Abstract
Background. Respiratory symptoms are usually underestimated in patients with chronic kidney disease undergoing maintenance hemodialysis. Therefore, we set out to investigate the prevalence of patients chronic dyspnea and the relationship of the symptom to lung function indices. Methods. Twenty-five clinically stable hemodialysis patients were included. The mMRC dyspnea scale was applied before and after hemodialysis. Spirometry, single breath nitrogen test, arterial blood gases, static maximum inspiratory (P imax) and expiratory (P emax) muscle pressures, and mouth occlusion pressure (P 0.1) were also measured. Results. Despite normal spirometry, all patients (100%) reported mild to moderate degree of chronic dyspnea pre which was reduced after hemodialysis. The sole predictor of (Δ) mMRC was the (Δ) P 0.1 (r = 0.71, P < 0.001). The P imax was reduced before and correlated with the duration of hemodialysis (r = 0.614, P < 0.001), whilst after the session it was significantly increased (P < 0.001). Finally (Δ) weight was correlated with the (Δ) P imax %pred (r = 0.533, P = 0,006) and with the (Δ) CV (%pred) (r = 0.65, P < 0.001). Conclusion. We conclude that dyspnea is the major symptom among the CKD patients that improves after hemodialysis. The neuromechanical dissociation observed probably is one of the major pathophysiologic mechanisms of dyspnea.
1. Introduction
Renal injury is not only a localized disease, eventually leading to a progressive and irreversible loss of nephron mass and functions, but also a syndrome affecting multiple organ systems. Among those, CKD and KRT options, such as hemodialysis and peritoneal dialysis, severely affect the respiratory system. Specifically, their effects are (a) acute: causing infections, pleural effusions, and ARDS and (b) chronic: leading to calcification of the lung parenchyma and finally respiratory impairment [1].
Despite the increasing interest in lung pathology during ESKD and the complications that may arise from the maintenance therapeutic options, respiratory symptoms are usually either underestimated or overlooked. The physical symptom burden though is the primary parameter defining the quality of life of CKD patients [2]. Furthermore, hemodialysis maintenance therapy seems to cause a definite regression of these symptoms. Specifically, symptoms concerning the respiratory system are not well documented [2, 3]. One of the commonest respiratory symptoms of ESKD is dyspnea [4], usually assessed with lung function testing. Several researchers explored CKD effects, the impact of hemodialysis on lung function, and dyspnea with ambiguous results. Anemia and gas transfer defects [5], fluid overload and premature airway closure [6], ventilation-perfusion mismatching [6], hypoxemia due to centrally driven hypoventilation [7], uremic respiratory muscle dysfunction, and severe mechanical loading [8] were reported in these patients. Several functional pulmonary abnormalities, including restriction, obstruction [9, 10], and impaired diffusion capacity [11], have also been described. Surprisingly, scarce reports exist for measuring, explaining, and categorizing chronic dyspnea in relation to lung function indices and the way the CKD and KRT affect it in these patients.
Thus we set out to investigate the following: (a) the prevalence, (b) the degree, and (c) the relationship of chronic dyspnea with lung function parameters, both before and after hemodialysis. Therefore, we studied the prevalence and the degree of chronic dyspnea in ESKD patients before and after hemodialysis and we wondered whether it is correlated with any of the commonly used lung function indices.
2. Materials and Methods
2.1. Subjects
The study population consisted of twenty-five (25) ambulatory Caucasian patients (15 men) with ESKD (GFR < 10 mL min−1) [12] undergoing maintenance hemodialysis every other day. These patients were candidates for renal transplantation and were referred to our laboratory for preoperative respiratory function assessment. Thus, this population was highly selected and neither inflammation nor malnutrition was observed. The causes of ESKD were diabetes mellitus type II (40%), arterial hypertension (20%), glomerulonephritis (20%), and cystic kidney disease (20%). Their anthropometric characteristics, blood serum, and lung function data are shown in Tables 1 and 2.
Table 1.
Anthropometric and renal function data of the 25 patients before undergoing hemodialysis. Values are mean ± SD.
| Subjects (n) | 25 |
| Age (yrs) | 52.0 ± 11.0 |
| Gender (M/F) | 15/10 |
| Height (cm) | 161.0 ± 0.3 |
| Weight (before hemodialysis) (Kg) | 74.0 ± 12.0 |
| Smokers/never smokers | 13/12 |
| Duration of dialysis (years) | 4.7 ± 4.0 |
| Hct (%) | 37.0 ± 3.0 |
| Urea (mg/dL) | 141.0 ± 26.0 |
| Creatinine (mg/dL) | 10.0 ± 2.0 |
| Potassium (mg/dL) | 5.5 ± 0.5 |
| Calcium adj (mg/dL) | 10.0 ± 0.5 |
n: number of subjects before and after hemodialysis; M/F: male/female; calcium is adjusted to the albumin levels.
Table 2.
Body weight, mMRC scale, SBN2 data (23 patients), and maximum static mouth pressure before and after hemodialysis.
| Before dialysis n = 25 |
After dialysis n = 25 |
P value | |
|---|---|---|---|
| Body weight (Kg) | 74.0 ± 12.0 | 71.0 ± 12.0 | <0.001 |
| BMI (Kg/m2) | 26.0 ± 3.0 | 25.0 ± 4.0 | <0.001 |
| mMRC (acc) | 2.0 ± 0.8 | 0.6 ± 0.7 | <0.001 |
| ΔN 2/ΔV, % pred. | 127.0 ± 25.0 | 86.0 ± 28.0 | <0.001 |
| CC % pred. | 90.0 ± 28.0 | 77.0 ± 22.0 | 0.03 |
| CV % pred. | 125.0 ± 39.0 | 92.0 ± 41.0 | <0.001 |
| FRC % pred. | 91 ± 27 | 88 ± 24 | NS |
| P imax % pred. | 70.0 ± 14.0 | 109.0 ± 15.0 | <0.001 |
| P emax % pred. | 103.0 ± 24.0 | 109.0 ± 27.0 | NS |
Values are mean ± SD obtained in the seated position. Unless otherwise specified, values are expressed as % predicted. Statistical significance for mMRC tested with Wilcoxon's signed rank test before and after dialysis. Student's paired t-test is used for all the other lung function parameters. BMI: body mass index; mMRC: modified medical research council dyspnea scale; n: number of subjects; ΔN 2/ΔV: slope of phase III; CC: closing capacity; CV: closing volume; FRC: functional residual capacity; P imax: maximum static inspiratory pressure; P emax: maximum static expiratory pressure.
Inclusion criteria were (a) age above 18 years, (b) ability to perform full lung function testing satisfactorily, and (c) stable clinical and functional state for at least four weeks before testing. Exclusion criteria were (a) cardiovascular disorders, ruled out by a cardiologist, (b) known lung disease, such as asthma or COPD, (c) neuromuscular disorders, (d) previous thoracic or abdominal surgery, (e) pleural disease, and (f) malnutrition. Twelve out of 25 patients have never been smokers and no patient was cachectic (BMI < 18).
The study had the approval of the hospital's ethics committee. All subjects gave informed consent.
2.2. Hemodialysis
All patients included in the study group suffered from established CKD according to the KOQI [12] with a predicted GFR < 10 mL min−1. They were receiving hemodialysis three times a week as maintenance therapy. This was applied via a forearm arteriovenous fistula with extracorporeal blood flow rates between 250 and 350 mL min−1 using a dialyzer (AK 200 Gambro, Stockholm, Sweden). Bicarbonate buffer with high concentration of HCO3 (35 mmol/L) and disposable synthetic biocompatible dialyzer membranes (polysulfone hollow fiber dialyzer membranes, Toray TS series, Japan) were used in all sessions. The duration of hemodialysis, the patients weight, and blood serum data are given in Table 1.
2.3. Chronic Dyspnea and Lung Function Testing
Chronic dyspnea was rated according to the mMRC 6-point scale [13]. Simple spirometry was measured with the V max apparatus (V max Encore 22: Sensor Medics, Yorba Linda, CA, USA) using the “fast inspiratory maneuver” [14]. Static lung volumes were measured with the multiple nitrogen washout technique [15] (V max Encore 22, Sensor Medics). The DLCO single breath technique was also determined (V max Encore 22, Sensor Medics) [16]. Predicted values for spirometry, static lung volumes, and DLCO were from the European Community for Coal and Steel [17]. The arterial pH and arterial partial pressures of oxygen (PaO2) and carbon dioxide (PaCO2) (mmHg) were measured with the Stat Profile Critical Care Xpress apparatus (Nova Biomedical, Waltham, MA, USA).
The SBN2 was also performed with the V max apparatus (V max Encore 22, Sensor Medics). Subjects were asked to exhale to RV, and after an inhalation of 100% oxygen to TLC, they were asked to slowly exhale (≤0.5 L/s) to RV again. The SBN2 technique was used to obtain the CC, the CV, and the slope ΔN 2/ΔV.
Static P imax and P emax were measured with a plastic semirigid flanged mouthpiece fitted to a metallic stem incorporating a 3-way tap manufactured according to the design of Ringqvist [18]. A differential pressure transducer (Validyne MP45-36-871, Validyne Co., Northridge, CA) with a range of ±340 cm H2O was connected to the 3-way tap with a 70 cm fine polyethylene catheter. The pressure transducer was calibrated before each study using a U-tube water manometer. Pressures signals were displayed on a computer screen. The subjects used their hands to hold the lips firmly onto the mouthpiece if a leak was noticed. Prior to a P emax or P imax effort, the operator closed the 3-way tap with the subject at TLC or RV, respectively. All subjects were given verbal encouragement. A period of learning the procedure preceded the definitive measurements [18, 19]. All measurements followed the criteria of Ringqvist [18] such that (a) no extra leakage occurred, (b) the three highest pressures were similar (within 5%) and later attempts did not yield higher results, and (c) the subjects felt that they had given a maximum effort. At least 1 min rest was allowed between efforts. Pressures maintained for less than 1 second were disregarded. The highest pressures generated by an individual were used for analysis. Predicted values for P emax and P imax standardized for age, height, and gender were obtained from Wilson et al. [19].
Pattern of breathing (V E, V T, T E, T I, T TOT, RR, V T/T I, T I/T TOT) was also assessed during resting breathing. Subjects with a nose clip on breathed through a heated pneumotachograph (Screenmate-Box, Erich Jaeger GmbH & Co., Germany) connected to a differential pressure transducer. Tidal volume was measured by integrating the flow signal. Inspiratory time and total breath cycle duration were also measured from the flow signal. In order to minimize the effects of anxiety, all indices were measured after the patient had become familiar with the procedure and actual measurements were made only when ventilation had remained constant for at least ten minutes [20]. Normal values for the pattern of breathing were obtained from Tobin et al. [21].
Respiratory drive as reflected in P 0.1 measurement was defined as the pressure change during the first 100 msec from the onset of airflow of a patient's spontaneously initiated breath [22]. It was measured using a three-way helium valve (Hans—Rudolph, Kansas City, MO, USA). The latter was occluded randomly at the end of expiration by a helium pneumatic valve. The valve was connected to a heated pneumotachograph (Screenmate-Box, Erich Jaeger GmbH & Co., Germany) while patients were seated comfortably in a quiet room with a nose clip on. A learning period preceded the definitive measurements. To eliminate stress and any other external influences, patients wore earplugs to prevent any acoustic stimuli while the operator was standing behind the patient in order to avoid any optic interference. Patients were unable to anticipate occlusions, which were applied silently and randomly according to the technique of Whitelaw et al. [23]. Normal values were obtained from the ATS/ERS statement on respiratory muscle testing [24]. At least 5 measurements were obtained for each subject. The mean value of the 5 best efforts was used for analysis.
2.4. Procedure
Patients had undergone a CXR, biochemistry, and a cardiovascular assessment obtained no more than 7 days before the examination day. On the day of examination each of the 25 patients signed an informed consent form. Then medical history was recorded and full physical examination, weighing of the patient, and arterial blood gases were performed; these were followed by full lung function testing (spirometry, static lung volumes, DLCO, SBN2, P imax and P emax pattern of breathing, and P 0.1) before and immediately after hemodialysis maintenance.
2.5. Statistical Analysis
Data are expressed as mean ± (SD), unless otherwise stated. For comparisons paired t test or Wilcoxon's nonparametric test for paired data was used where appropriate. Correlations between mMRC dyspnea scale and various variables were calculated using Spearman's correlation coefficient. Multiple linear regression and backward stepwise regression analysis were used to identify the significant variables. A P ≤ 0.05 value was considered as significant. Statistical analysis was performed using SigmaStat (V3.5) and SigmaPlot (V10.0) (Jandel Scientific, CA, USA) statistical software.
3. Results
Anthropometric, hemodialysis, and blood serum data are presented in Table 1. We included smokers (n = 13, pack years 31 ± 23), who were advised to abstain from their habit for at least 24 hours before performing full lung function testing, and nonsmokers (n = 12). Electrolytic disturbances were noted, namely, hypercalcemia (adjusted to the albumin levels) and hyperkalemia.
Body weight was reduced significantly by 3 Kgs (P < 0.001, 74 ± 12 before to 71 ± 12 after dialysis) (Table 2). The mMRC 6-point scale was also significantly reduced (Figure 1(a)). Routine lung function indices were within normal limits and they did not change significantly after hemodialysis.
Figure 1.
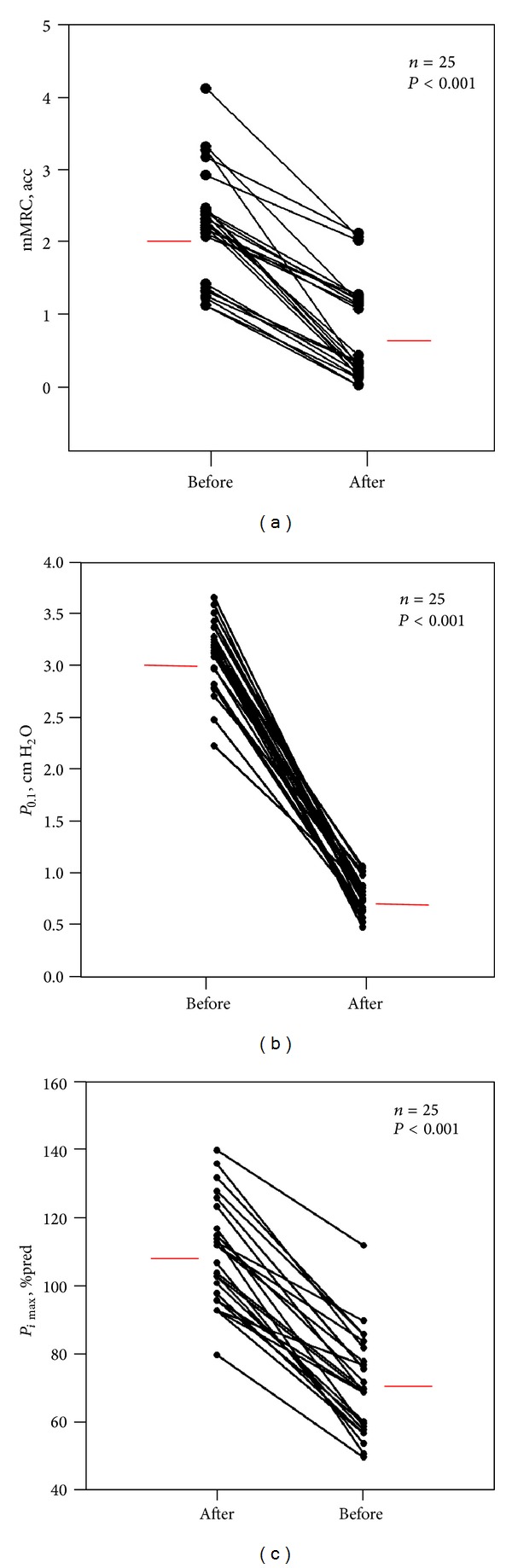
(a) mMRC, (b) P 0.1—ventilatory drive, and (c) P imax before and after hemodialysis are shown. Black dots represent the individual patients and red lines represent the mean values before and after hemodialysis.
An analysis of the SBN2 technique performed (23 subjects) is shown at Table 2 (in 2 patients CC and CV could not be calculated). The ΔN 2/ΔV and CV were increased before hemodialysis but were significantly reduced returning to normal limits after hemodialysis in all but 3 patients. The CC was increased before hemodialysis, but was also significant decreased after the hemodialysis session. There were no significant differences between groups, when SBN2 data were tested separately for smokers and nonsmokers. The P imax before and after hemodialysis (r = 0.614, P < 0.001 and r = 0.766, P < 0.001, resp.) was well correlated with the duration of hemodialysis maintenance therapy whilst the same result was observed for the P emax before and after the session (r = 0.489, P = 0.013 and r = 0.701, P < 0.001, resp.) (Figures 2(a) and 2(b)). Additionally P imax was reduced significantly (Figure 1(c)), whilst P emax remained unchanged after hemodialysis (Table 2).
Figure 2.
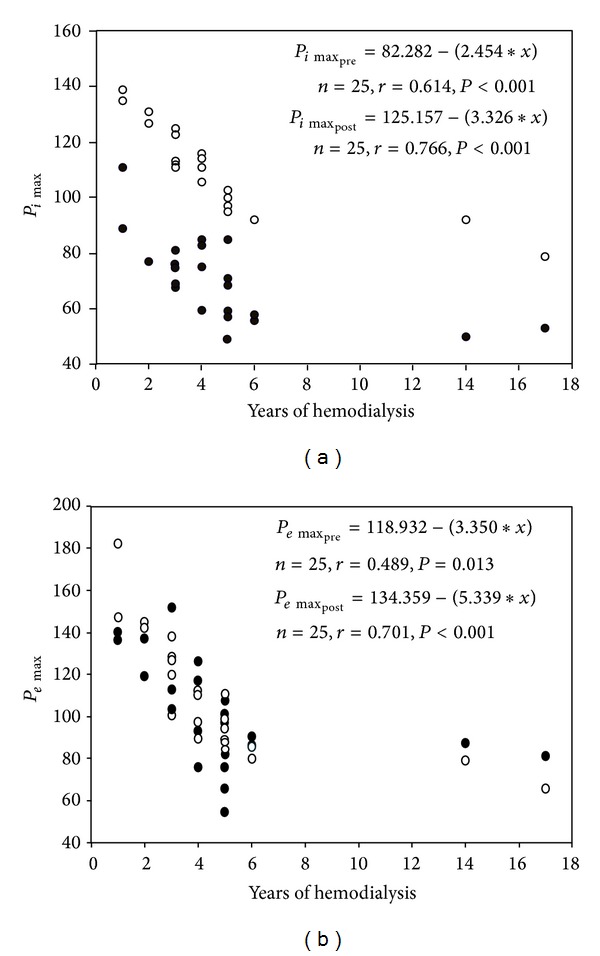
(a) Relationship of the P imax with the duration of hemodialysis for the 25 patients before and after hemodialysis (r = 0.614, P < 0.001 and r = 0.766, P < 0.001, resp.). Black dots: P imax before hemodialysis. White dots: P imax after hemodialysis. (b) Relationship of the P emax with the duration of hemodialysis for the 25 patients before and after hemodialysis (r = 0.489, P = 0.013 and r = 0.701, P < 0.001, resp.). Black dots: P emax before hemodialysis. White dots: P emax after hemodialysis.
Pattern of breathing and arterial blood gases data are shown in Table 3. All patients were hyperventilating as indicated by the high V E and RR before and after hemodialysis. The P 0.1 was increased before hemodialysis and decreased significantly after hemodialysis in all patients (Figure 1(b)). The P 0.1/V T % pred VC ratio was also significantly reduced. The pH became alkalotic whilst PaCO2 tension was significantly increased but remained below the normal limits. The PaO2 was decreased after the session but no hypoxemia was observed.
Table 3.
Pattern of breathing and arterial blood gases of the 25 ESKD patients before and after dialysis.
| Before dialysis n = 25 |
After dialysis n = 25 |
P value | |
|---|---|---|---|
| V T (lt) | 0.7 ± 0.2 | 0.6 ± 0.2 | NS |
| T E (sec) | 1.8 ± 0.5 | 1.7 ± 0.5 | NS |
| T I (sec) | 1.5 ± 0.3 | 1.4 ± 0.3 | NS |
| V T/T I (lt/sec) | 0.5 ± 0.2 | 0.4 ± 0.2 | NS |
| T I/T TOT (sec) | 0.4 ± 0.04 | 0.5 ± 0.03 | NS |
| V E (lt/min) | 13.0 ± 4.0 | 12.0 ± 5.0 | NS |
| RR (breaths/min) | 19.0 ± 5.0 | 20.0 ± 4.0 | NS |
| P 0.1 (cm H2O) | 3.0 ± 0.3 | 0.7 ± 0.2 | <0.001 |
| P 0.1/V T% VC pred., acc | 0.2 ± 0.1 | 0.05 ± 0.02 | <0.001 |
| pH | 7.4 ± 0.1 | 7.5 ± 0.1 | <0.001 |
| PaO2 (mm Hg) | 98.0 ± 11.0 | 97.0 ± 14.0 | NS |
| PaCO2 (mm Hg) | 31.0 ± 4.0 | 33.0 ± 5.0 | 0.02 |
| HCO3 (mmol) | 20.0 ± 4.0 | 25.0 ± 3.0 | <0.001 |
Values are mean ± SD obtained in the seated position. Unless otherwise specified, values are expressed as % predicted. Statistical significance tested with Student's paired t-test before and after dialysis. V T: tidal volume; V E: minute ventilation; T E: duration of expiration; T I: duration of inspiration; T TOT: total cycle duration; V T/T I: mean inspiratory flow; T I/T TOT: duty cycle; f: frequency of breathing; P 0.1: mouth occlusion pressure; % pred. VC: vital capacity % predicted; PaO2: arterial pressure tension of oxygen; PaCO2: arterial pressure tension of carbon dioxide; HCO3: bicarbonate anion.
The (Δ) mMRC was correlated with the (Δ) P 0.1 (P < 0.001) before and after dialysis. Multiple regression analysis showed that the sole predictor of (Δ) mMRC grade was the (Δ) P 0.1 (r = 0.71, P < 0.001) (Figure 3). Furthermore, the observed (Δ) Wt was correlated with the (Δ) P imax % pred. (r = 0.533, P = 0,006) and (Δ) CV (% pred.) (P = 0.0008) (Figures 4(a) and 4(b)). Backward stepwise regression revealed that (Δ) CV (% pred.) was proportionally reduced to (Δ) weight (r = 0.65, P < 0.001). Finally, a weak correlation was detected between the (Δ) V E and the (Δ) pH (r = 0.44, P = 0.03) before and after hemodialysis.
Figure 3.

Box plot showing the relationship of (Δ) mMRC with (Δ) P 0.1 before and after hemodialysis. Dashed line: regression line. Solid lines: median lines of the change (Δ) of P 0.1 at ΔmMRC values of 1 and 2, respectively. Regression equation: y = −0.736 + (0.905∗x) ± 2(0.44), n = 25, r = 0.71, and P < 0.001. The slope of the line indicates that the (Δ) mMRC decreases, on average, by one unit per 0.9 units decrease of the (Δ) P 0.1.
Figure 4.
(a) Relationship of (Δ) Wt with the (Δ) CV % pred. Solid line: regression line. Regression equation: y = 1.874 − 0.0145∗x ± 2(0.195) , n = 23, r = 0.65, P < 0.001. Subjects, represented as black dots, are 23 because closing volume could not be calculated in 2 patients. (b) Relationship of the (Δ) Wt to (Δ) P imax % pred. Solid line: regression line. Regression equation: y = 4.064 − (0.0424∗x) ± 2(0.581), n = 25, r = 0.533, and P = 0.006. The 25 subjects are represented as black dots.
4. Discussion
The main findings of the present study in patients with ESKD undergoing hemodialysis maintenance therapy, awaiting kidney transplantation, are the following. (1) All patients (100%) reported some degree of chronic dyspnea before hemodialysis maintenance, which was reduced significantly after the treatment. (2) P 0.1 was significantly reduced after hemodialysis. (3) P imax was reduced before and significantly increased after hemodialysis, whilst P emax remained unchanged.
4.1. Chronic Dyspnea
Scarce reports exist on the prevalence and grading of chronic dyspnea in ESKD patients receiving hemodialysis maintenance therapy. Dyspnea prevalence has not been adequately studied and the main reason is that no author has applied a widely accepted and validated tool such as the mMRC scale. Furthermore, the (Δ) mMRC has not been reported immediately after the hemodialysis maintenance sessions. Therefore dyspnea is either underestimated or overlooked. The reported prevalence of dyspnea was 20 to 60% [2–4]. In the present study, all patients (100%) reported mild to moderate degree of chronic dyspnea before and exhibited a significant reduction after hemodialysis (Figures 1(a) and 3). Dyspnea improvement was not correlated with any of the changes in the arterial blood gases. Finally after hemodialysis in half of the patients (13/25), the sensation of dyspnea was abolished.
4.2. Pattern of Breathing
To the best of our knowledge there are a few studies examining P 0.1 before and immediately after hemodialysis maintenance therapy [25, 26] in ESKD patients. In spontaneous breathing patients Sebert et al. [26] examined the patient's P 0.1 relationship with the V E at different CO2 concentrations, before and after hemodialysis maintenance. They found that for the same V E response CKD patients needed a higher neuromuscular ventilatory drive than normal subjects. The latter response was not corrected after the hemodialysis maintenance session. They attributed their results to a neuromuscular hypoexcitability that did not respond to hemodialysis therapy. Only one other study, that of Huang et al. [25], examined the effect of hemodialysis on P 0.1 in mechanically ventilated patients. They reported that P 0.1 was reduced after hemodialysis. Their finding was attributed to the reduced work of breathing and to the lung mechanics improvement after the hemodialysis session. In our spontaneously breathing patients P 0.1 was increased before but significantly decreased after hemodialysis (Table 3) (Figure 1(b)). To the best of our knowledge there is no report measuring P 0.1 before and after maintenance hemodialysis in spontaneously breathing patients. A significant correlation was detected between (Δ) mMRC grade and (Δ) P 0.1 (P < 0.001) (Figure 3) before and after hemodialysis. Multiple regression analysis (r = 0.71, P < 0.001) revealed that the sole predictor of the (Δ) mMRC chronic dyspnea magnitude was the change in the ventilatory drive assessed by the (Δ) P 0.1. Specifically the slope of the regression indicated that, for 1 unit reduction of mMRC after hemodialysis, the P 0.1 is changing also by approximately one unit (0.9). Additionally we observed a paired pattern of the (Δ) mMRC scale and the (Δ) P 0.1 after hemodialysis. Thus the patients with the higher (Δ) mMRC chronic dyspnea scale exhibited the largest (Δ) P 0.1 values.
After the hemodialysis maintenance the significant drop in P 0.1 can be attributed to many factors. (a) Better neuromechanical coupling: the P 0.1/V T % VC was significantly decreased after the hemodialysis maintenance therapy session (Table 3), reflecting a better neuromuscular coupling. Unfortunately this ratio was not correlated with the dyspnea scale change. (b) Depression of the ventilatory drive and profound hypoventilation because of the alkalization of body fluids after bicarbonate hemodialysis: hyperventilation due to the metabolic acidosis occurring during the interdialytic period is well documented in patients with ESKD on hemodialysis maintenance therapy [27]. After hemodialysis, breathing pattern irregularities and hypoventilation hypoxemia are frequently observed, due to the transient alkalization of the internal milieu and CO2 unloading into the dialytic regiment, as De Backer et al. noted [27, 28]. In the present study, using biocompatible membrane and bicarbonate enriched dialytic regiments, applied uniformly to all patients and thus preventing significant CO2 unloading, the pH became alkalotic as a result of the PaCO2 and HCO3 significant increase (Table 3). Despite the latter, patients still exhibited hyperventilation, albeit the small tendency to decrease after the hemodialysis session, as V E remained practically unchanged (Table 3). Furthermore, we did not note any hypoxemia nor any correlations of the P 0.1 with the pH or with any other blood gas exchange indices, in consistency with Herrera et al. [29]. Only a weak correlation was detected between the (Δ) V E and the (Δ) pH (r = 0.44, P = 0.03) before and after hemodialysis. The reason of the increased peripheral chemosensitivity after hemodialysis still remains controversial, and it is still under investigation. The disequilibrium syndrome observed in the uremic patients due to the abrupt changes of the blood internal milieu is proposed as a potential cause in the ventilatory responses of the central nervous system in ESKD patients on hemodialysis maintenance therapy [30]. Finally, it is not clear if the reduction of dyspnea resulted from the improvement of the ventilatory drive or vice versa. Further studies are needed to elucidate these findings.
4.3. Respiratory Muscles
Another explanation for the significant drop in P 0.1 immediately after hemodialysis in ESKD patients may arise from the suggested mechanical unloading of the respiratory muscles and the overall improvement of chest mechanics after the hemodialysis session. Skeletal muscle dysfunction is a well-described entity of uremia. Uremic myopathy [31] can be attributed to (a) structural changes, such as a decrease in the proportion of contractile tissue to collagen fibers in the sarcomere, resulting from malnutrition, uremic toxins, and acidosis, and (b) inefficient contractility because of hypercalcemia and secondary hyperparathyroidism. Several studies have investigated the respiratory muscle function of patients with ESKD [31–35] but with controversial results. On one hand, Bark et al. [31] suggested that the excitability-contractility coupling of respiratory muscles is deranged, resulting from the aluminum compounds frequently used in the hemodialysis regiments. The observed hypoexcitability was fully reversed in their cohort after the hemodialysis session. On the contrary, Karacan et al. [35] described a pronounced decrease in the inspiratory and expiratory muscle strength that was not reversed after a hemodialysis session. Our data show that P imax is compromised, whilst P emax is generally preserved. The P imax decreased (70 ± 14 cm H2O) in all patients before the hemodialysis (Figure 1(c)), whilst P emax was within normal limits (103 ± 24 cm H2O). After the hemodialysis session though all patients (Figure 1) exhibited a significant increase in P imax values (109 ± 15 cm H2O). Our findings are in agreement with the Kovelis et al. [32] study which showed that the reduction of the P imax is correlated with the duration of hemodialysis before and after the session (r = 0.614, P < 0.001 and r = 0.766, P < 0.001, resp.), whilst very few patients exhibited expiratory muscle weakness (Figure 2). The P emax was preserved, although a small tendency to decrease with the duration of dialysis was observed before and after the session (r = 0.489, P = 0.013 and r = 0.701, P < 0.001, resp.) (Table 3) (Figure 2). The surprising finding is that our data show a significant increase towards normal of the P imax after a single dialysis session whilst the P emax remained unchanged although it tended to increase (Table 3). A possible explanation is the fact that CKD affects selectively only the large nerve fibers such as the phrenic nerve which innervates the largest inspiratory muscle (the diaphragm), whilst the nerves innervating the expiratory muscles are not of large caliber nerves [36, 37]. In the preliminary study of Zifko et al. [34] delayed phrenic nerve latencies are observed in patients with CKD on maintenance hemodialysis, suggesting that phrenic neuropathy is an observed complication of uremia. Corroborative evidence of this selectivity is the fact that our group of patients had reduced P imax, but very few patients had reduced P emax. These patients with reduced P emax had also reduced P imax.
Additionally the (Δ) P imax % pred. was correlated with the (Δ) weight (r = 0.533, P = 0,006) (Figure 4(b)). The (Δ) weight was also correlated, but stronger, with the (Δ) CV (r = 0.65, P < 0.001). Backward stepwise regression analysis revealed that the sole predictor of the (Δ) CV was the (Δ) weight (Figure 4(a)), in accordance with the study of Zidulka et al. [6]. We assume that premature airway closure and gas trapping occur during the interdialytic period because of the accumulation of excess lung water and subclinical pulmonary edema. The latter increases the work of breathing and the load of the already impaired muscles by the uremic neuropathy and myopathy. Thus, the neuromuscular dissociation becomes apparent before the hemodialysis maintenance therapy. As a result a higher ventilatory drive (P 0.1) reflecting an augmented respiratory effort was necessary to overcome the disadvantaged mechanical load and the neuromechanical dissociation. This neuromechanical dissociation can be perceived as dyspnea from the patients [38]. The limitation of this theory is that the (Δ) weight is not a reliable marker of the total lung water as Wallin et al. [5] reported. Unfortunately, in the present study we did not measure the total lung water removed during hemodialysis. After the dialysis session the neuromechanical coupling is improved because of the unloading of the muscles expressed as the significant increase in P imax and the improvement of neuropathy because of the ultrafiltration of the middle sized molecules and the restoration of the electrolytic imbalances [37].
4.4. Clinical Implications
The disease symptoms burden affects therapy compliance and hence the therapeutic outcome [2–4]. Whether the removal of excess lung water with aggressive ultrafiltration could imply greater reduction of patient's chronic dyspnea and thus better compliance needs to be further explored.
Respiratory muscles dysfunction leads to breathlessness and ventilatory failure [39]. It should be noted that chronic hemodialysis maintenance therapy promotes gradual loss of inspiratory muscle strength, which deteriorates further with the duration of hemodialysis therapy [33]. Whether rehabilitation programs will prove to be beneficial for these patients' functional performance status is of interest and needs to be further investigated.
Finally, we noted that the CV % predicted reflecting small airway disease and maldistribution of ventilation [6, 7] and the P imax improved after hemodialysis. Both were correlated with the fluid depletion observed after the therapeutic session. This is beneficial for the patient's respiratory function. Whether aggressive hemodialysis treatment in preoperative patients can imply a better survival outcome after surgery needs also to be assessed.
5. Conclusions
We conclude that dyspnea is one of the most prominent symptoms in patients with CKD receiving hemodialysis maintenance therapy, which improves and often subsides after a therapeutic session. Dyspnea is correlated with the observed decrease in the neuromuscular drive as it is expressed by the mouth occlusion pressure (P 0.1). The ensuing decreased neuromechanical dissociation can be attributed to (a) better neuromechanical muscle coupling, (b) the hypoventilation due to the alkalization of body fluids, (c) the increased inspiratory muscle strength after hemodialysis, and (d) the improved function of the small airways as it is expressed by the increased CV in the majority of the study patients, correlated with the weight loss after the hemodialysis. Further studies are needed to elucidate the impact of CKD and hemodialysis maintenance therapy on the patient's symptoms and respiratory function in order to improve patient's care.
Acknowledgments
The authors would like to thank Mr Stelios Vechlidis for his valued technical support. This research was partly funded by the Greek NHS and University of Athens.
Abbreviations
- (Δ):
Change
- % pred.:
Percent predicted
- ARDS:
Adult respiratory distress syndrome
- ATS/ERS:
American Thoracic Society/European Respiratory Society
- BMI:
Body mass index
- CC:
Closing capacity
- CKD:
Chronic kidney disease
- CRF:
Chronic renal failure
- COPD:
Chronic obstructive pulmonary disease
- CSF:
Cerebrospinal fluid
- CV:
Closing volume
- CXR:
Chest X-ray
- DLCO:
Diffusing capacity for carbon monoxide
- ESKD:
End stage kidney disease
- FVC:
Forced vital capacity
- FRC:
Functional residual capacity
- IC:
Intensive care unit
- GFR:
Glomerular filtration rate
- KRT:
Kidney replacement therapy
- KOQI:
Kidney outcome quality initiative
- mMRC:
Modified medical research council dyspnea scale
- NECOSAD:
Netherlands Cooperative Study on Adequacy of Dialysis
- PaO2:
Arterial oxygen tension
- PaCO2:
Arterial carbon dioxide tension
- Pemax:
Maximum static expiratory mouth pressure
- Pimax:
Maximum static inspiratory mouth pressure
- P0.1:
Pressure 0.1 second
- P0.1/VT % pred. VC ratio:
The mouth occlusion pressure to tidal volume expressed as percent predicted of the vital capacity (VC) index.
- RR:
Respiratory rate
- RV:
Residual volume
- SD:
Standard deviation
- SE:
Standard error
- SBN2:
Single breath nitrogen test
- TLC:
Total lung capacity
- TE:
Duration of expiration
- TI:
Duration of inspiration
- TI/TTOT:
Duty cycle
- TTOT:
Total respiratory cycle duration
- VE:
Minute ventilation
- VT:
Tidal volume
- VT/TI:
Mean inspiratory flow
- Wt:
Weight
- ΔN2/ΔV:
Slope of phase III.
Conflict of Interests
The authors report no conflict of interests.
Authors' Contribution
A. F. Palamidas, S. A. Gennimata, F. Karakontaki, G. Kaltsakas, I. Papantoniou, A. Koutsoukou, and N. G. Koulouris made measurements on the subjects, analyzed the data, and contributed to lengthy discussions during the writing of the paper. J. Milic-Emili and D. V. Vlahakos made constructive criticisms. A. F. Palamidas, S.A. Gennimata, and N. G. Koulouris wrote the paper.
References
- 1.Lazarus JM, Brenner BM. Chronic renal failure. In: Fauci AS, Braunwald E, Isselbacher K, et al., editors. Harrison’s Principles of Internal Medicine. 14th edition. New York, NY, USA: Mc Grow-Hill; 1998. pp. 1513–1520. [Google Scholar]
- 2.Weisbord SD, Fried LF, Arnold RM, et al. Prevalence, severity, and importance of physical and emotional symptoms in chronic hemodialysis patients. Journal of the American Society of Nephrology. 2005;16(8):2487–2494. doi: 10.1681/ASN.2005020157. [DOI] [PubMed] [Google Scholar]
- 3.Merkus MP, Jager KJ, Dekker FW, De Haan RJ, Boeschoten EW, Krediet RT. Physical symptoms and quality of life in patients on chronic dialysis: results of the Netherlands Cooperative Study on Adequacy of Dialysis (NECOSAD) Nephrology Dialysis Transplantation. 1999;14(5):1163–1170. doi: 10.1093/ndt/14.5.1163. [DOI] [PubMed] [Google Scholar]
- 4.Murtagh FEM, Addington-Hall J, Higginson IJ. The prevalence of symptoms in end-stage renal disease: a systematic review. Advances in Chronic Kidney Disease. 2007;14(1):82–99. doi: 10.1053/j.ackd.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Wallin C-JB, Jacobson SH, Leksell LG. Subclinical pulmonary oedema and intermittent haemodialysis. Nephrology Dialysis Transplantation. 1996;11(11):2269–2275. doi: 10.1093/oxfordjournals.ndt.a027147. [DOI] [PubMed] [Google Scholar]
- 6.Zidulka A, Despas PJ, Milic Emili J, Anthonisen NR. Pulmonary function with acute loss of excess lung water by hemodialysis in patients with chronic uremia. American Journal of Medicine. 1973;55(2):134–141. doi: 10.1016/0002-9343(73)90161-7. [DOI] [PubMed] [Google Scholar]
- 7.Ralph DD, Ott SM, Sherrard DJ, Hlastala MP. Inert gas analysis of ventilation-perfusion matching during hemodialysis. The Journal of Clinical Investigation. 1984;73(5):1385–1391. doi: 10.1172/JCI111342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siafakas NM, Argyrakopoulos T, Andreopoulos K, Tsoukalas G, Tzanakis N, Bouros D. Respiratory muscle strength during continuous ambulatory peritoneal dialysis (CAPD) European Respiratory Journal. 1995;8(1):109–113. doi: 10.1183/09031936.95.08010109. [DOI] [PubMed] [Google Scholar]
- 9.Myers BD, Rubin AHE, Schey G. Functional characteristics of the lung in chronic uremia treated by renal dialysis therapy. Chest. 1975;68(2):191–194. doi: 10.1378/chest.68.2.191. [DOI] [PubMed] [Google Scholar]
- 10.Ferrer A, Roca J, Rodriguez-Roisin R, Lopez-Pedret J, Revert L. Bronchial reactivity in patients with chronic renal failure undergoing haemodialysis. European Respiratory Journal. 1990;3(4):387–391. [PubMed] [Google Scholar]
- 11.Herrero JA, Álvarez-Sala JL, Coronel F, et al. Pulmonary diffusing capacity in chronic dialysis patients. Respiratory Medicine. 2002;96(7):487–492. doi: 10.1053/rmed.2002.1346. [DOI] [PubMed] [Google Scholar]
- 12.Slinin Y, Guo H, Gilbertson DT, et al. Meeting KDOQI guideline goals at hemodialysis initiation and survival during the first year. Clinical Journal of the American Society of Nephrology. 2010;5(9):1574–1581. doi: 10.2215/CJN.01320210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones PW. Measurement of breathlessness. In: Hughes JMB, Pride NB, editors. Lung Function Tests: Physiological Principles and Clinical Applications. 1st edition. London, UK: Saunders; 1999. pp. 121–131. [Google Scholar]
- 14.D’Angelo E, Prandi E, Marazzini L, Milic-Emili J. Dependence of maximal flow-volume curves on time course of preceding inspiration in patients with chronic obstruction pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 1994;150(6):1581–1586. doi: 10.1164/ajrccm.150.6.7952618. [DOI] [PubMed] [Google Scholar]
- 15.Darling RC, Cournand A, Richards DW. Studies on the intrapulmonary mixture of gases. III. An open circuit method for measuring residual air. The Journal of Clinical Investigation. 1940;19(4):609–618. doi: 10.1172/JCI101163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacIntyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. European Respiratory Journal. 2005;26(4):720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 17.Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. report working party standardization of lung function tests, european community for steel and coal. Official statement of the european respiratory society. The European Respiratory Journal. Supplement. 1993;16:5–40. [PubMed] [Google Scholar]
- 18.Ringqvist T. The ventilator capacity in healthy subjects: an analysis of casual factors with special reference to the respiratory forces. Scandinavian Journal of Clinical & Laboratory Investigation. 1966;18(88):8–170. [PubMed] [Google Scholar]
- 19.Wilson SH, Cooke NT, Edwards RHT, Spiro SG. Predicted normal values for maximal respiratory pressures in caucasian adults and children. Thorax. 1984;39(7):535–538. doi: 10.1136/thx.39.7.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milic-Emili J. Recent advances in clinical assessment of control of breathing. Lung. 1981;160(1):1–17. [Google Scholar]
- 21.Tobin MJ, Chadha TS, Jenouri G. Breathing patterns. 1. Normal subjects. Chest. 1983;84(2):202–205. doi: 10.1378/chest.84.2.202. [DOI] [PubMed] [Google Scholar]
- 22.Whitelaw WA, Derenne JP, Milic Emili J. Occlusion pressure as a measure of respiratory center output in conscious man. Respiration Physiology. 1975;23(2):181–199. doi: 10.1016/0034-5687(75)90059-6. [DOI] [PubMed] [Google Scholar]
- 23.Whitelaw WA, Derenne J-P. Airway occlusion pressure. Journal of Applied Physiology. 1993;74(4):1475–1483. doi: 10.1152/jappl.1993.74.4.1475. [DOI] [PubMed] [Google Scholar]
- 24.American Thoracic Society/European Respiratory Society. Review ATS/ERS Statement on respiratory muscle testing. American Journal of Respiratory and Critical Care Medicine. 2002;15166(4):518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 25.Huang CC, Tsai YH, Lin MC, Yang CT, Hsieh MJ, Lan RS. Respiratory drive and pulmonary mechanics during haemodialysis with ultrafiltration in ventilated patients. Anaesthesia and Intensive Care. 1997;25(5):464–470. doi: 10.1177/0310057X9702500502. [DOI] [PubMed] [Google Scholar]
- 26.Sebert P, Bellet M, Girin E. Ventilatory and occlusion pressure responses to hypercapnia in patients with chronic renal failure. Respiration. 1984;45(3):191–196. doi: 10.1159/000194618. [DOI] [PubMed] [Google Scholar]
- 27.De Backer WA, Heyrman RM, Wittesaele WM, Van Waeleghem J-P, Vermeire PA, De Broe ME. Ventilation and breathing patterns during hemodialysis-induced carbon dioxide unloading. American Review of Respiratory Disease. 1987;136(2):406–410. doi: 10.1164/ajrccm/136.2.406. [DOI] [PubMed] [Google Scholar]
- 28.De Backer WA, Verpooten GA, Borgonjon DJ, Van Waeleghem JP, Vermeire PA, De Broe ME. Hypoxia during haemodialysis, effects of different membranes and dialysate compositions. Kidney International. 1983;23:738–743. doi: 10.1038/ki.1983.87. [DOI] [PubMed] [Google Scholar]
- 29.Herrera M, Blasco J, Venegas J. Mouth occlusion pressure (P0.1) in acute respiratory failure. Intensive Care Medicine. 1985;11(3):134–139. doi: 10.1007/BF00258538. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy AC, Linton AL, Eaton JC. Urea levels in cerebrospinal fluid after hemodialysis. The Lancet. 1962;279(7226):410–411. doi: 10.1016/s0140-6736(62)91365-x. [DOI] [PubMed] [Google Scholar]
- 31.Bark H, Heimer D, Chaimovitz C, Mostoslovski M. Effect of chronic renal failure on respiratory muscle strength. Respiration. 1988;54(3):153–161. doi: 10.1159/000195516. [DOI] [PubMed] [Google Scholar]
- 32.Kovelis D, Pitta F, Probst VS, et al. Pulmonary function and respiratory muscle strength in chronic renal failure patients on hemodialysis. Jornal Brasileiro de Pneumologia. 2008;34(11):907–912. doi: 10.1590/s1806-37132008001100004. [DOI] [PubMed] [Google Scholar]
- 33.Rocha C, Araujo S. Evaluation of maximum respiratory pressures in chronic renal patients at the pre and post hemodialysis moment. Jornal Brasileiro de Nefrologia. 2010;32(1):105–111. [PubMed] [Google Scholar]
- 34.Zifko U, Auinger M, Albrecht G, et al. Phrenic neuropathy in chronic renal failure. Thorax. 1995;50(7):793–794. doi: 10.1136/thx.50.7.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karacan Ö, Tutal E, Çolak T, Sezer S, Eyüboğlu FÖ, Haberal M. Pulmonary function in renal transplant recipients and end-stage renal disease patients undergoing maintenance dialysis. Transplantation Proceedings. 2006;38(2):396–400. doi: 10.1016/j.transproceed.2005.12.068. [DOI] [PubMed] [Google Scholar]
- 36.Ramirez B, Gomez BPA. Uremic neuropathy: a review. International Journal of Genetics and Molecular Biology. 2012;3(11):155–160. [Google Scholar]
- 37.Krishnan AV, Kiernan MC. Uremic neuropathy: clinical features and new pathophysiological insights. Muscle and Nerve. 2007;35(3):273–290. doi: 10.1002/mus.20713. [DOI] [PubMed] [Google Scholar]
- 38.American Thoracic Society. Dyspnea. Mechanisms, assessment, and management: a consensus statement. American Journal of Respiratory and Critical Care Medicine. 1999;159(1):321–340. doi: 10.1164/ajrccm.159.1.ats898. [DOI] [PubMed] [Google Scholar]
- 39.Koulouris N, Mulvey DA, Laroche CM, Green M, Moxham J. Comparison of two different mouthpieces for the measurement of PImax and PEmax in normal and weak subjects. European Respiratory Journal. 1988;1(9):863–867. [PubMed] [Google Scholar]



