuum, that is, some individuals are more psychopathic than others. Historically, most investigations of psychopathy have been carried out in a categorical fashion; however, there has been an increasing trend to implement continuous analyses. Our preference is to report both continuous and grouped analyses.
Comorbidity and other intervening variables
Psychopathy very often occurs together with substance abuse, and care should be taken to control for this as a mediating factor, particularly in physiological outcomes. Other common subject variables that may need to be controlled for are intelligence, level of education, socioeconomic status, gender, and age.
multiple comparisons
Neuroimaging investigations are particularly prone to type I errors if care is not taken to control for the large number of multiple comparisons inherent in this field. This has become an important standard in empirical reporting of these data, but one that has not always been adhered to.
Redundant samples
Neuroimaging provides rich data sets that often allow for investigation and reporting of several distinct hypotheses using the same subjects; however, investigators do not always make it clear that a given report utilizes the same subjects as a previous report. This can artificially reinforce effects investigated across multiple studies, particularly in large-scale reviews and in meta-analyses.
Small sample size
Neuroimaging investigations require considerable resources and thus reports have often been published for very small sample sizes. Generalization of effects is virtually impossible for very small sample sizes because the idiosyncratic characteristics of small groups are less likely to average out across a larger number of subjects. The consequences of this are often apparent in their lack of replicability.
Amygdala
The amygdala is located in the medial temporal lobe and has an important role in the acquisition of stimulus reinforcement learning and the recognition of emotionally salient information, such as the detection of threat cues [41] and salient auditory or visual stimuli [42]. Because of its integral role in threat detection, the amygdala was thought to be dysfunctional in psychopaths even before recent support from neuroimaging research [43]. This hypothesis has been confirmed in several fMRI studies, which have revealed that psychopathic traits are associated with abnormalities in hemodynamic activity in the amygdala [33]. In recent investigations, relative to controls, psychopaths demonstrated lower levels of amygdala activation when viewing pictures depicting moral violations [44] and fearful faces [45]. With the use of scores for psychopathy measures as continuous variables, higher levels of psychopathy have been associated with reduced amygdala activity during aversive conditioning [46], and while viewing pictures of aversive stimuli [47]. Youths with callous/unemotional traits and conduct disorder also show lower amygdala activity when engaged in passive avoidance learning [48].
In terms of anatomical structure, scores for psychopathy measures have been associated with gray-matter volume reductions in the amygdala. In a large-scale assessment involving nearly 300 incarcerated subjects, Ermer and colleagues reported reduced volumes in the amygdala, along with several other regions discussed further below [49]. This effect has also been demonstrated in those with criminal records compared to those without self-reported criminal records (but who reported that they engaged in as much criminal behavior as those with records) [50]. Still others have found this effect more reliably associated with affective/interpersonal facets of psychopathy than with implusive/behavioral symptoms [51]. Comparing psychopathic criminal offenders to controls, Boccardi et al. examined specific anatomical nuclei of the amygdaloid complex and found significant reductions in the basolateral amygdalae of psychopaths [52]. The basolateral nucleus shares reciprocal connections with the orbitofrontal cortex and is thought to be important for updating reinforcement value [53,54]. Boccardi et al. also found that central and lateral nuclei were enlarged in psychopaths compared to controls. The central and lateral nuclei are integral components of the basic threat circuit and are strongly implicated in fear conditioning [54,55]. It should be noted that in this particular sample, all the psychopaths also met criteria for substance abuse, but none of the controls did, leaving open the possibility for substance use as an intervening factor. In by far the largest sample used to examine these features, Ermer and colleagues still found reductions in amygdala volumes after controlling for substance, brain size and age [49].
The amygdala remains a prominent fixture in the investigation of neural underpinnings of psychopathy. Most contemporary models of psychopathy, including the Kiehl and Blair models, acknowledge the involvement of the amygdala in the development of psychopathic traits, and the most recent neuroimaging data continue to support existing models. What remain to be shown are the reasons why the amygdala may fail to show appropriate activation in psychopaths. It could be a physical deficiency in the ability of the amygdala to encode certain forms of salient information, but it remains possible that it is a failure in other networks governing automated attention to salient information that are impaired. Further investigations into these possibilities should be pursued.
Prefrontal cortex
Areas of the prefrontal cortex are important for monitoring ongoing behavior, estimating consequences and incorporating emotional learning into decision-making [56,57]. Like the amygdala, the prefrontal cortex has featured prominently in theories of psychopathy. Before recent neuroimaging evidence emerged, the prefrontal cortex was implicated in psychopathy because of indications that damage here is capable of instigating patterns of poor moral judgment and impulsivity similar to those observed in psychopathy [58]. Recent evidence suggests that damage to the ventromedial portion of the prefrontal cortex is also associated with specific types of utilitarian moral judgments (e.g. sacrificing one life to save two) [59], which exemplifies the type of non-empathic rationality that characterizes decision-making by psychopaths.
The orbitofrontal/ventromedial prefrontal cortex remains the most common prefrontal region implicated in recent neuroimaging investigations of psychopathy. Reductions in orbitofrontal gray matter have been consistently observed in comparing psychopaths to non-psychopaths [52,60,61], along with reductions in the most anterior frontopolar regions of the prefrontal cortex [60,61]. It has also been reported that cortical thickness in the orbitofrontal region of psychopaths is inversely related to response perseveration, a classic behavioral correlate of psychopathy [62]. The orbitofrontal cortex of those with high psychopathic traits and criminal convictions shows reduced gray-matter volume and thickness compared to those without self-reported criminal convictions [50].
Functional imaging studies examining the orbitofrontal cortex are consistent with structural findings. Using a prisoner’s dilemma task, which involves complex decisions about social cooperation, psychopaths evince lower orbito-frontal activity when choosing to cooperate and lower dorsolateral prefrontal activity when choosing to defect than do non-psychopaths [46]. Reduced orbitofrontal activity has also been observed in adolescents with callous-unemotional traits and conduct disorder during reinforcement stages of a passive avoidance learning task [48]. In an emotional Simon paradigm, which depends on the integration of emotional information into ongoing behavioral outcomes, adult criminal psychopaths demonstrated no prefrontal cortical activation in scenarios of emotional integration [63]. Similarly, contrary to controls, medial prefrontal areas in psychopaths were inactive when retaliating against an opponent during a competitive reinforcement task; however, psychopaths demonstrated relatively increased activity in this region when observing an opponent being punished, but this was specifically associated with impulsivity and antisocial behavior on Hare’s measure [64]. Reduced activity in the medial prefrontal cortex has also been observed while psychopaths were engaged in moral reasoning [65] and viewing pictures depicting moral violations [44,47]. Furthermore, complex relationships between specific facets of psychopathy and activation patterns in the orbitofrontal cortex and amygdala while viewing pictures of facial affect have been suggested [45].
Not all tasks induce reduced prefrontal activity in psychopaths; for instance, it has been reported that healthy individuals engage the mirror neuron system (supramarginal and superior frontal gyrus) while attributing emotional states to others, whereas psychopaths conversely engage the orbitofrontal and medial frontal cortex and the temporoparietal junction [66]. This may indicate that structures commonly labeled as dysfunctional in psychopaths are not necessarily inoperative, but may simply be utilized differently, processing information under abnormal contexts. This also highlights the importance of attending to task requirements in the evaluation of functional imaging reports, because ostensible abnormalities are only interpretable with regard to the cognitive function a particular task is accessing.
In sum, the most recent data support longstanding claims that psychopaths present with irregular structural features of the prefrontal cortex accompanied by atypical functional activity in prefrontal regions during various tasks, often marked by hypofunctioning in the ventromedial/orbitofrontal cortex. Again here, contemporary models of psychopathy including Blair’s and Kiehl’s generally acknowledge these abnormalities, implicating these regions in the apparent deficits in updating reinforcement values, appraising emotional states for behavioral regulation and engaging in moral evaluations by psychopaths. A recent meta-analysis by Yang and Raine [67] emphasizes a specific link between the antisocial facets of psychopathy and prefrontal abnormalities, and the most recent research continues to report rather complex relationships in this regard. In the future, more rigorous attention to these elements may help to specify such relationships with greater clarity.
Extended paralimbic and additional structures
The amygdala and areas of the prefrontal cortex have well-understood relationships with emotional learning and the estimation of rewards and consequences, but several other brain regions with subtler supportive roles in these processes have also been shown to be abnormal in psychopaths [11]. The parahippocampal gyrus, temporal pole, insula and cingulate cortex (both anterior and posterior) have been emphasized by Kiehl as particularly relevant to psychopathy, in addition to the amygdala and orbitofrontal cortex. The most recent data have continued to support the indication of dysfunction beyond primary limbic structures in psychopaths, which may suggest impairments carried up in a hierarchical fashion from primary limbic regions or broader cognitive impairments in general.
Contemporary techniques for whole-brain structural analysis have revealed abnormalities in many of these key regions. For instance, Boccardi and colleagues used cortical distance mapping and radial distance mapping to measure precise regions of interest in psychopaths [52]. In addition to differences in the orbitofrontal cortex and amygdala, they found volume reductions in the anterior cingulate, parahippocampal gyrus and superior frontal gyrus, along with smaller volumes in the primary visual cortex and precuneus, which plays a role in episodic memory and self-referential experiences [68]. In related work, this group found that abnormal morphological features of the hippocampus were correlated with psychopathy [69]. Others have reported relative tissue reductions in the temporal pole [70]. In a large-scale assessment involving nearly 300 incarcerated subjects, Ermer and colleagues reported that associations between psychopathy and decreased gray matter in the amygdala, orbitofrontal cortex, posterior cingulate, parahippocampal region and the temporal pole [49], all components of the paralimbic system. This study importantly quantified and controlled for moderating variables including substance abuse, age and relative brain size, and included over 40 individuals who scored in the clinical range of psychopathy (PCL-R score ≥30 out of 40), and thus stands as a good example of the methodological rigor needed to advance the field. It represents a significant leap forward in our understanding of the neural systems underlying the full manifestation of psychopathy and highlights the need to control for potentially moderating or confounding variables.
A number of studies have examined relationships between widespread structural features of the brain and the individual factor elements of psychopathy. For instance, the primary affective deficits of psychopathy have been linked to expansive reductions in gray-matter thickness in the anterior and medial temporal lobe [71], as well as reductions in the superior temporal regions and the insula [61]. Relatively larger striatum volumes have also been linked to component factors of psychopathy, because caudate body volume was reportedly associated with affective facets and caudate head volume was more consistently associated with impulsivity and stimulation-seeking [72]. The striatum as a whole plays an important role in reinforcement learning [73], which is a potential neuroanatomical basis for the increased reward sensitivity observed in psychopaths [23].
Abnormal task-specific functional activation patterns in psychopaths are also commonly found in widespread regions of the brain, echoing some of these structural findings. While engaging in moral dilemmas, psychopaths exhibited reduced activation in the hippocampal formation and posterior cingulate, in addition to the medial prefrontal cortex and amygdala [65]. Other reports indicate that psychopathic traits are associated with reduced cingulate cortex activity during defection in the prisoner’s dilemma task [46] and exaggerated activity in the mesolimbic reward system (ventral striatum) in response to a psychostimulant [74]. Reduced anterior temporal cortex activity was also observed in criminal psychopaths while they were looking at pictures depicting moral violations [44]. Furthermore, several investigations examining youths with psychopathic traits revealed abnormal function in the superior temporal gyrus [48,75–77], superior frontal gyrus [75,77], cingulate [76] and the parahippocampal gyrus [48,76].
Some neurobiological models of psychopathy, such as Blair’s, have limited the scope of emphasis to the amygdala and orbitofrontal cortex; however, accumulating evidence indicates that functional abnormalities also occur in other paralimbic structures and extended regions that share close connections with the limbic system and that contribute to a broader range of cognitive functions. Owing to the intimate connections between these structures, abnormal functional activity in the extended paralimbic network may be partially attributable to primary dysfunction in core limbic regions. However, considerable evidence suggests that damage to these regions can produce some symptoms congruent with psychopathy, such as impairments in affective recognition following lesions of the anterior cingulate [78], disruptions in social exchange reasoning following lesions to the temporal pole [79], and abnormal risky decision-making after damage to the insula [80]. As this evidence accumulates, it seems clear that continued attention should be paid to these functional units, and future investigations of psychopathy should devise tasks to parse out the variable activity in these regions. What may be necessary to clarify the roles of these anatomical components in psychopathy is the development of detailed models of connectivity and path models emphasizing the specific influence that these regions exert on one another.
Concluding remarks
The neuroscience of psychopathy is a field undergoing rapid growth and, like any field in its nascent stages, is vulnerable to methodological inconsistencies and subsequent interpretive variation. Particularly relevant are issues surrounding the operationalization of psychopathy, such as the assessment tools implemented and the cutoffs used to designate psychopathy. Other issues include variation in control groups, proper statistical control over potentially confounding variables including substance abuse, and small sample sizes. Although standards are still being established, we briefly summarize these issues and offer a number of recommendations in Box 2. Nevertheless, despite these growing pains, notable consistency is emerging in the field.
The structures most commonly implicated in psychopathy are those that serve functions in the evaluation of emotional information, including basic processing of threat and reward contingencies, utilization of this information in the modification of ongoing behavior, and extension of these contingencies into the realm of higher cognitive processing. Converging evidence from a number of imaging modalities and across a diverse range of populations supports the hypothesis that psychopathic traits are associated with abnormalities in the amygdala and orbitofrontal cortex. In addition, accumulating research supports the implication of additional brain regions in psychopathy, prominently the anterior cingulate, posterior cingulate, hippocampus and temporal pole (superior temporal gyrus).
Several neurobiological models of psychopathy agree on the core functional elements involved, but differ mainly in the scope of their focus. Blair currently favors a model proposing dysfunction of the amygdala and orbital frontal cortex [30], whereas Kiehl’s model also includes other closely related paralimbic structures (Figure 1) [11]. As more neuroimaging evidence accumulates, it seems less likely that deficits in psychopaths are confined to the amygdala and orbitofrontal cortex. The extant variety in brain regions implicated also leaves open the possibility of multiple neurodevelopmental pathways leading to similar behavioral outcomes. A limitation of current models may be that they primarily rely on personality traits and behavioral styles to operationalize a construct for which we are trying to describe a specific neurobiological origin, when physical problems in a number of brain regions – which may occur independently – could potentially manifest as symptoms consistent with familiar psychopathic traits. Although the Blair and Kiehl models are both supported by the data reviewed here, these still ultimately describe correlational relationships between behavior, neural structure and functional activity. Determination of a causal directional relationship between biology and behavior or personality is more elusive, but emerging research in determining path models of connectivity and genetic analyses is a promising enterprise in this regard.
With respect to the heritability of psychopathy [12], a promising resource for future investigations will be neurogenetic analyses that seek out allelic (and perhaps epigenetic) variations that determine structural and neurochemical abnormalities, which in turn contribute to divergent brain activity already reported in neuroimaging studies. This would certainly provide resources for devising more focused treatment and intervention strategies. Along these lines, Meyer-Lindenberg and colleagues found evidence that a low-expression variant polymorphism influencing monoamine oxidase (MAOA-L) was linked to structural volume reductions in the amygdala, cingulate cortex, insula, hypothalamus and orbitofrontal cortex [81]. Furthermore, these structural abnormalities were associated with impulsive reactive aggression, which often characterizes secondary psychopathy and the impulsive/antisocial facets of the construct. As evidence suggesting that psychopathy is a persistent neurocognitive disorder strongly influenced by genetic factors accumulates, it has become a pressing concern in forensic settings to decide whether a designation of psychopathy is a mitigating factor in determining responsibility and sentencing, and neuroscientists are now joining the debate [82,83].
In the future, two issues will be instrumental in maintaining the momentum of progress we are currently witnessing in neuroimaging investigations of psychopathy and in refining this knowledge to inform practical issues such as treatment, intervention strategies and legal responsibility. Attention to methodological issues that contribute to variability in the literature will be paramount, as well as application of current knowledge to more diverse interdisciplinary fields including neurogenetics and legal ethics. This knowledge will serve to inform our understanding of antisocial behavior, moral decision-making, and the routine integration of emotion into rational thought and behavior; therefore, continued progress in this field is extremely valuable. However, further progress will not be achieved through simple accumulation of data, but will require persistence accompanied by adaptive strategies of investigation and a commitment to the most rigorous experimental methods available.
Table 1.
| Item | Two-factor model | Three-factor model | Four-factor model | |
|---|---|---|---|---|
| 1 | Glibness or superficial charm | 1 | 1 | 1 |
| 2 | Grandiose sense of self-worth | 1 | 1 | 1 |
| 3 | Need for stimulation | 2 | 3 | 3 |
| 4 | Pathological lying | 1 | 1 | 1 |
| 5 | Conning or manipulative | 1 | 1 | 1 |
| 6 | Lack of remorse or guilt | 1 | 2 | 2 |
| 7 | Shallow affect | 1 | 2 | 2 |
| 8 | Callous or lack of empathy | 1 | 2 | 2 |
| 9 | Parasitic lifestyle | 2 | 3 | 3 |
| 10 | Poor behavioral controls | 2 | – | 4 |
| 11 | Promiscuous sexual behavior | – | – | – |
| 12 | Early behavioral problems | 2 | – | 4 |
| 13 | Lack of realistic long-term goals | 2 | 3 | 3 |
| 14 | Impulsivity | 2 | 3 | 3 |
| 15 | Irresponsibility | 2 | 3 | 3 |
| 16 | Failure to accept responsibility | 1 | 2 | 2 |
| 17 | Many marital relationships | – | – | – |
| 18 | Juvenile delinquency | 2 | – | 4 |
| 19 | Revocation of conditional release | 2 | – | 4 |
| 20 | Criminal versatility | – | – | 4 |
Items corresponding to the early two-factor conceptualization of psychopathy [94,98], the subsequent three-factor model [96] and the current four-factor model are listed [17]. The two-factor model labels are interpersonal/affective (factor 1) and social deviance (factor 2). The three-factor model labels are arrogant and deceitful interpersonal style (factor 1), deficient affective experience (factor 2), and impulsive and irresponsible behavioral style (factor 3). The four-factor model labels are interpersonal (factor 1), affective (factor 2), lifestyle (factor 3) and antisocial (factor 4). Items with ‘–’ did not load on any factor.
References
- 1.Cleckley H. The Mask of Sanity: An Attempt to Reinterpret the So-Called Psychopathic Personality. Mosby; 1941. [Google Scholar]
- 2.Leistico AR, et al. A large-scale meta-analysis relating the Hare measures of psychopathy to antisocial conduct. Law Hum Behav. 2008;32:28–45. doi: 10.1007/s10979-007-9096-6. [DOI] [PubMed] [Google Scholar]
- 3.Walters GD. Predicting institutional adjustment and recidivism with the psychopathy checklist factor scores: a meta-analysis. Law Hum Behav. 2003;27:541–558. doi: 10.1023/a:1025490207678. [DOI] [PubMed] [Google Scholar]
- 4.Hemphill JF, et al. Psychopathy and recidivism: a review. Legal Criminol Psychol. 1998;3:139–170. [Google Scholar]
- 5.Gretton H, et al. Psychopathy and recidivism in adolescent sex offenders. Crim Justice Behav. 2001;28:427–449. [Google Scholar]
- 6.Garrido V, et al. The effectiveness in the treatment of psychopathy: a meta-analysis. Issues Crim Legal Psychol. 1995;24:57–59. [Google Scholar]
- 7.Rice ME, et al. An evaluation of a maximum security therapeutic community for psychopaths and other mentally disordered offenders. Law Hum Behav. 1992;16:399–412. [Google Scholar]
- 8.Seto MC, Barbaree HE. Psychopathy, treatment behavior, and sex offender recidivism. J Interpers Violence. 1999;14:1235–1248. doi: 10.1177/0886260505278262. [DOI] [PubMed] [Google Scholar]
- 9.Skeem JL, Cooke DJ. Is criminal behavior a central component of psychopathy? Conceptual directions for resolving the debate. Psychol Assess. 2010;22:433–445. doi: 10.1037/a0008512. [DOI] [PubMed] [Google Scholar]
- 10.Blair RJR. The emergence of psychopathy: implications for the neuropsychological approach to developmental disorders. Cognition. 2006;101:414–442. doi: 10.1016/j.cognition.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Kiehl KA. A cognitive neuroscience perspective on psychopathy: evidence for paralimbic system dysfunction. Psychiatry Res. 2006;142:107–128. doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsson H, et al. A genetic factor explains most of the variation in the psychopathic personality. J Abnorm Psychol. 2006;115:221–230. doi: 10.1037/0021-843X.115.2.221. [DOI] [PubMed] [Google Scholar]
- 13.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 2. American Psychiatric Association; 1968. [Google Scholar]
- 14.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; 2000. text revision. [Google Scholar]
- 15.Cunningham MD, Reidy TJ. Antisocial personality disorder and psychopathy: diagnostic dilemmas in classifying patterns of antisocial behavior in sentencing evaluations. Behav Sci Law. 1998;16:333–351. doi: 10.1002/(sici)1099-0798(199822)16:3<333::aid-bsl314>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 16.Hare RD. The Hare Psychopathy Checklist-Revised. Multi-Health Systems; 1991. [Google Scholar]
- 17.Hare RD. The Hare Psychopathy Checklist. 2. Multi-Health Systems; 2003. revised. [Google Scholar]
- 18.Viding E, et al. Evidence for substantial genetic risk for psychopathy in 7-year-olds. J Child Psychol Psychiatry. 2005;46:592–597. doi: 10.1111/j.1469-7610.2004.00393.x. [DOI] [PubMed] [Google Scholar]
- 19.Glenn AL, et al. Evolutionary theory and psychopathy. Aggress Violent Behav. 2011;16:371–380. [Google Scholar]
- 20.Babiak P, et al. Corporate psychopathy: talking the walk. Behav Sci Law. 2010;28:174–193. doi: 10.1002/bsl.925. [DOI] [PubMed] [Google Scholar]
- 21.Hall JR, Benning SD. The ‘successful’ psychopath: adaptive and subclinical manifestations of psychopathy in the general population. In: Patrick CJ, editor. Handbook of Psychopathy. Guilford Press; 2006. pp. 459–478. [Google Scholar]
- 22.Murphy JM. Psychiatric labeling in cross-cultural perspective. Science. 1976;191:1019–1028. doi: 10.1126/science.1251213. [DOI] [PubMed] [Google Scholar]
- 23.Blair RJR, et al. Passive avoidance learning in individuals with psychopathy: modulation by reward but not by punishment. Pers Indiv Differ. 2004;37:1179–1192. [Google Scholar]
- 24.Birbaumer N, et al. Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Arch Gen Psychiatry. 2005;62:799–805. doi: 10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- 25.Lykken DT. A study of anxiety in the sociopathic personality. J Abnorm Soc Psychol. 1957;55:6–10. doi: 10.1037/h0047232. [DOI] [PubMed] [Google Scholar]
- 26.Lykken DT. The Antisocial Personalities. Lawrence Earlbaum Associates; 1995. [Google Scholar]
- 27.Gray JA. A critique of Eysenck’s theory of personality. In: Eysenck HJ, editor. A Model for Personality. Springer-Verlag; 1981. pp. 246–276. [Google Scholar]
- 28.Fowles DC. The three arousal model: implications of Gray’s two-factor learning theory for heart rate, electrodermal activity, and psychopathy. Psychophysiology. 1980;17:87–104. doi: 10.1111/j.1469-8986.1980.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 29.Damasio AR. Descartes’ Error: Emotion, Reason, and the Human Brain. Avon; 1994. [Google Scholar]
- 30.Blair RJR. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends Cogn Sci. 2007;11:387–392. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Brodmann K. Localisation in the Cerebral Cortex. Smith-Gordon; 1909. [Google Scholar]
- 32.Mesulam MM. Behavioral neuroanatomy: large-scale networks, association cortex, frontal lobe syndromes, the limbic system, and hemispheric specializations. In: Mesulam MM, editor. Principles of Behavioral and Cognitive Neurology. 2. Oxford University Press; 2000. pp. 1–120. [Google Scholar]
- 33.Kiehl KA, et al. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biol Psychiatry. 2001;50:677–684. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- 34.Newman JP, Lorenz AR. Response modulation and emotion processing: implications for psychopathy and other dysregulatory psychopathology. In: Davidson RJ, et al., editors. Handbook of Affective Sciences. Oxford University Press; 2003. pp. 904–929. [Google Scholar]
- 35.Newman JP, et al. Passive avoidance in psychopaths: the effects of reward. Pers Indiv Differ. 1990;11:1101–1114. [Google Scholar]
- 36.Newman JP, et al. The impact of motivationally neutral cues on psychopathic individuals: assessing the generality of the response modulation hypothesis. J Abnorm Psychol. 1997;106:563–575. doi: 10.1037//0021-843x.106.4.563. [DOI] [PubMed] [Google Scholar]
- 37.Newman JP, et al. Attention moderates the fearlessness of psychopathic offenders. Biol Psychiatry. 2010;67:66–70. doi: 10.1016/j.biopsych.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blair RJR. Neuroimaging of psychopathy and antisocial behavior: a targeted review. Curr Psychiatry Rep. 2010;12:76–82. doi: 10.1007/s11920-009-0086-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harenski C, et al. Neurodevelopmental bases of psychopathy: a review of brain imaging studies. In: Malatesti L, McMillan J, editors. Responsibility and Psychopathy: Interfacing Law, Psychiatry and Philosophy. Oxford University Press; 2010. pp. 125–154. [Google Scholar]
- 40.Müller JL. Psychopathy – an approach to neuroscientific research in forensic psychiatry. Behav Sci Law. 2010;28:129–147. doi: 10.1002/bsl.926. [DOI] [PubMed] [Google Scholar]
- 41.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 42.Kiehl KA, et al. An adaptive reflexive processing model of neurocognitive function supporting evidence from a large scale (n = 100) fMRI study of an auditory oddball task. Neuroimage. 2005;25:899–915. doi: 10.1016/j.neuroimage.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 43.Patrick CJ, et al. Emotion in the criminal psychopath: startle reflex modulation. J Abnorm Psychol. 1993;102:82–92. doi: 10.1037//0021-843x.102.1.82. [DOI] [PubMed] [Google Scholar]
- 44.Harenski CL, et al. Aberrant neural processing of moral violations in criminal psychopaths. J Abnorm Psychol. 2010;119:863–874. doi: 10.1037/a0020979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dolan MC, Fullam RS. Psychopathy and functional magnetic resonance imaging blood oxygenation level-dependent responses to emotional faces in violent patients with schizophrenia. Biol Psychiatry. 2009;66:570–577. doi: 10.1016/j.biopsych.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 46.Rilling JK, et al. Neural correlates of social cooperation and non-cooperation as a function of psychopathy. Biol Psychiatry. 2007;61:1260–1271. doi: 10.1016/j.biopsych.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 47.Harenski CL, et al. Neuroticism and psychopathy predict brain activation during moral and nonmoral emotion regulation. Cogn Affect Behav Neurosci. 2009;9:1–15. doi: 10.3758/CABN.9.1.1. [DOI] [PubMed] [Google Scholar]
- 48.Finger EC, et al. Disrupted reinforcement signaling in the orbitofrontal cortex and caudate in youths with conduct disorder or oppositional defiant disorder and a high level of psychopathic traits. Am J Psychiatry. 2011;168:152–162. doi: 10.1176/appi.ajp.2010.10010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ermer E, et al. Aberrant paralimbic gray matter in criminal psychopathy. J Abnorm Psychol. doi: 10.1037/a0026371. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Y, et al. Morphological alterations in the prefrontal cortex and the amygdala in unsuccessful psychopaths. J Abnorm Psychol. 2010;119:546–554. doi: 10.1037/a0019611. [DOI] [PubMed] [Google Scholar]
- 51.Yang Y, et al. Localization of deformations within the amygdala in individuals with psychopathy. Arch Gen Psychiatry. 2009;66:986–994. doi: 10.1001/archgenpsychiatry.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boccardi M, et al. Cortex and amygdala morphology in psychopathy. Psychiatry Res. 2011;193:85–92. doi: 10.1016/j.pscychresns.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 53.Schoenbaum G, et al. Encoding predicted outcome and acquired value during cue sampling depends on input from basolateral amygdala. Neuron. 2003;39:855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- 54.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 55.LeDoux JE, et al. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 57.Salzman DC, Fusi S. Emotion, cognition, and mental state representation in amygdala and prefrontal cortex. Annu Rev Neurosci. 2010;33:173–202. doi: 10.1146/annurev.neuro.051508.135256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anderson SW, et al. Long-term sequelae of prefrontal cortex damage acquired in early childhood. Dev Neuropsychol. 2000;18:281–296. doi: 10.1207/S1532694202Anderson. [DOI] [PubMed] [Google Scholar]
- 59.Koenigs M, et al. Damage to the prefrontal cortex increases utilitarian moral judgments. Nature. 2007;446:908–911. doi: 10.1038/nature05631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tiihonen J, et al. Brain anatomy of persistent violent offenders: more rather than less. Psychiatry Res. 2008;163:201–212. doi: 10.1016/j.pscychresns.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 61.de Oliveira-Souza R, et al. Psychopathy as a disorder of the moral brain: fronto temporo-limbic grey matter reductions demonstrated by voxel-based morphometry. NeuroImage. 2008;40:1202–1213. doi: 10.1016/j.neuroimage.2007.12.054. [DOI] [PubMed] [Google Scholar]
- 62.Yang Y, et al. Abnormal structural correlates of response perseveration in individuals with psychopathy. J Neuropsychiatry Clin Neurosci. 2011;23:107–110. doi: 10.1176/appi.neuropsych.23.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Müller JL, et al. Disturbed prefrontal and temporal brain function during emotion and cognition interaction in criminal psychopathy. Behav Sci Law. 2008;26:131–150. doi: 10.1002/bsl.796. [DOI] [PubMed] [Google Scholar]
- 64.Veit R, et al. Aberrant social and cerebral responding in a competitive reaction time paradigm in criminal psychopaths. NeuroImage. 2010;49:3365–3372. doi: 10.1016/j.neuroimage.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 65.Pujol J, et al. Breakdown in the brain network subserving moral judgment in criminal psychopathy. Soc Cogn Affect Neurosci. 2011 doi: 10.1093/scan/nsr075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sommer M, et al. In psychopathic patients emotion attribution modulates activity in outcome-related brain areas. Psychiatry Res. 2010;182:88–95. doi: 10.1016/j.pscychresns.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 67.Yang Y, Raine A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: a meta-analysis. Psychiatry Res. 2009;174:81–88. doi: 10.1016/j.pscychresns.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 69.Boccardi M, et al. Abnormal hippocampal shape in offenders with psychopathy. Hum Brain Mapp. 2010;31:438–447. doi: 10.1002/hbm.20877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Müller JL, et al. Gray matter changes in right superior temporal gyrus in criminal psychopaths: evidence from voxel based morphometry. Psychiatry Res. 2008;163:213–222. doi: 10.1016/j.pscychresns.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 71.Yang Y, et al. Abnormal temporal and prefrontal cortical gray matter thinning in psychopaths. Mol Psychiatry. 2009;14:561–562. doi: 10.1038/mp.2009.12. [DOI] [PubMed] [Google Scholar]
- 72.Glenn AL, et al. Increased volume of the striatum of psychopathic individuals. Biol Psychiatry. 2010;67:52–58. doi: 10.1016/j.biopsych.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haruno M, et al. A neural correlate of reward-based behavioral learning in caudate nucleus: a functional magnetic resonance imaging study of a stochastic decision task. J Neurosci. 1994;24:1660–1665. doi: 10.1523/JNEUROSCI.3417-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buckholtz JW, et al. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat Neurosci. 2010;13:419–421. doi: 10.1038/nn.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Finger EC, et al. Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Arch Gen Psychiatry. 2008;65:586–594. doi: 10.1001/archpsyc.65.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marsh AA. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry. 2008;165:712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- 77.Jones AP, et al. Amygdala hypoactivity to fearful faces in boys with conduct problems and callous unemotional traits. Am J Psychiatry. 2009;166:95–102. doi: 10.1176/appi.ajp.2008.07071050. [DOI] [PubMed] [Google Scholar]
- 78.Hornak J, et al. Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain. 2003;126:1691–1712. doi: 10.1093/brain/awg168. [DOI] [PubMed] [Google Scholar]
- 79.Stone VE, et al. Reasoning about social exchange in patient with bilateral limbic system damage. Pro Natl Acad Sci USA. 2002;99:11531–11536. doi: 10.1073/pnas.122352699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weller JA, et al. The effects of insula damage on decision-making for risky gains and losses. Soc Neurosci. 2009;4:347–358. doi: 10.1080/17470910902934400. [DOI] [PubMed] [Google Scholar]
- 81.Meyer-Lindenberg A, et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci USA. 2006;103:6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Glenn AL, et al. Is it wrong to criminalize and punish psychopaths? Emot Rev. 2011;3:302–304. [Google Scholar]
- 83.Harenski C, et al. Neuroimaging, genetics, and psychopathy: implications for the legal system. In: Malatesti L, McMillan J, editors. Responsibility and Psychopathy: Interfacing Law, Psychiatry & Philosophy. Oxford University Press; 2010. pp. 125–154. [Google Scholar]
- 84.Karpman B. On the need for separating psychopathy in to two distinct clinical subtypes: symptomatic and idiopathic. J Criminol Psychopathol. 1941;3:112–137. [Google Scholar]
- 85.Lykken DT. The Antisocial Personalities. Lawrence Erlbaum; 1995. [Google Scholar]
- 86.Skeem J, et al. Two subtypes of psychopathic violent offenders that parallel primary and secondary variants. J Abnorm Psychol. 2007;116:395–409. doi: 10.1037/0021-843X.116.2.395. [DOI] [PubMed] [Google Scholar]
- 87.Gao Y, Raine AD. Successful and unsuccessful psychopaths: a neurobiological model. Behav Sci Law. 2010;28:194–210. doi: 10.1002/bsl.924. [DOI] [PubMed] [Google Scholar]
- 88.Ullrich S, et al. Psychopathic personality traits and life-success. Pers Indiv Differ. 2008;44:1162–1171. [Google Scholar]
- 89.Hare RD. Comparison of procedures for the assessment of psychopathy. J Consult Clin Psychol. 1985;53:7–16. doi: 10.1037//0022-006x.53.1.7. [DOI] [PubMed] [Google Scholar]
- 90.Levenson M, et al. Assessing psychopathic attributes in a noninstitutionalized population. J Pers Soc Psychol. 1995;68:151–158. doi: 10.1037//0022-3514.68.1.151. [DOI] [PubMed] [Google Scholar]
- 91.Lilienfeld SO, Widows M. Professional Manual for the Psychopathic Personality Inventory – Revised. Psychological Assessment Resources; 2005. [Google Scholar]
- 92.Lilienfeld SO, Fowler KA. The self-report assessment of psychopathy: problems, pitfalls, and promises. In: Patrick CJ, editor. Handbook of Psychopathy. Guilford Press; 2006. pp. 107–132. [Google Scholar]
- 93.Butcher JN, et al. Minnesota Multiphasic Personality Inventory-2 (MMPI-2) Manual for Administration and Scoring. University of Minnesota Press; 1989. [Google Scholar]
- 94.Hare RD, et al. The revised psychopathy checklist: reliability and factor structure. Psychol Assess. 1990;2:338–341. [Google Scholar]
- 95.Benning SD, et al. Factor structure of the psychopathic personality inventory: validity and implications for clinical assessment. Psychol Assess. 2003;15:340–350. doi: 10.1037/1040-3590.15.3.340. [DOI] [PubMed] [Google Scholar]
- 96.Cooke DJ, Michie C. Refining the construct of psychopathy: towards a hierarchical model. Psychol Assess. 2001;13:171–188. [PubMed] [Google Scholar]
- 97.Koenigs M, et al. Investigating the neural correlates of psychopathy: a critical review. Mol Psychiatry. 2011;16:792–799. doi: 10.1038/mp.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Harpur TJ, et al. Factor structure of the psychopathy checklist. J Consult Clin Psychol. 1988;56:741–747. doi: 10.1037//0022-006x.56.5.741. [DOI] [PubMed] [Google Scholar]