Abstract
Background
There is increasing concern about the appropriateness of intensive medical care near the end of life in ICUs throughout the United States. As a result of hospice expansion in the 1990s, we hypothesized that ICU use decreased over time in older adults with advanced lung cancer.
Methods
Retrospective analysis using the linked Surveillance, Epidemiology and End Results Medicare database. There were 45,627 Medicare beneficiaries > 66 years of age with confirmed stage IIIB or IV lung cancer between January 1, 1992, and December 31, 2002, who died within a year of their cancer diagnosis from 1993 through 2002.
Results
ICU use in the last 6 months of life increased from 17.5% in 1993 to 24.7% in 2002 (p < 0.001). After adjusting for patient characteristics, there was a 6.6% annual increase in ICU use from 1993 to 2002. During the same period, hospice use had risen from 28.8 to 49.9% (p < 0.001). A total of 6.2% of patients received both end-of-life ICU care and hospice care, a percentage that increased over time. The total health-care cost for Medicare fee-for-service patients during last 6 months was $40,929 for ICU users and $27,160 for non-ICU users (p < 0.001).
Conclusion
Despite increasing hospice use, ICU utilization among older adults dying with advanced lung cancer continued to rise in the United States during the 1990s.
Keywords: end-of-life care, hospice use, ICU care, lung cancer, older adults, utilization
Lung cancer is the leading cause of cancer-related death in the United States. It is predominantly a disease of older adults, with a median age of 68 years at diagnosis.1 The best treatment for early stage non-small cell lung cancer is surgical resection. Unfortunately, most patients present with advanced disease (stage IIIB or IV), for which treatment options are limited. Despite technological and chemotherapeutic advances, the overall 5-year relative survival rate for advanced lung cancer patients is < 5%.2
Over the past 2 decades, there has been growing concern about care near the end of life in individuals with advanced cancer and other terminal illnesses.3 Barnato et al4 showed that the proportion of fee-for-service Medicare beneficiaries with one or more ICU admissions in the last year of life increased from 30.5% in 1985 to 35% in 1999. One in five to one in eight decedents received ICU care during a terminal hospitalization; end-of-life ICU use increased with increasing age and comorbidity.5–7 This terminal ICU admission consumed 80% of all terminal hospitalization costs.5
In particular, it has been suggested3,8 that ICU use and mechanical ventilation may be ineffective in this setting. The in-hospital mortality rate, especially in mechanically ventilated lung cancer patients, has been reported9 –12 to be as high as 75% in patients with advanced lung cancer. The financial cost, emotional burden, and failed expectations of this scenario can exact a substantial toll from patients, family members, and society.
An increasing number of health-care alternatives, such as home hospice, have become available for patients with advanced lung cancer. These aim at providing less aggressive but more patient-centered care during the last months of life. Moreover, among Medicare beneficiaries, enrolling lung cancer patients in hospice has been shown to provide the largest cost savings for any terminal illness.13,14 It is unclear, however, how increased hospice enrollment may have affected patterns of ICU use near the end of life in such lung cancer patients.
This study examines time trends in ICU utilization in the last 6 months of life in individuals in whom advanced lung cancer had been diagnosed. We hypothesized that, with the growth of the hospice movement, end-of-life ICU utilization would decrease over time.
Materials and Methods
Data Source
This is a retrospective study of lung cancer patients identified from the linked Surveillance, Epidemiology and End Results (SEER)-Medicare database for the years from 1992 to 2002.15 Over this period, the SEER program supported population-based tumor registries in the following 12 geographic regions: Connecticut; Hawaii; Utah; New Mexico; California; Kentucky; New Jersey; Louisiana; Iowa; Detroit, MI; Seattle, WA; and Atlanta, GA. For all incident cancers that are diagnosed in these areas, the SEER registries collect information on patient demographics, tumor characteristics, stage at diagnosis, date of diagnosis, therapy received within 4 months of diagnosis, and date and cause of death.
Through a collaborative project between the National Cancer Institute (NCI) and the Centers for Medicare and Medicaid Services (CMS), entitlement information and claims data from the Medicare program were linked to the SEER data for cancer patients ≥ 65 years of age.16 Medicare eligibility could be identified for 94% of persons ≥ 65 years of age appearing in the SEER records.
The following data from multiple files were used for this study: (1) the Patient Entitlement and Diagnosis File (SEER registry data and Medicare entitlement information); (2) four Medicare claims files (the Medicare Provider Analysis and Review file [for hospital inpatient and skilled nursing facility stays], the Outpatient Standard Analytic File [for hospital outpatient services], the Hospice Standard Analytic File, and the 100% Physician/Supplier File [for physician and other medical services]); and (3) a hospital file created by the NCI with information on hospital characteristics from the CMS Provider of Service survey and the Health-care Cost Report.
Study Cohort
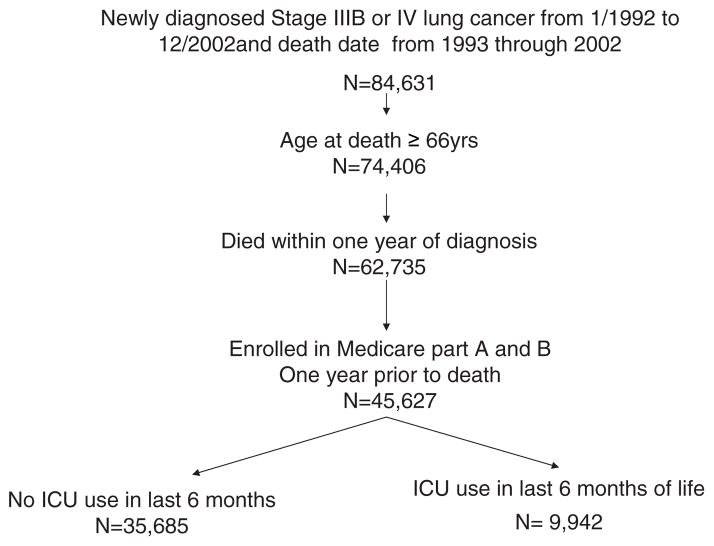
Eligible subjects were selected from the Patient Entitlement and Diagnosis File and included the following patients: (1) those in whom stage III B or stage IV lung cancer had been diagnosed from 1992 to 2002; (2) those ≥ 66 years of age at the time of diagnosis; (3) those who died within 1 year of receiving a diagnosis over the period 1993 to 2002; and (4) those who were enrolled in Medicare parts A and B during the year prior to death (Fig 1). The stage of lung cancer was ascertained from the SEER registry data through the modified American Joint Committee on Cancer. Individuals enrolled in health maintenance organizations (HMOs) at any time from the date of diagnosis through the date of death were excluded from the study because of concerns about the completeness of information in the Medicare files of these patients.
Figure 1.
Establishment of a study cohort of patients in whom stage IIIB or IV lung cancer was diagnosed from 1992 to 2002, and who died within 1 year of diagnosis between 1993 and 2002.
Measures
Information on patients’ sociodemographic characteristics was obtained from the SEER data, as follows: age (66 to 74, 75 to 84, and ≥ 85 years of age); race (non-Hispanic white, black, His-panic, and other); gender (male or female); and marital status at the time of diagnosis (married or not married). Tumor stage, vital status, cause of death, and geographic region were also derived from SEER data. Residence was dichotomized into large metropolitan area vs other.17 A large metropolitan area was defined as a concentrated urban area with an average population of > 1 million people based on the 1990 census. Socioeconomic status was based on whether the patient had applied for and received supplemental insurance from Medicaid (ie, the state buy-in coverage). One month or more of having state buy-in coverage was considered to be an indicator of low socioeconomic income status. Comorbidity was measured with a score developed by Klabunde18 using all Medicare claims from the year prior to diagnosis.
The primary outcome was ICU use in the last 6 months of life and was ascertained from inpatient hospital claims in the Medicare Provider Analysis and Review file. Subjects were divided into the following two groups: ICU users, who were those patients with at least one ICU hospital claim in the last 6 months of life in the year after the diagnosis of lung cancer; and non-ICU users, who were those patients who did not have an ICU claim in the last 6 months of life. Likewise, subjects were considered to be hospice users if they had a hospice claim in the Standard Analytic File in the last 6 months of life. Mechanical ventilation during an ICU stay was determined from International Classification of Diseases, 9th edition-clinical modification (ICD-9-CM), procedure codes 9670, 9671, and 9672.
Principal diagnoses among ICU users were obtained from diagnosis-related hospital billing (ICD-9-CM) codes. Pneumonia (ICD-9-CM codes 486, 482.3, 482.1, 507.0, 482.3, 482.4, 482.0, and 481); COPD (ICD-9-CM code 496); renal failure/electrolyte abnormalities (ICD-9-CM codes 101.1 and 584.9); GI (ICD-9-CM codes 127, 569.83, and 531.0); deep vein thrombosis/pulmonary embolism (ICD-9-CM codes 453.8 and 415.19); coronary artery disease (ICD-9-CM codes 187, 410.11, 410.91, 427.5, and 410.41); neurologic (ICD-9-CM codes 436, 431, and 434.91); malignancy and/or metastatic disease (ICD-9-CM codes 162.9, 162.3, 162.8, 162.5, 186.7, 121.0, 197.0, 198.5, and 192.7); congestive heart failure (ICD-9-CM codes 428.0 and 497); atrial fibrillation/flutter (ICD-9-CM codes 427.31 and 427.32); sepsis (ICD-9-CM codes 0389, 599.0, 0384.2, 0383, 0384.9, and 0381); pathologic fracture (ICD-9-CM codes 733.15, 820.09, and 733.13); pneumothorax (ICD-9-CM code 512.1); pericardial diseases (ICD-9-CM code 423.9); pleural diseases (ICD-9-CM codes 511.8 and 510.9); hematologic causes (ICD-9-CM code 284.8); and other. For the purpose of this study, we defined potentially irreversible diagnoses as those diagnosis-related groups (DRGs) related to malignancy and/or metastatic disease, and all other DRGs were considered as potentially reversible conditions.
We calculated hospital expenditures using Medicare DRG reimbursement plus a per diem charge, as listed in each hospital claim. The total cost during the last 6 months includes the hospital cost, facility charges and the physician reimbursement by Medicare.
Hospitals were dichotomized into teaching or nonteaching hospitals. Teaching hospitals were defined as hospitals with a major medical school affiliation. Medical school affiliation was ascertained from the Provider of Service data in the NCI hospital file. For ICU users, we used the affiliation of the hospital associated with their last ICU claim. For non-ICU users, affiliation was based on the last hospital discharge within 6 months of death. The research proposal was approved by the representatives from the NCI and SEER.
Statistical Analysis
The likelihood ratio χ2 statistic was used to compare rates of ICU use by subject characteristics. Changes in the rates of ICU and hospice use over time (ie, year of diagnosis) were initially evaluated with the Cochran-Armitage trend test. Multivariate logistic regression analysis was used to assess whether changes in ICU use over time varied by subject and hospital characteristics. All statistical analyses were performed using a statistical software package (SAS, version 9.1; SAS Institute Inc; Cary, NC).
Results
Table 1 describes the baseline characteristics of eligible subjects age ≥66 years of age and the percentage of subjects who had received ICU care in the last 6 months of life. Among 45,627 patients with stage IIIB or IV lung cancer who died between January 1993 and December 2002, 9,942 patients (21.8%) had received ICU care in the last 6 months of life. ICU use was more common among individuals < 85 years old and those with less advanced disease (ie, stage IIIB disease). Nonwhite subjects, married subjects, and subjects with more comorbid illnesses were more likely to receive ICU care in the last 6 months of life. ICU use did not differ substantially by gender. There was a large variation in ICU use by SEER site. Patients residing in large metropolitan areas or receiving care at teaching hospitals were more likely to have ICU care in the last 6 months of life.
Table 1.
Baseline Characteristics of Subjects With Advanced Lung Cancer Who Died Between 1993 and 2002 and the Percentage of Those Who Received ICU Care in Last 6 Months of Life by Subject Characteristics*
| Variables | Patients, No. | Received ICU Care, % | p Value |
|---|---|---|---|
| Overall cohort | 45,627 | 21.8 | |
| Age at diagnosis | |||
| 66–74 yr | 21,545 | 23.5 | |
| 75–84 yr | 19,246 | 21.1 | |
| ≥85 yr | 4,836 | 17.1 | < 0.001 |
| Gender | |||
| Male | 26,021 | 21.9 | |
| Female | 19,606 | 21.7 | 0.58 |
| Race | |||
| Non-Hispanic white | 38,004 | 20.9 | |
| Black | 4,088 | 25.6 | |
| Hispanic | 1,513 | 28.2 | |
| Other | 2,022 | 26.5 | < 0.001 |
| Married | |||
| Yes | 22,534 | 22.5 | < 0.001 |
| Others | 23,093 | 21.1 | |
| SEER site | |||
| Atlanta, GA | 2,130 | 17.4 | |
| Connecticut | 4,443 | 17.3 | |
| Detroit | 6,285 | 25.6 | |
| Hawaii | 916 | 17.9 | |
| Iowa | 4,907 | 13.4 | |
| Kentucky | 2,325 | 22.3 | |
| Louisiana | 1,877 | 20.8 | |
| New Mexico | 1,294 | 22.9 | |
| New Jersey | 3,431 | 27.8 | |
| Seattle | 3,921 | 15.5 | |
| Utah | 964 | 14.7 | |
| California | 13,124 | 26.4 | < 0.001 |
| Low socioeconomic status | |||
| No | 35,549 | 20.9 | |
| Yes | 8,078 | 25.9 | < 0.001 |
| Comorbidity score | |||
| 0 | 14,336 | 14.0 | |
| 1 | 14,741 | 21.1 | |
| ≥ 2 | 16,550 | 29.9 | < 0.001 |
| Diagnosis year | |||
| 1992 | 1,014 | 16.7 | |
| 1993 | 3,513 | 17.4 | |
| 1994 | 3,463 | 18.8 | |
| 1995 | 3,490 | 18.5 | |
| 1996 | 3,439 | 19.6 | |
| 1997 | 3,331 | 21.2 | |
| 1998 | 3,368 | 19.9 | |
| 1999 | 3,230 | 22.8 | < 0.001 |
| 2000 | 7,316 | 23.4 | |
| 2001 | 7,764 | 24.7 | |
| 2002 | 5,697 | 25.4 | |
| Teaching hospital† | |||
| Yes | 9,398 | 28.6 | |
| No | 27,284 | 26.6 | < 0.001 |
| Cause of death | |||
| Lung cancer | 38,555 | 20.6 | < 0.001 |
| Other | 7,072 | 28.1 | |
| AJCC stage | |||
| IIIB | 14,131 | 26.1 | |
| IV | 31,496 | 19.9 | < 0.001 |
| Residence | |||
| Large metropolitan area | 26,851 | 24.9 | < 0.001 |
| Others | 18,776 | 17.2 | |
AJCC = American Joint Committee on Cancer.
A total of 8,945 subjects were not hospitalized in the last 6 months of life.
Among ICU users, 2,484 patients (25%) received mechanical ventilation during the ICU stay. The median and mean days of ICU use were 4.0 and 5.9 days, respectively. The majority of subjects (87%) received ICU care only once during the last 6 months of life.
Figure 2 outlines the subsequent clinical trajectory for the 9,942 patients with advanced lung cancer who were admitted to an ICU. Approximately two fifths of the patients died during that hospitalization, and three fifths were discharged from the hospital alive. The median survival time for the patients discharged from the hospital alive was 33 days. Of those discharged from the hospital alive, 43.2% were discharged to an institutional setting and 56.8% were discharged to home. The median survival time for those discharged from the hospital to home was 50 days. Approximately half of those discharged from the hospital to home were rehospitalized, and one quarter were subsequently in a nursing home or related institution. Of the 5,841 patients discharged from the hospital alive after an ICU stay, 48.7% were enrolled in hospice prior to death.
Figure 2.
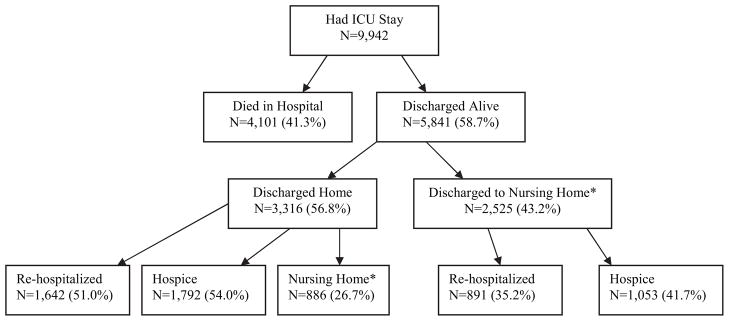
The clinical trajectory of patients admitted to an ICU with advanced lung cancer. The categories of re-hospitalized, hospice, and nursing home are not mutually exclusive.
Some 38.1% of ICU users had three or more hospitalizations in the last 6 months of life compared to 26.1% of non-ICU users (p < 0.0001). The mean (± SD) total health-care cost reimbursed by Medicare in the last 6 months of life for ICU users was $40,929 ± $30,854 compared to $27,160 ± $20,089 for non-ICU users (p < 0.001). The mean hospital costs alone for ICU users and non-ICU users were $25,929 ± $23,576 and $12,133 ± $11,349, respectively (p < 0.001). Among ICU users, there was no difference in the total cost between those who died and those who were discharged from the hospital alive.
Table 2 shows the distribution of primary diagnoses for patients receiving ICU care. The most common diagnoses were related to cancer. We also examined the trajectory of care after ICU use, depending on whether the primary diagnosis for the hospitalization suggested a potentially reversible cause (eg, pneumonia, deep vein thrombosis, pulmonary embolism, or congestive heart failure) for the ICU stay or a potentially irreversible cause (eg, lung cancer). There were no meaningful differences in the place of hospital discharge between the groups of patients with potentially reversible and potentially irreversible disease. The median survival times were also similar (potentially reversible disease group, 33 days; nonreversible disease group, 34 days). The rates of hospice use and rehospitalization were also similar.
Table 2.
Distribution of Primary Diagnosis for ICU Admissions (n = 9,942)
| Primary Diagnosis | No. (%) |
|---|---|
| Malignancy* | 5,059 (50.8) |
| Respiratory failure | 599 (6) |
| COPD | 164 (1.6) |
| Pneumonia | 794 (7.9) |
| Arrhythmias | 306 (3) |
| Sepsis | 292 (2.9) |
| Coronary artery disease | 253 (2.5) |
| Congestive heart failure | 215 (2.2) |
| GI† | 193 (1.9) |
| Venous thromboembolism | 160 (1.6) |
| Renal/electrolyte abnormalities | 140 (1.4) |
| Neurologic‡ | 131 (1.3) |
| Pathologic fracture | 120 (1.2) |
| Pericardial diseases | 72 (0.7) |
| Pleural effusion | 60 (0.6) |
| Pneumothorax | 55 (0.5) |
| Hematologic | 81 (0.8) |
| Others | 1,248 (12.4) |
Includes patients with lung cancer and/or metastatic disease.
Includes patients with GI bleeding and diverticulitis.
Includes patients with cerebrovascular accident and intracranial hemorrhage.
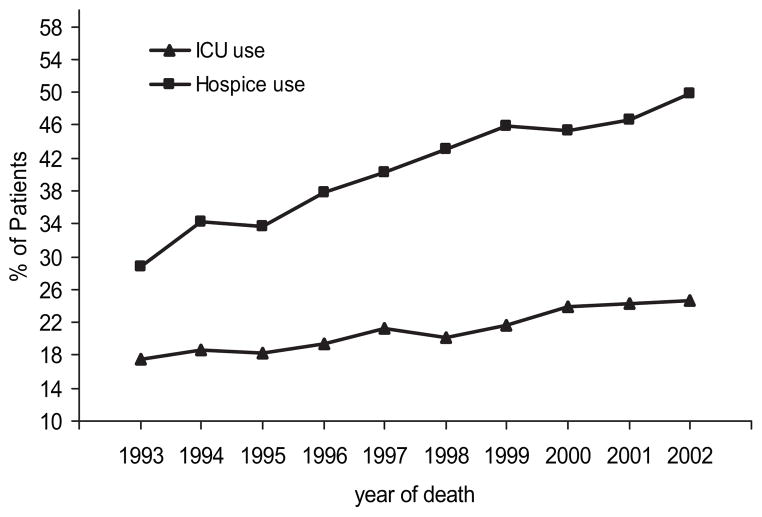
ICU use in the last 6 months of life increased from 17.5% in 1993 to 24.7% in 2002 (p < 0.001) [Fig 3]. There were no significant changes in mean or median length of stay in the ICU among ICU users over time. The percentage of advanced-stage lung cancer patients who were enrolled in hospice prior to death can also be seen in Figure 3. Hospice enrollment increased from 28.8% in 1993 to 49.9% in 2002 (p < 0.001). In a multivariate analysis controlling for all of variables listed in Table 1, hospice use in the last 6 months of life by patients in whom advanced lung cancer had been diagnosed increased by 9.7% each year from 1993 to 2002 (odds ratio, 1.097 for each year; 95% confidence interval, 1.090 to 1.104).
Figure 3.
Unadjusted percentages of ICU use and hospice use in the last 6 months of life among patients with advanced lung cancer by year of death. Cochran-Armitage trend test for ICU use from 1993 to 2002, p < 0.001; Cochran-Armitage trend test for hospice use from 1993 to 2002, p < 0.001.
In order to understand the increase in ICU use in the last 6 months of life, other measures of aggressive care, such as chemotherapy, used during the study period were investigated. Overall, 30.8% of patients received chemotherapy in the last 6 months of life. The patients receiving chemotherapy increased from 26.8% in 1993 to 33.3% in 2002 (p < 0.001). ICU use among the chemotherapy group and nonchemotherapy group was 24.2% and 20.7%, respectively (p < 0.001).
Table 3 presents the results of a multivariate analysis of factors associated with ICU use in the last 6 months of life among patients with advanced lung cancer. After controlling for other relevant factors, there was a 6.6% increase in the odds of receiving ICU care each year from 1993 to 2002. Other significant predictors of ICU use included younger age, Hispanic ethnicity, low socioeconomic status, treatment at a teaching hospital, nonreceipt of chemotherapy, death from non-lung cancer causes, presence of comorbidity, being married, living in a large metropolitan area, and stage IIIB disease at diagnosis.
Table 3.
Multivariate Analysis of Trend in ICU Use in Last 6 Months of Life*
| Variables | Model 1 | Model 2 |
|---|---|---|
| Year of death (each increasing year) | 1.052 (1.044–1.060) | 1.066 (1.058–1.075) |
| Age at death (each increasing year) | 0.970 (0.966–0.973) | |
| Gender | ||
| Male | 1.0 | |
| Female | 0.965 (0.918–1.015) | |
| Race | ||
| Non-Hispanic white | 1.0 | |
| Black | 1.070 (0.985–1.162) | |
| Hispanic | 1.380 (1.216–1.565) | |
| Others | 1.291 (1.154–1.444) | |
| Low socioeconomic status | ||
| No | 1.0 | |
| Yes | 1.094 (1.025–1.167) | |
| Teaching hospital | ||
| No | 1.0 | |
| Yes | 1.078 (1.021–1.167) | |
| Cause of death | ||
| Other than lung cancer | 1.0 | |
| Lung cancer | 0.697 (0.655–0.742) | |
| Comorbidity score | ||
| 0 | 1.0 | |
| 1 | 1.200 (1.124–1.281) | |
| ≥ 2 | 1.622 (1.525–1.7425) | |
| Married | ||
| No | 1.0 | |
| Yes | 1.119 (1.063–1.179) | |
| Place of residence | ||
| Nonlarge metropolitan area | 1.0 | |
| Large metropolitan area | 1.556 (1.479–1.636) | |
| AJCC stage | ||
| IIIB | 1.0 | |
| IV | 0.685 (0.652–0.720) | |
| Chemotherapy use | ||
| No | 1.0 | |
| Yes | 0.915 (0.869–0.964) | |
Values are presented as odds ratio (95% confidence interval). Model 1 = unadjusted odds of ICU use in the last 6 months of life by death year; model 2 = model 1 + age at death, gender, low socioeconomic status, cause of death, comorbidity score, marital status, AJCC stage, race, place of residence, chemotherapy use, and major medical school affiliation. See Table 1 for abbreviation not used in the text.
Finally, we calculated the percentage of patients with advanced lung cancer who received both ICU and hospice services in the last 6 months of life. During the entire period from 1993 to 2002, 21.8% of patients received ICU care, 42.3% of patients received hospice care, and 6.2% of patients received both ICU and hospice care. The percentage of patients receiving both ICU and hospice care increased from 3.3% in 1993 to 9.0% in 2002 (p < 0.001 for trend over all years).
Discussion
From 1993 to 2002, an increasing proportion of patients in whom advanced lung cancer had been diagnosed received ICU care near the end of life. Of those patients receiving ICU care, one in four received mechanical ventilation. Two thirds of patients died either during or within 1 month of hospitalization. This increase coincided with the marked increase in hospice care for these same patients.
Earle et al19 reported an increase in the number of indicators of aggressive care near death during a 4-year period (1993 to 1996) in patients with advanced cancer. We extended these findings by showing that the increase was accentuated in certain groups of patients (ie, those patients who had been treated at teaching hospitals and resided in a large metropolitan area). In addition, an increase in ICU use occurred in conjunction with a marked increase in end-of-life hospice use.
During the study period, end-of-life ICU use was greater in large metropolitan areas. These findings are consistent with the hypotheses of Wennberg and coworkers20,21 that ICU utilization is driven by inter-hospital variation of medical resources and practice patterns. Specifically, compared to other settings, large metropolitan areas are characterized both by a greater supply of ICU beds relative to other acute care beds, by a greater supply of specialists compared to primary care physician services, and by a greater emphasis on the use of aggressive therapeutic interventions.
Across US hospitals of all sizes, between 1985 and 2000 the number of critical care medicine beds increased by 26.1% (from 69,300 to 87,400).22 During the same period, ICU use near the end of life increased from 30.5 to 35%.4 Seferian and Afessa6 showed in a population-based study that end-of-life ICU use in Olmsted County, MN, during the year 1998 was 0.26 per 1,000 person-years in those persons 18 to 44 years of age compared to 18.5 per 1,000 person-years in those persons ≥ 85 years of age. Earle et al19 showed an increase in ICU use in the last month of life from 9.9% in 1993 to 11.7% in 1996 in patients with cancer. Overall, hospice use among cancer patients increased from 28.3% in 1993 to 38.8% in 1996. However, in the increasing proportion of patients who received hospice care services, they were initiated only in the last 3 days of life.19
Our results suggested that over a third of patients receiving ICU care had a potentially reversible condition. Although ICU users had a median survival time 11 days longer than non-ICU users, the quality of life for those ICU survivors remains unexplored. The trajectory of their subsequent care suggests that half are rehospitalized and most are placed in a nursing home or other institutional setting prior to death.
The increasing use of ICU care near the end of life in the face of a large increase in hospice services was contrary to our hypothesis, and is puzzling. The hospice movement has two major justifications. First, it is a more humane and appropriate way to care for terminally ill patients. Second, it is far less costly than usual care.13 Both justifications rely to a certain extent on the assumption that hospice care is an alternative to or substitute for more aggressive end-of-life care. Our findings show that while most patients admitted to an ICU in the last 6 months of life are less likely to enroll in hospice care, and vice versa, a growing proportion of patients use both services. Thus, in many instances hospice use simply adds to ICU care near the end of life rather than abbreviating intensive management. Presumably, these patients are enrolled in hospice just before death, but for most of the last 6 months of life there is ongoing aggressive care. The median duration of hospice care for patients with advanced lung cancer was merely 15 days.
This study is subject to the limitations of any retrospective study. Moreover, the administrative data used in this investigation did not contain information on the attitudes of patients, family, and treating physicians, and on preferences regarding care near the end of life. It is difficult for physicians to predict the life span of an individual with advanced lung cancer, even though the median survival time of such patients has not changed much over the span of the study. In other words, ICU use in the last 6 months of life is always defined in retrospect. Traditional severity-of-illness scores lack accuracy, making it difficult for physicians to predict survival.23 Treating physicians may be assuming longer survival times than those that result. Lamont and Christakis24 reported that, for two thirds of cancer patients, treating physicians provide either no estimate, or consciously underestimate or overestimate the survival time. Cancer patients often choose treatments based on prognosis, which may be overestimated.25
These results reflect the Medicare population of persons ≥ 66 years of age and may not be applicable to other settings or populations. Subjects with HMO coverage were excluded from the study. Increasing HMO enrollments during the study period might have influenced the analysis of the time trend of ICU usage.
In summary, there was a commensurate increase in ICU care near the end of life with hospice utilization among patients with advanced lung cancer during the 1990s. A third of the patients were discharged from the hospital to home; however, the quality of life remains unknown. Efforts to enroll such patients in hospice earlier in the course of disease might reverse the trend in increasing end-of-life ICU use.
Acknowledgments
The authors thank Dr. Sarah Toombs Smith for help in preparation of the manuscript. The authors thank the anonymous reviewers for their helpful comments. This study used the linked SEER-Medicare database. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services, Inc; and the SEER Program tumor registries in the creation of the SEER-Medicare database.
Abbreviations
- CMS
Centers for Medicare and Medicaid Services
- DRG
drug-related group
- HMO
health maintenance organization
- ICD-9-CM
International Classification of Diseases, 9th edition-clinical modification
- NCI
National Cancer Institute
- SEER
Surveillance, Epidemiology, and End Results
Footnotes
The interpretation and reporting of these data are the sole responsibility of the authors.
The authors have reported to the ACCP that no significant conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/misc/reprints.shtml).
References
- 1.Spiro SS, Silvestri GA. One hundred years of lung cancer. Am J Respir Crit Care Med. 2005;172:523–529. doi: 10.1164/rccm.200504-531OE. [DOI] [PubMed] [Google Scholar]
- 2.Ries LAG, Melbert D, Krapcho M, et al., editors. [Accessed November 14, 2007];SEER cancer statistics review, 1975–2004. Available at: http://seer.cancer.gov/csr/1975_2004/
- 3.Griffin JP, Nelson JE, Koch KA, et al. End-of-life care in patients with lung cancer. Chest. 2003;123:312S–331S. doi: 10.1378/chest.123.1_suppl.312s. [DOI] [PubMed] [Google Scholar]
- 4.Barnato AE, McClellan MB, Kagay CR, et al. Trends in inpatient treatment intensity among Medicare beneficiaries at the end of life. Health Serv Res. 2004;39:363–375. doi: 10.1111/j.1475-6773.2004.00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angus DC, Barnato AE, Linde-Zwirble WT, et al. Use of intensive care at the end of life in the United States: an epidemiologic study. Crit Care Med. 2004;32:638–643. doi: 10.1097/01.ccm.0000114816.62331.08. [DOI] [PubMed] [Google Scholar]
- 6.Seferian EG, Afessa B. Adult intensive care unit use at the end of life: a population-based study. Mayo Clin Proc. 2006;81:896–901. doi: 10.4065/81.7.896. [DOI] [PubMed] [Google Scholar]
- 7.Seferian EG, Afessa B. Demographic and clinical variation of adult intensive care unit utilization from a geographically defined population. Crit Care Med. 2006;34:2113–2119. doi: 10.1097/01.CCM.0000227652.08185.A4. [DOI] [PubMed] [Google Scholar]
- 8.Nelson J, Danis M. End-of-life care in the intensive care unit: where are we now? Crit Care Med. 2001;29:N2–N9. doi: 10.1097/00003246-200102001-00002. [DOI] [PubMed] [Google Scholar]
- 9.Ewer MS, Ali MK, Atta MS, et al. Outcome of lung cancer patients requiring mechanical ventilation for pulmonary failure. JAMA. 1986;256:3364–3366. [PubMed] [Google Scholar]
- 10.Lin YC, Tsai YH, Huang CC, et al. Outcome of lung cancer patients with acute respiratory failure requiring mechanical ventilation. Respir Med. 2004;98:43–51. doi: 10.1016/j.rmed.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Boussat S, El’rini T, Dubiez A, et al. Predictive factors of death in primary lung cancer patients on admission to the intensive care unit. Intensive Care Med. 2002;26:1811–1816. doi: 10.1007/s001340000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groeger JS, White P, Jr, Nierman DM, et al. Outcome for cancer patients requiring mechanical ventilation. J Clin Oncol. 1999;17:991–997. doi: 10.1200/JCO.1999.17.3.991. [DOI] [PubMed] [Google Scholar]
- 13.Campbell DE, Lynn J, Louis TA, et al. Medicare program expenditures associated with hospice use. Ann Intern Med. 2004;140:269–277. doi: 10.7326/0003-4819-140-4-200402170-00009. [DOI] [PubMed] [Google Scholar]
- 14.Christakis NA, Escarce JJ. Survival of Medicare patients after enrollment in hospice programs. N Engl J Med. 1996;3355:172–178. doi: 10.1056/NEJM199607183350306. [DOI] [PubMed] [Google Scholar]
- 15.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(suppl):3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 16.National Cancer Institute. [Accessed November 14, 2007];SEER-Medicare: provider files. http://healthservices.cancer.gov/seermedicare/aboutdata/provider.html.
- 17.Ghelfi LM, Parker TS. A county level measure of urban influence. Rural Dev Perspect. 1997;12:32–41. [Google Scholar]
- 18.Klabunde CN. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 19.Earle CC, Neville BA, Landrum MB, et al. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22:315–321. doi: 10.1200/JCO.2004.08.136. [DOI] [PubMed] [Google Scholar]
- 20.Wennberg JE, Cooper MM, editors. The Dartmouth atlas of health care 1999. Chicago, IL: American Hospital Association Press; 1999. The quality of medical care in the United States: a report on the Medicare program. [PubMed] [Google Scholar]
- 21.Wennberg JE, Fischer ES, Stukel YA, et al. Use of hospitals, physician visits, and hospice care during last sixth months of life among cohorts loyal to highly respected hospitals in the United States. BMJ. 2004;328:607–611. doi: 10.1136/bmj.328.7440.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halpern NA, Pastores SM, Thaler HT, et al. Changes in critical care beds and occupancy in the United States 1985–2000: difference attributable to hospital size. Crit Care Med. 2006;34:2105–2112. doi: 10.1097/01.CCM.0000227174.30337.3E. [DOI] [PubMed] [Google Scholar]
- 23.Carlet J, Thijs LG, Antonelli M, et al. Challenges in end-of-life care in the ICU: statement of the 5th International Consensus Conference in Critical Care. Intensive Care Med. 2004;30:770–784. doi: 10.1007/s00134-004-2241-5. [DOI] [PubMed] [Google Scholar]
- 24.Lamont EB, Christakis NA. Prognostic disclosure to patients with cancer near the end of life. Ann Intern Med. 2001;134:1096–1105. doi: 10.7326/0003-4819-134-12-200106190-00009. [DOI] [PubMed] [Google Scholar]
- 25.Weeks JC, Cook EF, O’Day SJ, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA. 1998;279:1709–1714. doi: 10.1001/jama.279.21.1709. [DOI] [PubMed] [Google Scholar]