Abstract
Impulsivity and aggressiveness are trait dispositions associated with the vulnerability to suicidal behavior across diagnoses. They are associated with structural and functional abnormalities in brain networks involved in regulation of mood, impulse and behavior. They are also core characteristics of borderline personality disorder (BPD), a disorder defined, in part, by recurrent suicidal behavior. We assessed the relationships between personality traits, brain structure and lethality of suicide attempts in 51 BPD attempters using multiple regression analyses on structural MRI data. BPD was diagnosed by the Diagnostic Interview for Borderline Patients-revised, impulsivity by the Barratt Impulsiveness Scale (BIS), aggression by the Brown-Goodwin Lifetime History of Aggression (LHA), and high lethality by a score of 4 or more on the Lethality Rating Scale (LRS). Sixteen High Lethality attempters were compared to 35 Low Lethality attempters, with no significant differences noted in gender, co-morbidity, childhood abuse, BIS or LHA scores. Degree of medical lethality (LRS) was negatively related to gray matter volumes across multiple fronto-temporal-limbic regions. Effects of impulsivity and aggression on gray matter volumes discriminated High from Low Lethality attempters and differed markedly within lethality groups. Lethality of suicide attempts in BPD may be related to the mediation of these personality traits by specific neural networks.
Keywords: Magnetic resonance imaging, Impulsive-aggression, Suicidal Behavior, Borderline personality disorder
1. Introduction
Personality traits such as impulsivity and aggressiveness are associated with suicidal behavior across diagnoses. In a stress-diathesis model of suicide, they represent vulnerable temperaments, predispositions to impulsive and aggressive behavior in response to specific trigger events (for review, see Mann et al.,1999, 2003). Trait dispositions such as impulsivity and aggressiveness may be heritable (e.g., as endophenotypes), or acquired in the course of development (e.g., through childhood abuse). In neuroimaging studies, they have been associated with variations in the structure and function of brain networks that regulate mood, impulse and behavior. At times of emotional stress, dysfunction in these neural networks may result in interference with executive cognitive functions, such as response inhibition, conflict resolution, and recall of episodic memory (for review, see Fertuck et al., 2006). As a result, problem solving and adaptive coping are impaired, increasing the likelihood of impulsive or aggressive behavior. We study the relationship between personality characteristics, brain function and suicidal behavior in the context of borderline personality disorder, a personality disorder defined, in part, by recurrent suicidal behavior, impulsivity and aggression. With a suicide rate of 3%–10% and a community prevalence estimated at 1% of the population, BPD is a clinically relevant model for the study of suicide (Swartz et al., 1990).
There is a paucity of neuroimaging studies in BPD subjects ascertained specifically for suicidal behavior. In a voxel-based morphometry study (VBM) comparing BPD suicide attempters with BPD non-attempters, we recently reported specific structural differences in BPD subjects associated with suicidal behavior, and differences between High Lethality and Low Lethality suicide attempters (Soloff et al., 2012). BPD attempters had diminished gray matter concentrations in left insular cortex compared with BPD non-attempters. High Lethality attempters had diminished gray matter compared with Low Lethality attempters in an extensive fronto-limbic network including the following regions: right middle-inferior orbital frontal cortex, right middle-superior temporal cortex, right insular cortex, left fusiform gyrus, left lingual gyrus, and right parahippocampal gyrus. These areas are broadly involved in emotion regulation, behavioral control, and adaptive responding to social situations. Suicide researchers have long maintained that suicide attempters and completers represent separate but overlapping populations, with differing clinical characteristics (Maris et al., 2000). High Lethality attempters share many clinical characteristics with patients who complete suicide and may share neurobiological vulnerabilities related to high-risk personality traits such as impulsivity and aggressiveness.
To assess the relationships between personality traits, brain structure, and suicidal behavior, we used a multiple regression analysis of VBM data in High and Low Lethality BPD attempters to map the relationships between impulsivity, aggression and gray matter in key brain structures.
2. Methods
2.1. Subjects
Subjects for this study were recruited by advertisement from the outpatient programs of the Western Psychiatric Institute and Clinic and surrounding community to participate in a longitudinal study of suicidal behavior in BPD. The study was approved by the Institutional Review Board of the University of Pittsburgh, and funded by the National Institute of Mental Health. All subjects gave written informed consent for participation.
Diagnoses were determined by Master’s prepared research raters using structured interviews. Axis I disorders were diagnosed using the Structured Clinical Interview for DSM III-R or DSM IV (SCID, Spitzer et al., 1998; First et al., 2005) (Because this is a longitudinal study, DSM-IV was added when it first became available.) Axis II diagnoses were established using the International Personality Disorders Examination (IPDE), which has a lifetime framework (Loranger et al., 1997). The Diagnostic Interview for Borderlines (DIB) (Gunderson et al., 1981) was administered as an independent measure of diagnosis and recent symptom severity, with a timeframe of 3 months to 2 years for individual subscales. (The DIB was used to preserve continuity with the longitudinal study; however, the Diagnostic Interview for Borderlines-Revised (DIB-R) was added and scored concurrently when it became available (Zanarini et al.,1989).) For inclusion, participants had to meet diagnostic criteria for BPD on the IPDE (probable or definite), have a score of 7 or more (definite) on the DIB, and 8 or more (definite) on the DIB-R. Exclusion criteria included any past or current Axis I diagnosis of schizophrenia, delusional (paranoid) disorder, schizoaffective disorder, bipolar disorder, or psychotic depression. Subjects were also excluded for physical disorders of known psychiatric consequence (e.g., hypothyroidism, seizure disorder, or brain injury) and borderline mental retardation. Medical records were reviewed where available to confirm inclusion and exclusion criteria. Final diagnoses were determined by consensus of raters using all available data. Control subjects were free of all Axis I and II disorders. Attempter status and medical lethality of attempts were obtained by interview using the Columbia Suicide History Form and Lethality Rating Scale (Oquendo et al., 2003). Scans were obtained from newly recruited subjects and from subjects already enrolled in the longitudinal study at the time of their annual follow-up assessment. As a result, all subjects had updated SCID interviews for current diagnoses. Aggression was assessed by interview on the Brown-Goodwin Lifetime History of Aggression (LHA, Brown et al., 1979) and trait impulsivity by self-report questionnaire using the Barratt Impulsiveness Scale (BIS, Barratt 1965). The 24 item Hamilton Rating Scale for Depression (HamD) was obtained before the scan as a measure of depressed mood (Guy, 1976). High Lethality status among attempters was defined as a lifetime maximum Lethality Rating Scale score (LRS) of 4 or more. (For example, for a suicide attempt by overdose with sedative drugs, an LRS score of 4 is defined as “comatose; injury sufficient for hospitalization.”)
All subjects were physically healthy, free of drugs of abuse and alcohol for at least 1 week before the scan. Female subjects were required to have a negative screen for pregnancy. All subjects had a negative urine toxicology screen for drugs of abuse immediately before the scan. Some BPD subjects were taking psychoactive medication.
2.2. Imaging method
Magnetic resonance imaging (MRI) was performed with a 1.5T GE Signa Imaging System running version Signa 5.4.3 software (General Electric Medical Systems, Milwaukee, WI, USA). A T1-weighted sagittal scout image was obtained for graphic prescription of the coronal and axial images. Three-dimensional gradient echo imaging (Spoiled Gradient Recalled Acquisition, SPGR) was performed in the coronal plane (repetition time=25 ms, echo time=5 ms, nutation angle=40°, field of view=24 cm, slice thickness=1.5 mm, number of excitations=1, matrix size=256×192) to obtain 124 images covering the entire brain. Additionally, a double echo-spin echo sequence was used to obtain T2 and proton density images in the axial plane to screen for neuroradiological abnormalities.
Structural MR images were pre-processed using the Statistical Parametric Mapping (SPM) diffeomorphic image registration algorithm (DARTEL) in SPM8 (Friston et al., 1995; Ashburner, 2007; Diwadkar et al., 2011). DARTEL optimizes the fidelity of shape-based deformations applied to fit native images in stereotactic space, and performs favorably relative to other non-linear deformation algorithms (Klein et al., 2009). It is therefore optimized for assessing structural changes within a stereotactic framework, and well suited for VBM analyses. Following re-sampling (2 mm3) and segmentation of T1-weighted images, a rigid gray matter template was created representing the average shape and size of the brains of all the subjects included in the study. Subjects’ gray matter maps were then warped to the coordinate system of the template, with Jacobian modulation used to scale native gray matter volume from native to Montreal Neurological Institute (MNI) space (Good et al., 2001). This procedure has been extensively used in voxel-based analyses of gray matter images within the framework of random field methods. Structural data for 44 BPD attempters were previously included in a larger study comparing BPD attempters, non-attempters and healthy control subjects (Soloff et al., 2012). Our findings from that structural analysis, defining deficits associated with lethality, were used to define regions of interest in this analysis. First, we defined a regional mask corresponding to clusters of significance (p<0.05, cluster level) identifying reductions in gray matter volume in High (relative to Low) Lethality attempters (Ward, 2000). This regional mask spanned nine regions of interest including the following: the middle-inferior orbital frontal cortex, anterior cingulate cortex, middle-superior temporal cortex, insula, hippocampus, parahippocampus, fusiform gyrus, lingual gyrus and amygdala (Soloff et al., 2008; Leichsenring et al., 2011).
Personality trait variables (LHA, BIS) were entered individually in multiple regression analyses as co-variates of interest to investigate the positive and negative effects of these clinical measures on brain structures in High and Low Lethality suicidal subjects (Friston et al., 1995). These methods follow previous investigations of effects of symptom or personality dimensions on regional brain structure (Banissy et al., 2012). Cluster level correction (pc<0.05) was used to optimize sensitivity to detect clusters with minimal extent (cluster forming threshold, pthr<0.05) (Ward, 2000).
3. Results
3.1. Sample characteristics
There were 51 BPD attempters subdivided as follows: 16 High Lethality (5 male, 11 female) and 35 Low Lethality attempters (5 male, 30 female), with no significant group differences by gender, race or socioeconomic status (Table 1). The mean (S.D.) age of the sample was 30.1 (8.1) years with a range of 18 to 47 years. High Lethality attempters were significantly older (36.1 (9.2) years) than Low Lethality attempters (27.4 (5.9) years), t = 3.47, df = 20.95, P=0.002). The two groups did not differ significantly in proportion of subjects with current or lifetime co-morbid Axis I disorders, including the following: major depressive disorder (MDD), alcohol abuse or dependence, other drug abuse or dependence, any anxiety disorder, or post-traumatic stress disorder. The most frequent current co-morbid diagnosis, MDD, was found in 12 High Lethality (75%) and 21 Low Lethality attempters (60%), with no significant difference between groups in severity of depressed mood (HamD) at the time of the scan (Table 1). Similarly, there were no significant differences between groups for BIS and LHA scores, and no significant correlation between these scores across all attempters. A history of childhood abuse was found in 7 High Lethality (43.8%) and 13 Low Lethality attempters (37.1%), with no significant difference between groups. Of the 51 subjects, 21 (41.5%) were taking psychoactive medication at the time of the scan, with no proportional difference between High and Low Lethality subjects.
Table 1.
Characteristics of the Sample*
| High Lethality | Low Lethality | Statistic (t,df,P or X2,df,P)** | |
|---|---|---|---|
| N (M/F) | 16 (5M/11F) | 35 (5M/30F) | X2 = 2.00, df =1, P.n.s. |
| Age (yrs, s.d) | 36.1 (9.2) | 27.4 (5.9) | t =3.47, df =20.95, P.= 0.002 |
| Race (%Cau.) | 75 | 74.3 | X2 = 0.003, df = 1, P.n.s. |
| Educ. (% > 12 yrs.) | 56.3 | 62.9 | X2 = 0.20, df =1, P.n.s. |
| SES (low = 2+3) | 10 | 23 | |
| (high = 4+5) | 6 | 12 | X2 = 0.05, df = 1, P.n.s |
| Abused (%yes) | 43.8 | 37.1 | X2 = 0.20 df =1, P.n.s. |
| MDD (%yes) | 75 | 60 | X2=1.08, df =1, P.n.s. |
| Alcohol (%yes) | 6.3 | 17.1 | X2= 1.10, df = 1, P.n.s |
| Other Drugs (%yes) | 31.3 | 14.3 | X2 = 2.01, df =1, P.n.s. |
| Anxiety Dx. (%yes) | 50 | 65.7 | X2 = 1.14, df =1, P.n.s. |
| PTSD (%yes) | 18.8 | 8.6 | X2 = 1.10, df =1, P.n.s. |
| Psych.Meds (%yes) | 50.0 | 37.1 | X2 = 0.75, df =1, P.n.s. |
| HamD | 18.1 (10.0) | 16.3 (7.9) | t = 0.68, df = 49, P.n.s |
| BIS | 73.5 (5.2) | 74.7 (4.8) | t = 0.82, df = 49, P.n.s. |
| LHA | 15.6 (8.4) | 16.5 (6.3) | t = 0.39, df = 49, P.n.s |
SCID Axis I co-morbidity at time of scan. Alcohol and Other Drugs include abuse and/or dependence, Anxiety Dx includes any anxiety disorder except PTSD.
t,df,P: Student’s t-test, 2 tailed. X2,df,P: Chi Square test, 2 tailed
Among all attempters, Lethality Rating Scale (LRS) scores ranged from 0 to 7 with a mean (S.D.) = 2.80 (1.9) and a median of 3.0. There were no significant correlations between LRS scores, BIS scores and LHA scores across all attempters. The number of lifetime suicide attempts differed greatly between High and Low Lethality attempters. High Lethality subjects had a mean (S.D.) of 7.1 (7.0) lifetime attempts compared with 2.3 (1.4) lifetime attempts for Low Lethality subjects (t = 2.76, df = 15.5, P = 0.014). However, the groups did not differ significantly in violence of suicide method. Overdose, a non-violent method, was the sole means used by 41 attempters (80.4%), while 10 subjects used violent methods on at least one occasion (e.g. cutting (5), hanging (3), jumping (1), drowning (1)). The mean (S.D.) time from the last attempt to the scan did not significantly differ between groups (High Lethality: 50.3 (56.2) months; Low Lethality: 72.6 (78.9) months, t = −1.01, df = 48, P = NS).
3.2. Lethality Rating Scale scores and gray matter volumes
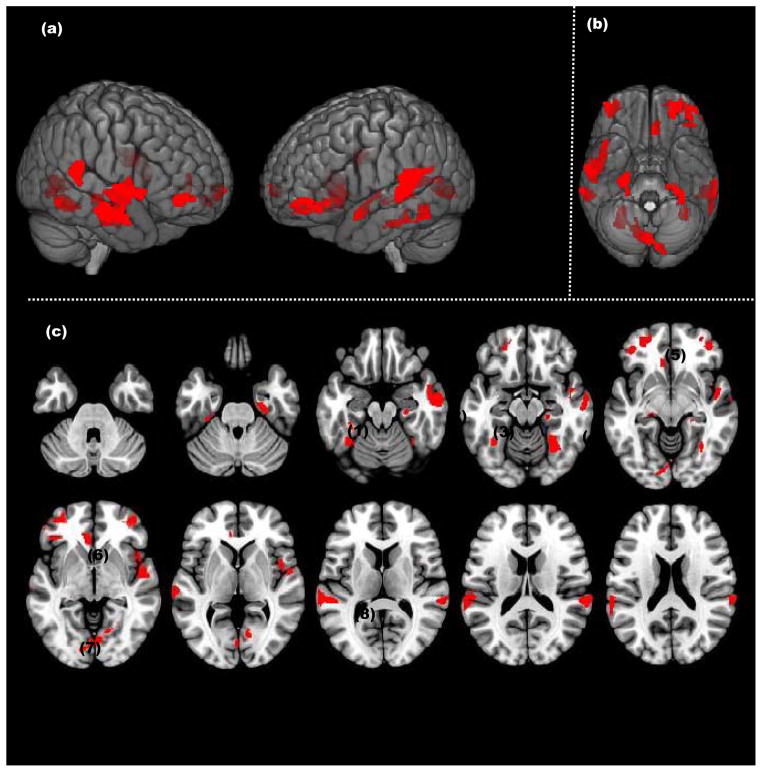
The relationship between LRS scores and gray matter volumes was assessed by regression analysis in all regions of interest (ROIs). LRS scores were negatively related to gray matter volumes in 8 ROIs, with some variations in laterality. Higher degrees of lethality were significantly associated with diminished gray matter volumes across multiple fronto-temporal-limbic regions, which included (in order of cluster size) the following: bilateral middle-superior temporal cortex, left lingual gyrus, bilateral middle-inferior orbitofrontal cortes, right insula, bilateral fusiform gyrus, right parahippocampal gyrus, left anterior cingulate, and left hippocampus (Table 2). There were no significant positive correlations between LRS scores and gray matter volumes.
Table 2.
Relationship between Lethality Rating Scale scores and grey matter concentrations in BPD attempters
| Anat. ROI (TAL) | Cluster Ext. p< 0.05 | Ind. Cluster Ext. | P value | Voxel Peak (x, y, z) |
|---|---|---|---|---|
| ROIs with significant negative correlations*. | ||||
| Mid-Sup Tp | 419 | 1139 | <0.002 | (45, −10, −16) |
| Mid-Sup Tp(L) | 419 | 920 | <0.002 | (−63, −35, 23) |
| Lingual g (L) | 238 | 801 | <0.003 | (−14, −85, 0) |
| Mid-Inf OFC(L) | 274 | 686 | <0.004 | (−21, 49, −9) |
| Mid-Inf OFC | 274 | 321 | <0.001 | (38, 44, −3) |
| Insula | 123 | 439 | <0.002 | (45, −3, 2) |
| Fusiform g | 183 | 421 | <0.009 | (30, −57, −5) |
| Fusiform g (L) | 183 | 409 | <0.006 | (−24, −31, −21) |
| Parahipp. g | 99 | 330 | <0.004 | (25, −24, −22) |
| ACC(L) | 160 | 281 | <0.007 | (−9, 26, −3) |
| Hipp.(L) | 43 | 64 | <0.013 | (−19, −28, −6) |
No significant positive correlations were found between LRS scores and grey matter concentrations.
Mid-Sup Tp = middle superior temporal cortex, mid-inf OFC = middle inferior orbital frontal cortex, Parahipp. g = parahippocampal gyrus, ACC = anterior cingulate cortex, Hipp. = hippocampus
3.3. Personality interactions in High Lethality attempters (Table 3)
Table 3a.
Relationships between Aggression (LHA) and Grey Matter Concentrations in High and Low Lethality Attempters (*)
| Anat. ROI (TAL) | Cluster Ext. p< 0.05 | Ind. Cluster Ext. | P value | Voxel Peak (x, y, z) |
|---|---|---|---|---|
| A. High Lethality LHA-Positive Correlations | ||||
| Mid-Inf OFC | 352 | 4019 | <0.003 | (24, 37, −11) |
| [Mid-Inf OFC(L) | 352 | 1011 | <0.003 | (−27, 29, −19)] |
| ACC | 176 | 3572 | <0.002 | (9, 37, −4) |
| ACC(L) | 176 | 3572 | <0.002 | (−11, 42, 0) |
| Mid Sup Tp | 398 | 771 | <0.001 | (58, −46, 5) |
| Insula | 173 | 568 | <0.007 | (45,11, 2) |
| Lingual | 165 | 277 | <0.006 | (13, −79, −2) |
| Fusiform(L) | 136 | 230 | <0.001 | (−36, −76, −10) |
| [Fusiform] | 136 | 189 | <0.009 | (32, −7, −30)] |
| Parahipp. | 76 | 201 | <0.009 | (23, −46, −2) |
| B. High Lethality LHA-Negative correlations: NONE | ||||
| C. Low Lethality LHA-Positive Correlations | ||||
| Insula | 166 | 868 | <0.006 | (35, 19, 9) |
| Fusiform(L) | 156 | 643 | <0.001 | (−35, −24, −19) |
| [Fusiform] | 156 | 601 | <0.005 | (37, −24, −19) |
| Hippocampus | 127 | 479 | <0.014 | (32, −23, −10) |
| Mid Sup Tp(L) | 390 | 438 | <0.001 | (−45, −18, −17) |
| Parahipp | 101 | 347 | <0.008 | (34, −23, −19) |
| Mid-Inf OFC(L) | 213 | 327 | <0.016 | (−27, 38, −16) |
| [Mid-Inf OFC] | 213 | 255 | <0.002 | (27, 49, −15) |
| Lingual(L) | 173 | 206 | <0.007 | (−4, −57, 5) |
| Amygdala | 59 | 64 | <0.019 | (23, −8, −6) |
| D. Low Lethality LHA-Negative Correlations: NONE | ||||
ROIs are listed in order of descending Ind. Cluster Ext., except for bilateral ROIs, which remain paired.
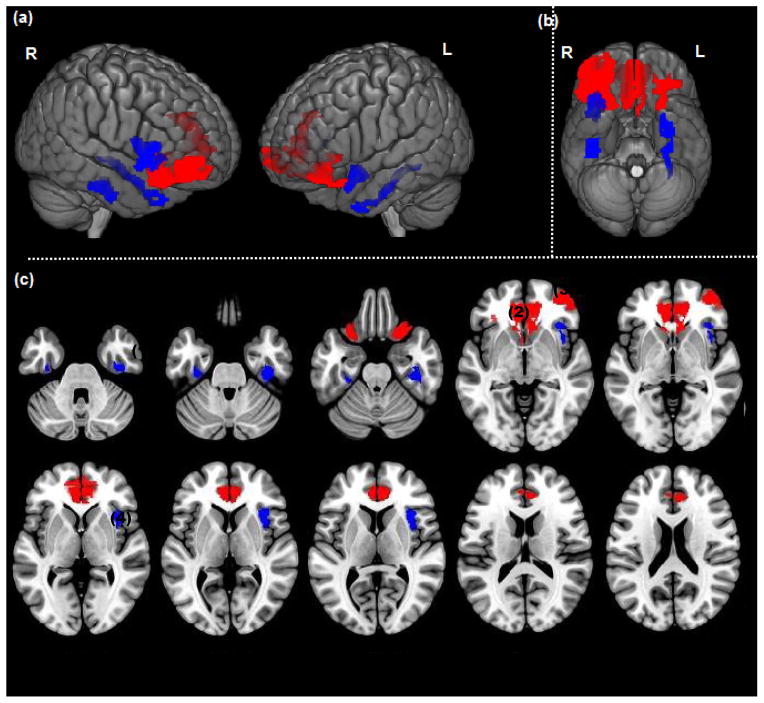
Among High Lethality attempters, aggression (LHA) was positively associated with gray matter volumes in large bilateral areas of the middle-inferior orbital frontal cortex (BA 11) and the anterior cingulate cortex. Significant, though much smaller, positive effects were also noted in the right middle-superior temporal cortex (BA 22), right insula, right lingual gyrus, bilateral fusiform gyrus, and right parahippocampus (Table 3). There were no significant negative effects of aggression on gray matter volumes among High Lethality attempters.
Impulsivity (BIS) had a positive effect on gray matter volumes in the right middle-superior temporal cortex, with lesser effects on the left fusiform gyrus and bilateral parahippocampus. A small negative effect of impulsivity on gray matter was noted in the right insula.
3.4. Personality interactions in Low Lethality attempters
Among Low Lethality attempters, aggression was also positively associated with gray matter volumes, although differing greatly from High Lethality attempters in anatomical locations and cluster sizes (i.e., size of correlated area). The most robust findings were in the right insula, and bilateral fusiform gyrus. Smaller areas of positive correlation with aggression were also noted in the right hippocampus, left middle-superior temporal lobe (BA 21), right parahippocampus, bilateral middle-inferior orbital frontal cortex (BA 11), left lingual gyrus, and right amygdala (Table 3). As with the High Lethality attempters, there were no significant negative correlations between aggression and gray matter volumes among Low Lethality attempters.
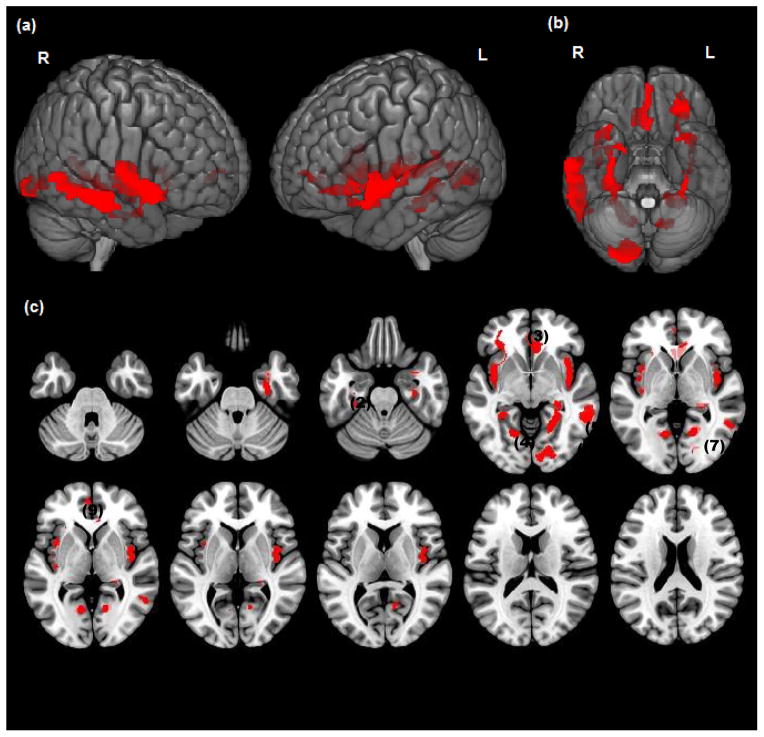
Impulsivity (BIS) was negatively associated with gray matter volumes in nine ROIs among Low Lethality attempters, most widely in the right middle-superior temporal cortex, bilateral insula, and bilateral lingual gyrus. Smaller negative effects of impulsivity on gray matter were also noted in the left anterior cingulate, left fusiform gyrus, bilateral parahippocampus, left middle-inferior orbitofrontal cortex, right hippocampus, and right amygdala (Table 3). There were no positive effects of impulsivity on gray matter volumes among Low Lethality attempters. Thus, High and Low Lethality attempters differed in the effects of impulsivity on gray matter in direction of association (e.g. negative vs. positive correlations), anatomical locations, and cluster sizes (e.g., insula).
4. Discussion
4.1. Overview
Neuroimaging studies, using MRI, positron emission tomography (PET), and functional MRI (fMRI) techniques, have demonstrated abnormalities in structure, metabolism and function in subjects with BPD compared with healthy controls (for review, see Schmahl and Bremner, 2006). Structural (MRI) and metabolic (PET) abnormalities have been described in impulsive subjects with BPD in areas of the prefrontal cortex (PFC), especially orbitofrontal and ventromedial PFC, anterior cingulate, and other fronto-limbic structures involved in regulation of mood, impulse and behavior (Goyer et al., 1994; de la Fuente et al., 1997; Juengling et al., 2002; Schmahl et al., 2003; Tebarz van Elst et al., 2003; Soloff et al., 2003, 2008; Hazlett et al., 2005). Subjects with BPD (and other impulsive PDs), have diminished metabolic responses to serotonergic pharmacologic activation by fenfluramine (FEN) or meta-chloropiperazine (mCPP) in related areas, suggesting a neurobiological basis for impulsivity and aggression in these patients (Siever et al., 1999; Soloff et al., 2000, 2005; New et al., 2002). Among PD subjects ascertained specifically for impulsive-aggression (e.g., subjects with intermittent explosive disorder), fMRI studies using angry faces demonstrate increased activation of the amygdala and diminished activation in orbitofrontal cortex (OFC). Among aggressive subjects, there is a loss of the normal amygdala-OFC coupling that facilitates cognitive control over affective arousal (Coccaro et al., 2007). Functional MRI studies in BPD subjects also demonstrate hyper-arousal of amygdala in response to negatively valenced faces or pictures (Herpertz et al., 2001; Donegan et al., 2003), and loss of connectivity between the amygdala and the OFC (New et al., 2007). Affective interference with executive cognitive functions in BPD is associated with a failure of “top down” cortical inhibition in the presence of “bottom up” limbic hyper-arousal resulting in the characteristic emotional dysregulation of the BPD patient (Silberzweig et al., 2007). Emotional dysregulation increases the vulnerability to impulsive, aggressive and suicidal behavior in BPD.
Our main aim in this study was to map the relationship between impulsivity, aggression and suicidal behavior in key brain structures among suicidal sub-groups distinguished by the lethality of their attempts. We found that higher degrees of medical lethality were related to diminished gray matter volumes across multiple fronto-temporal-limbic regions. The relationships between impulsivity, aggression and brain structure differed markedly between High and Low Lethality attempters. Similarly, the effects of impulsivity and aggression on brain structure differed markedly within lethality groups.
4.2. Aggression
The effects of aggression on brain structure differed by lethality status in anatomical location and cluster size (a measure of the area of significant correlation). Since there were no significant differences between groups in baseline aggression scores, lethality of suicidal behavior in BPD may be related to the mediation of aggression by specific neural networks. Among High Lethality attempters, increased aggression is most widely associated with increased gray matter volumes in the middle-inferior OFC and the anterior cingulate gyrus. The OFC is associated with executive cognitive functions such as response inhibition, selective attention, conflict resolution, and monitoring and regulating the limbic system (Bonelli and Cummings, 2007). It is part of a complex neural network (along with the fusiform gyrus and amygdala) that assesses emotion in facial expression and is activated by angry faces (Blair, 1999, 2004). Injury to the OFC results in disinhibition and in impulsive and aggressive behavior. (The famous 1848 case of Phineas Gage illustrates this point [Damasio et al., 1994].) Structural MRI studies in BPD subjects have reported diminished volumes in the OFC in adults and adolescents compared with healthy controls, and an association between increased impulsivity (BIS), diminished gray matter (BA 10), and increased white matter (BA 47) in areas of the OFC (Tebarz van Elst et al., 2003; Hazlett et al., 2005; Chanen et al., 2008). PET studies in BPD subjects report diminished fluorodeoxyglucose uptake in medial OFC (BA 9,10,11) compared with healthy controls. Covarying for impulsivity (BIS) rendered these differences non-significant (Soloff et al., 2003).
BPD subjects also have diminished alpha [C-11] mTrptophan trapping (mTrp) (a measure of serotonin synthesis) in the OFC compared with healthy controls (Leyton et al., 2001) (in these studies, impulsivity was inversely related to alpha[C-11] mTrp trapping in the medial frontal gyrus, anterior cingulate, temporal cortex and striatum). Structural and functional imaging studies suggest a neural mediation for impulsive-aggressive behavior in BPD, related, in part, to diminished serotonergic function in the OFC.
The OFC has extensive connectivity with the amygdala and, in concert with other prefrontal structures (such as the DLPFC), moderates amygdala hyper-arousal (Silberzweig et al., 2007). At times of negative emotional stress, prefrontal cortical inhibition from the OFC is decreased in subjects with BPD compared with control subjects (Silberzweig et al., 2007; New et al., 2009; Kraus et al., 2010). OFC activation decreases in BPD subjects while imagining self-injurious acts (Kraus et al., 2010). The resulting affective dysregulation of cognitive control increases the likelihood of impulsive-aggressive behavior in response to trigger events.
Among High Lethality attempters, increased aggression was also related to increased grey matter volumes in the anterior cingulate cortex. The anterior cingulate is activated by tasks requiring choices between competing stimuli (e.g., conflict resolution in the X version of the continuous performance task) (Carter et al., 2000). It is also involved in recognition of affective states, execution of affect-related operations, and self-regulation of emotion (Beauregard et al., 2001; Devinsky et al., 1995; Hazlett et al., 2005). In concert with the amygdala, the anterior cingulate is activated by negative emotions in healthy subjects (e.g., especially in dorsal, middle cingulate, perigenual and subgenual cingulate) where it acts to modulate emotion. Among subjects with BPD, negative emotion activates the posterior cingulate gyrus, with decreased activation of dorsal and right subgenual divisions (for review, see Ruocco et al., 2012b). When presented with fearful faces in an fMRI task, BPD subjects show greater deactivation (compared with controls) in the subgenual anterior cingulate cortex bilaterally, while demonstrating increased activation in the right amygdala. This result supports the hypothesis of fronto-limbic dysfunction in BPD with exaggerated amygdala response and diminished emotion regulation by the anterior cingulate (Minzenberg et al., 2007). (Unfortunately, this study defined only two ROIs: amygdala and anterior cingulate. Other fMRI studies with BPD subjects have also noted diminished activation of the OFC with negative affective stimuli (Silberzweig et al., 2007; New et al., 2009; Kraus et al., 2010).
Some, but not all, structural MRI studies in BPD subjects have reported diminished volumes in anterior and ventral cingulate compared to healthy control subjects (de la Fuente et al., 1997; Tebartz van Elst et al., 2003; Hazlett et al., 2005; Soloff et al., 2008 ). Hazlett et al. (2005) found that diminished gray matter volume in the left anterior cingulate (BA 25), and diminished white matter volume in the right posterior cingulate (BA 23), correlated with increased impulsivity (BIS) in subjects with BPD. Although these results appear contrary to our own, our VBM analyses did not define separate ROIs within the cingulate gyrus.
In marked contrast, aggression among Low Lethality attempters was most widely associated with gray matter volumes in the right insula and bilateral fusiform gyrus. The insula is a limbic “integration area” that is involved in recognition of one’s own internal emotional state and perceived emotion in others (i.e. “empathy”) (New et al., 2008). It is also involved in representing negative emotional states, such as disgust (Augustine, 1996). In structural studies, BPD subjects have diminished gray matter in insular cortex compared with healthy controls (Soloff et al., 2008). High lethality BPD attempters have diminished gray matter in the right insula compared with Low Lethality attempters (Soloff et al., 2012). In fMRI studies of BPD subjects, the insula is activated by negative emotional stimuli (for review, see Ruocco et al., 2012), by paradigms that mimic social exclusion (Eisenberger et al., 2003), by aversive memories (Schnell et al., 2007), and by recall of unresolved autobiographical memories (Beblo et al., 2006). Social tasks requiring co-operation activate the anterior insula, which is responsive to violations of social norms (King-Casas et. al., 2008). BPD subjects show diminished activation in the anterior insula compared with control subjects during an economic exchange game requiring social co-operation (King-Casas et al., 2008). Impairment in the function of the insula could lead to misjudging others’ intentions and disinhibited responses to perceived rejection, a core characteristic of BPD and common precipitant for impulsive suicide attempts.
Aggression among Low Lethality attempters also had a significant effect on the fusiform gyrus, bilaterally. The fusiform gyrus is primarily associated with facial recognition, and is a component part of a complex face-processing system that includes the OFC and the superior temporal cortex, lingual and parahippocampal gyrii, and the amygdala (Radua et al., 2010). This network processes facial recognition and emotion, and it analyzes bodily movement to assess the intentions of others. (The parahippocampus is involved in memory encoding and retrieval of information concerning familiarity of social settings, complementing the function of the fusiform face area by adding social and emotional context to a scene [Radua et al., 2010].) Deficits in these functions would impair social functioning.
Abnormalities in the structure and function of the fusiform gyrus have previously been reported in BPD compared with control subjects. In previous VBM studies, fusiform and parahippocampal gyrii were both diminished in volume in High compared with Low Lethality BPD attempters (Soloff et al., 2012). In some PET studies (though not all), BPD subjects have demonstrated diminished metabolism or cerebral blood flow in the fusiform gyrus compared with healthy controls (Leyton et al., 2001; Schmahl et al., 2003; Lange et al., 2005). The fusiform gyrus is activated bilaterally (with the amygdala) in BPD subjects compared with healthy controls in response to aversive stimuli in fMRI protocols (Herpertz et al., 2001).
Although there is considerable overlap in areas positively associated with aggression in High and Low Lethality attempters, regions with the largest cluster sizes differ markedly between groups. Prefrontal regions primarily associated with regulation of affect and impulsive-aggressive behavior are correlated with aggression in High Lethality attempters, while aggression in Low Lethality attempters is associated with limbic areas involved in empathy, social acceptance and co-operation. High Lethality suicide attempts are characterized by a subjective intent to die, while Low lethality attempts are generally communicative gestures intended to convey emotional distress and coerce a caring response.
4.3. Impulsivity
Impulsivity in both High and Low Lethality attempters was correlated with gray matter volumes in the middle-superior temporal cortex, but in opposite directions and markedly differing cluster sizes. The superior temporal cortex (with the insula and the lingual and fusiform gyrii) is involved in facial processing, analysis of intentions of others through bodily movement (Frith and Frith, 1999; Allison and McCarthy, 2000), and hyper-vigilance in attachment relationships (Buchheim et al., 2008). The superior temporal cortex is part of a complex circuit that mediates reflexive responses to visual social inputs, especially negative visual stimuli, such as angry, disgruntled faces (Koenigsberg et al., 2009; IIidaka et al., 2001).
The effects of impulsivity and aggression on brain structure discriminated High from Low Lethality attempters, despite the absence of significant differences on psychological measures of impulsivity and aggression (BIS, LHA). Differences between High and Low Lethality attempters are found in the neural mediation of these personality traits, supporting the view that they represent two separate but overlapping groups. High Lethality attempters are a commonly used model for completed suicides and may share with them specific neurobiological vulnerabilities associated with personality risk factors such as impulsivity and aggression. We also demonstrated differences in the effects of impulsivity and aggression on brain structure within each lethality group. These differences in affected regions and direction of association may suggest separate neural mediation for these traits.
Impulsivity and aggressiveness are related trait dispositions, but not identical constructs. Distinctions between these traits have been obscured in the literature through the use of terms such as impulsive-aggression or aggressive impulsivity, implying a unitary behavioral dimension for non-premeditated aggression. Coccaro et al. (1992, 1998) have advanced the view that impulsive-aggression is a single dimensional trait, a dysregulation of impulse control related to diminished serotonergic function in the brain. In this view, impulsive-aggression is an endophenotype that increases the likelihood of aggressive behavior given environmental triggers (McCloskey et al., 2009). In studies of BPD patients, and in large-sample surveys of non-clinical subjects, impulsivity and aggressiveness have been shown to be separable traits. Impulsivity is a higher order trait predisposing to non-premeditated aggression, which is a lower order observable behavior (Garcia-Forero et al., 2008; Critchfield et al., 2004). Impulsivity and aggression are risk factors for suicidal behavior in BPD, with differing effects on specific neural networks. Our results provide evidence of the neural substrates of impulsivity and aggression in the context of suicidality. Specific neural substrates may functionally mediate these personality traits, a hypothesis that can be more strongly tested using fMRI studies. Lethality of suicidal behavior in BPD may be determined, in part, by the mediation of these personality traits by specific neural networks involved in the regulation of mood, impulse and behavior.
4.4. Limitations
This study focused on effects of impulsivity and aggression on gray matter volumes in suicidal subjects with BPD and may not be generalizable to suicidal behavior in other disorders. Similarly, personality traits associated with suicidal behavior in other disorders (e.g., hopelessness/pessimism in MDD) may have their own unique relationships to neural structures and lethality, not found in BPD.
Could differences between High and Low Lethality attempters be related to brain injury incurred in high lethality attempts? This is a valid concern in all imaging studies of high lethality suicidal subjects. We are unable to resolve this issue. Although we did not conduct formal neuropsychological testing, all of our subjects were able to give informed consent, demonstrated normal cognitive functioning during diagnostic interviews, self-reports and scanning procedures, and had no apparent cognitive impairment in their everyday lives.
This was a correlational study. Correlation does not prove causation. Functional MRI studies are needed to test effects of impulsivity and aggression on brain activation in High and Low Lethality suicide attempters with BPD.
Fig. 1.
In ALL BPD attempters: Significant clusters (p<.05, cluster level) showing a negative association between Lethality Rating Scale scores and grey matter are rendered on a) bilateral lateral cortical surface views, b) ventral view of the cortical surface, and c) successive axial slices (z=−36 to z=20). Significant decreases were observed in Mid-Inf OFC(1), Fusiform(1), Insula(2), Hippocampus (3), Parahippocampus(4), Mid-Inf OFC(5), ACC(6), Lingual(7), Mid-Sup Temp(8)
Fig. 2.
Significant clusters (p<.05, cluster level) in High Lethality attempters (RED) and Low Lethality attempters (BLUE) showing a positive association between gray matter and aggression (LHA) are rendered on a) bilateral lateral cortical surface views, b) ventral view of the cortical surface and c) successive axial slices (z=−36 to z=20). Significant associations shown in Fusiform(1), ACC(2), Mid-Inf OFC(3), Insula(4).
Fig. 3. Low Lethality subjects.
Significant clusters (p<.05, cluster level) showing a negative association between impulsivity (BIS) and grey matter are rendered on a) bilateral lateral cortical surface views, b) ventral view of the cortical surface and c) successive axial slices (z=−36 to z=20). Significant decreases are shown in : Insula(1), Fusiform(2), Mid-Inf OFC(3), Parahippocampus(4), Hippocampus(5), Lingual(6), Amygdala (7), Mid-Sup Temp(8), ACC(9)
Table 3b.
Relationships between Impulsivity (BIS) and Grey Matter Concentrations in High and Low Lethality Attempters
| Anat. ROI (TAL) | Cluster Ext. p< 0.05 | Ind. Cluster Ext. | P value | Voxel Peak (x, y, z) |
|---|---|---|---|---|
| A. High Lethality BIS–Positive Correlations | ||||
| Mid Sup Tp | 390 | 771 | <0.001 | (58, −46, 5) |
| Parahipp.(L) | 65 | 232 | <0.004 | (−16, −28, −8) |
| [Parahipp.] | 65 | 140 | <0.001 | (14, −28, −6)] |
| Fusiform(L) | 140 | 208 | <0.005 | (−20, −4, −35) |
| B. High Lethality BIS-Negative Correlations: | ||||
| Insula | 162 | 162 | <0.008 | (42, 10, 1) |
| C. Low Lethality BIS-Positive: NONE | ||||
| D. Low Lethality BIS-Negative Correlations | ||||
| Mid Sup Tp | 466 | 1709 | <0.001 | (52, −48, −2) |
| Insula | 177 | 1158 | <0.002 | (40, −3, 1) |
| [Insula(L)] | 177 | 775 | <0.007 | (−35, 2, −1)] |
| Lingual | 237 | 1020 | <0.002 | (22, −88, −7) |
| [Lingual(L)] | 237 | 327 | <0.012 | (−17, −63, 0)] |
| ACC(L) | 151 | 534 | <0.008 | (−2, 48, 0) |
| Fusiform(L) | 149 | 503 | <0.008 | (−27, −46, −2) |
| Parahipp | 105 | 451 | <0.007 | (31, −28, −7) |
| [Parahipp(L)] | 105 | 159 | <0.008 | (−27, −45, −2)] |
| Mid-Inf OFC(L) | 148 | 336 | <0.001 | (−28, 31, −3) |
| Hippocampus | 96 | 271 | <0.006 | (26, −31, 2) |
| Amygdala | 60 | 176 | <0.019 | (32, −4, −19) |
Acknowledgments
The research reported was supported by NIMH grant MH 48463 (PHS), the Brain Behavior Research Foundation, the Children’s Hospital Foundation, and the Prechter Pediatric Bipolar Program World Heritage Foundation (VAD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allison T, McCarthy G. Social perception from visual cues: role of the STS region. Trends in Cognitive Science. 2000;4 (7):267–277. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Research Reviews. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Banissy MJ, Kanai R, Walsh V, Rees G. Inter-individual differences in empathy are reflected in human brain structure. NeuroImage. 2012;62 (3):2034–2039. doi: 10.1016/j.neuroimage.2012.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt ES. Factor analysis of some psychometric measures of impulsiveness and anxiety. Psychological Reports. 1965;16:547–554. doi: 10.2466/pr0.1965.16.2.547. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. Journal of Neuroscience. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beblo T, Driessen M, Mertens M, Wingenfeld K, Piefke M, Rullkoetter N, Silva-Saavedra A, Mensebach C, Reddemann L, Rau H, Markowitsch HJ, Wulff H, Lange W, Berea C, Ollech I, Woermann FG. Functional MRI correlates of the recall of unresolved life events in borderline personality disorder. Psychological Medicine. 2006;36:845–856. doi: 10.1017/S0033291706007227. [DOI] [PubMed] [Google Scholar]
- Blair RJ. The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain and Cognition. 2004;55:198–208. doi: 10.1016/S0278-2626(03)00276-8. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Morris JS, Frith CD, Perrett DJ, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122:883–893. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- Bonelli RM, Cummings JL. Frontal subcortical circuitry and behavior. Dialogues in Clinical Neuroscience. 2007;9 (2):141–151. doi: 10.31887/DCNS.2007.9.2/rbonelli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GL, Goodwin FK, Ballenger JC, Goyer PF, Major LK. Aggression in humans correlates with cerebrospinal fluid amine metabolites. Psychiatry Research. 1979;1:131–139. doi: 10.1016/0165-1781(79)90053-2. [DOI] [PubMed] [Google Scholar]
- Buchheim A, Erk S, George C, Kachele H, Kircher T, Martius P, Pokorny D, Ruchsow M, Spitzer M, Walter H. Neural correlates of attachment trauma in borderline personality disorder: A functional magnetic resonance imaging study. Psychiatry Research: Neuroimaging. 2008;163:223–235. doi: 10.1016/j.pscychresns.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinik M, Ross LL, Stenger VA, Noll D, Cohen JD. Parsing executive processes: strategic versus evaluative functions of the anterior cingulate cortex. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanen AM, Velakoulis D, Carison K, Gaunson K, Wood SJ, Yuen HP, Yücel M, Jackson HJ, McGorry PD, Pantelis C. Orbitofrontal, amygdala and hippocampal volumes in teenagers with first presentation borderline personality disorder. Psychiatry Research: Neuroimaging. 2008;163:116–125. doi: 10.1016/j.pscychresns.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Coccaro E. Impulsive aggression and central serotonergic system function in humans: an example of a dimensional brain-behavior relationship. International Clinical Psychopharmacology. 1992;7:3–12. doi: 10.1097/00004850-199200710-00001. [DOI] [PubMed] [Google Scholar]
- Coccaro EF. Impulsive aggression: a behavior in search of a clinical definition. Harvard Review of Psychiatry. 1998;5:1–4. doi: 10.3109/10673229809003583. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biological Psychiatry. 2007;62:168–178. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Critchfield K, Levy K, Clarkin J. The relationship between impulsivity, aggression, and impulsive-aggression in borderline personality disorder: an empirical analysis of self-report measures. Journal of Personality Disorders. 2004;18:555–570. doi: 10.1521/pedi.18.6.555.54795. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski T, Frank R, Galaburda A, Damasio AR. The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science. 1994;264:1102–1105. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- De La Fuenta JM, Goldman S, Stanus E, Vizuete C, Morlan I, Bobes J, Mendlewicz J. Brain glucose metabolism in borderline personality disorder. Journal of Psychiatric Research. 1997;31:531–541. doi: 10.1016/s0022-3956(97)00001-0. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell M, Vogt B. Contributions of anterior cingulate cortex to behavior. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Donegan NH, Sanislow CA, Blumberg HP, Fulbright RK, Lacadie C, Skudlarski P, Gore JC, Olson IR, McGlashan TH, Wexler BE. Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation. Biological Psychiatry. 2003;54 (11):1284–1293. doi: 10.1016/s0006-3223(03)00636-x. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Pruitt P, Goradia D, Murphy E, Bakshi N, Keshavan MS, Rajan U, Reid A, Zajac-Benitez C. Fronto-parietal hypo-activation during working memory independent of structural abnormalities: conjoint fMRI and sMRI analyses in adolescent offspring of schizophrenia patients. NeuroImage. 2011;58 (1):234–241. doi: 10.1016/j.neuroimage.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302 (5643):290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Fertuck EA, Lenzenweger MF, Clarkin JF, Hoermann S, Stanley B. Executive neurocognition, memory systems and borderline personality disorder. Clinical Psychology Review. 2006;26:346–375. doi: 10.1016/j.cpr.2005.05.008. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition (SCID-I/P, 4/2005 revision) Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 2005. [Google Scholar]
- Frith CD, Frith U. Interacting minds- a biological basis. Science. 1999;286:1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsely KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Garcia-Forero C, Gallardo-Pujol D, Maydeu-Olivares A, Andres-Pueyo A. Disentangling impulsiveness, aggressiveness and impulsive aggression: an empirical approach using self-report measures. Psychiatry Research. 2009;168:40–49. doi: 10.1016/j.psychres.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Goyer PF, Andreason PJ, Semple WE, Clayton AH, King AC, Compton-Toth BA, Schultz SC, Cohen RM. Positron-emission tomography and personality disorders. Neuropsychopharmacology. 1994;10:21–28. doi: 10.1038/npp.1994.3. [DOI] [PubMed] [Google Scholar]
- Gunderson JG, Kolb JE, Austin V. The Diagnostic Interview for Borderlines. American Journal of Psychiatry. 1981;138:896–903. doi: 10.1176/ajp.138.7.896. [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU Assessment Manual of Psychopharmacology-Revised. DHEW Publication No. ADM. 76-338. NIMH; Rockville, MD: 1976. [Google Scholar]
- Hazlett EA, New AS, Newmark R, Haznedar MM, Lo JN, Speiser LJ, Chen AD, Mitropoulou V, Minzenberg M, Siever LJ, Buchsbaum MS. Reduced anterior and posterior cingulate gray matter in borderline personality disorder. Biological Psychiatry. 2005;58:614–623. doi: 10.1016/j.biopsych.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Dietrich TM, Wenning B, Krings T, Erberich SG, Willmes K, Thron A, Sass H. Evidence of abnormal amygdala functioning in borderline personality disorder: a functional MRI study. Biological Psychiatry. 2001;50:292–298. doi: 10.1016/s0006-3223(01)01075-7. [DOI] [PubMed] [Google Scholar]
- Iidaka T, Omori M, Murata T, Kosaka H, Yonekura Y, Okada T, Sadato N. Neural interaction of the amygdala with the prefrontal and temporal cortices in the processing of facial expressions as revealed by fMRI. Journal of Cognitive Neuroscience. 2001;13 (8):1035–1047. doi: 10.1162/089892901753294338. [DOI] [PubMed] [Google Scholar]
- Juengling FD, Schmahl C, Hesslinger B, Ebert D, Bremner JD, Gostomzyk J, Bohus M, Lieb K. Positron emission tomography in female patients with borderline personality disorder. Journal of Psychiatric Research. 2003;37:109–115. doi: 10.1016/s0022-3956(02)00084-5. [DOI] [PubMed] [Google Scholar]
- King-Casas B, Sharp C, Loman-Breqam L, Lohrenz T, Fonagy P, Montague PR. The rupture and repair of co-operation in borderline personality disorder. Science. 2008;321:806–810. doi: 10.1126/science.1156902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. NeuroImage. 2009;46 (3):786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg HW, Siever LJ, Lee H, Pizzarello S, New AS, Goodman M, Cheng H, Flory J, Prohovnik I. Neural correlates of emotion processing in borderline personality disorder. Psychiatry Research: Neuroimaging. 2009;172:192–199. doi: 10.1016/j.pscychresns.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus A, Valerius G, Seifritz E, Ruf M, Bremner JD, Bohus M, Schmahl C. Script-driven imagery of self-injurious behavior in patients with borderline personality disorder: a pilot fMRI study. Acta Psychiatrica Scandinavica. 2010;121:41–51. doi: 10.1111/j.1600-0447.2009.01417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C, Kracht L, Herholz K, Sachsse U, Irle E. Reduced glucose metabolism in temporo-parietal cortices of women with borderline personality disorder. Psychiatry Research: Neuroimaging. 2005;139:115–126. doi: 10.1016/j.pscychresns.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Leichsenring F, Leibing E, Kruse J, New AS, Leweke F. Borderline personality disorder. Lancet. 2011;377 (9759):74–84. doi: 10.1016/S0140-6736(10)61422-5. [DOI] [PubMed] [Google Scholar]
- Leyton M, Okazawa H, Diksic M, Paris J, Rosa P, Mzengeza S, Young SN, Blier B, Benkelfat C. Brain regional alpha-[11C] methyl-l-tryptophan trapping in impulsive subjects with borderline personality disorder. American Journal of Psychiatry. 2001;158:775–782. doi: 10.1176/appi.ajp.158.5.775. [DOI] [PubMed] [Google Scholar]
- Loranger AW, Janca A, Sartorius N. Assessment and Diagnosis of Personality Disorder: The ICD-10 International Personality Disorder Examination (IPDE) Cambridge University Press; Cambridge: 1997. [Google Scholar]
- Mann JJ. Neurobiology of suicidal behavior. Nature Reviews. 2003;4:819–828. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Waternaux C, Haas GL, Malone KM. Toward a clinical model of suicidal behavior in psychiatric patients. American Journal of Psychiatry. 1999;156:181–189. doi: 10.1176/ajp.156.2.181. [DOI] [PubMed] [Google Scholar]
- Maris RW, Berman AL, Silverman MM. Comprehensive Textbook of Suicidology. Guildford Press; New York: 2000. p. 19. [Google Scholar]
- McCloskey MS, New AS, Siever LJ, Goodman M, Koenigsberg HW, Flory JD, Coccaro EF. Evaluation of behavioral impulsivity and aggression tasks as endophenotypes for borderline personality disorder. Journal of Psychiatric Research. 2009;43:1036–1048. doi: 10.1016/j.jpsychires.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Fan J, New AS, Tang CY, Siever LJ. Fronto-limbic dysfunction in response to facial emotion in borderline personality disorder: an event-related fMRI study. Psychiatry Research: Neuroimaging. 2007;155:231–243. doi: 10.1016/j.pscychresns.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New AS, Goodman M, Triebwasser J, Siever LJ. Recent advances in the biological study of personality disorders. Psychiatric Clinics of North America. 2008;31:441–461. doi: 10.1016/j.psc.2008.03.011. [DOI] [PubMed] [Google Scholar]
- New AS, Hazlett EA, Buchsbaum MS, Goodman M, Mitelman SA, Newmark R, Trisdorfer R, Haznedar MM, Koenigsberg HW, Flory J, Siever LJ. Amygdala-prefrontal disconnecrtion in borderline personality disorder. Neuropsychopharmacology. 2007;32:1629–1640. doi: 10.1038/sj.npp.1301283. [DOI] [PubMed] [Google Scholar]
- New AS, Hazlett EA, Buchsbaum MS, Goodman M, Reynolds D, Mitropoulou V, Sprung L, Shaw RB, Koeningsberg H, Platholi J, Silverman J, Siever LJ. Blunted prefrontal cortical 18-Flurodeoxyglucose positron emission tomography response to meta-chlorophenylpiperazine in impulsive aggression. Archives of General Psychiatry. 2002;59:621–629. doi: 10.1001/archpsyc.59.7.621. [DOI] [PubMed] [Google Scholar]
- New AS, Hazlett EA, Newmark RE, Zhang J, Triebwasser J, Meyerson D, Lazarus S, Trisdorfrer R, Goldstein KE, Goodman M, Koenigsberg HW, Flory JD, Siever LJ, Buchsbaum MS. Laboratory induced aggression: a positron emission tomography study of aggressive individuals with borderline personality disorder. Biological Psychiatry. 2009;66:1107–1114. doi: 10.1016/j.biopsych.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oquendo MA, Halberstam B, Mann JJ. Risk factors for suicidal behavior. In: First MB, editor. Standardized Evaluation in Clinical Practice. Review of Psychiatry. Vol. 22. American Psychiatric Publishing, Inc; Washington, DC: 2003. pp. 103–129. [Google Scholar]
- Radua J, Phillips ML, Russell T, Lawrence NS, Marshall N, Kalidindi S, El-Hage W, McDonald C, Giampietro V, Brammer MJ, David AS, Surguladze SA. Neural response to specific components of fearful faces in healthy and schizophrenic adults. NeuroImage. 2010;49:939–946. doi: 10.1016/j.neuroimage.2009.08.030. [DOI] [PubMed] [Google Scholar]