Abstract
Early selective attention skills are a crucial building block for cognitive development, as attention orienting serves as a primary means by which infants interact with and learn from the environment. Although several studies have examined infants’ attention orienting using the spatial cueing task, relatively few studies have examined neurodevelopmental factors associated with attention orienting during infancy. The present study examined the relationship between normative genetic polymorphisms affecting dopamine and acetylcholine signaling and attention orienting in 7-month-old infants during a spatial cueing task. We focused on 3 genes, including the CHRNA4 C1545T SNP (rs10344946), DAT1 3′ UTR VNTR, and COMT Val158Met SNP (rs4680), as previous adult research has linked spatial attention skills to these polymorphisms. Behavioral measures included both facilitation of orienting at the cued location as well as inhibition of return (IOR), in which attention orienting is suppressed at the cued location. Results indicated that COMT Val carriers showed robust IOR relative to infants with the Met/Met genotype. However, COMT was unrelated to infants’ facilitation responses, and there were no effects of CHRNA4 or DAT1 on either facilitation or IOR. Overall, this study suggests that variations in dopamine signaling, likely in prefrontal cortex, contribute to individual differences in orienting during early development.
Keywords: attention infancy, inhibition of return (IOR), dopamine, genetics
Attention orienting supports effective learning and updating of cognitive representations, which allows individuals to remain sensitive to changing contexts and alter their behavior accordingly (Itti & Koch, 2001; Ristic & Giesbrecht, 2011). Attention orienting is especially crucial early in life, as it serves as a primary means by which young infants interact with and learn from their environment (Gibson, 2003). Furthermore, attention orienting is often disrupted in neurodevelopmental disorders, including attention-deficit/hyperactivity disorder, schizophrenia, and autism (Berger & Posner, 2000; Mushquash, Fawcett, & Klein, 2012; Nation & Penny, 2008), suggesting that early orienting may be a crucial building block for the development of more complex cognitive skills. Examining the mechanisms underlying attention orienting early in life can thus inform researchers’ understanding of both typical and atypical cognitive development. The present study considered the neural mechanisms underlying early attention orienting by examining links between cholinergic and dopaminergic genetic polymorphisms and infant orienting during a spatial cueing task.
Attention orienting has been extensively studied across development using the spatial cueing task (Posner, 1980). In this task, attention is engaged at a central location while a peripheral cue appears, a brief delay is imposed, and a target is subsequently presented in either the cued or noncued location. The timing of the cue-to-target delay (or stimulus-onset asynchrony [SOA]) is critical, as varying this delay can elicit different orienting responses. Individuals typically respond faster to targets in the cued location when the SOA is very short (< 250 ms; Posner, 1980; Posner & Cohen, 1984). This facilitation effect at short SOAs is mediated by a covert orienting mechanism in which attention is engaged at the cued location prior to target onset, allowing for more efficient responding to targets appearing in the same location (Posner & Cohen, 1984). However, when the SOA is extended, responses become inhibited at the cued location and orienting is instead faster to targets in the noncued location, an effect known as inhibition of return (IOR; Klein, 2000; Posner & Cohen, 1984). The IOR effect has been interpreted as an updating mechanism that biases orienting toward novel information (Klein, 1988; Posner, Rafal, Choate, & Vaughan, 1985). Proposed mechanisms underlying this novelty bias include habituation/sensory adaptation at the cued location (Dukewich, 2009; Wang, Satel, & Klein, 2012), inhibitory tagging of the cued location (Houghton & Tipper, 1996; Milliken, Tipper, Houghton, & Lupianez, 2000), or updating of attentional saliency maps (Colzato & Hommel, 2009; Itti & Koch, 2001; Vivas, Humphreys, & Fuentes, 2006). These mechanisms are not mutually exclusive and may interact in driving IOR (Berlucchi, 2006).
Infant researchers have used the spatial cueing task to identify developmental changes in attention orienting during the first year of life. Although some studies have found spatial cueing effects between birth and 3 months of age (Clohessy, Posner, Rothbart, & Vecera, 1991; Valenza, Simion, & Umilta, 1994), these effects were based on overt orienting, in which eye gaze and attention shift together, making it difficult to disentangle attention orienting from eye movements. Other studies converge on 3–4 months as the age at which the ability to shift attention independently of eye gaze, or covert orienting, first emerges (Johnson, Posner, & Rothbart, 1994; Richards, 2000). Facilitation effects are also first evident at this age. In contrast, IOR takes more time to develop, with robust IOR evident by the age of 6 months (Butcher, Kalverboer, & Geuze, 1999; Hood, 1993, 1995; Johnson & Tucker, 1996; Varga, Frick, Kapa, & Dengler, 2010). However, it is important to note that individual differences in spatial attention and orienting remain, despite robust group-level facilitation and IOR effects (Butcher et al., 1999). Overall, this trajectory from early emergence of facilitation to gradual development of IOR reflects increasing control over attention during the first year of life.
Based on these findings from infant spatial cueing studies, researchers have also made inferences about the developing neural systems that support attention orienting during infancy. In particular, it has been noted that the functional maturation of posterior parietal cortex around 3 months of age (Chugani, 1994) mirrors the timing of development of covert orienting and facilitation cueing effects. This observation led to the hypothesis that the parietal cortex is crucial for spatial cueing effects based on covert orienting and, more broadly, that cortical networks become increasingly relevant as infants develop greater control over attention orienting (Hood, 1993, 1995; Johnson, 1990; Johnson et al., 1994; Johnson & Tucker, 1996). This hypothesis was supported by event-related potential (ERP) studies (Richards, 2000, 2001, 2005) that showed both increasing IOR between 3.5 and 6.5 months old and changes in cortical activity. These changes in cortical activity were localized over frontal regions involved in saccade planning (e.g., frontal eye fields), suggesting that frontoparietal attention networks may become more engaged as attentional control develops.
These findings linking early attention orienting to possible developmental changes in frontal and parietal regions are consistent with work in adults highlighting the role of frontoparietal attention networks in classic spatial cueing effects (Corbetta, Kincade, Ollinger, McAvoy, & Shulman, 2000; Lepsien & Pollmann, 2002; Mayer, Dorflinger, Rao, & Seidenberg, 2004; Mayer, Seidenberg, Dorflinger, & Rao, 2004; Posner & Petersen, 1990; Snyder & Chatterjee, 2006). However, further examination of these links has remained difficult due to the relatively limited repertoire of noninvasive methods for studying brain– behavior relations in infancy. One relatively new approach to examining neural mechanisms underlying cognitive processes is to link individual differences in cognitive processing to normative genetic polymorphisms that affect signaling within relevant networks. One important caveat when taking this approach is that complex cognitive processes, including those available in early infancy, are mediated by numerous genes that interact with each other and with a wide range of contextual/environmental factors. Because it is difficult to capture this complexity in a single study, it can be difficult to replicate genetic studies. One way researchers have tried to counter this challenge is by identifying candidate genes that have repeatedly been linked to attention orienting.
Very few studies have examined genetic effects on attention processes in infancy; however, a number of previous adult studies have linked spatial attention to genetic polymorphisms that modulate acetylcholine and dopamine signaling. Specifically, the cholinergic CHRNA4 C1545T SNP has been related to performance on a variety of attention tasks (Greenwood, Parasuraman, & Espeseth, 2012), with the C/C genotype often associated with increased orienting efficiency during endogenous cueing tasks (Greenwood, Fossella, & Parasuraman, 2005; Parasuraman, Greenwood, Kumar, & Fossella, 2005). Similar to the results of these adult studies, the results of Sheese, Voelker, Posner, and Rothbart (2009) identified a link between rates of anticipatory looking, believed to be a precursor to executive attention (Sheese, Rothbart, Posner, White, & Fraundorf, 2008), and CHRNA4 genotype at 6–7–months old. Though only one study, this result suggests that CHRNA4 may be similarly related to spatial attention during infancy.
Adult work has also related dopaminergic polymorphisms to spatial attention processes. In particular, two recent studies showed that the DAT1 VNTR polymorphism predicted performance on the spatial cueing task (Colzato, Pratt, & Hommel, 2010; Rokem et al., 2011), with the 9-R genotype associated with enhanced IOR relative to the 10-R genotype. A second polymorphism, the COMT Val158Met SNP, affects dopamine signaling in prefrontal cortex and has been studied in relation to performance on executive attention tasks. In these studies, the Met allele confers an advantage on tasks requiring controlled attention, whereas the Val allele is associated with improved performance on tasks requiring updating or sensitivity to novelty (Cools & D’Esposito, 2011; Weinshilboum, Otterness, & Szumlanski, 1999). However, COMT has been examined in the context of attention orienting in only two adult studies. These studies demonstrated that the Val allele was related to greater efficiency in orienting away from targets during an antisaccade task (Haraldsson et al., 2010) but also showed larger costs following invalid cues during a spatial cueing task (Lundwall, Guo, & Dannemiller, 2012). Together, these studies provide preliminary evidence that COMT may be relevant for attention orienting during adulthood; however, to date no study has examined potential relations between COMT and IOR.
A recent study examined relationships among the DAT1 and COMT polymorphisms, attention orienting, and inhibitory control among 9-month-old infants (Holmboe et al., 2010). In this study, infants completed a “freeze-frame” task that assessed inhibitory control over attention orienting. Infants fixated an animated stimulus while a distractor appeared in the periphery. If the infant oriented to the distractor, the animated stimulus froze, thus discouraging subsequent orienting to the periphery. Infants’ ability to inhibit orienting to the peripheral distractors in favor of maintaining the animated stimulus varied across genotype groups. Specifically, for the COMT polymorphism, the Val allele was associated with more frequent disengagement and reorienting to the periphery, while the Met allele was associated with increased inhibition over orienting. For the DAT1 VNTR, the 9-R genotype was associated with increased orienting to the peripheral distractors. Overall, this study suggests that dopaminergic signaling may be related to attention orienting and saccade inhibition at 9 months old (Holmboe et al., 2010).
In sum, adult genetic studies have demonstrated a role for cholinergic (CHRNA4) and dopaminergic (DAT1, COMT) systems in attention orienting, though few studies have specifically examined facilitation and IOR effects elicited by the spatial cueing task. Given that there are relatively few methods available for examining brain– behavior relations in infancy, genetic studies in infants can help elucidate whether similar mechanisms are relevant for normative attention orienting in early life. However, only three genetic studies (Holmboe et al., 2010; Sheese et al., 2009; Voelker, Sheese, Rothbart, & Posner, 2009) have examined early attention processes, and none of these studies has examined orienting during the spatial cueing task. Thus, though these early findings are promising, much more work is necessary for researchers to understand how genetic polymorphisms, and the neural systems they influence, contribute to early individual differences in attention orienting.
In the present study, we examined links between cholinergic and dopaminergic genes and infants’ orienting behavior during the spatial cueing task. Specifically, we examined whether CHRNA4, COMT, and DAT1 genotypes contributed to individual differences in facilitation or IOR orienting effects among 7-month-old infants. We selected this age group because both relevant attention effects are typically observed at the group level by 6 months of age. As with many infant studies, we used several task modifications to accommodate limitations in infant responses, including measures of saccade latencies, rather than manual reaction time and presentation of bilateral targets (i.e., one target appearing in the cued location and a second target simultaneously appearing in the noncued location) to allow for multiple measures of spatial cueing effects. Given that the CHRNA4 C/C genotype has been related to increased orienting efficiency among adults, we predicted that infants with the C/C genotype would show more robust facilitation of orienting following short SOAs. In addition, we predicted that the dopaminergic polymorphisms would be related to IOR in this age group, as IOR has previously been linked to DAT1 among adults. Finally, based on the high degree of structural and functional interconnectivity of frontostriatal networks (Casey, Durston, & Fossella, 2001; Cools, 2011), we also hypothesized that the two dopaminergic polymorphisms, COMT and DAT1, may interact in their contributions to infants’ orienting behaviors.
Method
Participants
Participants were 88 infants (43 boys, 45 girls; M age = 7.0 months; range = from 6 months 23 days to 7 months 8 days) who completed the spatial cueing task. An additional seven infants were tested but excluded due to fussiness (five) or experimenter error (two). Infants were recruited from a database of volunteers from the community. Based on parental report of race/ethnicity, 89% were White, 6% were Asian, 1% were African American, and 4% were other/unknown.1 Infants were excluded if they had been born early (< 37 weeks gestation), had low birth weight (< 5 lbs), or had a history of serious health problems. All families received an infant T-shirt as a thank-you gift.
Parents also reported basic socioeconomic (SES) data, including the mother’s and father’s highest levels of education and total household income bracket. In the present sample, maternal and paternal education ratings ranged from less than a high school education to professional degrees. Maternal and paternal education levels were averaged to generate a single composite parental education score. Total household income ratings corresponded to income brackets of $25,000 increments. In the present sample, the median income bracket was $75,000 –$100,000.
Spatial Cueing Task
Materials
All stimuli were presented on a 42-in. LCD screen using Macromedia Director MX 2004 for Windows (Microsoft Corp., Redmond, Washington). Infants sat on their parent’s lap and parents were asked to refrain from pointing to or talking about any of the stimuli. A digital video camera (Sony DCR-SR45; Tokyo, Japan) was placed on the table below the screen to provide live feed to the experimenter’s monitor, which was used for online data coding. The video output was also burned to DVD for subsequent offline coding.
Task stimuli included a central fixation, a peripheral cue, and a target shape. The central fixation loomed in and out (from 3.2 cm2 to 8.9 cm2) to maintain infants’ interest. The cue was a yellow ring (5.1-cm diameter), and the targets were identical green hearts (8.9 cm2) presented as static images. Cue and target stimuli were presented 18 degrees to the left or right of the fixation.
Procedure
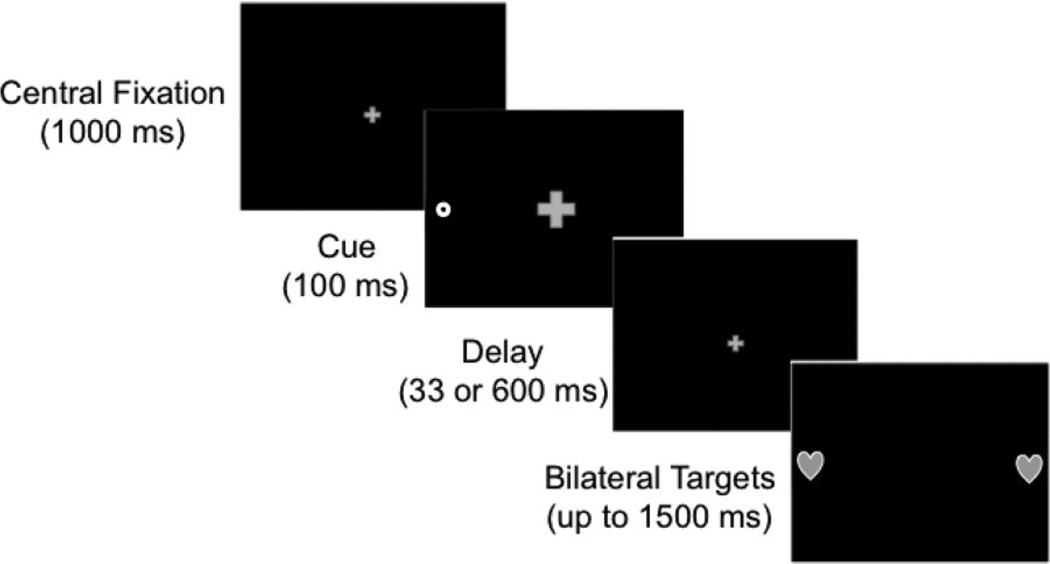
A schematic depiction of the task is presented in Figure 1. Short- and long-SOA trials were interleaved and presented in random order. Each trial began with presentation of the fixation stimulus. After 1,000 ms, the cue appeared on the left or right side for 100 ms. Following a short (33-ms) or long (600-ms) delay, the targets simultaneously appeared on both the left and right sides (i.e., in the cued and noncued locations). The fixation remained visible through the cue presentation and subsequent delay and disappeared at target onset. The targets remained visible up to 1,500 ms or until the infant looked away for longer than 500 ms.
Figure 1.
Schematic depiction of the spatial cueing task.
The experimenter monitored the infant’s eye movements and indicated when the infant was looking center, left, right, or away from the screen. The experimenter was kept blind to all stimuli. The computer program utilized the experimenter’s input to calculate the cumulative duration of looking during each trial. Trials were scored as invalid if the infant looked away before target onset or if the infant failed to look at a target within the 1,500-ms time window. Invalid trials were replaced to maximize the amount of usable data from each infant. Trials continued until the infant became too fussy to continue or completed a maximum of 48 valid trials (24 trials per trial type).
Data processing
Videos were coded for the timing and direction (e.g., center, left, right, or away) of each eye movement during the task. These coded data were used to compute the latency of the infant’s first look following target onset. In most cases, additional trials were excluded after this more accurate offline coding. Individual trials were discarded if the infant looked away before looking at a target (M = 28%, SD = 13.4%), if the infant broke fixation from the central fixation prior to target onset (M = 8.6%, SD = 7.8%), or if the infant never oriented to a target (M = 1.1%, SD = 2.7%). Genotype groups did not differ in the proportion of trials that were excluded. Trials were further filtered to exclude those with latencies that were less than 200 ms or greater than two SDs above the individual’s mean. Approximately half of the videos were coded for reliability; all latency measures were highly reliable (r ≥ .95, p < .001). Finally, latency values were standardized based on each individual’s mean latency to account for potential differences in baseline response times.
Standardized latency values were averaged to determine each infant’s mean reaction time (RT) to the peripheral targets. We computed RT difference scores by subtracting the mean standardized latency to the noncued location from the mean standardized latency to the cued location. RT difference scores were calculated separately for short- and long-SOA trials in order to assess facilitation and IOR, respectively. For ease of interpretation, all analyses utilized the absolute value of RT difference scores so that stronger facilitation and IOR effects would both be indicated by more positive values. Three infants oriented to the expected location during all trials, which precluded calculating the RT difference score. In addition, one infant had an extreme RT difference score for long-SOA trials (> 3 SDs above the group mean) and was excluded for all analyses of IOR. Thus, final N = 87 for short-SOA trials and N = 84 for long-SOA trials.
Genotyping
Procedure
DNA samples were collected from participants with the BuccalAmp DNA Extraction Kit (BQ0901SCR; EpiCentre Biotechnologies; Madison, Wisconsin). Seven samples became contaminated before genotyping was complete; five of these samples were recollected when the infants were 12 months old.
The DNA-containing solution was diluted to a working concentration for genotype testing. The CHRNA4 C1545T (rs10344946) and COMT Val158Met (rs4680) SNPs were genotyped using the TaqMan SNP Genotyping Assays C__25746809_10 and C__25746809_50 (Applied Biosystems; Grand Isle, New York), respectively. Assay-specific reagents were combined with TaqMan Genotyping Master Mix (4371353; Applied Biosystems) and amplified per kit instructions, which was followed by end-point fluorescence detection on an Infinite M200 (Tecan; Durham, NC) with allelic determinations made with JMP 8.0 (SAS). The DAT1 VNTR length measurements were completed using the previously reported primers TGTGGTGTAGGGAACGGCCTGAG and CTTCCTGGAGGTCACGGCTCAAGG (Barr et al., 2001; Vandenbergh et al., 1992). Following amplification, the fragments were analyzed on a 3130xl Genetic Analyzer (Applied Biosystems).
All DNA samples were genotyped in duplicate for quality control. DNA from cell lines was purchased from Coriell Cell Repositories (Camden, NJ) for all representative genotypes in duplicate. Genotypes were confirmed by sequencing using dye terminator cycle sequencing chemistry (DTCS chemistry) on an ABI 3130×1 (Applied Biosystems).
Results
Genotype Distributions
Eighty-seven of the samples (99%) were successfully genotyped for the CHRNA4 SNP; all 88 samples were successfully genotyped for the COMT Val158Met SNP; and 82 samples (93%) were successfully genotyped for the DAT1 VNTR. The number of infants with each genotype is presented in Table 1. These allele distributions were consistent with expected frequencies derived from the Hardy–Weinberg equilibrium—CHRNA4: χ2(2) = 0.19, p = .911; COMT: χ2(2) = 0.04, p = .983; DAT1: χ2(2) = 0.19, p = .911. Due to the low frequency of infants with the 9/9 variant for DAT1, infants with the 9/10 and 9/9 genotypes were grouped together, resulting in a 10-R group composed of infants with the 10/10 genotype (41 infants, 50%) and a 9-R group composed of infants with at least one 9-repeat allele (41 infants). Similarly, in order to maximize power to detect interactions between COMT and DAT1, we examined COMT effects across two groups, infants with the Met/Met genotype (N = 21) and those with at least one Val allele (N = 67).
Table 1.
Genotype Distributions
| Genotype | Total (%) | Grouping |
|---|---|---|
| CHRNA4 | ||
| T/T | 26 (29.9) | T/T |
| T/C | 41 (47.1) | T/C |
| C/C | 20 (23.0) | C/C |
| COMT Val158Met | ||
| Met/Met | 21 (23.9) | Met/Met |
| Met/Val | 44 (50.0) | Val carrier |
| Val/Val | 23 (26.1) | Val carrier |
| DAT1 3′ VNTR | ||
| 9/9 | 6 (7.3) | 9-R |
| 9/10 | 35 (42.7) | 9-R |
| 10/10 | 41 (50.0) | 10-R |
Demographics
Preliminary analyses were conducted to determine whether demographic measures varied across genotype. Age, gender, ethnicity (White vs. non-White), SES–parental education, and SES–total household income were entered into a multivariate analysis of variance, with CHRNA4, COMT, and DAT1 genotypes as independent variables. Results indicated a trend-level effect of CHRNA4 genotype on SES–total income, F(2, 62) = 2.57, p = .084, with higher income ratings within the T/T group, M = 5.04, SD = 2.38, relative to the T/C group, M = 4.33, SD = 1.93; t(57) = 2.23, p = .03. Results also indicated a marginal effect of COMT genotype on SES–total income, F(1, 62) = 2.67, p = .108, with higher income ratings for the Val carriers (M = 4.88, SD = 1.98) compared with the Met/Met group (M = 4.11, SD = 2.52). There was also a trend-level effect of COMT on SES–parental education, F(1, 62) = 3.20, p = .079, with higher ratings for Val carriers (M = 3.45, SD = 0.79) compared with the Met/Met group (M = 2.63, SD = 1.03). No effects reached significance for any of the other variables. In order to account for potential confounds between genotype and SES, we included both SES variables (total income and parental education) as covariates in subsequent analyses of genetic effects.
Spatial Cueing Effects
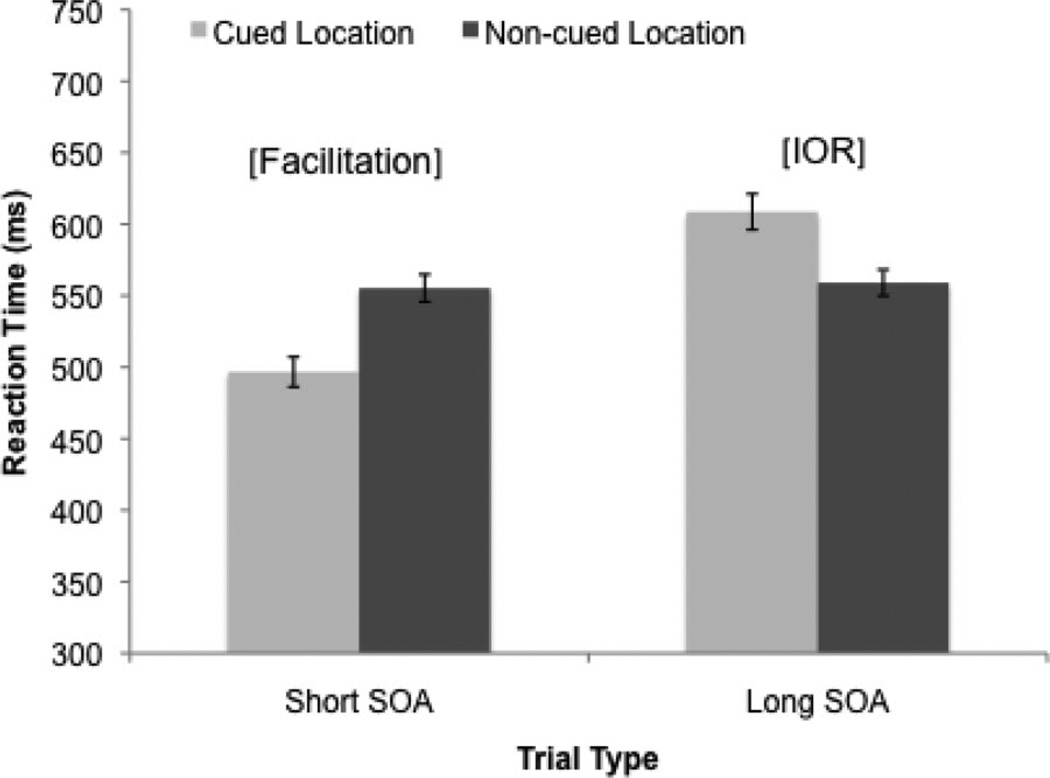
We first verified that infants showed the expected cueing effects on orienting. On average, infants initially oriented to the cued location on 54.6% (SD = 15.3%) of short SOA trials and oriented to the noncued location on 61.7% (SD = 17.7%) of long SOA trials. One-sample t tests indicated that these orienting rates were significantly above chance: tShort(85) = 2.89, p = .005; tLong(85) = 6.04, p < .001. Cueing effects on RT were assessed using a 2 (trial type: short, long SOA) × 2 (target: cued, non-cued) repeated-measures analysis of variance (ANOVA) with mean saccade latency as the dependent variable. Results indicated a main effect of trial type, F(1, 83) = 46.64, p < .001; η2 = .36, with faster responses during short-SOA trials (M = 528.51 ms, SD = 82.75 ms) compared with long-SOA trials (M = 582.04 ms, SD = 83.0 ms). There was also a significant Trial Type × Target interaction, F(1, 83) = 50.59, p < .001; Figure 2. Follow-up analyses indicated that infants showed the expected facilitation of orienting to targets in the cued location during short-SOA trials, MCued = 496.69 ms, SD = 98.4 ms; MNoncued = 555.30 ms, SD = 89.54 ms; F(1, 86) = 36.93, p < .001; η2 = .30, as well as the expected inhibition of orienting to targets in the cued location during long-SOA trials, MCued = 604.47 ms, SD = 109.82 ms; MNoncued = 559.61 ms, SD = 87.16 ms; F(1, 83) = 14.38, p < .001; η2 = .15. Thus, infants showed the predicted response facilitation during short-SOA trials and the predicted IOR during long-SOA trials.
Figure 2.
Reaction times to the cued and noncued locations during short- and long-stimulus-onset-asynchrony (SOA) trials. Facilitation is indicated by faster responses to the cued location during short-SOA trials. Inhibition of return (IOR) is indicated by faster responses to the noncued location during long-SOA trials.
Genetic Effects
Facilitation and IOR scores (i.e., RT difference scores) for all genotype groups are presented in Table 2. For each gene, we initially examined basic effects of genotype on overall RT using a one-way ANOVA with genotype as a between-subjects factor. Preliminary analyses were also conducted to determine whether facilitation and IOR scores were above chance (zero) within each genotype. Our primary goal was to determine whether the extent of facilitation or IOR varied across genotype groups. To do so, we conducted separate analyses of covariance (ANCOVAs) with standardized facilitation/IOR scores as the dependent variable, genotype as the primary independent variable, and SES–parental education and SES–household income treated as covariates.
Table 2.
Reaction Time (RT) Difference Scores in Milliseconds for All Genotypes
| RT difference score (SD) |
z RT difference score (SD) |
||||
|---|---|---|---|---|---|
| Genotype | N | Short SOA (Facilitation) | Long SOA (IOR) | Short SOA (Facilitation) | Long SOA (IOR) |
| COMT | |||||
| Met/Met | 20 | 72.58 (113.88) | 14.63 (114.12) | 0.36 (0.51) | −0.05 (0.49) |
| Val carrier | 66 | 54.74 (82.46) | 54.94 (105.50) | 0.29 (0.49) | 0.25 (0.49) |
| CHRNA4 | |||||
| T/T | 26 | 49.51 (101.30) | 51.37 (102.56) | 0.25 (0.55) | 0.26 (0.60) |
| T/C | 41 | 60.69 (84.84) | 32.45 (110.84) | 0.30 (0.50) | 0.15 (0.49) |
| C/C | 18 | 72.76 (91.18) | 63.06 (113.95) | 0.43 (0.36) | 0.19 (0.40) |
| DAT1 | |||||
| 9-R | 41 | 54.87 (99.09) | 45.15 (101.33) | 0.26 (0.45) | 0.22 (0.48) |
| 10-R | 39 | 53.70 (80.90) | 35.92 (98.53) | 0.34 (0.56) | 0.16 (0.47) |
| COMT × DAT1 | |||||
| Met/Met × 9-R | 8 | 64.27 (87.42) | 29.1 (69.35) | 0.22 (0.32) | 0.16 (0.28) |
| Met/Met × 10-R | 11 | 66.55 (129.90) | 22.81 (127.40) | 0.43 (0.62) | −0.07 (0.36) |
| Val carrier × 9-R | 33 | 51.13 (80.47) | 37.68 (105.63) | 0.27 (0.48) | 0.24 (0.52) |
| Val carrier × 10-R | 28 | 49.86 (84.98) | 55.08 (88.36) | 0.29 (0.56) | 0.25 (0.48) |
Note. SOA = stimulus-onset-asynchrony; IOR = inhibition of return.
COMT Val158Met SNP (rs4680)
Met/Met infants showed slower overall response times (M = 595.96 ms, SD = 88.36 ms) compared with Val carriers (M = 540.79 ms; SD = 65.42 ms), F(1, 86) = 9.54, p = .003; η2 = .10. Facilitation scores were above chance for all COMT genotypes, tMet/Met(20) = 2.92, p = .008; tVal carrier (66) < 5.42, p < .001). IOR scores were above chance among Val carriers, t(62) = 4.13, p < .001, but were at chance levels within the Met/Met group, t(20) = 0.59, p = .563.
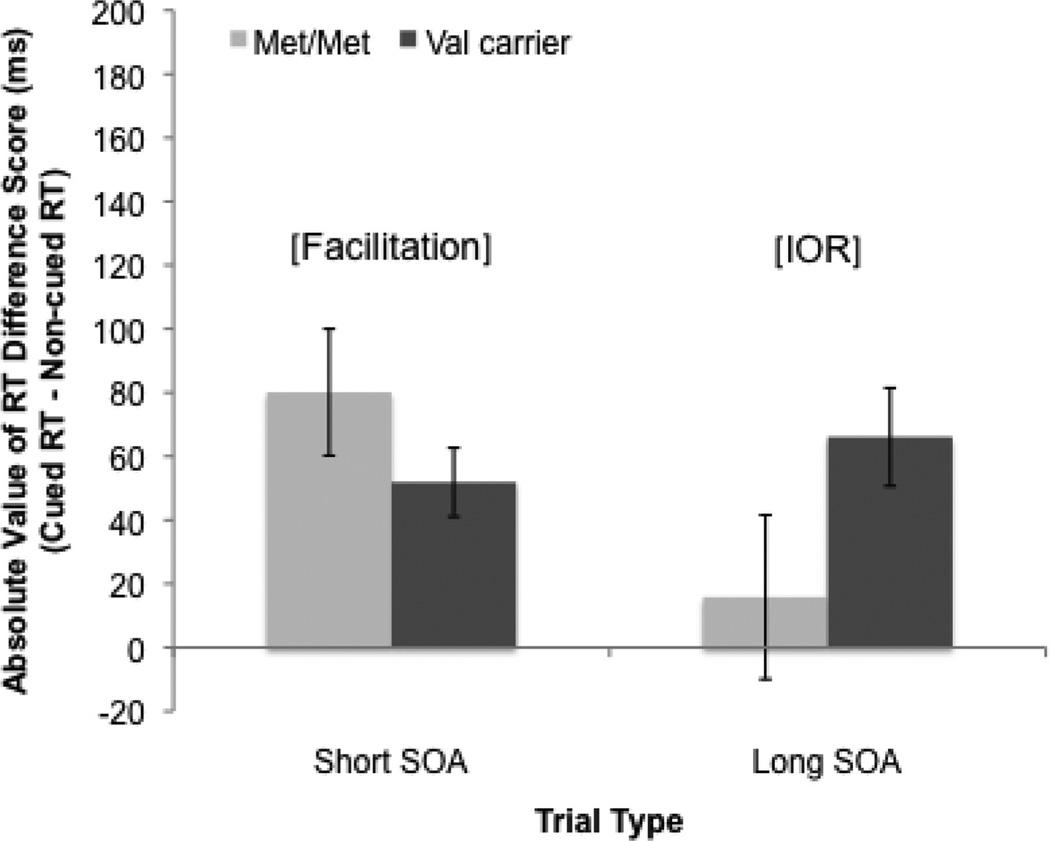
We conducted separate ANCOVAs to examine whether facilitation and IOR scores varied across COMT genotypes. Results indicated that COMT genotype was not related to infants’ facilitation scores, F(1, 73) = 0.48, p = .49. In contrast, a second ANCOVA indicated that COMT genotype was related to IOR scores, F(1, 75) = 5.12, p = .027, η2 = .06, with Val carriers showing stronger IOR effects (M = 54.94 ms, SD = 105.50 ms) compared with infants with the Met/Met genotype (M = 14.63 ms, SD = 114.12 ms; Figure 3). Thus, COMT genotype predicted individual differences in infants’ IOR scores but was unrelated to the extent to which they demonstrated facilitation.
Figure 3.
Absolute value of reaction time (RT) difference scores during short- and long-stimulus-onset-asynchrony (SOA) trials across COMT genotype groups. Positive scores indicate stronger cueing effects. IOR = inhibition of return.
CHRNA4 C1454T SNP (rs1044396)
Overall RT did not vary by CHRNA4 genotype, F(2, 84) = 0.81, p = .446. Facilitation scores were above chance for all of the CHRNA4 genotype groups: tT/T(25) = 2.49, p = .02; tT/C(40) = 4.58, p < .001; and tC/C(19) = 3.5, p = .002. IOR scores were above chance in the T/T and C/C genotype groups, tT/T(25) = 2.55, p = .017; and tC/C(18) = 2.56, p = .02, but were only marginally significant in the T/C group, M = 32.45 ms, SD = 110.84 ms, t(39) = 1.85, p = .072.
We again conducted separate ANCOVAs to examine infants’ facilitation and IOR scores across CHRNA4 genotype groups. Results indicated that there were no significant effects of CHRNA4 on either facilitation, F(2, 72) = 0.60, p = .552, or IOR scores, F(2, 73) = 0.26, p = .772.
DAT1 3′ VNTR
Overall RT did not differ between the 10-R and 9-R groups, F(1, 80) = 0.08, p = .776. Infants in both groups showed significant facilitation scores (t10-R(40) = 3.53, p = .001; t9-R(40) = 4.25, p < .001). Similarly, IOR scores were above chance in both groups (t10-R(38) = 2.78, p = .008; t9-R(38) = 2.27, p = .029). Finally, results of the ANCOVA analyses indicated that there were no significant effects of DAT1 on infants’ facilitation, F(1, 68) = 0.35, p = .559 or IOR scores, F(1, 70) = 0.40, p = .529.
Combined effects of COMT Val158Met and DAT1 3′ VNTR polymorphisms
The combined genotype distribution of COMT Val158Met and DAT1 VNTR resulted in group sizes ranging from eight to 31 infants, allowing us to examine potential interactions between these two polymorphisms. We limited this analysis to IOR scores, since the previous analyses revealed no significant effects of COMT or DAT1 on facilitation scores. One-sample t tests indicated that Val carriers had above-chance IOR scores regardless of their DAT1 genotype: t10-R(26) = 3.24, p = .003; and t9-R = (30) = 1.99, p = .056. Conversely, infants with the Met/Met genotype did not show significant IOR effects, regardless of their DAT1 genotype, t10-R(11) = 0.62, p = .548; and t9-R(7) = 1.19, p = .274. A final ANCOVA similarly indicated that there was no significant COMT × DAT1 interaction effect on infants’ IOR scores, F(1, 68) = 0.67, p = .417.
Discussion
The present study indicated that normative genetic polymorphisms were related to individual differences in attention orienting among 7 month-old infants during the spatial cueing task. Specifically, the COMT Val158Met SNP was associated with individual differences in IOR, as infants with at least one Val allele showed a stronger IOR effect than those with the Met/Met genotype. Moreover, COMT was the only gene that was related to spatial cueing performance in this study; we did not find evidence of any effects of CHRNA4 or DAT1 genotype on orienting behavior, nor did we find an interaction between the dopaminergic genes COMT and DAT1.
Previous developmental studies of spatial cueing have shown that facilitation effects associated with spatial cueing emerge around the age of 3–4 months, while IOR develops more slowly, becoming robust around 6 months of age (Butcher et al., 1999; Hood, 1993, 1995; Johnson & Tucker, 1996; Varga et al., 2010). In addition, neurodevelopmental studies have shown that as IOR emerges, this increasing control over attention orienting is associated with greater contributions from cortical networks, particularly in frontal and parietal regions (Richards, 2000, 2001, 2005). The present study adds to this work by demonstrating that normative differences in COMT, which impacts dopaminergic signaling in prefrontal cortex, were related to the extent to which infants showed IOR in the spatial cueing task.
As noted earlier, candidate gene studies are notoriously difficult to replicate because of the complexities resulting from gene– gene interactions and the influence of contextual factors. There have only been a very small number of previous studies examining genetic effects on attention processing in infancy, all with relatively small sample sizes (Ns = 45–104). As such, this infant genetic data must be interpreted with caution and on a preliminary basis. Nonetheless, careful consideration of the present data reveals both intriguing consistencies and discrepancies with existing studies of genetic effects on orienting during both adulthood and infancy.
Broadly speaking, evidence for a link between IOR and COMT corroborates previous work demonstrating a role for frontoparietal networks in attention orienting in both adulthood and infancy (Lepsien & Pollmann, 2002; Mayer, Dorflinger, et al., 2004; Richards, 2001, 2005; Snyder & Chatterjee, 2006). It is important to note that the COMT effect was specific to IOR and not facilitation, which is consistent with other dissociations between facilitation and IOR. First, adult studies have shown that while both facilitation and IOR rely on basic orienting networks, IOR requires additional cortical recruitment, particularly in frontal regions (Lepsien & Pollmann, 2002; Mayer, Dorflinger, et al., 2004; Mayer, Seidenberg, et al., 2004; Ro, Farne, & Chang, 2003; Snyder & Chatterjee, 2006). Second, previous infant studies have found that the development of facilitation and IOR follow different time courses, with IOR developing several months later than facilitation. Moreover, this later-developing IOR is associated with changes in cortical activity that are localized over frontal regions (Richards, 2000, 2001, 2005), suggesting a link between the emergence of IOR and developing frontal/parietal attention networks. The specificity of the present data linking COMT to IOR, but not facilitation, gives further credence to the idea that the development of IOR is particularly dependent on cortical activity in developing frontal regions.
The present results are also consistent with two studies that previously examined COMT effects on attention orienting. In a previous infant study, the Met allele was associated with greater focused attention at a central fixation and reduced orienting to peripheral distractors (Holmboe et al., 2010). In the present study, infants with the Met/Met genotype showed the slowest latencies to orient to the peripheral targets, regardless of SOA. Using an antisaccade task,Haraldsson et al. (2010) found that adults with the Val allele were most efficient in reorienting attention away from a target and toward a novel location. Similarly, in the present study, infants with the Val allele showed faster orienting away from the cued location and toward novel locations during long-SOA trials (i.e., showed the strongest IOR effects). Together, these studies suggest a role for COMT in attention orienting and highlight a need for additional research examining this relationship both in adulthood and across development.
The consistencies across the present study and Haraldsson et al. (2010) are especially intriguing since the antisaccade task and IOR effect may share component mechanisms. Although the precise mechanism(s) underlying IOR are not fully understood, common accounts include inhibition at the cued location and updating of salience maps to generate an oculomotor/attentional bias toward novel locations (Colzato & Hommel, 2009; Houghton & Tipper, 1996; Itti & Koch, 2001). Similarly, effective antisaccade performance requires both inhibition of reflexive saccades to targets as well as updating of saccade trajectories toward the opposite location (Munoz & Everling, 2004). Many other complex cognitive processes also involve this interplay between control/inhibition and flexible updating (see Cools & D’Esposito, 2011, for review). One model relates dopamine function to these inhibition and updating processes (Durstewitz & Seamans, 2008) and suggests a plausible mechanism that may contribute to more effective IOR/ antisaccade performance among Val carriers. In this model, balanced cognitive control is mediated by relative activation of D1 versus D2 dopamine networks, with D1 activation conferring an advantage for control/inhibition and D2 activation conferring an advantage for flexible updating (Bilder, Volavka, Lachman, & Grace, 2004; Ettinger et al., 2008; Nolan, Bilder, Lachman, & Volavka, 2004). This model further posits that the COMT Val allele is associated with greater D2 activity and, thus, greater updating efficiency (Durstewitz & Seamans, 2008). Additional work has shown that IOR is dependent on D2 activation in frontostriatal networks (Colzato & Hommel, 2009; Fillmore, Rush, & Abroms, 2005; Rokem et al., 2011) and that antisaccade performance may be mediated by similar dopaminergic mechanisms (Allman et al., 2010). Although much more work is needed to elucidate the precise mechanisms, it is possible that enhanced IOR/antisaccade performance among Val carriers is related to an advantage in the updating component necessary for these tasks.
Although the present COMT finding is consistent with previous work, it is also important to note that, unlike previous adult studies, the present study did not find effects of CHRNA4 or DAT1 on orienting behavior. One possibility is that these null findings are due to task differences across adult and infant studies. Infant studies typically use several task modifications in order to accommodate limitations in infant responses, including measures of saccade latencies rather than manual RT and presentation of bilateral targets. In addition, while adult studies often utilize endogenous cueing parameters, which elicit more voluntary, controlled orienting, infant tasks predominantly utilize exogenous cues, which elicit reflexive orienting. Exogenous and endogenous attention processes are associated with different underlying neural mechanisms (Kincade, Abrams, Astafiev, Shulman, & Corbetta, 2005; Landau, Esterman, Robertson, Bentin, & Prinzmetal, 2007), and several studies have found that cholinergic agonists affected endogenous orienting but had no impact on exogenous orienting of attention (Meinke, Thiel, & Fink, 2006; Rokem, Landau, Garg, Prinzmetal, & Silver, 2010). Thus, CHRNA4 polymorphisms could have very different effects on endogenous orienting among adults compared with exogenous orienting in infants. Similarly, links between DAT1 and IOR performance among adults have only been observed following relatively short SOAs (< 750 ms; Colzato et al., 2010; Rokem et al., 2011), suggesting that potential effects of DAT1 genotype on IOR may have emerged in the present study if we had a used a wider variety of delay lengths.
In addition to these methodological considerations, our divergent results may also be due to developmental differences in the functioning of acetylcholine and dopamine systems. Although all of the major neurotransmitter systems are functional at birth, they continue to develop well into postnatal life (de Graaf-Peters & Hadders-Algra, 2006; Levitt, 2003). For example, expression of DAT in the striatum gradually increases from birth until 9–10 years of age (Meng, Ozawa, Itoh, & Takashima, 1999), while expression of D2 receptors peaks in early infancy and begins to decline in late childhood (Meng, Obonai, & Takashima, 1998; Meng et al., 1999). As such, the specific effects of genetic polymorphisms on signaling within these systems may be highly dependent on developmental timing. Furthermore, these developing neurotransmitter systems interact in a dynamic manner. Numerous studies have highlighted a reciprocal relationship between prefrontal and striatal dopamine functioning, whereby increased dopamine function in one region leads to reduced dopamine function in another (Bertolino et al., 2000; Cools, 2011; Cools, Miyakawa, Sheridan, & D’Esposito, 2010; Jackson, Frost, & Moghaddam, 2001; Kellendonk et al., 2006; Roberts et al., 1994). Similarly, dopaminergic polymorphisms can indirectly influence cholinergic processes (Sarter, Bruno, & Turchi, 1999), perhaps making it more difficult to identify main effects of a single candidate gene. As such, much more research is necessary to understand the intricate interactions between dopaminergic and cholinergic systems, how genetic polymorphisms modulate signaling in these systems, and how these interactions vary across development.
Despite the variability inherent to studies of candidate genes, there is growing evidence that normal variations in genes that regulate dopaminergic and cholinergic systems contribute to individual differences in attention processing among adults, including attention orienting. Attention orienting is a fundamental cognitive skill that supports early learning and likely serves as a building block for development of more complex attention/cognitive skills. As yet only a handful of studies have examined links between genetic polymorphisms and normative attention development in infancy. The present study adds to this work by highlighting a relationship between the dopaminergic COMT polymorphism and young infants’ spatial attention/orienting behavior. These results broadly corroborate previous adult studies of genetic polymorphisms and spatial attention and provide converging evidence for the role of developing frontoparietal networks in early attention processing.
Acknowledgment
The authors gratefully acknowledge the National Institutes of Health (Grant T32-HD007151 to Julie Markant), the Spunk Fund Inc., and the Institute of Child Development for their generous support of this work. We would also like to thank Amanda Hodel and Sara Van Den Heuvel for their help with data collection and coding.
This report represents a portion of Julie Markant’s dissertation, which was completed at the University of Minnesota under the direction of Kathleen M. Thomas.
Footnotes
Unless otherwise noted, analyses were repeated with only White infants, and all findings were consistent with the reported results.
Contributor Information
Julie Markant, Department of Cognitive, Linguistic, and Psychological Sciences, Brown University.
Dante Cicchetti, Institute of Child Development, University of Minnesota.
Susan Hetzel, Institute of Child Development, University of Minnesota.
Kathleen M. Thomas, Institute of Child Development, University of Minnesota
References
- Allman A-A, Benkelfat C, Durand F, Sibon I, Dagher A, Leyton M, O’Driscoll GA. Effect of D-amphetamine on inhibition and motor planning as a function of baseline performance. Psychopharmacology. 2010;211:423–433. doi: 10.1007/s00213-010-1912-x. [DOI] [PubMed] [Google Scholar]
- Barr CL, Xu C, Kroft J, Feng Y, Wigg K, Zai G, Kennedy JL. Haplotype study of three polymorphisms at the dopamine transporter locus confirm linkage to attention-deficit/hyperactivity disorder. Biological Psychiatry. 2001;49:333–339. doi: 10.1016/s0006-3223(00)01053-2. [DOI] [PubMed] [Google Scholar]
- Berger A, Posner MI. Pathologies of brain attentional networks. Neuroscience and Biobehavioral Reviews. 2000;24:3–5. doi: 10.1016/s0149-7634(99)00046-9. [DOI] [PubMed] [Google Scholar]
- Berlucchi G. Inhibition of return: A phenomenon in search of a mechanism and a better name. Cognitive Neuropsychology. 2006;23:1065–1074. doi: 10.1080/02643290600588426. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Breier A, Callicott JH, Adler C, Mattay VS, Shapiro M, Weinberger DR. The relationship between dorsolateral prefrontal neuronal N-acetylaspartate and evoked release of striatal dopamine in schizophrenia. Neuropsychopharmacology. 2000;22:125–132. doi: 10.1016/S0893-133X(99)00096-2. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphims: Relations to the tonicphasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29:1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- Butcher PR, Kalverboer AF, Geuze RH. Inhibition of return in very young infants: A longitudinal study. Infant Behavior & Development. 1999;22:303–319. [Google Scholar]
- Casey BJ, Durston S, Fossella JA. Evidence for a mechanistic model of cognitive control. Clinical Neuroscience Research. 2001;1:267–282. [Google Scholar]
- Chugani HT. Development of regional brain glucose metabolism in relation to behavior and plasticity. In: Dawson G, Fischer K, editors. Human behavior and the developing brain. New York, NY: Guilford Press; 1994. pp. 153–175. [Google Scholar]
- Clohessy AB, Posner MI, Rothbart MK, Vecera SP. The development of inhibition of return in early infancy. Journal of Cognitive Neuroscience. 1991;3:345–350. doi: 10.1162/jocn.1991.3.4.345. [DOI] [PubMed] [Google Scholar]
- Colzato LS, Hommel B. Recreational use of cocaine eliminates inhibition of return. Neuropsychology. 2009;23:125–129. doi: 10.1037/a0013821. [DOI] [PubMed] [Google Scholar]
- Colzato LS, Pratt J, Hommel B. Dopaminergic control of attentional flexibility: Inhibition of return is associated with the dopamine transporter gene (DAT1) Frontiers in Human Neuroscience. 2010;4:53–59. doi: 10.3389/fnhum.2010.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R. Dopaminergic control of the striatum for high-level cognition. Current Opinion in Neurobiology. 2011;21:402–407. doi: 10.1016/j.conb.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Cools R, D’Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biological Psychiatry. 2011;69:e113–e125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Miyakawa A, Sheridan M, D’Esposito M. Enhanced frontal function in Parkinson’s disease. Brain. 2010;133:225–233. doi: 10.1093/brain/awp301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy, Shulman G. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- de Graaf-Peters VB, Hadders-Algra M. Ontogeny of the human central nervous system: What is happening when? Early Human Development. 2006;82:257–266. doi: 10.1016/j.earlhumdev.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Dukewich KR. Reconceptualizing inhibition of return as habituation of the orienting response. Psychonomic Bulletin & Review. 2009;16:238–251. doi: 10.3758/PBR.16.2.238. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK. The dual-state theory of prefrontal cortex dopamine function with relevance to catechol-O-methyltransferase genotypes and schizophrenia. Biological Psychiatry. 2008;64:739–749. doi: 10.1016/j.biopsych.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Kumari V, Collier DA, Powell J, Luzi S, Michel TM, Williams SCR. Catechol-O-methyltransferase (COMT) Val158Met genotype is associated with BOLD response as a function of task characteristic [Special theme] Neuropsychopharmacology. 2008;33:3046–3057. doi: 10.1038/sj.npp.1301658. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR, Abroms BD. d-Amphetamine-induced enhancement of inhibitory mechanisms involved in visual search. Experimental and Clinical Psychopharmacology. 2005;13:200–208. doi: 10.1037/1064-1297.13.3.200. [DOI] [PubMed] [Google Scholar]
- Gibson EJ. The world is so full of a number of things: On specification and perceptual learning. Ecological Psychology. 2003;15:283–287. [Google Scholar]
- Greenwood PM, Fossella JA, Parasuraman R. Specificity of the effect of a nicotinic receptor polymorphism on individual differences in visuospatial attention. Journal of Cognitive Neuroscience. 2005;17:1611–1620. doi: 10.1162/089892905774597281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood PM, Parasuraman R, Espeseth T. A cognitive phenotype for a polymorphism in the nicotinic receptor gene CHRNA4. Neuroscience and Biobehavioral Reviews. 2012;36:1331–1341. doi: 10.1016/j.neubiorev.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Haraldsson HM, Ettinger U, Magnusdottir BB, Sigmundsson T, Sigurdsson E, Ingason A, Petursson H. Catechol-O-methyltransferase Val158Met polymorphism and antisaccade eye movements in schizophrenia. Schizophrenia Bulletin. 2010;36:157–164. doi: 10.1093/schbul/sbn064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmboe K, Nemoda Z, Fearon RMP, Csibra G, Sasvari-Szekely M, Johnson MH. Polymorphisms in dopamine system genes are associated with individual differences in attention in infancy. Developmental Psychology. 2010;46:404–416. doi: 10.1037/a0018180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood BM. Inhibition of return produced by covert shifts of visual attention in 6-month-old infants. Infant Behavior & Development. 1993;16:245–254. [Google Scholar]
- Hood BM. Shifts of visual attention in the human infant: A neuroscientific approach. Advances in Infancy Research. 1995;10:163–216. [Google Scholar]
- Houghton G, Tipper SP. Inhibitory mechanisms of neural and cognitive control: Applications to selective attention and sequential action. Brain and Cognition. 1996;30:20–43. doi: 10.1006/brcg.1996.0003. [DOI] [PubMed] [Google Scholar]
- Itti L, Koch C. Computational modelling of visual attention. Nature Reviews Neuroscience. 2001;2:194–203. doi: 10.1038/35058500. [DOI] [PubMed] [Google Scholar]
- Jackson ME, Frost AS, Moghaddam B. Stimulation of prefrontal cortex at physiologically relevant frequencies inhibits dopamine release in the nucleus accumbens. Journal of Neurochemistry. 2001;78:920–923. doi: 10.1046/j.1471-4159.2001.00499.x. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Cortical maturation and the development of visual attention in early infancy. Journal of Cognitive Neuroscience. 1990;2:81–95. doi: 10.1162/jocn.1990.2.2.81. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Posner MI, Rothbart MK. Facilitation of saccades toward a covertly attended location in early infancy. Psychological Science. 1994;5:90–93. [Google Scholar]
- Johnson MH, Tucker LA. The development and temporal dynamics of spatial orienting in infants. Journal of Experimental Child Psychology. 1996;63:171–188. doi: 10.1006/jecp.1996.0046. [DOI] [PubMed] [Google Scholar]
- Kellendonk C, Simpson EH, Polan HJ, Winiger V, Moore H, Kandel ER. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Kincade JM, Abrams RA, Astafiev SV, Shulman G, Corbetta M. An event-related functional magnetic resonance imaging study of voluntary and stimulus-driven orienting of attention. The Journal of Neuroscience. 2005;25:4593–4604. doi: 10.1523/JNEUROSCI.0236-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RM. Inhibitory tagging system facilitates visual search [Letter] Nature. 1988 Aug 4;334:430–431. doi: 10.1038/334430a0. [DOI] [PubMed] [Google Scholar]
- Klein RM. Inhibition of return. Trends in Cognitive Sciences. 2000;4:138–147. doi: 10.1016/s1364-6613(00)01452-2. [DOI] [PubMed] [Google Scholar]
- Landau AN, Esterman M, Robertson LC, Bentin S, Prinzmetal W. Different effects of voluntary and involuntary attention on EEG activity in the gamma band. The Journal of Neuroscience. 2007;27:11986–11990. doi: 10.1523/JNEUROSCI.3092-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepsien J, Pollmann S. Covert reorienting and inhibition of return: An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14:127–144. doi: 10.1162/089892902317236795. [DOI] [PubMed] [Google Scholar]
- Levitt P. Structural and functional maturation of the developing primate brain. Journal of Pediatrics. 2003:35–45. doi: 10.1067/s0022-3476(03)00400-1. 143Review of General Psychology. [DOI] [PubMed] [Google Scholar]
- Lundwall RA, Guo D-C, Dannemiller JL. Exogenous visual orienting is associated with specific neurotransmitter genetic markers: A population-based genetic association study. PLoS ONE. 2012;7:e30731. doi: 10.1371/journal.pone.0030731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Dorflinger JM, Rao SM, Seidenberg M. Neural networks underlying endogenous and exogenous visual-spatial orienting. NeuroImage. 2004;23:534–541. doi: 10.1016/j.neuroimage.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Seidenberg M, Dorflinger JM, Rao SM. An event-related fMRI study of exogenous orienting: Supporting evidence for the cortical basis of inhibition of return? Journal of Cognitive Neuroscience. 2004;16:1262–1271. doi: 10.1162/0898929041920531. [DOI] [PubMed] [Google Scholar]
- Meinke A, Thiel CM, Fink GR. Effects of nicotine on visuo-spatial selective attention as indexed by event-related potentials. Neuroscience. 2006;141:201–212. doi: 10.1016/j.neuroscience.2006.03.072. [DOI] [PubMed] [Google Scholar]
- Meng SZ, Obonai T, Takashima S. A developmental study of the dopamine D2R receptors in the human basal ganglia and thalamus. Early Human Development. 1998;51:23–30. doi: 10.1016/s0378-3782(97)00071-6. [DOI] [PubMed] [Google Scholar]
- Meng SZ, Ozawa Y, Itoh M, Takashima S. Developmental and age-related changes of dopamine transporter, and dopamine D1 and D2 receptors in human basal ganglia. Brain Research. 1999;843:136–144. doi: 10.1016/s0006-8993(99)01933-2. [DOI] [PubMed] [Google Scholar]
- Milliken B, Tipper SP, Houghton G, Lupianez J. Attending, ignoring, and repetition: On the relation between negative priming and inhibition of return. Perception & Psychophysics. 2000;62:1280–1296. doi: 10.3758/bf03212130. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: The anti-saccade task and the voluntary control of eye movement. Nature Reviews Neuroscience. 2004;5:218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Mushquash AR, Fawcett JM, Klein RM. Inhibition of return and schizophrenia: A meta-analysis. Schizophrenia Research. 2012;135:55–61. doi: 10.1016/j.schres.2011.11.034. [DOI] [PubMed] [Google Scholar]
- Nation K, Penny S. Sensitivity to eye gaze in autism: Is it normal? Is it automatic? Is it social? Development and Psychopathology. 2008;20:79–97. doi: 10.1017/S0954579408000047. [DOI] [PubMed] [Google Scholar]
- Nolan KA, Bilder RM, Lachman HM, Volavka J. Catechol-O-methyltransferase Val158Met polymorphism in schizophrenia: Differential effects of Val and Met alleles on cognitive stability and flexibility. The American Journal of Psychiatry. 2004;161:359–361. doi: 10.1176/appi.ajp.161.2.359. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Greenwood PM, Kumar R, Fossella JA. Beyond heritability: Neurotransmitter genes differentially modulate visuospatial attention and working memory. Psychological Science. 2005;16:200–207. doi: 10.1111/j.0956-7976.2005.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. The Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Cohen Y. Components of visual orienting. In: Bouma H, Bouwhuis D, editors. Attention and performance: Vol. X: Control of language processes. Hillsdale, NJ: Erlbaum; 1984. pp. 531–556. [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rafal RD, Choate LS, Vaughan J. Inhibition of return: Neural basis and function. Cognitive Neuropsychology. 1985;2:211–228. [Google Scholar]
- Richards JE. Localizing the development of covert attention in infants with scalp event-related potentials. Developmental Psychology. 2000;36:91–108. [PubMed] [Google Scholar]
- Richards JE. Cortical indexes of saccade planning following covert orienting in 20-week-old infants. Infancy. 2001;2:135–157. [Google Scholar]
- Richards JE. Localizing cortical sources of event-related potentials in infants’ covert orienting. Developmental Science. 2005;8:255–278. doi: 10.1111/j.1467-7687.2005.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristic J, Giesbrecht B. Electrophysiological evidence for spatiotemporal flexibility in the ventrolateral attention network. PLoS ONE. 2011;6:e24436. doi: 10.1371/journal.pone.0024436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro T, Farne A, Chang E. Inhibition of return and the human frontal eye fields. Experimental Brain Research. 2003;150:290–296. doi: 10.1007/s00221-003-1470-0. [DOI] [PubMed] [Google Scholar]
- Roberts AC, De Salvia MA, Wilkinson LS, Collins P, Muir JL, Everitt BJ, Robbins TW. 6-Hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analog of the Wisconsin Card Sort Test: Possible interactions with subcortical dopamine. The Journal of Neuroscience. 1994;14:2531–2544. doi: 10.1523/JNEUROSCI.14-05-02531.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokem A, Landau AN, Garg D, Prinzmetal W, Silver MA. Cholinergic enhancement increases the effects of voluntary attention but does not affect involuntary attention. Neuropsychopharmacology. 2010;35:2538–2544. doi: 10.1038/npp.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokem A, Landau AN, Prinzmetal W, Wallace DL, Silver MA, D’Esposito M. Modulation of inhibition of return by the dopamine D2 receptor agonist bromocriptine depends on individual DAT1 genotype. Cerebral Cortex. 2011;22:1124–1138. doi: 10.1093/cercor/bhr185. Retrieved from doi: 10.1093/cercor/bhr185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Bruno JP, Turchi J. Basal forebrain afferent projections modulating cortical acetylcholine, attention, and implications for neuropsychiatric disorders. Annals of the New York Academy of Sciences. 1999;877:368–382. doi: 10.1111/j.1749-6632.1999.tb09277.x. [DOI] [PubMed] [Google Scholar]
- Sheese BE, Rothbart MK, Posner MI, White LK, Fraundorf SH. Executive attention and self-regulation in infancy. Infant Behavior & Development. 2008;31:501–510. doi: 10.1016/j.infbeh.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Sheese BE, Voelker P, Posner MI, Rothbart MK. Genetic variation influences on the early development of reactive emotions and their regulation by attention. Cognitive Neuropsychiatry. 2009;14:332–355. doi: 10.1080/13546800902844064. [DOI] [PubMed] [Google Scholar]
- Snyder JJ, Chatterjee A. The frontal cortex and exogenous attentional orienting. Journal of Cognitive Neuroscience. 2006;18:1913–1923. doi: 10.1162/jocn.2006.18.11.1913. [DOI] [PubMed] [Google Scholar]
- Valenza E, Simion F, Umilta C. Inhibition of return in newborn infants. Infant Behavior & Development. 1994;17:293–302. [Google Scholar]
- Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, Uhl GR. Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR. Genomics. 1992;14:1104–1106. doi: 10.1016/s0888-7543(05)80138-7. [DOI] [PubMed] [Google Scholar]
- Varga K, Frick JE, Kapa LL, Dengler MJ. Developmental changes in inhibition of return from 3 to 6 months of age. Infant Behavior & Development. 2010;33:245–249. doi: 10.1016/j.infbeh.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Vivas AB, Humphreys GW, Fuentes LJ. Abnormal inhibition of return: A review and new data on patients with parietal lobe damage. Cognitive Neuropsychology. 2006;23:1049–1064. doi: 10.1080/02643290600588400. [DOI] [PubMed] [Google Scholar]
- Voelker P, Sheese BE, Rothbart MK, Posner MI. Variations in catechol-O-methyltransferase gene interact with parenting to influence attention in early development. Neuroscience. 2009;164:121–130. doi: 10.1016/j.neuroscience.2009.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Satel J, Klein RM. Sensory and motor mechanisms of oculomotor inhibition of return. Experimental Brain Research. 2012;218:441–453. doi: 10.1007/s00221-012-3033-8. [DOI] [PubMed] [Google Scholar]
- Weinshilboum RM, Otterness DM, Szumlanski CL. Methylation pharmacogenetics: Catechol-O-methyltransferase, thiopurine methyltransferase, and histamine-N-methyltransferase. Annual Review of Pharmacology and Toxicology. 1999;39:19–52. doi: 10.1146/annurev.pharmtox.39.1.19. [DOI] [PubMed] [Google Scholar]