Abstract
Background
Formoterol is a long‐acting beta2‐agonist but because it has a fast onset of action it can also be used as a relief medication.
Objectives
To asses the efficacy and safety of formoterol as reliever therapy in comparison to short‐acting beta2‐agonists in adults and children with asthma.
Search methods
We searched the Cochrane Airways Group Specialised Register and websites of clinical trial registers (for unpublished trial data), and we checked the Food and Drug Administration (FDA) submissions in relation to formoterol. The date of the most recent search was February 2010.
Selection criteria
Randomised, parallel‐arm trials of at least 12 weeks duration in patients of any age and severity of asthma. Studies randomised patients to any dose of as‐needed formoterol versus short‐acting beta2‐agonist. Concomitant use of inhaled corticosteroids or other maintenance medication was allowed, as long as this was not part of the randomised treatment regimen.
Data collection and analysis
Two authors independently selected trials for inclusion in the review. Outcome data were extracted by one author and checked by the second author. We sought unpublished data on primary outcomes.
Main results
This review includes eight studies conducted in 22,604 participants (mostly adults). Six studies compared formoterol as‐needed to terbutaline whilst two studies compared formoterol with salbutamol as‐needed. Background maintenance therapy varied across the trials. Asthma exacerbations and serious adverse events showed a direction of treatment effect favouring formoterol, of which one outcome reached statistical significance (exacerbations requiring a course of oral corticosteroids). In patients on short‐acting beta2‐agonists, 117 people out of 1000 had exacerbations requiring oral corticosteroids over 30 weeks, compared to 101 (95% CI 93 to 108) out of 1000 for patients on formoterol as‐needed. In patients on maintenance inhaled corticosteroids there were also significantly fewer exacerbations requiring a course of oral corticosteroids on formoterol as‐needed (Peto OR 0.75; 95% CI 0.62 to 0.91). There was one death per 1000 people on formoterol or on short‐acting beta2‐agonists.
Authors' conclusions
In adults, formoterol was similar to short‐acting beta2‐agonists when used as a reliever, and showed a reduction in the number of exacerbations requiring a course of oral corticosteroids. Clinicians should weigh the relatively modest benefits of formoterol as‐needed against the benefits of single inhaler therapy and the potential danger of long‐term use of long‐acting beta2‐agonists in some patients. We did not find evidence to recommend changes to guidelines that suggest that long‐acting beta2‐agonists should be given only to patients already taking inhaled corticosteroids.
There was insufficient information reported from children in the included trials to come to any conclusion on the safety or efficacy of formoterol as relief medication for children with asthma.
Plain language summary
Formoterol versus short‐acting beta‐agonists as symptom relief for adults and children with asthma
Short‐acting beta‐agonists are traditionally used to ease symptoms when people experience wheezing and breathlessness during asthma exacerbations. Formoterol is a bronchodilator that works quickly to relieve symptoms and the effect lasts longer. We are interested in whether there are any benefits or disadvantages associated with using formoterol instead of more traditional treatments to relieve symptoms.
We found eight trials involving a total of 22,604 patients. We found that taking formoterol reduced the risk of having an exacerbation that was treated with oral corticosteroids, but none of the other benefits from taking formoterol were statistically significant. Guidelines suggest that long‐acting beta‐agonists should be given only to patients already taking an inhaled corticosteroid.
We could not find enough trials conducted in children to reach a conclusion on the benefits and harms in children, so we do not recommend using the results to make recommendations on treatment of children with asthma.
Summary of findings
Summary of findings for the main comparison. Formoterol versus short‐acting beta2‐agonist as relief medication for asthma.
| Formoterol versus short‐acting beta2‐agonist as relief medication for asthma | ||||||
| Patient or population: Patients with asthma Settings: International studies Intervention: Formoterol versus short‐acting beta2‐agonist | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Formoterol versus short‐acting beta2‐agonist | |||||
| Patients with an exacerbation requiring hospitalisation Follow up: mean 30 weeks | 16 per 10001 | 13 per 1000 (11 to 17) | OR 0.84 (0.67 to 1.04) | 22236 (7 studies) | ⊕⊕⊕⊝ moderate2 | |
|
Patients with an exacerbation requiring a course of oral corticosteroids Follow up: mean 30 weeks |
117 per 10001 | 101 per 1000 (93 to 108) | OR 0.84 (0.77 to 0.91) | 21591 (6 studies) | ⊕⊕⊕⊝ moderate3 | Exacerbations were still significantly reduced when results were confined to double‐blind studies. |
|
Fatal serious adverse events (all‐cause) Follow up: mean 30 weeks |
1 per 10001 | 1 per 1000 (1 to 2) | OR 1.08 (0.51 to 2.3) | 21629 (5 studies) | ⊕⊕⊝⊝ low4 | There were few deaths in participants on either medication. A larger trial is unlikely to be powered to detect a difference in mortality. |
|
Patients with a serious adverse event (all cause) Follow‐up: mean 30 weeks |
35 per 10001 | 33 per 1000 (29 to 38) | OR 0.94 (0.81 to 1.08) | 22538 (7 studies) | ⊕⊕⊝⊝ low2,3 | |
|
Patients with a serious adverse event (asthma related) Follow up: mean 30 weeks |
14 per 10001 | 13 per 1000 (10 to 16) | OR 0.91 (0.72 to 1.15) | 21986 (6 studies) | ⊕⊕⊝⊝ low2,3 | |
|
Withdrawals (any reason) Follow up: mean 30 weeks |
72 per 10001 | 80 per 1000 (73 to 87) | OR 1.12 (1.02 to 1.24) | 22541 (7 studies) | ⊕⊕⊝⊝ low3,5 | Confining the analysis to double‐blind studies changed the direction of the treatment effect. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Mean control event rate. 2 Confidence interval includes the possibility of benefit or harm. 3 One study was open‐label. 4 Few participants died which led to wide confidence intervals. 5 There was significant heterogeneity in this outcome, probably due to the open‐label design and population‐wide nature of RELIEF.
Background
Description of the condition
There is currently no universally accepted definition of the term asthma. This is in part due to an overlap of symptoms with other diseases such as chronic bronchitis but is also due to the probable existence of more than one underlying pathophysiological process. There are, for example, wide variations in the age of onset, symptoms, triggers, association with allergic disease and the type of inflammatory cell infiltrate seen in patients diagnosed with asthma (Miranda 2003). Patients with all forms and severity of disease will typically have intermittent symptoms of cough, wheeze and/or breathlessness. Underlying these symptoms there is a process of variable, at least partially reversible, airway obstruction, airway hyper‐responsiveness and chronic inflammation.
Description of the intervention
People with persistent asthma can use preventer therapy (usually low‐dose inhaled corticosteroid (ICS)) to maintain symptom control, improve lung function and reduce emergency care requirement (Adams 2008). However, when symptoms deteriorate, reliever medication in the form of short‐acting beta2‐agonists such as salbutamol or terbutaline (BTS/SIGN 2008) is required. An alternative long‐acting beta2‐agonist (LABA), formoterol, has the potential to be used as reliever therapy, as it has an onset of action that is as fast as salbutamol and terbutaline, unlike another long‐acting beta2‐agonist, salmeterol (Palmqvist 2001).
How the intervention might work
Formoterol can be used to relieve bronchospasm and may have advantages over using salbutamol and terbutaline as reliever medication, since the benefit lasts for 12 hours (Lötvall 2008). Concerns have been raised about the use of regular salmeterol and formoterol in asthma, in particular where it is used without a regular inhaled corticosteroid, in relation to the possible increased risk of severe adverse events and asthma‐related death (Cates 2008; Cates 2008a; Walters 2007).
Why it is important to do this review
The only large worldwide safety study on formoterol has been done on its use as relief medication (RELIEF 2003). This trial was not considered in a previous systematic review which evaluated the use of regular formoterol compared to placebo (Cates 2008a) rather than as a relief medication; the review showed that there was an increased risk of serious adverse events in patients on maintenance formoterol. Although the use of single inhaler therapy has been advocated as a new approach to asthma care (Barnes 2007), and as way of increasing compliance with inhaled corticosteroids (Delea 2008; Sovani 2008), others have pointed out limitations in the current research evidence on formoterol alone in children and adults with less severe asthma (Bisgaard 2003; Lipworth 2007).
Although there are existing reviews on formoterol combined with an inhaled corticosteroid used for maintenance and relief of asthma symptoms (Cates 2009; Cates 2009a), there is currently no systematic review of the efficacy and safety of formoterol alone as reliever therapy.
Objectives
To assess the efficacy and safety of formoterol as reliever therapy in asthma in comparison to short‐acting beta2‐agonists for relief of symptoms.
Methods
Criteria for considering studies for this review
Types of studies
Randomised trials of parallel‐group design of at least 12 weeks duration were included in the review. Open‐label and double‐blind study designs were eligible. We excluded cross‐over trials.
Types of participants
Adults and children with a diagnosis of asthma. We accepted trialist‐defined asthma and recorded both the definition of asthma used in the studies and the entry criteria. Studies on patients with acute asthma or exercise‐induced bronchospasm were not included.
Types of interventions
Eligible treatment group intervention
Studies which assessed the effects of using any dose of formoterol for the relief of asthma symptoms were eligible. Other maintenance treatments were allowed provided they were not part of the as‐needed randomisation regime.
Eligible control group treatment
The control groups for the studies in this review consisted of short‐acting beta2‐agonists (salbutamol or terbutaline) for relief of symptoms. Studies that compared different doses of formoterol, or different delivery devices or propellants were not included.
Types of outcome measures
Primary outcomes
Patients with exacerbations requiring hospitalisation
Patients with exacerbations requiring oral corticosteroids
Fatal serious adverse events (all‐cause)
Non‐fatal serious adverse events (all‐cause and asthma‐related)
Secondary outcomes
Diary card morning and evening peak expiratory flow (PEF)
Clinic spirometry (FEV1)
Symptoms/symptom‐free days
Nocturnal awakenings
Quality of life
Search methods for identification of studies
Electronic searches
We identified trials using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearching of respiratory journals and meeting abstracts (please see the Airways Group Module for further details). All records in the Specialised Register coded as 'asthma' were searched using the following terms:
(formoterol or eformoterol or oxis or foradil) and (relie* or "as need*" or as‐need* or prn)
Searching other resources
We contacted the manufacturer in order to confirm data and to establish whether other unpublished or ongoing studies are available for assessment. We handsearched clinical trial websites (www.clinicalstudyresults.org; www.clinicaltrials.gov; www.fda.gov) and the clinical trial websites of the manufacturer of formoterol (www.astrazenecaclinicaltrials.com).
Data collection and analysis
Selection of studies
Following electronic literature searches, two review authors (CC and EJW) independently selected articles on the basis of title and/or abstract for full‐text scrutiny. We agreed a list of articles to be retrieved and subsequently assessed each study to determine whether it was a secondary publication of a primary study publication and whether the study met the entry criteria of the review.
Data extraction and management
We extracted information from each study for the following characteristics:
Design (description of randomisation, blinding, number of study centres and location, number of study withdrawals).
Participants (N, mean age, age range of the study, baseline lung function, % on maintenance ICS or ICS/LABA combination and average daily dose of steroid (beclomethasone dipropionate equivalent), entry criteria).
Intervention (type and dose of component ICS and LABA, control limb dosing schedule, intervention limb dose adjustment schedule, inhaler device, study duration and run‐in)
Outcomes (type of outcome analysis, outcomes analysed).
Assessment of risk of bias in included studies
We assessed the risk of bias in the included studies as either high, low or unclear using the Cochrane Collaboration's 'Risk of bias' tool (Higgins 2008) and the following headings 1) sequence generation; 2) allocation concealment; 3) blinding; 4) incomplete outcome data; 5) selective outcome reporting; and 6) other bias.
Measures of treatment effect
We extracted data, where possible, for each of the outcomes listed above from the trial publication(s) and contacted trialists and manufacturers for further information. We entered exacerbations into the meta‐analysis by subtype (hospitalisation and courses of oral steroids), rather than as a composite outcome and figures were calculated from other outcome data and verified by the manufacturer where necessary. We considered serious adverse events separately as fatal and non‐fatal events.
Unit of analysis issues
We used or requested data from the trial sponsors that were reported with patients (rather than events) as the unit of analysis for the primary outcomes. Some patients suffer more than one exacerbation over the course of a study and these events are not independent. Where it was not possible to obtain these data, we entered events and discussed any effects this may have on the results of individual meta‐analyses.
Dealing with missing data
The proportion of randomised patients who provided data for the main outcomes was reported and compared with the number of patients with events in each outcome category.
Assessment of heterogeneity
We measured statistical variation between combined studies by the I2 statistic (Higgins 2003). We investigated possible causes of any heterogeneity that were found.
Assessment of reporting biases
We inspected funnel plots to see if there was evidence of publication bias where there were enough studies to render this meaningful. Where possible we compared the outcomes suggested in the trial protocol with those reported for each trial.
Data synthesis
We combined data with Review Manager 5 (RevMan 2008) using a fixed‐effect mean difference (calculated as a weighted mean difference) for continuous data variables, and a fixed‐effect odds ratio for dichotomous variables. For the primary outcomes of exacerbations and serious adverse events we calculated a number needed to treat (NNT) (benefit or harm) for the different levels of risk as represented by control group event rates over a specified time period using the pooled odds ratio and its confidence interval using an on‐line calculator, Visual Rx. The Peto odds ratio was used for subgroup analysis as there were no important differences in the results when compared to the Mantel‐Haenszel odds ratio and Peto allows for a test of subgroup interaction to be calculated in Review Manager 5.
We constructed 'Summary of findings' tables for the four primary outcomes.
Subgroup analysis and investigation of heterogeneity
We intended to pool data from adults and children separately and requested separate information on outcomes in order to compare adults and children using subgroup analysis, but it was not possible to obtain separate results on children from the trials that included adults and children. We also intended to perform subgroup analyses based on use of maintenance inhaled corticosteroids and long‐acting beta2‐agonists, and asthma severity.
Sensitivity analysis
We conducted sensitivity analyses on the basis of risk of bias in studies and methods of data analysis (OR, RR, RD with fixed and random‐effects models).
Results
Description of studies
Results of the search
We conducted an all‐years search of the Airways Group Register in February 2010. There was no restriction on language of the search. The search yielded a total of 140 references. We examined the reference list of titles and abstracts and assessed each reference against eligibility criteria. We retrieved full text articles of 35 references. We identified 8 included studies and 6 excluded studies and complete agreement was reached between authors. A search of www.astrazenecaclinicaltrials.com yielded five study reports corresponding to five of the included clinical trials and an AstraZeneca Briefing Document was found on the FDA website. We asked AstraZeneca if there were any additional study reports or references to studies that they had sponsored, but none were returned.
Included studies
Full details can be found in the Characteristics of included studies tables.
Participants
A total of 22,604 participants were randomised to eight eligible studies (Ind 2002; Jain 2004; Rabe 2006; RELIEF 2003; SD‐037‐0714; SD‐037‐0716; Tattersfield 2001; Villa 2002). The largest trial was RELIEF 2003 with 17,862 participants, whilst Rabe 2006 had 2281 participants and the remaining six trials had between 60 and 675 participants. The trials were also of different lengths with a mean duration of 29.5 weeks; three trials were 12 months long (Rabe 2006; SD‐037‐0714; SD‐037‐0716), three were six months long (Jain 2004; RELIEF 2003; Villa 2002) and two were three months long (Ind 2002; Tattersfield 2001).
Two trials (Ind 2002; Tattersfield 2001) were conducted in adults, one in children (Villa 2002), four trials (Rabe 2006; RELIEF 2003; SD‐037‐0714; SD‐037‐0716) were conducted in adults and children and it was unclear in what population Jain 2004 was conducted. Participants with a range of different asthma severities across studies were enrolled. The largest study (RELIEF 2003) allowed any severity of asthma, whilst Rabe 2006 allowed moderate to severe, Tattersfield 2001 and Villa 2002 allowed mild‐moderate, SD‐037‐0714 participants had mild asthma and SD‐037‐0716 had intermittent asthma.
Interventions
All eight trials compared formoterol as‐needed with one of two short‐acting beta2‐agonists (Table 2) and most were designed to show that formoterol was as safe as the short‐acting beta2‐agonist in question. Formoterol was compared with terbutaline in six trials (Ind 2002; Rabe 2006; SD‐037‐0714; SD‐037‐0716; Tattersfield 2001; Villa 2002) and salbutamol in two trials (Jain 2004; RELIEF 2003). In addition to the as‐needed medications, participants in some trial were permitted to take, or required to be on, additional maintenance medication (Table 2). Although this review addresses formoterol used as‐needed rather than as maintenance, three trials (Ind 2002; Rabe 2006; Tattersfield 2001) reported mean daily doses of 1.9 to 3.9 puffs a day (8.5 to 17.5 μg) which is of the order of recommended maintenance formoterol doses (12 μg twice daily, BNF).
1. Randomised as‐needed medication and maintenance therapies.
| Study ID | Intervention as‐needed medication [mean daily puffs (range)] | Control medication as‐needed [mean daily puffs (range)] | Maintenance medication [mean daily ICS dose] |
| Ind 2002 | Formoterol, 4.5 μg DPI [2.16 (0.00 to 6.3)] |
Terbutaline, 0.5 mg DPI [2.34 (0.1 to 7.5)] |
All on formoterol , 9 μg DPI, twice a day and maintenance inhaled or oral corticosteroids |
| Jain 2004 | Formoterol, 4.5 μg DPI | Salbutamol, 100 μg DPI | Not stated |
| Rabe 2006 | Formoterol, 4.5 μg DPI [1.9 (0.0 to 9.1)] |
Terbutaline, 0.4 mg DPI [1.9 (0.3 to 9.7)] |
Budesonide/formoterol, 160/4.5 μg DPI combined inhaler |
| RELIEF 2003 | Formoterol, 4.5 μg DPI | Salbutamol, 200 μg DPI (6 countries) or PMDI (18 countries) | Any ordinary asthma medication apart from other relief medication, changes in maintenance medication allowed [76% participants on 760 μg] |
| SD‐037‐0714 | Formoterol, 4.5 μg DPI | Terbutaline, 0.5 mg DPI | All on inhaled corticosteroids ([380 μg] 200 to 500 μg per day), but not long‐acting beta2‐agonists |
| SD‐037‐0716 | Formoterol, 4.5 μg DPI | Terbutaline, 0.5 mg DPI | Not inhaled corticosteroids or other anti‐inflammatories |
| Tattersfield 2001 | Formoterol, 4.5 μg DPI [3.92] |
Terbutaline, 0.5 mg DPI [4.89] |
All on inhaled corticosteroids [875 μg]. No beta2‐agonists allowed but other asthma medications at constant dosage permitted |
| Villa 2002 | Formoterol, 4.5 μg DPI | Terbutaline, 0.25 mg DPI | Inhaled corticosteroids [410 μg], disodium cromoglycate or nedocromil |
DPI ; Dry power inhaler; PMDI: pressurised metered dose inhaler.
All participants in Ind 2002 were on maintenance formoterol as a study medication in addition to constant dose of inhaled corticosteroids and randomised as‐needed formoterol or terbutaline. All participants in Rabe 2006 were originally on inhaled corticosteroids and were then moved to budesonide/formoterol at a dose on which they were symptomatic in addition to randomised formoterol or terbutaline. Participants in SD‐037‐0714 were on inhaled corticosteroids at different but constant doses and were not permitted other long‐acting beta2‐agonists. Participants in Tattersfield 2001 stayed on the same dose of inhaled corticosteroids or other maintenance medications and participants in Villa 2002 were on inhaled corticosteroids, disodium cromoglycate or nedocromil at a constant dose. Any ordinary asthma medication apart from relievers was permitted in RELIEF 2003, and subgroup data by background medication were reported for serious adverse events, discontinuations due to serious adverse events and exacerbations. Patients in RELIEF 2003 were able to have their prescriptions for maintenance medication changed in response to changing asthma. Participants in SD‐037‐0716 were not on maintenance medication. It was not stated whether patients were on any sort of maintenance medication in the abstract located for Jain 2004.
RELIEF 2003 was the only trial to employ pressurised metered dose inhalers; formoterol was delivered via dry powder inhaler in all countries whereas salbutamol was delivered via a dry powder inhaler in six countries and by pressurised metered dose inhaler in 18 countries. The other six trials employed dry powder inhalers for both formoterol and short‐acting beta2‐agonist.
Participants were instructed to take their relief inhalers as needed and to tell the investigators if they took more than 10 puffs in a day (Rabe 2006) or more than 12 puffs (Tattersfield 2001) or more than 12 puffs in adults and eight puffs in children (RELIEF 2003).
Usage of relief inhalers was an inclusion criteria in six of the studies, this was not stated by Jain 2004 and not a criteria for RELIEF 2003. To be eligible for randomisation, participants in Ind 2002 had to have taken between two and five puffs of terbutaline per day during run‐in, those in Tattersfield 2001 had to have taken between three and eight puffs a day on at least seven days in the run‐in period. Patients in the other trials took fewer inhalations; those in Rabe 2006 had to have used relief medication on five out of seven days; participants in SD‐037‐0714 participants used fewer than four inhalations per day on at least three occasions per week; SD‐037‐0716 used their inhalers on between two and six occasions during run‐in and participants in Villa 2002 used an average of at least one puff per day during the run‐in period. Asthma severity in the studies is summarised in Table 3 with details of the duration and number of centres for each study.
2. Study characteristics.
| Study ID | Number of participants | Duration | Mean age (range) | Locale centres (countries) | Asthma severity | Sponsor |
| Ind 2002 | 375 | 12 weeks | 47 | 42 (5) | stable on ICS | AZ |
| Jain 2004 | 60 | 6 months | ? | ? | ? | ? |
| Rabe 2006 | 2281 | 12 months | 42 (12 to 81) | 289 (20) | moderate to severe | AZ |
| RELIEF 2003 | 17,862 | 6 months | 39 (4 to 91) | 1139 (24) | intermittent, mild, moderate or severe | AZ |
| SD‐037‐0714 | 455 | 12 months | 25 (6 to 75) | 48 (4) | mild | AZ |
| SD‐037‐0716 | 675 | 12 months | 24 (6 to 87) | 54 (8) | intermittent | AZ |
| Tattersfield 2001 | 362 | 12 weeks | 47 (18 to 75) | 35 (4) | mild to moderate | AZ |
| Villa 2002 | 552 | 6 months | 11 (5 to 19) | 77 (9) | mild or moderate persistent | AZ |
Patients were withdrawn from the studies if their daily use of relief medication exceeded certain thresholds. These were eight puffs per day (N = 2), 10 puffs (N = 1) and 12 puffs (N = 2).
Outcomes
The primary outcomes for the studies did not necessarily match ours because the aim of individual trials was to show that formoterol is as effective as short‐acting beta2‐agonists and there was some variation across studies. Time until first asthma exacerbation as the primary outcome was used in four studies (Rabe 2006; RELIEF 2003; Tattersfield 2001; Villa 2002). Peak expiratory flow was employed as the primary outcome by SD‐037‐0714 and SD‐037‐0716 whilst Ind 2002 used serum potassium levels, ECG, vital signs, lung function and adverse events.
However, data for our primary outcomes were well‐reported and so we were able to use these in our review. Patients with exacerbations requiring hospitalisation were reported in seven studies; patients with exacerbations requiring oral corticosteroids in six studies and fatal serious adverse events in four studies and non‐fatal serious adverse events in seven studies. Our secondary outcomes were also well‐reported. We did not find separate details of results from children in those studies that included both adults and children.
Excluded studies
Full details can be found in the Characteristics of excluded studies tables.
Risk of bias in included studies
A summary of the risk of bias in the included studies is shown in Figure 1
1.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
All eight trials were described as randomised. Three trials (Rabe 2006; RELIEF 2003; Tattersfield 2001) gave detailed descriptions of satisfactory sequence generation and allocation concealment. Four trials did not provide such clear descriptions (Ind 2002; SD‐037‐0714; SD‐037‐0716; Villa 2002); however the sponsor provided details of adequate randomisation. Jain 2004 was described as randomised with no further details, and so sequence generation and allocation concealment remains at unclear risk of bias.
Blinding
Six trials overall were described as double‐blind; neither patient nor investigator knew to which as‐needed medication an individual was randomised. Blinding was preserved by delivering medications via identical inhalers. Three studies provided detailed descriptions of how the patients were blinded (Ind 2002; Rabe 2006; Tattersfield 2001) and the sponsors provided suitable descriptions of the blinding for the remaining three trials (SD‐037‐0714; SD‐037‐0716; Villa 2002). In three trials (Tattersfield 2001; SD‐037‐0714; SD‐037‐0716) the blinding was lifted in the case of a serious adverse event and so was judged as unclear risk of bias for the subjective outcomes.
RELIEF 2003 was an open‐label study that did not attempt to blind the participants or investigators. This is unlikely to have affected objective outcome measures (hospitalisations, all‐cause serious adverse events, deaths) which was judged to be at low risk of bias. However, the open‐label design may have affected subjective outcomes and was judged to be at unclear risk of bias for this domain. Bias may result from having unblinded investigators, who may consciously or subconsciously make different decisions on whether to give a patients a course of oral corticosteroids or in judging whether or not a serious adverse event was related to asthma. In addition, knowledge of the study drug may affect a patient's decision to withdraw from the study.
Incomplete outcome data
Six trials were judged to be at low risk of bias from incomplete outcome reporting (Rabe 2006; RELIEF 2003; SD‐037‐0714; SD‐037‐0716; Tattersfield 2001; Villa 2002) and all trials were analysed on an intention‐to‐treat basis. Five trials reported reasons for withdrawals and were balanced between treatment arms (Rabe 2006; RELIEF 2003; SD‐037‐0714; SD‐037‐0716; Tattersfield 2001). Although Villa 2002 did not provide reasons for withdrawal, it was judged to be low risk of bias because the numbers of withdrawals were similar to those in other trials in this review and balanced between treatment arms (Table 4). Ind 2002 was judged to be at unclear risk of bias due to incomplete outcome data, because although numbers of withdrawals were reported it was not clear how many withdrawals corresponded to each treatment arm. Jain 2004 was judged to be at unclear risk of incomplete outcome data bias because the number of withdrawals, if any, was not disclosed in the abstract.
3. Withdrawals.
| Study ID | Eligibility criteria | Discontinuations due to adverse events | Lost to follow up | Total numbers of withdrawals | N | |||||
| Formoterol | SABA | Formoterol | SABA | Formoterol | SABA | Formoterol | SABA | Formoterol | SABA | |
| Ind 2002 | 8 | 9 | 14 | 14 | 1 | 8 | 28 (15.9%) | 34 (18.8%) | 176 | 181 |
| Rabe 2006 | 50 | 56 | 22 (1.9%) | 19 (1.6%) | 14 (1.2%) | 9 (1.6%) | 132 (11.5%) | 151 (13%) | 1140 | 1141 |
| RELIEF 2003 | 12 | 21 | 213 (2.4%) | 119 (1.3%) | 221 (2.5%) | 204 (3.2%) | 664 (7.4%) | 525 (5.9%) | 8924 | 8938 |
| SD‐037‐0714 | 2 | 3 | 2 | 3 | ‐ | ‐ | 11 (4.8%) | 20 (8.8%) | 228 | 227 |
| SD‐037‐0716 | ‐ | ‐ | 0 | 2 | ‐ | ‐ | 23 (6.9%) | 28 (8.3%) | 333 | 339 |
| Tattersfield 2001 | ‐ | ‐ | 8 | 18 | ‐ | ‐ | 21 (6.3%) | 32 (17.8%) | 182 | 180 |
| Villa 2002 | ‐ | ‐ | 3% | 3% | ‐ | ‐ | 17 (7.5%) | 18 (6.5%) | 227 | 275 |
Additionally, Ind 2002 reported only run‐in data for FEV1 or PEF and stated that this remained unchanged throughout the treatment period. We felt it was unlikely that the mean and standard deviation stayed constant throughout this whole period, but since there were no data to enter into the meta‐analysis this judgement did not effect the outcome of our meta‐analysis.
Selective reporting
Six trials were judged to be of low risk of selective outcome reporting bias (Ind 2002; Rabe 2006; RELIEF 2003; SD‐037‐0714; SD‐037‐0716; Tattersfield 2001). Villa 2002 was judged to be at unclear risk of selective outcome reporting bias because some key data (PEF, number of inhalations, night‐time awakenings, days restricted activity, FEV1, quality of life, adverse events) relevant to our study or stipulated as outcomes in the study report, were missing from the study report. Jain 2004 was also at unknown risk of bias in this domain but since there was a single abstract published and we cannot be sure of the missing results.
Other potential sources of bias
Villa 2002 was judged to be at high risk of publication bias because the study has only been published as a study report and an abstract and therefore lacks information on study characteristics and outcome data. Jain 2004 was also at high risk of publication bias since it was published as a single abstract. Although it is debatable whether trials that have only been reported as abstract should be included in Cochrane systematic reviews, these two trials were small and did not have a meaningful effect on the results of the meta‐analysis and so they remain in the review as a record.
Exacerbations were assessed subjectively by the investigator in some of the trials (Ind 2002; Rabe 2006; RELIEF 2003; SD‐037‐0714; SD‐037‐0716; Tattersfield 2001), although a drop in PEF of > 30% was also considered an exacerbation by Ind 2002 and Tattersfield 2001. None of the trials reported explicit definitions of asthma‐related serious adverse events, and they used patient reported asthma aggravated events where described.
All the trials apart from Jain 2004 were sponsored by AstraZeneca.
Effects of interventions
See: Table 1
There was only one trial conducted in children (Villa 2002, N = 552), and trials that were conducted in children and adults did not provide separate paediatric data. Therefore there was insufficient paediatric data presented to merit a full subgroup analysis. We also found that subgroup analysis by asthma severity was not feasible due to the overlap in asthma severities in the various trials.
In the majority of the meta‐analyses, heterogeneity was not encountered. The I2 statistic is only mentioned in the discussion below when it is not equal to zero. All meta‐analyses were compared with both the Peto odds ratio and/or the Mantel‐Haenszel random‐effects model. There was no difference in these sensitivity analyses except for withdrawals.
Primary outcomes
Patients with an exacerbation requiring hospitalisation
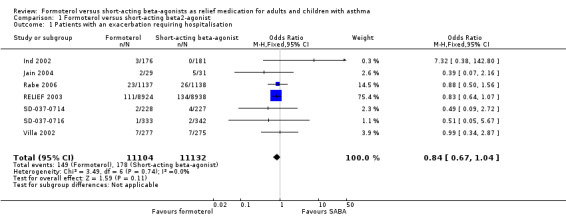
Overall seven trials provided data on hospital admissions for 22,236 participants (Ind 2002; Jain 2004; Rabe 2006; RELIEF 2003; SD‐037‐0714; SD‐037‐0716; Villa 2002). There were fewer hospitalisations in participants on formoterol than in those on short‐acting beta2‐agonist (OR 0.84; 95% CI 0.67 to 1.04), however this was not statistically significant (Figure 2). Sixteen patients on short‐acting beta2‐agonists out of 1000 had hospitalisations over 30 weeks, compared to 13 (95% CI 11 to 17) out of 1000 in patients on formoterol but this confidence interval includes the possibility that there is no difference between the treatments.
2.
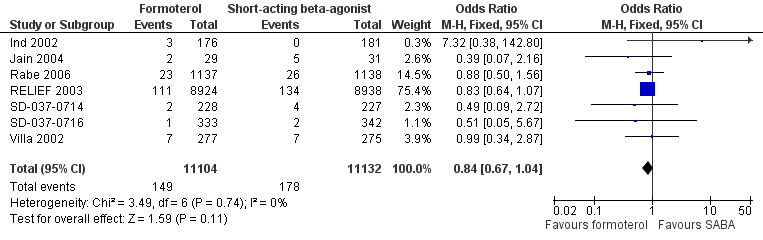
Forest plot of comparison: 1 Formoterol versus short‐acting beta2‐agonist, outcome: 1.1 Patients with an exacerbation requiring hospitalisation.
Rabe 2006 reported serious adverse events reported as asthma and this was used as a proxy measure for hospitalisations. Two trials (Ind 2002; Jain 2004) reported events rather than the number of participants experiencing an event, which could lead to tighter confidence intervals than representative of the true treatment effect if any participants had experienced more than one hospital admission.
Patients with an exacerbation requiring a course of oral corticosteroids
Six trials contributed data on exacerbations requiring a course of oral corticosteroids for 21,591 participants (Ind 2002; Jain 2004; Rabe 2006; RELIEF 2003; SD‐037‐0716; Villa 2002). There were fewer exacerbations requiring a course of oral corticosteroids in patients of formoterol than those on short‐acting beta2‐agonists (OR 0.84; 95% CI 0.77 to 0.91) which was a statistically significant difference (Figure 3; Analysis 1.2). In patients on short‐acting beta2‐agonists, 117 people out of 1000 had exacerbations requiring oral corticosteroids over 30 weeks, compared to 101 (95% CI 93 to 108) out of 1000 for patients on formoterol as‐needed (Figure 4).
3.
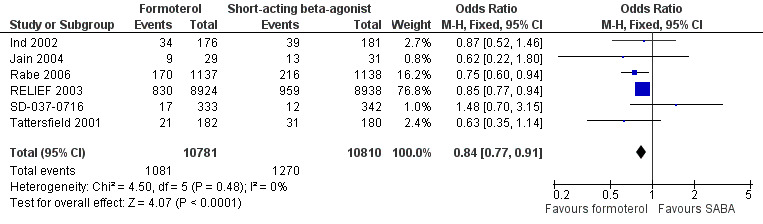
Forest plot of comparison: 1 Formoterol versus short‐acting beta2‐agonist, outcome: 1.2 Patients with an exacerbation requiring a course of oral corticosteroids.
1.2. Analysis.
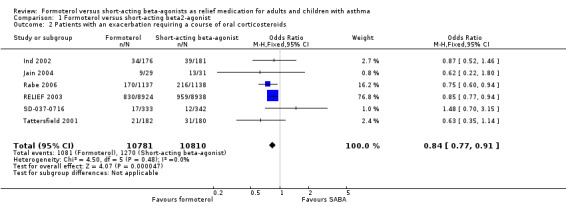
Comparison 1 Formoterol versus short‐acting beta2‐agonist, Outcome 2 Patients with an exacerbation requiring a course of oral corticosteroids.
4.

In patients on short‐acting beta2‐agonists, 117 people out of 1000 had exacerbations requiring oral corticosteroids over 30 weeks, compared to 101 (95% CI 93 to 108) out of 1000 for patients on formoterol as‐needed.
We calculated data for RELIEF 2003 by subtracting hospitalisations from severe exacerbations, but the reduction in exacerbations is still significant when data from RELIEF 2003 are excluded. Jain 2004 reported events rather than the number of participants experiencing an event, which could again lead to an over‐precise estimate of the treatment effect, however performing a sensitivity analysis by removing this study did not significantly alter the estimate of treatment effect. Data were provided by the sponsors for Rabe 2006.
Exacerbations requiring a course of oral corticosteroids in relation to maintenance medication use
Four studies contributed to a subgroup analysis for exacerbations requiring a course of oral corticosteroids according to maintenance inhaled corticosteroid use (Figure 5; Analysis 2.1) on 3669 patients. Patients in Ind 2002, Rabe 2006 and Tattersfield 2001 were on maintenance inhaled corticosteroids as either a randomised dose of budesonide/formoterol (Rabe 2006) or non‐randomised inhaled corticosteroids at a stable dose (Ind 2002; Tattersfield 2001). Among these patients, there were fewer exacerbations requiring a course of oral corticosteroids in patients on formoterol than those on short‐acting beta2‐agonists (Peto OR 0.75; 95% CI 0.62 to 0.91) which was a statistically significant improvement. There was only one trial that we could ascertain was conducted in patients who were not taking inhaled corticosteroids (SD‐037‐0716) and there was no statistically significant difference in exacerbations requiring oral corticosteroids for this study (Peto OR 1.47; 95% CI 0.70 to 3.10). Although these treatment effects were in opposite directions, there was no significant difference in the test for subgroup differences (Chi² = 2.94, df = 1 (P = 0.09)) so a relationship between exacerbations requiring oral corticosteroids and maintenance inhaled corticosteroids was neither proved or disproved.
5.
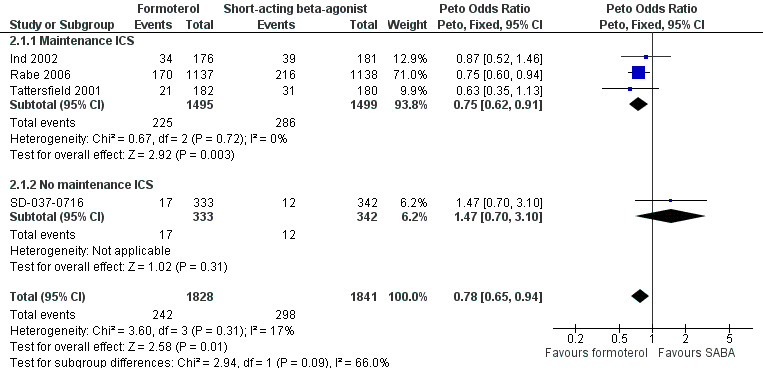
Forest plot of comparison: 2 Formoterol versus short‐acting beta2‐agonist (background ICS use), outcome: 2.1 Patients with an exacerbation requiring a course of oral corticosteroids.
2.1. Analysis.
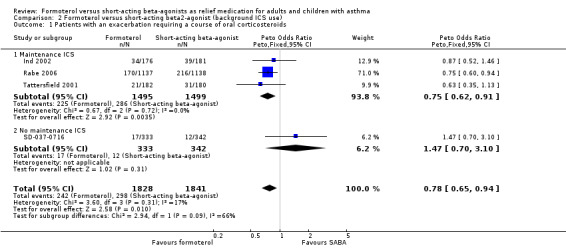
Comparison 2 Formoterol versus short‐acting beta2‐agonist (background ICS use), Outcome 1 Patients with an exacerbation requiring a course of oral corticosteroids.
Fatal serious adverse events (all‐cause)
Five trials on 21,629 participants provided mortality data (Figure 6, Analysis 1.3). There was one death per 1000 people on both formoterol and on short‐acting beta2‐agonists used for relief of symptoms (OR 1.08; 95% CI 0.51 to 2.30). These trials are underpowered to detect a difference in mortality rates and an unfeasibly large trial would be required to do this.
6.
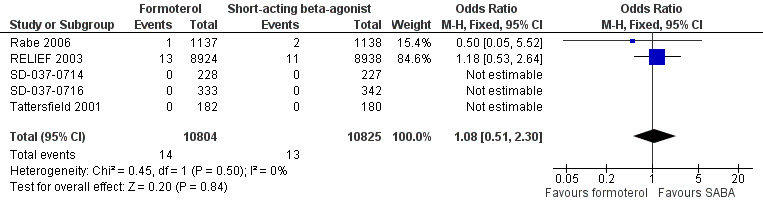
Forest plot of comparison: 1 Formoterol versus short‐acting beta2‐agonist, outcome: 1.3 Fatal serious adverse events (all‐cause).
1.3. Analysis.
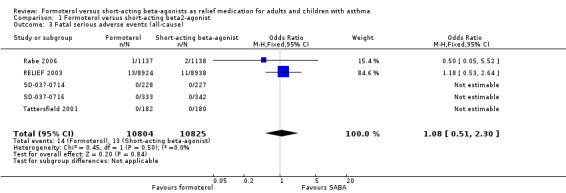
Comparison 1 Formoterol versus short‐acting beta2‐agonist, Outcome 3 Fatal serious adverse events (all‐cause).
There were three deaths in Rabe 2006, one on formoterol as‐needed and two on terbutaline as‐needed, but none of these were judged by the study investigator to be related to the study drug and none were reported as asthma. In RELIEF 2003, there were 13 deaths in patients on formoterol as‐needed of which three were judged to be related to asthma, and 11 deaths in patients on salbutamol as‐needed, of which two were deemed related to asthma.
Patients with a serious adverse event (all‐cause)
Seven trials provided data on serious adverse events in 22,538 participants (Ind 2002; Rabe 2006; RELIEF 2003; SD‐037‐0714; SD‐037‐0716; Tattersfield 2001; Villa 2002). Overall there were fewer serious adverse events from any cause in patients on formoterol than in patients on short‐acting beta2‐agonists but this difference did not reach statistical significance (OR 0.94; 95% CI 0.81 to 1.08), see Figure 7 (Analysis 1.4). In patients on short‐acting beta2‐agonists, 35 people out of 1000 had serious adverse events (all‐cause) over 30 weeks, compared to 33 (95% CI 29 to 38) out of 1000 in patients on formoterol but the confidence interval includes the possibility that there is no difference between the treatments. There was a small amount of statistical heterogeneity (I2 = 9%). Data were entered into the meta‐analysis as the number of patients experiencing one or more serious adverse events in six cases (Rabe 2006; RELIEF 2003; SD‐037‐0714; SD‐037‐0716; Tattersfield 2001; Villa 2002) and as the total number of events in Ind 2002, although performing a sensitivity analysis without this trial did not significantly alter the estimate of the treatment effect. Three trials reported patients experiencing more than one exacerbation (Rabe 2006; RELIEF 2003; Villa 2002) and further details can be found in the Characteristics of included studies.
7.
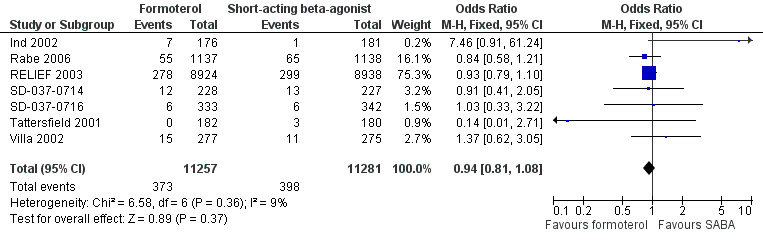
Forest plot of comparison: 1 Formoterol versus short‐acting beta2‐agonist, outcome: 1.4 Patients with a serious adverse event (all‐cause).
1.4. Analysis.
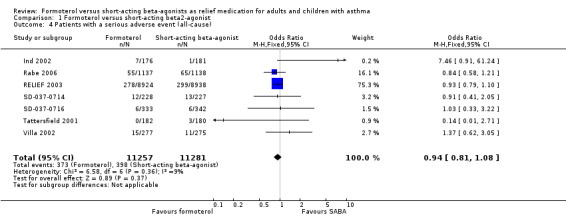
Comparison 1 Formoterol versus short‐acting beta2‐agonist, Outcome 4 Patients with a serious adverse event (all‐cause).
Serious adverse events (all‐cause) in relation to maintenance medication use
All seven trials provided data and could be analysed by subgroup according to maintenance inhaled corticosteroids, or lack thereof (Figure 8, Analysis 2.2). Five trials were conducted in patients who were on maintenance inhaled corticosteroids (Ind 2002; Rabe 2006; SD‐037‐0714; Tattersfield 2001; Villa 2002) and separate data were available for this outcome in RELIEF 2003. There were fewer all‐cause serious adverse events in patients randomised to formoterol who were also on maintenance inhaled corticosteroids, although this difference did not reach statistical significance (OR 0.91; 95% CI 0.78 to 1.06). There was a small amount of statistical heterogeneity found (I2 = 25%). In patients who were not taking inhaled corticosteroids (RELIEF 2003; SD‐037‐0716) there were fewer serious adverse events in patients on short‐acting beta2‐agonists although this difference did not reach statistical significance (OR 1.14; 95% CI 0.77 to 1.69). Although there was a difference in the direction of the treatment effects for each subgroup, the test for subgroup differences (Chi² = 1.17, df = 1 (P = 0.28)) did not show a significant interaction between maintenance inhaled corticosteroids and all‐cause serious adverse events.
8.
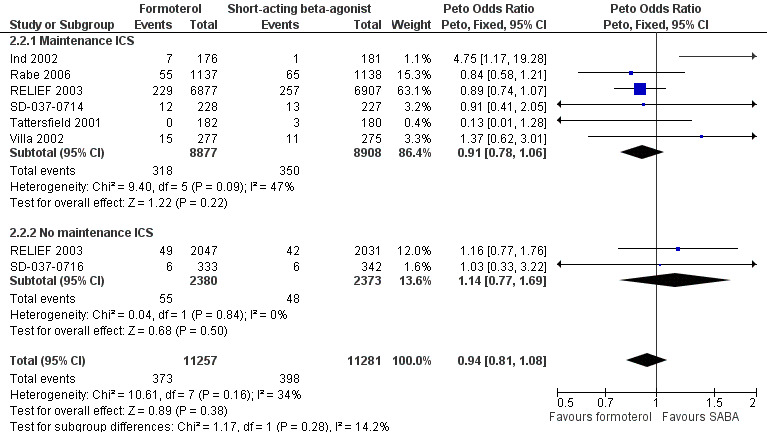
Forest plot of comparison: 2 Formoterol versus short‐acting beta2‐agonist (background ICS use), outcome: 2.2 Patients with a serious adverse event (all‐cause).
2.2. Analysis.
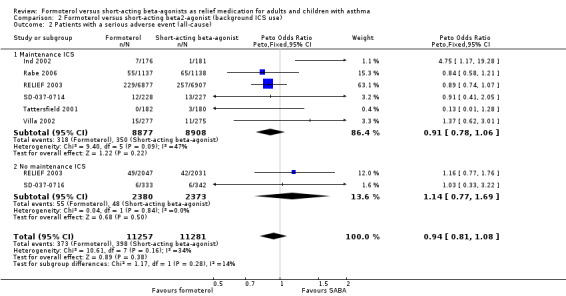
Comparison 2 Formoterol versus short‐acting beta2‐agonist (background ICS use), Outcome 2 Patients with a serious adverse event (all‐cause).
We also performed a subgroup analysis on the basis of maintenance long‐acting beta2‐agonist use or lack thereof (Analysis 3.1). Three trials contributed data for patients who were taking maintenance long‐acting beta2‐agonists (Ind 2002; Rabe 2006; RELIEF 2003). There were fewer all‐cause serious adverse events in patients on formoterol compared to those on short‐acting beta2‐agonist, although this did not reach statistical significance (OR 0.84; 95% CI 0.68 to 1.03). In patients who were not taking long‐acting beta2‐agonist as maintenance, there was no significant difference in serious adverse events in those on formoterol or short‐acting beta2‐agonists (OR 1.06; 95% CI 0.86 to 1.30). The test for subgroup difference did not show a statistically significant difference in the treatment effects in patients on background long‐acting beta2‐agonists compared to those on none (Chi² = 2.44, df = 1 (P = 0.12)).
3.1. Analysis.
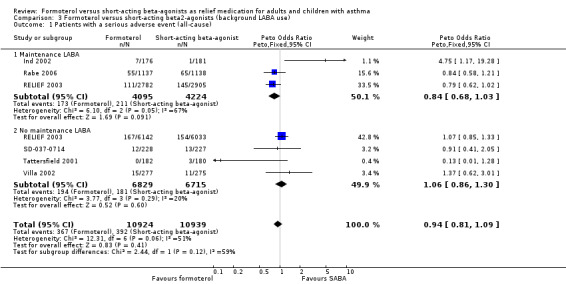
Comparison 3 Formoterol versus short‐acting beta2‐agonists (background LABA use), Outcome 1 Patients with a serious adverse event (all‐cause).
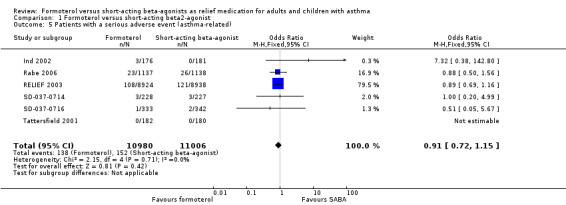
Patients with a serious adverse event (asthma‐related)
Six trials reported asthma‐related serious adverse events in 21,986 participants (Ind 2002; Rabe 2006; RELIEF 2003; SD‐037‐0714; SD‐037‐0716; Tattersfield 2001). These trials showed fewer asthma‐related serious adverse events in patients on formoterol than in patients on short‐acting beta2‐agonists, although this difference did not reach statistical significance (OR 0.91; 95% CI 0.72 to 1.15), see Figure 9. In patients on short‐acting beta2‐agonists, 14 people out of 1000 had asthma‐related serious adverse events over 30 weeks, compared to 13 (95% CI 10 to 16) out of 1000 for patients on formoterol as‐needed. Although number of events was reported, the sponsors provided data on the number of patients experiencing an event from three trials (Ind 2002; SD‐037‐0714; SD‐037‐0716).
9.
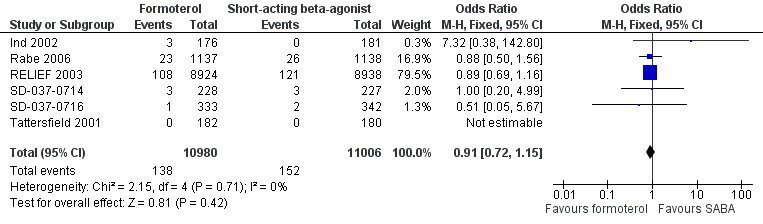
Forest plot of comparison: 1 Formoterol versus short‐acting beta2‐agonist, outcome: 1.5 Patients with a serious adverse event (asthma‐related).
Secondary outcomes
Peak expiratory flow (PEF)
Patients on formoterol showed a greater improvement in morning PEF than those on short‐acting beta2‐agonists (MD 3.88 L/min; 95% CI 1.29 to 6.46), and this was a small but statistically significant result (Analysis 1.6). There was a small amount of statistical heterogeneity (I2 = 17%). Again, patients on formoterol showed a greater improvement in evening PEF than those on short‐acting beta2‐agonists (MD 2.05 L/min; 95% CI ‐0.50 to 4.60), however this difference was not statistically significant (Analysis 1.7).
1.6. Analysis.
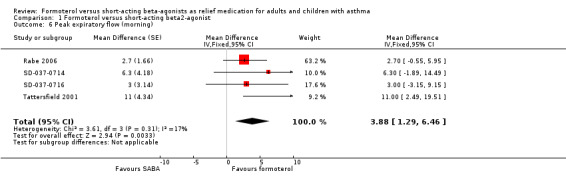
Comparison 1 Formoterol versus short‐acting beta2‐agonist, Outcome 6 Peak expiratory flow (morning).
1.7. Analysis.
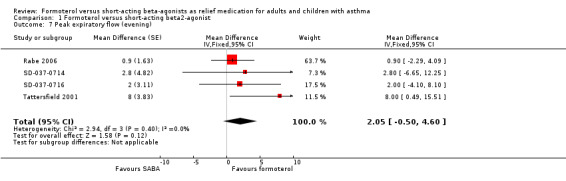
Comparison 1 Formoterol versus short‐acting beta2‐agonist, Outcome 7 Peak expiratory flow (evening).
Fixed expiratory flow in one second (FEV1)
One study reported a modest change in FEV1 in litres (Rabe 2006). There was an improvement in FEV1 of 30 mL (MD 0.03 L; 95% CI 0.00 to 0.06).
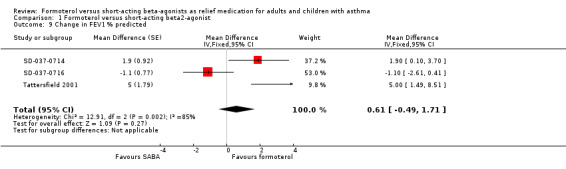
Three studies reported change in % predicted FEV1 (SD‐037‐0714; SD‐037‐0716; Tattersfield 2001). These studies favoured formoterol (MD 0.61%; 95% CI ‐0.49 to 1.71), but this difference was not statistically significant. There was a large amount of heterogeneity in this result (I2 = 85%).There was clinical heterogeneity in the baseline values which might explain the statistical heterogeneity observed; two studies had mean baseline FEV1 % predicted close to 100% (SD‐037‐0714; SD‐037‐0716), whilst Tattersfield 2001 had a lower mean FEV1 % predicted at baseline (74%).
Symptoms (day‐time)
Five studies provided information on symptoms (Rabe 2006; RELIEF 2003; SD‐037‐0714; SD‐037‐0716; Tattersfield 2001), but the unit of analysis varied. Rabe 2006 reported change from run‐in on an asthma symptom score scale from zero to six. Participants in Rabe 2006 on formoterol showed an improvement in symptoms of ‐0.58 and participants on terbutaline showed an improvement of ‐0.57 which did not result in a significant difference between the two treatments (MD 0.1; 95% CI ‐0.05 to 0.07) (Analysis 1.8). SD‐037‐0714, SD‐037‐0716 and Tattersfield 2001 reported symptom scores on a scale of zero to four, but there was no significant difference between scores.
1.8. Analysis.
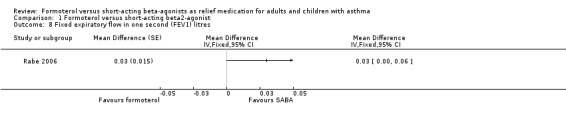
Comparison 1 Formoterol versus short‐acting beta2‐agonist, Outcome 8 Fixed expiratory flow in one second (FEV1) litres.
Nocturnal awakenings
Two studies reported nocturnal awakenings. Rabe 2006 reported no significant difference in the adjusted mean change from run‐in; patients on formoterol reported an improvement of ‐14.0% and patients on formoterol a ‐13.5% reduction in awakenings (MD ‐0.60; 95% CI ‐2.25 to 1.05). Tattersfield 2001 also reported no significant difference in nocturnal awakenings (MD 0.00, 95% CI ‐0.10 to 0.10).
Quality of life
Tattersfield 2001 reported data for quality of life, using the Asthma Quality of Life Questionnaire (AQLQ) measured on a scale of zero to seven. There was an improvement of 0.41 units in patients on formoterol as‐needed and 0.17 units in patients on terbutaline as‐needed which was a statistically significant difference (MD 0.24; 95% CI 0.09 to 0.39), but the minimally important difference to the individual is 0.5 units.
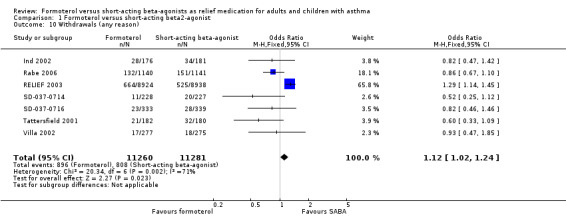
Withdrawals (any reason)
Seven trials provided data for the number of withdrawals (Ind 2002; Rabe 2006; RELIEF 2003; SD‐037‐0714; SD‐037‐0716; Tattersfield 2001; Villa 2002). The numbers of withdrawals varied between 4.8% and 13% per treatment arm across all the studies (Table 4). There were more withdrawals in patients on formoterol compared to short‐acting beta2‐agonists (OR fixed‐effect 1.12; 95% CI 1.02 to 1.24) and this was statistically significant. However, there is statistical heterogeneity present which merits further discussion (I2 = 71%).
This meta‐analysis is dominated by RELIEF 2003, a trial in which the majority of withdrawals were from patients on formoterol, in contrast to the other studies that have more withdrawals in patients on short‐acting beta2‐agonists. The population‐wide, open‐label design of RELIEF 2003 in comparison to the double‐blind nature of the other trails may account for part of this difference. Performing sensitivity analysis by removing trials at high risk of bias for this outcome, which in this case is RELIEF 2003, gives a statistically significant result in favour of short‐acting beta2‐agonists (OR 0.80; 95% CI 0.67 to 0.96).
The RELIEF 2003 trialists report a statistically significant treatment interaction for asthma‐related discontinuations by inhaler type. In the countries where both medications were delivered by dry powder inhalers, the numbers of discontinuations due to asthma‐related adverse events were balanced across both treatments (28 (1.1%) in patients on formoterol and 24 (1.0%) in patients on salbutamol, P = 0.61). However in countries where salbutamol was delivered by pressurised metered dose inhaler there were more discontinuations due to asthma‐related adverse events in patients on formoterol (61 (1%) compared to 25 (0.4%), P < 0.001). Discontinuation may therefore have been related to the change from metered dose inhaler to dry powder delivery for patients who used formoterol as a reliever in RELIEF 2003.
Discussion
There were eight included studies, of which two were reported only as an abstract. Participants in five of the studies were on a maintenance inhaled corticosteroid (one with maintenance formoterol plus and inhaled corticosteroid and one on a budesonide/formoterol combined inhaler) and patients in the largest trial were allowed to take any normal medication. The short‐acting beta2‐agonist was terbutaline in six trials and salbutamol in two. Most of the studies employed dry powder inhalers. All the studies apart from a large open‐label effectiveness study were double‐blind. Despite these differences we judged that it was possible to look at the major endpoints laid out in our protocol.
Summary of main results
Asthma exacerbations and serious adverse events showed a direction of treatment effect favouring formoterol, of which one primary outcome reached statistical significance (exacerbations requiring a course of oral corticosteroids). In patients on short‐acting beta2‐agonists, 117 people out of 1000 had exacerbations requiring oral corticosteroids over 30 weeks, compared to 101 (95% CI 93 to 108) out of 1000 for patients on formoterol as‐needed. There were fewer exacerbations requiring a course of oral corticosteroids in the subgroup of patients taking maintenance inhaled corticosteroids on formoterol as‐needed than those on short‐acting beta2‐agonists. Although study participants not on background inhaled steroids appeared to be at a greater risk of exacerbations than those on inhaled steroids (Analysis 2.1), the subgroup difference did not reach statistical significance. We remain uncertain as to the nature and strength of the relationship between concurrent inhaled steroid exposure and the risk of exacerbations requiring oral corticosteroids. There were few deaths in the studies and consequently there were wide confidence intervals around the risk of death. In the control group one person out of 1000 died over 30 weeks, compared to one (95% CI 1 to 3) out of 1000 for the active treatment group.
Overall completeness and applicability of evidence
There were few studies of formoterol versus short‐acting beta2‐agonists as‐needed conducted solely in children and a lack of separate paediatric data in other trials. Therefore in order to apply the results of this systematic review to children, one would have to assume that children have the same response to these drugs as adults. A review of maintenance formoterol in patients who were not taking maintenance inhaled corticosteroids showed an increase in adverse events in serious adverse events in children compared to adults (Cates 2008a). Our results therefore cannot be safely applied to children.
There were limited data for subgroups according to background maintenance inhaled corticosteroid or long‐acting beta2‐agonist therapy.
There was a broad range of asthma severities included in the trials and it was not possible to separate outcome data by asthma severity, so we cannot apply evidence in this review to populations with specific asthma severities.
Participants in three studies (Rabe 2006; Tattersfield 2001; Villa 2002) demonstrated reversibility to terbutaline, whereas participants were not tested for reversibility in four trials (Ind 2002; RELIEF 2003; SD‐037‐0714; SD‐037‐0716) and we are not sure whether or not reversibility was tested in Jain 2004. This might limit the applicability of our findings.
Quality of the evidence
Most of the studies we found were good quality trials in terms of randomisation and blinding, although Jain 2004 was reported as a single abstract the author did not provide more information. Removing this trial from the meta‐analysis did not markedly affect the results. Although there is a risk of detection bias from RELIEF 2003 being open‐label, particularly with respect to subjective outcomes, excluding it from the meta‐analyses did not actually change the direction or statistical significance of the pooled treatment effects. Its impact on the estimate of withdrawals was more substantial, with the direction of the result moving in favour of short‐acting beta2‐agonist.
The studies and also our systematic review were underpowered to detect a difference in mortality. Because of the low incidence of death in asthma clinical trials, an unfeasibly large trial would be required to demonstrate a difference in mortality (Rodrigo 2010; Wijesinghe 2009).
The studies employed different as‐needed medications, inhalers and background medication. In addition the major trial was open‐label in comparison to the other trials which were double‐blind. The considerable differences between the trials may make the combined results harder to interpret.
Summary of findings table
We downgraded evidence for the subjective outcomes (exacerbations requiring oral corticosteroids, asthma‐related serious adverse events and withdrawals) because we felt that these were subject to bias due to the large open‐label trial. Although this trial might be more like "real life", a double‐blind trial of the same size might change the results of the review. Exacerbations leading to hospitalisations was downgraded because the confidence interval included the possibility of no difference in treatment effect. Deaths were downgraded twice for imprecision due to the sparsity of events and the width of the confidence interval. Withdrawals was downgraded by an additional point because there was significant heterogeneity for this outcome.
Potential biases in the review process
The review process was protected from bias by following a pre‐published protocol. We minimised bias by assessing studies independently and resolving differences of opinion by discussion. Data extraction was also performed in duplicate. We consulted the manufacturer of formoterol and asked if they could identify other published or unpublished reports of their trials, and provide unpublished data and clarification of data that we calculated from available information. We only performed subgroup analyses that were specified a priori in the protocol.
Agreements and disagreements with other studies or reviews
Of current concern in asthma management is whether treatment with regular long‐acting beta2‐agonists such as formoterol masks deterioration in asthma due to non‐control of underlying inflammation with inhaled corticosteroids (Pavord 2009). The average dose used of formoterol as‐needed (8.5 to 17.5 μg) was of the order of the recommended maintenance formoterol dosage (12 μg twice daily). Patients on formoterol "as‐needed" may therefore be subject to increased risks of serious adverse events if they are not taking regular inhaled corticosteroids. It is not recommended to take formoterol without taking inhaled corticosteroids (BTS/SIGN 2008; Cates 2008a; Cates 2009b; FDA website).
Patients tend to increase their reliever therapies rather than their inhaled corticosteroids when their asthma worsens. Therefore, a more pertinent clinical question than whether formoterol as‐needed is better than short‐acting beta2‐agonists as‐needed, at least in high‐income countries, is whether single inhaler therapy is superior to separate inhalers. The studies described in this review were designed by the sponsors to demonstrate whether formoterol as a reliever is as safe and effective as short‐acting beta2‐agonists, and this allowed development of single inhaler therapy for the maintenance and relief of symptoms.
Authors' conclusions
Implications for practice.
In adults, formoterol was similar to short‐acting beta2‐agonists when used as a reliever and showed a reduction in the number of exacerbations requiring a course of oral corticosteroids. Clinicians should weigh the relatively modest benefits of formoterol as‐needed against the benefits of single inhaler therapy and the potential danger of long‐term use of long‐acting beta2‐agonists in some patients. We did not find evidence to recommend changes to guidelines that suggest that long‐acting beta2‐agonists should be given only to patients already taking inhaled corticosteroids.
There was insufficient information reported from children in the included trials to come to any conclusion on the safety or efficacy of formoterol as relief medication for children with asthma.
Implications for research.
Further research is required to clarify the safety and efficacy of formoterol as a reliever in children.
Assessing differences in mortality rates in a study comparing formoterol to short‐acting beta2‐agonists is hampered by the requirement for very large numbers of patients. A double‐blind trial of the same size as RELIEF 2003 may offer further, more reliable, information on the differences in efficacy in relation to adverse events and exacerbations although it is difficult to recommend that a trial of this nature should be conducted in patients who are not already receiving maintenance inhaled corticosteroids. It is also questionable whether there would be sufficient interest in the results of such a study, in view of the advent of maintenance and reliever therapy with combined inhaled corticosteroid and formoterol inhalers.
What's new
| Date | Event | Description |
|---|---|---|
| 11 April 2013 | Amended | NIHR acknowledgement inserted |
Acknowledgements
We are grateful to Susan Hansen for assistance with designing the search strategy. We also thank Joe Gray from AstraZeneca for providing data and information on studies and Toby Lasserson for editing our review and ensuring that our editing reflected peer review comments.
CRG Funding Acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Airways Group.
Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. Formoterol versus short‐acting beta2‐agonist.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patients with an exacerbation requiring hospitalisation | 7 | 22236 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.67, 1.04] |
| 2 Patients with an exacerbation requiring a course of oral corticosteroids | 6 | 21591 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.77, 0.91] |
| 3 Fatal serious adverse events (all‐cause) | 5 | 21629 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.51, 2.30] |
| 4 Patients with a serious adverse event (all‐cause) | 7 | 22538 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.81, 1.08] |
| 5 Patients with a serious adverse event (asthma‐related) | 6 | 21986 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.72, 1.15] |
| 6 Peak expiratory flow (morning) | 4 | Mean Difference (Fixed, 95% CI) | 3.88 [1.29, 6.46] | |
| 7 Peak expiratory flow (evening) | 4 | Mean Difference (Fixed, 95% CI) | 2.05 [‐0.50, 4.60] | |
| 8 Fixed expiratory flow in one second (FEV1) litres | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 9 Change in FEV1 % predicted | 3 | Mean Difference (Fixed, 95% CI) | 0.61 [‐0.49, 1.71] | |
| 10 Withdrawals (any reason) | 7 | 22541 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [1.02, 1.24] |
1.1. Analysis.
Comparison 1 Formoterol versus short‐acting beta2‐agonist, Outcome 1 Patients with an exacerbation requiring hospitalisation.
1.5. Analysis.
Comparison 1 Formoterol versus short‐acting beta2‐agonist, Outcome 5 Patients with a serious adverse event (asthma‐related).
1.9. Analysis.
Comparison 1 Formoterol versus short‐acting beta2‐agonist, Outcome 9 Change in FEV1 % predicted.
1.10. Analysis.
Comparison 1 Formoterol versus short‐acting beta2‐agonist, Outcome 10 Withdrawals (any reason).
Comparison 2. Formoterol versus short‐acting beta2‐agonist (background ICS use).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patients with an exacerbation requiring a course of oral corticosteroids | 4 | 3669 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.65, 0.94] |
| 1.1 Maintenance ICS | 3 | 2994 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.75 [0.62, 0.91] |
| 1.2 No maintenance ICS | 1 | 675 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.47 [0.70, 3.10] |
| 2 Patients with a serious adverse event (all‐cause) | 7 | 22538 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.94 [0.81, 1.08] |
| 2.1 Maintenance ICS | 6 | 17785 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.91 [0.78, 1.06] |
| 2.2 No maintenance ICS | 2 | 4753 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.14 [0.77, 1.69] |
Comparison 3. Formoterol versus short‐acting beta2‐agonists (background LABA use).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patients with a serious adverse event (all‐cause) | 6 | 21863 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.94 [0.81, 1.09] |
| 1.1 Maintenance LABA | 3 | 8319 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.68, 1.03] |
| 1.2 No maintenance LABA | 4 | 13544 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [0.86, 1.30] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ind 2002.
| Methods | Study design: randomised, double‐blind, parallel‐group Study duration: 12 weeks Number of study centres and location: 42 centres in 5 countries (the UK, Spain, Israel, Finland and Hungary) |
|
| Participants | N randomised (males): formoterol maintenance plus formoterol as‐needed 176 (67), formoterol maintenance plus terbutaline as‐needed 181 (76) Withdrawals: formoterol maintenance plus formoterol as‐needed 7 and formoterol maintenance plus terbutaline as‐needed 1 Age mean (range): 47 Asthma severity: patients had to have been stable on an adequate constant dose of ICS for > 4 weeks. Patients were also included if ≤ 10 mg per day of oral prednisolone or equivalent. Diagnostic criteria: ATS Baseline ICS use: formoterol maintenance plus formoterol as‐needed 1034 μg (200 to 2900), formoterol maintenance plus terbutaline as‐needed1030 μg (200 to 3200) Baseline lung function, FEV1 (% predicted): formoterol maintenance plus formoterol as‐needed 2.23 L (76%), formoterol maintenance plus terbutaline as‐needed 2.24 L (76%) Inclusion criteria: patients > 18 years with FEV1 > 50% predicted normal. Patients requiring 2 to 5 inhalations per day of as‐needed terbutaline during run‐in. Patients must have completed the run‐in according to protocol. Exclusion criteria: patients with significant cardiovascular disease, pregnant or breastfeeding women or patients with hypersensitivity to lactose or beta2‐agonists. Beta2‐agonist, anticholinergics, leukotriene receptor agonists, cromones or immunotherapy were not permitted. Patients who used > 8 inhalations during a single day during run‐in. |
|
| Interventions | Run‐in: 2 weeks on formoterol 9 μg twice a day and terbutaline Turbuhaler 0.5 mg as‐needed Intervention: formoterol 9 μg twice a day plus formoterol Turbuhaler 4.5 μg as‐needed Control: formoterol 9 μg twice a day plus terbutaline Turbuhaler 0.5 mg as‐needed Instructions provided for as‐needed therapy: "use as‐needed medication for either relief of asthma symptoms or prevention of bronchoconstriction (e.g. before exercise) and to appraise the effect of each inhalation before proceeding with as second" Average puffs per day used, mean (range): formoterol as‐needed 2.16 (0.0 to 6.3), terbutaline as‐needed 2.34 (0.1 to 7.5) Co‐medication: all on inhaled or oral corticosteroids at a constant dose |
|
| Outcomes | Primary outcomes: serum potassium levels, ECG, vital signs, lung function, adverse events Secondary outcomes: number of inhalations of as‐needed medication, severe asthma exacerbations, lung function, asthma symptoms Time points: attended clinic on 5 occasions with telephone calls to check on usage of reliever medication and adverse events between visits Definition of severe asthma exacerbation: either a requirement for oral glucocorticosteroids, either as judged by the investigator or following a drop in PEF on 2 consecutive days to < 70% of mean baseline value. Treated with 30 mg/day oral prednisolone for 10 days reducing dose by 5 mg/day over the next 5 days. Patients withdrawn after a second exacerbation. |
|
| Funding | AstraZeneca | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomised" |
| Allocation concealment (selection bias) | Low risk | From correspondence: "Patients received an enrolment code in consecutive order per centre at visit 1. Eligible patients... were allocated a randomised patient No. in consecutive order, per centre, at visit 2." |
| Blinding (performance bias and detection bias) Objective outcomes; hospitalisation, deaths, SAEs | Low risk | "Double blind". Both study drugs administered by identical inhalers. |
| Blinding (performance bias and detection bias) subjective outcomes; exacerbations requiring OCS, asthma‐related SAEs, withdrawal | Low risk | "Double blind" |
| Incomplete outcome data (attrition bias) | Unclear risk | The numbers of withdrawals in each treatment arm were not described adequately in the text |
| Selective reporting (reporting bias) | Low risk | Outcomes reported, although numerical data not given for PEF and FEV1 apart from a graph that no data could be obtained from |
| Other bias | Low risk | None noted |
Jain 2004.
| Methods | Study duration: 6 months | |
| Participants | N completed (males): formoterol 29, salbutamol 31 | |
| Interventions | Intervention: formoterol 4.5 μg as‐needed Control: salbutamol 100 μg as‐needed Instructions provided for as‐needed therapy: formoterol 1 puff as‐needed, salbutamol 2 puffs as‐needed Average puffs per day used, mean (range): |
|
| Outcomes | Time points: 30, 90 and 180 days | |
| Funding | — | |
| Notes | Completed diary card for 2 weeks prior to 3 data collection visits This study was reported as an abstract and we were not provided with further details on request and so the details reported here are limited. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" Comment: not stated, possibly done |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated, possibly done |
| Blinding (performance bias and detection bias) Objective outcomes; hospitalisation, deaths, SAEs | Unclear risk | Comment: not stated, possibly done |
| Blinding (performance bias and detection bias) subjective outcomes; exacerbations requiring OCS, asthma‐related SAEs, withdrawal | Unclear risk | Comment: not stated, possibly done |
| Incomplete outcome data (attrition bias) | Unclear risk | Withdrawals not stated |
| Selective reporting (reporting bias) | Unclear risk | "data were analysed for safety and efficacy variables" Comment: since we do not know what outcomes the trialists were planning to measure, we cannot assess this. It was not clear whether hospitalisations or courses of oral corticosteroids were per event or per individual. |
| Other bias | High risk | Publication bias. There was only a single abstract published on this trial |
Rabe 2006.
| Methods | Study design: randomised, double‐blind, parallel‐group, active‐controlled, phase IIIB Study duration: 2‐week run‐in plus 12‐month study Number of study centres and location: 289 centres from 20 countries (Belgium, Bulgaria, China, Czech Republic, Germany, Greece, Hungary, Indonesia, Italy, Malaysia, the Netherlands, Norway, the Philippines, Poland, Romania, Russia, Slovakia, South Africa, South Korea and Vietnam) Date of study: 10 April 2003 to 21 December 2004 |
|
| Participants | N randomised (males): budesonide/formoterol for maintenance plus formoterol as‐needed 1140 (458), budesonide/formoterol for maintenance plus terbutaline as‐needed 1141 (450) Withdrawals: budesonide/formoterol single inhaler plus formoterol as‐needed 132, budesonide/formoterol single inhaler plus terbutaline as‐needed 122 Age, mean (range): 42 (12 to 81) Asthma severity: moderate to severe asthma and documented symptoms despite use of ICS Diagnostic criteria: ATS Baseline ICS use: all on ICS. Budesonide/formoterol single inhaler plus formoterol 758 μg (320 to 1600), budesonide/formoterol single inhaler plus terbutaline 751 μg (250 to 1600) Baseline lung function, FEV1 [range] (% predicted): budesonide/formoterol single inhaler plus formoterol 2.20 L [0.74 to 4.58] (72%), budesonide/formoterol single inhaler plus terbutaline 2.16 L [0.68 to 4.58] (72%) Inclusion criteria: outpatients > 12 years, clinical diagnosis of asthma for ≥ 6 months with > 1 severe asthma exacerbation in the 12 months before entry. All patients used ICS for ≥ 3 months and at a constant dose for 4 weeks prior to study. FEV1 ≥ 50% predicted with ≥ 12% reversibility after inhalation of 1 mg terbutaline. Used reliever medication on 5 or more of the last 7 days of run‐in. Exclusion criteria: any respiratory infection affecting the patients asthma or use of OCS within 1 month of study entry |
|
| Interventions | Run‐in: 2 weeks. Symbicort (budesonide/formoterol) Turbuhaler 160/4.5 μg 1 inhalation twice a day as maintenance and terbutaline turbuhaler 0.5 mg per inhalation as‐needed Intervention: budesonide/formoterol Turbuhaler 160/4.5 μg 1 inhalation twice a day as maintenance and formoterol turbuhaler 4.5 μg as‐needed Control: budesonide/formoterol Turbuhaler 160/4.5 μg 1 inhalation twice a day as maintenance and terbutaline turbuhaler 0.4 mg per inhalation as‐needed Instructions provided for as‐needed therapy: "patients were instructed to use their reliever medication for asthma symptoms, but not prophylaxis. During treatment, patients were not allowed to use more than ten inhalations of reliever medication a day." Average puffs per day used, mean (range): formoterol as‐needed 1.90 (0.00 to 9.14), terbutaline as needed 1.91 (0.30 to 9.73) Co‐medication: participants stopped taking ICS at baseline and started taking budesonide/formoterol Definition of severe asthma exacerbation: deterioration in asthma resulting in emergency treatment or hospitalisation or the need for oral steroids for 3 days or more (as judged by the investigator) |
|
| Outcomes | Primary outcome: time to first severe asthma exacerbation (hospitalisation of ER/ED visit, course of OCS lasting at least 3 days as judged by the investigator). Days with OCS recorded. Secondary outcomes: number of severe and mild asthma exacerbations, number of hospitalisations/ED visits, intake of maintenance medication, FEV1, FVC, morning and evening PEF, asthma symptom score, inhalations of as‐needed medication, night awakenings due to asthma symptoms, as‐needed free days, time to first mild exacerbation, patient recorded outcomes and asthma control questionnaire, health economics resource utilisation and sick days. Percentage of asthma control days (24 hours with no symptoms, no intake of as‐needed medication and no night‐time awakening due to asthma). Safety variables were nature, incidence and severity of adverse events Time points: beginning and end of run‐in and after 1, 4, 8 and 12 months of study treatment |
|
| Funding | AstraZeneca | |
| Notes | There were 71 serious adverse events in 55 patients on formoterol compared to 83 events in 65 patients on terbutaline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation schedule computer generated at AstraZeneca Research and Development, Charnwood UK, by a person independent of the study team." |
| Allocation concealment (selection bias) | Low risk | "Within each study centre, eligible patients were sequentially assigned a randomisation code by the investigator from the computer generated list." |
| Blinding (performance bias and detection bias) Objective outcomes; hospitalisation, deaths, SAEs | Low risk | "as all needed study medication was given via identical turbuhalers, all matched in appearance." |
| Blinding (performance bias and detection bias) subjective outcomes; exacerbations requiring OCS, asthma‐related SAEs, withdrawal | Low risk | As above |
| Incomplete outcome data (attrition bias) | Low risk | Full analysis set included all randomised patients who provided data after randomisation. Reasons given for withdrawal similar across arms |
| Selective reporting (reporting bias) | Low risk | All the outcomes that we were interested in were reported |
| Other bias | Low risk | None noted |
RELIEF 2003.
| Methods | Study design: Multi‐national, multi‐centre, randomised, open, parallel‐group Study duration: 6 months Number of study centres and location: 1139 in 24 countries Date of study: 17 April 2000 to 24 June 2001 |
|
| Participants | N randomised (males): formoterol 8924 (3924), salbutamol 8938 (3798) Withdrawals: formoterol 664, salbutamol 525 Age, mean (range): 39 (4 to 91) Asthma severity: any allowed, defined by use of maintenance treatment at entry as intermittent (no maintenance treatment), mild (ICS < 500 μg per day or regular LABA, cromone, theophylline or leukotriene modifier), moderate (ICS alone ≥ 500 μg per day or ICS 500 to 800 μg per day in combination with LABA, theophylline or leukotriene modifier) and severe (ICS > 800 μg per day in combination with LABA, theophylline or leukotriene modifier, or oral corticosteroids). Intermittent: 16%, mild: 35%, moderate: 35%, severe: 15% Diagnostic criteria: judged by asthma medication levels, GINA Baseline ICS use: 76% using ICS. Mean usage at baseline 753 μg (formoterol group), 763 μg (salbutamol group) Baseline LABA use: 31% Baseline lung function, FEV1 (% predicted): not reported Inclusion criteria: ≥ 6 years, previous use of or candidates for beta2‐agonist reliever therapy Exclusion criteria: women who were pregnant, breast‐feeding or not using appropriate contraception. Patients with concomitant cardiovascular diseases were included at physicians' discretion. |
|
| Interventions | Run‐in: none Intervention: formoterol 4.5 μg, Turbuhaler DPI Control: salbutamol 200 μg delivered by Turbuhaler dry powder inhaler in 6 countries and by pressurised metered dose inhaler in 18 countries Instructions provided for as‐needed therapy: patients instructed to contact investigator if they used more that 12 puffs reliever medication in adults and 8 in children in 1 day, with lower limits for those on LABA Average puffs per day used, mean (range): not reported Co‐medication: any ordinary asthma maintenance medication, except other reliever medication was allowed and investigators could change the maintenance medication according to clinical judgement Definition of asthma exacerbation: any of: 1) increase in maintenance asthma medication, 2) course of ICS ≥ 5 days, 3) emergency treatment with nebulised beta2‐agonist or corticosteroid injection, 4) hospitalisation Definition of severe asthma exacerbation: any of: 1) course of ICS ≥ 5 days, 2) emergency treatment with nebulised beta2‐agonist or corticosteroid injection, 3) hospitalisation |
|
| Outcomes | Efficacy outcomes collected: primary efficacy variable was time to first asthma exacerbation. Secondary variables: change in concomitant maintenance asthma medication, number of inhalations of study drug, number of days with asthma symptoms, health care resource utilisation, days restricted activity. Safety outcomes collected: primary safety variables were asthma‐related and non‐asthma‐related serious adverse events and adverse events resulting in discontinuations Time points: 1, 3 and 6 months |
|
| Funding | AstraZeneca | |
| Notes | There were 305 serious adverse events in 278 patients on formoterol compared to 327 events in 299 patients on salbutamol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated code" |
| Allocation concealment (selection bias) | Low risk | "At entry, patients were randomised in chronological order at each site, according to a computer generated code and treatment communicated via code envelope" |
| Blinding (performance bias and detection bias) Objective outcomes; hospitalisation, deaths, SAEs | Low risk | "Open label" Comment: the study was open‐label, but knowing the assignment of medication is unlikely to make a difference when judging when a participant experienced death, hospitalisation or all‐cause serious adverse event |
| Blinding (performance bias and detection bias) subjective outcomes; exacerbations requiring OCS, asthma‐related SAEs, withdrawal | Unclear risk | Comment: because the study was open‐label, this may introduce bias when investigators were subjectively judging whether a serious adverse event was related to asthma or required a course or oral corticosteroids. Knowledge of the treatment drug might influence a patient's decision to withdraw from the study. |
| Incomplete outcome data (attrition bias) | Low risk | All analyses were performed on intention‐to‐treat population and there were few withdrawals |
| Selective reporting (reporting bias) | Low risk | The number of outcomes measured was kept to a minimum as RELIEF was a large study and they were all reported |
| Other bias | Low risk | None noted |
SD‐037‐0714.
| Methods | Study design: double‐blind, parallel‐group, non‐inferiority, therapeutic confirmatory Study duration: 12 months Number of study centres and location: 48 centres in Czech Republic, Portugal, Slovak Republic and South Africa Date of study: 22 December 2000 to 9 July 2002 |
|
| Participants | N randomised (males): formoterol 228 (113), terbutaline 227 (119) Withdrawals: formoterol 11, terbutaline 20 Age, mean (range): 25 (6 to 75) Asthma severity: mild Diagnostic criteria: GINA Baseline ICS use, mean (range): on a regular stable dose of ICS formoterol group 376 μg (200 to 900) daily, terbutaline 388 μg (200 to 800) daily Baseline lung function, FEV1 mean [range] (% predicted): formoterol 2.91 [1.12 to 5.38] (101%), terbutaline 2.92 [0.96 to 5.77] (100%) Inclusion criteria: Visit 1: ≥ 6 years old with a diagnosis of asthma (ATS). Baseline FEV1 ≥ 80% predicted normal. Stable inhaled steroid dose of ≥ 200 but ≤ 500 μg/day, nedocromyl or cromoglycate treatment for at least 4 weeks prior to enrolment. Visit 2: use of as‐needed medication drug between ≥ 3 inhalation occasions/week and ≤ 4 inhalations/day during the run‐in period. Exclusion criteria: Visit 1. Use of LABA 3 months prior. Use of a beta‐blocker including eye drops. Respiratory infection affecting the asthma within 4 weeks prior to enrolment, as judged by the investigator. Smoking history ≥10 pack‐years. Women who were pregnant, breastfeeding or not using an acceptable method of contraception. Visit 2. < 16 morning PEF values in the diary, any significant respiratory infection, change in prescribed asthma medication during run‐in. |
|
| Interventions | Run‐in: 3 weeks terbutaline turbuhaler 0.5 mg single‐blind Intervention: formoterol turbuhaler 4.5 μg Control: terbutaline sulfate turbuhaler 0.5 mg Co‐medication: ICS, not LABA Definition of severe asthma exacerbation: the need for oral corticosteroid course or hospitalisation due to asthma |
|
| Outcomes | Efficacy outcomes collected: primary variable: average morning PEF over the entire 12‐month period. Secondary variables: FEV1 pre‐ and post‐bronchodilator, evening PEF, day‐ and night‐time use of study medication, day‐ and night‐time asthma symptoms, time to first asthma exacerbation, provocative cumulative dose of metacholine giving a 20% fall in FEV1 (PD20). Safety outcomes collected: adverse events, clinical chemistry, haematology and urinalysis, ECG, systolic and diastolic blood pressure Time points: start and end of run‐in, at 1, 2, 4, 6, 8, 10 and 12 months plus telephone call between visits |
|
| Funding | AstraZeneca | |
| Notes | Full text: Chuchalin A, Kasl M, Bengtsson T, Nihlen U, Rosenborg J. Formoterol used as needed in patients with intermittent or mild persistent asthma. Respiratory medicine 2005;99(4):461‐70 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomised" Subjects stratified according to age (6 to 11, 12 to 17, ≥18 years) and a different randomisation list was used for each group |
| Allocation concealment (selection bias) | Low risk | From correspondence: patients "who fulfilled all the inclusion and none of the exclusion criteria were given a randomisation number at visit 2." The "randomisation number was allocated in sequential order. If a subject discontinued participation in the study, the number was not re‐used." |
| Blinding (performance bias and detection bias) Objective outcomes; hospitalisation, deaths, SAEs | Low risk | "double blind" From correspondence: "The study was double blind and all inhalers were identical in appearance. The treatment was not to be prematurely broken unless in an emergency situation when the appropriate management of the subject necessitated knowledge of the treatment allocation. Prior to breaking treatment codes, all decisions taken on data validation for each individual subject had to be documented." |
| Blinding (performance bias and detection bias) subjective outcomes; exacerbations requiring OCS, asthma‐related SAEs, withdrawal | Unclear risk | From correspondence: the drug safety department at AstraZeneca could break the treatment codes if serious adverse events were suspected to be causally related to the study medications, if expedited reporting to authorities was required or in exceptional circumstances for other safety reasons |
| Incomplete outcome data (attrition bias) | Low risk | The results were analysed on an intention‐to‐ treat basis. The withdrawals were balanced between arms and in line with other studies and reasons were provided. |
| Selective reporting (reporting bias) | Low risk | None noted |
| Other bias | Low risk | None noted |
SD‐037‐0716.
| Methods | Study design: randomised, double‐blind, multi‐centre, parallel‐group Study duration: 12 months Number of study centres and location: 54 centres in 8 countries (Estonia, Germany, Latvia, Lithuania, Poland, Russia, the United Kingdom and Ukraine) Date of study: February 2001 to June 2002 |
|
| Participants | N randomised (males): formoterol 333 (194), terbutaline 342 (208) Withdrawals: formoterol 23, terbutaline 28 Age, mean (range): formoterol 23 (6 to 73), terbutaline 24 (6 to 87) Asthma severity: intermittent Diagnostic criteria: ATS Baseline ICS use: not on ICS or LABA Baseline lung function, FEV1 mean [range] (% predicted): formoterol 3.11 L [0.98 to 5.56] (98%), terbutaline 3.15 L [1.14 to 6.80] (97%) Inclusion criteria: Visit 1. ≥ 6 years old with a diagnosis of asthma according to the ATS. Baseline FEV1 ≥ 80% predicted normal. Informed consent. Visit 2. Use of SABA on between 2 and 6 occasions during the last 2 weeks of the run‐in. Exclusion criteria: Visit 1. Use of ICS, other anti‐inflammatory treatment or LABA 3 months prior. Use of a beta‐blocker including eye drops. Respiratory infection affecting the asthma within 4 weeks prior to enrolment, as judged by the investigator. Smoking history ≥ 10 pack‐years. Use of unallowed medication. Women who were pregnant, breastfeeding or not using an acceptable method of contraception. Visit 2. < 16 morning PEF values in the diary, any significant respiratory infection, change in prescribed asthma medication during run‐in. |
|
| Interventions | Run‐in: 3 weeks on Bricanyl terbutaline Turbuhaler 0.5 mg as‐needed. Single‐blind. Intervention: Oxis formoterol Turbuhaler 4.5 μg as‐needed Control: Bricanyl terbutaline Turbuhaler 0.5 mg Co‐medication: not ICS or LABA Definition of severe asthma exacerbation: the need for oral corticosteroid course or hospitalisation due to asthma |
|
| Outcomes | Efficacy outcomes collected: primary variable: average morning PEF over the entire 12‐month period. Secondary variables: FEV1 pre‐ and post‐bronchodilator. Evening PEF, average daily number of inhalations of as‐needed, day‐ and night‐time asthma symptoms, time to first asthma exacerbation, provocative cumulative dose of metacholine giving a 20% fall in FEV1 (PD20). Safety outcomes collected: adverse events, clinical chemistry, haematology and urinalysis, ECG, systolic and diastolic blood pressure Time points: 1 screening visit, 1 at the end of run‐in and after 1, 2, 4, 6, 8, 10 and 12 months treatment. Subjects contacted by phone between visits to check adverse events and compliance. |
|
| Funding | AstraZeneca | |
| Notes | Full text: Chuchalin A, Kasl M, Bengtsson T, Nihlen U, Rosenborg J. Formoterol used as needed in patients with intermittent or mild persistent asthma. Respiratory medicine 2005;99(4):461‐70 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomised" Subjects stratified according to age (6 to 11, 12 to 17, ≥18 years) and a different randomisation list was used fro each group |
| Allocation concealment (selection bias) | Low risk | From correspondence: "At visit one all subjects received an enrolment code. The subjects who fulfilled all inclusion and none of the exclusion criteria were given a subject number at visit two. Both the enrolment and subject numbers were allocated in consecutive order. If a subject discontinued participation in the study, this number was not to be re‐used." |
| Blinding (performance bias and detection bias) Objective outcomes; hospitalisation, deaths, SAEs | Low risk | From correspondence: "The study was double blind. All inhalers were identical in appearance" |
| Blinding (performance bias and detection bias) subjective outcomes; exacerbations requiring OCS, asthma‐related SAEs, withdrawal | Unclear risk | The drug safety department at AstraZeneca could break the treatment codes if an serious adverse events were suspected to be causally related to the study medications, if expedited reporting to authorities was required or in exceptional circumstances for other safety reasons |
| Incomplete outcome data (attrition bias) | Low risk | The results were analysed on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | None noted |
| Other bias | Low risk | None noted |
Tattersfield 2001.
| Methods | Study design: double‐blind, randomised, parallel‐group Study duration: 12 weeks Number of study centres and location: 35 centres in 4 countries (Greece, the Netherlands, Norway and Sweden). |
|
| Participants | N randomised: formoterol 182, terbutaline 180 Withdrawals: 21 formoterol, 32 terbutaline Age, mean (range): 47 (18 to 75) Asthma severity: FEV1 > 50% predicted (mild‐moderate according to GOLD definition by FEV1) Baseline ICS use: formoterol 890 μg (200 to 2800), terbutaline 860 (100 to 2400) Baseline lung function, FEV1 mean [range] (% predicted): formoterol 2.36 L [1.13 to 4.30] (74%), terbutaline 2.27 L [1.00 to 4.65] (74%) Inclusion criteria: ≥ 18 years, asthma for 6 months or more and been treated with ICS for > 4 weeks (mean dose 870 μg daily). FEV1 > 50% predicted, and increase in FEV1 of ≥ 12% after inhalation of 1.5 mg terbutaline dry‐powder inhaler and used the relief terbutaline turbuhaler on average 3 to 8 times per day on at least 7 days in the run‐in period Exclusion criteria: patients who needed more than 12 inhalations per day of relief medication during the run‐in period. Patients with a serum potassium value outside the reference range. |
|
| Interventions | Run‐in: 2 weeks on terbutaline Turbuhaler Intervention: formoterol 4.5 μg (metered dose 6 μg) Control: terbutaline Turbuhaler 0.5 mg Instructions provided for as‐needed therapy: patients told to take medication only when needed. Patients taking more than 12 inhalations per day were withdrawn. Average puffs per day used, mean: formoterol as‐needed 3.92, terbutaline as‐needed 5.52 Co‐medication: Patients were all on ICS. Patients were not allowed to take any oral or inhaled beta2‐agonists during the study period apart from the study medication. Other asthma medications (xanthines, sodium cromoglycate, nedocromil, antihistamines and diuretics) were allowed provided that they were kept at a constant dosage throughout the study. Definition of severe asthma exacerbation: the need for oral corticosteroid course, as judged by investigator, or decreased PEF of more than 30% from baseline on 2 consecutive days. All severe exacerbations were treated with a 7‐day course of oral prednisolone. |
|
| Outcomes | Primary: time to first severe exacerbation Secondary outcome measures included: morning/evening PEF, FEV1, symptoms, number of relief medication and safety data, including serum potassium concentration and changes in electrocardiogram Time points: start of run‐in, start of treatment and after 4, 8 and 12 weeks of treatment. Contacted by telephone between the last 4 visits to check for adverse events and study drug consumption. |
|
| Funding | AstraZeneca | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to treatment groups in computer‐generated blocks. The randomisation sequence was generated by AstraZeneca research and Development, Lund." |
| Allocation concealment (selection bias) | Low risk | "Investigators assigned a number to each patient in order. The study drugs were sent to each centre’s pharmacy with a number allocated by randomisation before shipping." |
| Blinding (performance bias and detection bias) Objective outcomes; hospitalisation, deaths, SAEs | Low risk | "Investigators were unaware of study drug assignment throughout the study unless a SAE occurred." |
| Blinding (performance bias and detection bias) subjective outcomes; exacerbations requiring OCS, asthma‐related SAEs, withdrawal | Unclear risk | "double blind" |
| Incomplete outcome data (attrition bias) | Low risk | Analysed data on an intention‐to‐treat basis. Reasons for withdrawal provided, more withdrawals due to adverse events in the terbutaline group. |
| Selective reporting (reporting bias) | Low risk | None noted |
| Other bias | Unclear risk | None noted |
Villa 2002.
| Methods | Study design: randomised, double‐blind, parallel‐group, reference controlled study, phase IIIb Study duration: 6 months Number of study centres and location: 77 centres in 9 countries Date of study: 27 Jan 2000 to 26 March 2001 |
|
| Participants | N randomised (males): formoterol 277 (178), terbutaline 275 (180) Withdrawals: 17 formoterol, 18 terbutaline Age, mean (range): 11 (5 to 19). Stratified in to 2 age groups 6 to 11 and 12 to 17 years. Asthma severity: mild or moderate persistent. Stable on dose of anti‐inflammatory and more than one dose of as‐needed medication. Diagnostic criteria: Baseline ICS use, mean (range): patients on ICS at baseline, formoterol group 395 μg (50 to 1400) daily, terbutaline group 406 μg (100 to 1000) daily Baseline lung function, FEV1 [range] (% predicted): formoterol 1.94 L [0.75 to 4.12] (83%), terbutaline 1.86 L [0.77 to 3.92] (80%) Inclusion criteria: patients with bronchial asthma on ICS, disodium cromoglycate or nedocromil. Visit 1: reversibility in FEV1 (12% from baseline of 9% predicted), on a stable dose of anti‐inflammatory treatment and with a demonstrated need for ≤ 1 inhalation per day of SABA during run‐in. Visit 2: average need of > 1 inhalation of study medication during the last 14 days of run‐in and compliant with the electronic diary. Exclusion criteria: women who were pregnant, breastfeeding or not on acceptable contraceptives. Subjects who used > 8 inhalations of study medication on any single day, had more than 3 days with a missing value for number of inhalations or those who had a respiratory tract infection |
|
| Interventions | Run‐in: 3 weeks Intervention: formoterol Turbuhaler 4.5 μg as‐needed Control: terbutaline Turbuhaler 0.25 mg as‐needed Co‐medication: on ICS, disodium cromoglycate or nedocromil |
|
| Outcomes | Primary variable: time to first asthma exacerbation (mild or serious) Secondary: morning and evening PEF, number of inhalations of study medication, night‐time awakenings due to asthma, days avoiding activity due to asthma symptoms, restrictions in activity (all collected days in electronic diary), FEV1 and paediatric Quality of Life Questionnaire (PAQLQ(S)). Adverse events, ECG variables, pulse and blood pressure. Time points: 6 months |
|
| Funding | AstraZeneca | |
| Notes | There were 16 serious adverse events in 15 patients in patients on formoterol compared to 13 events in 11 patients on terbutaline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomised" From correspondence: "Randomisation lists were computer generated at AstraZeneca. Lund." Stratified according to age groups. |
| Allocation concealment (selection bias) | Low risk | From correspondence: "At visit 1, all subjects received an enrolment code. The subjects who met the inclusion criteria and none of the exclusion criteria were also given a randomisation number at visit 2. If a subject discontinued, that number was not re‐used." |
| Blinding (performance bias and detection bias) Objective outcomes; hospitalisation, deaths, SAEs | Low risk | "double‐blind" From correspondence: "The run‐in was single blind (blind to the subject) |
| Blinding (performance bias and detection bias) subjective outcomes; exacerbations requiring OCS, asthma‐related SAEs, withdrawal | Low risk | "double‐blind" |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for withdrawal not given, although the numbers of withdrawals were in line with those in other trials in this review and balanced between treatment arms |
| Selective reporting (reporting bias) | Unclear risk | Missing data in study report from AZ. PEF, number of inhalations, night‐time awakenings, days restricted activity, FEV1, quality of life, adverse events, ECG, blood pressure |
| Other bias | High risk | Publication bias. Just study report and 2 conference abstracts. No full paper. |
ATS: American Thoracic Society; ECG: electrocardiogram; ER/ED: emergency room/emergency department; FEV1: forced expiratory volume in one second; GINA: Global Initiative for Asthma; GOLD: Global Initiative for Chronic Obstructive Lung Disease; ICS: inhaled corticosteroid; LABA: long‐acting beta2‐agonist; OCS: oral corticosteroid; PEF: peak expiratory flow; SABA: short‐acting beta2‐agonist; SAE: serious adverse event
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bisgaard 2005 | STAY trial; budesonide/formoterol in a single inhaler as maintenance and reliever compared to same dose single inhaler as maintenance and terbutaline as reliever |
| Boskovska 2001 | Formoterol twice daily versus salbutamol as‐needed |
| Cheung 2006 | Cross‐over |
| Kesten 1991 | Randomised to formoterol or albuterol twice daily plus albuterol as‐needed |
| O'Connor 2000 | Cross‐over |
| Richter 2007 | Formoterol as maintenance versus formoterol as‐needed |
Differences between protocol and review
We did not perform subgroup analyses on the basis of age or asthma severity.
We did not ask trialists for separate data for adults and children.
Contributions of authors
EJW extracted information for the characteristics of included studies and CJC checked them. CJC and EJW independently extracted the data and entered data into RevMan. EJW drafted the review with input from CJC.
CJC and EJW co‐authored the protocol.
Sources of support
Internal sources
St George's University of London, UK.
External sources
-
NIHR, UK.
Funding for research time for CJC and EW
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Ind 2002 {published data only}
- Ind P, Borszormenyi Nagy G, Pietinalho A, Shiner R, Villasante C, Brander R, et al. Formoterol 4.5 microgram used as needed via turbuhaler was as safe and well tolerated as terbutaline 0.5 mg. European Respiratory Society, Oct 9‐13; Madrid, Spain. 1999:1086.
- Ind PW, Villasante C, Shiner RJ, Pietinalho A, Boszormenyi NG, Soliman S, et al. Safety of formoterol by Turbuhaler as reliever medication compared with terbutaline in moderate asthma. European Respiratory Journal 2002;20(4):859‐66. [DOI] [PubMed] [Google Scholar]
Jain 2004 {published data only}
- Jain A, Raghuram J. Randomized controlled study of the safety and efficacy of PRN formoterol compared to PRN albuterol for the management of asthma. American Thoracic Society 100th International Conference, May 21‐26, Orlando. 2004:B36 Poster G17.
Rabe 2006 {published data only}
- Rabe KF, Atienza T, Magyar P, Larsson P, Jorup C, Lalloo UG. Effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: a randomised controlled, double‐blind study. Lancet 2006;368(9537):744‐53. [DOI] [PubMed] [Google Scholar]
- SD‐039‐0734. Efficay of Symbicort® Turbuhaler® 160/4.5 μg as needed versus Oxis® 4.5 μg as needed and Bricanyl® 0/4 mg as needed in adults and adolescents with asthma receiving Symbicort® Turbuhaler® 160/4.5 μg twice daily as maintenance treatment. A 12‐month, randomised, double‐blind, parallel group, active‐controlled, phase IIIB, multi‐centre study. AstraZeneca Study Report.
RELIEF 2003 {published data only}
- SD‐037‐0699. AstraZeneca Study Report.
- Lindgren B, Sears MR, Campbell M, Villasante C, Huang S, Lindh A, et al. Cost‐effectiveness of formoterol and salbutamol as asthma reliever medication in Sweden and in Spain. International Journal of Clinical Practice 2005;59(1):62‐8. [DOI] [PubMed] [Google Scholar]
- Lindgren B, Sears MR, Campbell M, Villasante C, Huang S, Lindh A, et al. Total costs according to reliever use of formoterol turbuhaler in asthma: results from the RELIEF worldwide randomised effectiveness study, stratified by maintenance medication levels. European Respiratory Journal 2002;20(Suppl 38):43s. [Google Scholar]
- Lindgren B, Sears MR, Compbell M, Villasante C, Huang S, Lindh A, et al. Cost‐effectiveness of formoterol turbuhaler versus salbutamol as reliever therapy in asthma: results from the RELIEF worldwide randomised effectiveness study. European Respiratory Journal. 2002:P389, 43s.
- Pauwels RA, Campbell M, Villasante C, Huang S, Lindh A, Petermann W. Formoterol turbuhaler compared with salbutamol as reliever medication in asthma: outcomes from the RELIEF study in patients across different severities and age groups. European Respiratory Journal. 2002; Vol. 20(Suppl 38):P395, 45s.
- Pauwels RA, Campbell M, Villasante C, Huang S, Lindh A, Petermann W, et al. Formoterol turbuhaler compared with salbutamol as reliever medication in asthma: a worldwide, randomised, effectiveness trial (RELIEF Study). European Respiratory Society Annual Congress. 2002:P391.
- Pauwels RA, Compbell M, Villasante C, Huang S, Lindh A, Petermann W, et al. Formoterol turbuhaler compared with salbutamol as reliever medication in asthma: an exploratory analysis of the RELIEF study in patients using formoterol as maintenance therapy. European Respiratory Journal. 2002:P392.
- Pauwels RA, Sears MR, Campbell M, Villasante C, Huang S, Lindh A, et al. Formoterol as relief medication in asthma: a worldwide safety and effectiveness trial. European Respiratory Journal 2003;22(5):787‐94. [DOI] [PubMed] [Google Scholar]
- Sears MR, Pauwels RA, Campbell M, Villasante C, Huang S, Lindhl A, et al. Safety of formoterol turbuhaler when used as a reliever therapy in asthma (the RELIEF study). European Respiratory Society Annual Congress 2002. 2002; Vol. 20(Suppl 38):P393.
- Sears MR, Pauwels RA, Campbell M, Villasante C, Uang SH, Lindh A, et al. Safety of formoterol turbuhaler used as reliever in asthma: relationship with age and baseline treatment including regular long‐acting beta2‐agonists (the RELIEF study). European Respiratory Journal. 2002; Vol. 20(Suppl 38):P394, 44S.
SD‐037‐0714 {published data only}
- A 12‐month comparison of Oxis® (formoterol) Turbuhaler® and Bricanyl® (terbutaline) Turbuhaler® both used as needed in patients with asthma on anti‐inflammatory treatment. AstraZeneca Study Report Vol. SD‐037‐0714.
- Chuchalin A, Kasl M, Bengtsson T, Nihlen U, Rosenborg J. Formoterol used as needed in patients with intermittent or mild persistent asthma. Respiratory Medicine 2005;99(4):461‐70. [DOI] [PubMed] [Google Scholar]
SD‐037‐0716 {published data only}
- A 12‐month comparison of Oxis® (formoterol) Turbuhaler® and Bricanyl® (terbutaline) Turbuhaler® both used as needed in patients with asthma not using anti‐inflammatory treatment. AstraZeneca Study Report Vol. SD‐037‐0716.
- Chuchalin A, Kasl M, Bengtsson T, Nihlen U, Rosenborg J. Formoterol used as needed in patients with intermittent or mild persistent asthma. Respiratory Medicine 2005;99(4):461‐70. [DOI] [PubMed] [Google Scholar]
- Chuchalin A, Makarova I, Bergqvist P. As‐needed formoterol has an improved safety profile compared with as‐needed terbutaline in mild intermittent asthma. European Respiratory Journal. 2003; Vol. 22(Suppl 45):P1571.
Tattersfield 2001 {published data only}
- Berggren F, Ekstrom T. A cost‐effectiveness study comparing the as‐needed use of formoterol Oxis and terbutaline Bricanyl in patients with moderate to severe asthma. Respiratory Medicine 2001;95(9):753‐8. [DOI] [PubMed] [Google Scholar]
- Berggren F, Ekstrom T. Formoterol was more cost effective in a modelling study than terbutaline in as needed treatment of patients with moderate asthma. European Respiratory Society. 1999:P2293.
- Berggren F, Svenson K, Rolnick M S, Alexander M, Mintz S. Twice‐daily formoterol Oxis® turbuhaler is more cost‐effective than twice‐daily salmeterol or as‐needed salbutamol. American Thoracic Society Meeting. 2001.
- Stahl E, Postma DS, Svensson K, Tattersfield AE, Eivindson A, Schreurs A, et al. Formoterol used as needed improves health‐related quality of life in asthmatic patients uncontrolled with inhaled corticosteroids. Respiratory Medicine 2003;96(9):1061‐6. [DOI] [PubMed] [Google Scholar]
- Tattersfield AE, Lofdahl CG, Postma DS, Eivindson A, Schreurs AG, Rasidakis A, et al. Comparison of formoterol and terbutaline for as‐needed treatment of asthma: a randomised trial. Lancet 2001;357(9252):257‐61. [DOI] [PubMed] [Google Scholar]
Villa 2002 {published data only}
- SD‐037‐0695. AstraZeneca Study report 2001.
- Villa J, Kuna P, Egner J, Brader R. Safety of formoterol reliever therapy compared with terbutaline in asthmatic children taking anti‐inflammatory therapy. European Respiratory Society Annual Congress. 2002:P2739.
- Villa J, Kuna P, Egner J, Brander R. A 6‐month comparison of the safety profiles of formoterol (Oxis® turbuhaler®) as needed and terbutaline (Bricanyl® turbuhaler®) as needed in asthmatic children on anti‐inflammatory medication. American Journal of Respiratory and Critical Care Medicine 2002;165(8 Suppl):A746. [Google Scholar]
References to studies excluded from this review
Bisgaard 2005 {published data only}
- Bisgaard H, Hultquist C. Efficacy and safety of budesonide/formoterol (Symbicort) Turbuhaler®as Single Therapy in patients with mild‐moderate asthma. Comparison with Symbicort Turbuhaler and Pulmicort® Turbuhaler as maintenance therapy, both complemented with Bricanyl® Turbuhaler (STAY). AstraZeneca Study Report 2005, issue SD‐039‐0673.
Boskovska 2001 {published data only}
- Boskovska MI, Gerovski BD, Dimitrovski TM, Arbutina SD, Jovkovsa Kaeva BM. Efficacy of long‐term formoterol therapy vs as‐needed salbutamol use in patients with moderate asthma. European Respiratory Journal 2001;18(Suppl 33):426s. [Google Scholar]
Cheung 2006 {published data only}
- Cheung D, Klink HCJ, Aalbers R. Improved lung function and symptom control with formoterol on demand in asthma. European Respiratory Journal 2006;27(3):504‐10. [DOI] [PubMed] [Google Scholar]
- Cheung D, Klink RCJ, Aalbers R. Improved asthma control with formoterol "as needed" compared to salbutamol in patients with asthma. American Thoracic Society 99th International Conference. 2003:B036 Poster H73.
- Klink HCJ, Cheung D, Aalbers R. Formoterol as relief medication is increasingly more effective than salbutamol in patients with increasing need for reliever therapy. European Respiratory Journal 2003;22(Suppl 45):P1572. [Google Scholar]
Kesten 1991 {published data only}
O'Connor 2000 {published data only}
- O'Connor B, McSorley L, Turbitt M. Does treatment with eformoterol turbohaler® prn allow a reduction in the number of inhalers used to treat moderate to severe asthma? [Abstract]. American Journal of Respiratory and Critical Care Medicine 2000;161(Suppl 3):A191. [Google Scholar]
- O'Connor B, McSorley LC, Turbitt ML. Use of formeterol (Oxis®) Turbuhaler ® as needed in moderate to severe asthma reduces the number of inhalers used whilst maintaining effectiveness. American Journal of Respiratory and Critical Care Medicine. 2001; Vol. 107(2):S108.
Richter 2007 {published data only}
- Richter K, Hartmann U, Metzenauer P, Magnussen H. Randomised trial comparing as‐needed versus regular treatment with formoterol in patients with persistent asthma. Respiratory Medicine 2007;101(3):467‐75. [DOI] [PubMed] [Google Scholar]
Additional references
Adams 2008
- Adams NP, Bestall JC, Lasserson TJ, Jones PW, Cates CJ. Fluticasone versus placebo for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003135.pub4] [DOI] [PubMed] [Google Scholar]
AstraZeneca Briefing Document
- AstraZeneca Briefing Materials: Review of the Benefits and Risks of Formoterol‐containing Products. www.fda.gov/ohrms/dockets/ac/08/briefing/2008‐4398b1‐03‐AstraZeneca.pdf 3 November 2008.
Barnes 2007
- Barnes PJ. Using a combination inhaler (budesonide plus formoterol) as rescue therapy improves asthma control. BMJ 2007; Vol. 335, issue 7618:513‐17. [DOI] [PMC free article] [PubMed]
Bisgaard 2003
- Bisgaard H. Effect of long‐acting beta2 agonists on exacerbation rates of asthma in children. Pediatric Pulmonology 2003;36(5):391‐8. [DOI] [PubMed] [Google Scholar]
BNF
- British National Formulary 59. http://bnf.org/bnf/bnf/current/index.htm (accessed 14 May 2010).
BTS/SIGN 2008
- British Thoracic Society. British Guidelines on Asthma Management. Thorax 2008; Vol. 63, issue Suppl 1.
Cates 2008
- Cates CJ, Cates MJ. Regular treatment with salmeterol for chronic asthma: serious adverse events (Cochrane Review). Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD006363.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cates 2008a
- Cates CJ, Cates MJ, Lasserson TJ. Regular treatment with formoterol for chronic asthma: serious adverse events. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD006923.pub2] [DOI] [PubMed] [Google Scholar]
Cates 2009
- Cates CJ, Lasserson TJ. Combination formoterol and inhaled steroid versus beta2‐agonist as relief medication for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD007085.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cates 2009a
- Cates CJ, Lasserson TJ. Combination formoterol and budesonide as maintenance and reliever therapy versus inhaled steroid maintenance for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007313.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cates 2009b
- Cates CJ, Lasserson TJ, Jaeschke R. Regular treatment with formoterol and inhaled steroids for chronic asthma: serious adverse events. Cochrane Database of Systematic Reviews 2009, Issue 2. [10.1002/14651858. CD006924.pub2] [DOI] [PubMed] [Google Scholar]
Delea 2008
FDA website
- Information for Healthcare Professionals ‐ Formoterol fumarate (marketed as Foradil). http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm162677.htm (accessed 11 May 2010).
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008] Available from: www.cochrane‐handbook.org. The Cochrane Collaboration, 2008. [Google Scholar]
Lipworth 2007
- Lipworth BJ, Jackson C. A SMART choice for primary care asthma therapy?. http://www.bmj.com/cgi/eletters/335/7618/513#178078 13 October 2007.
Lötvall 2008
- Lötvall Jan, Ankerst Jaro. Long duration of airway but not systemic effects of inhaled formoterol in asthmatic patients. Respiratory Medicine 2008;102(3):449‐56. [DOI] [PubMed] [Google Scholar]
Miranda 2003
- Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. Journal of Allergy and Clinical Immunology 2003;113(1):101‐8. [DOI] [PubMed] [Google Scholar]
Palmqvist 2001
- Palmqvist M, Arvidsson P, Beckman O, Peterson S, Lotvall J. Onset of bronchodilation of budesonide/formoterol vs. salmeterol/fluticasone in single inhalers. Pulmonary Pharmacology & Therapeutics 2001;14(1):29‐34. [DOI] [PubMed] [Google Scholar]
Pavord 2009
- Pavord ID, Jeffery PK, Qiu Y, Zhu J, Parker D, Carlsheimer A, et al. Airway inflammation in patients with asthma with high‐fixed or low‐fixed plus as‐needed budesonide/formoterol. Journal of Allergy and Clinical Immunology. 2009;123(5):1083‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Rodrigo 2010
- Rodrigo Gustavo J. Increased risk of asthma death with salmeterol monotherapy compared with placebo, but not with salmeterol plus inhaled corticosteroids compared with inhaled corticosteroids alone. Evidence Based Medicine 2010;15(2):37‐8. [DOI] [PubMed] [Google Scholar]
Sovani 2008
- Sovani MP, Whale CI, Oborne J, Cooper S, Mortimer K, Ekström T, et al. Poor adherence with inhaled corticosteroids for asthma: can using a single inhaler containing budesonide and formoterol help?. British Journal of General Practice 2008;58(546):37‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walters 2007
- Walters EH, Gibson PG, Lasserson TJ, Walters JAE. Long‐acting beta2‐agonists for chronic asthma in adults and children where background therapy contains varied or no inhaled corticosteroid. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD001385.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wijesinghe 2009
- Wijesinghe M, Weatherall M, Perrin K, Harwood M, Beasley R. Risk of mortality associated with formoterol: a systematic review and meta‐analysis. Postgraduate Medical Journal 2009;34(4):803‐11. [DOI] [PubMed] [Google Scholar]


