Abstract
Introduction
i.v. bisphosphonates reduce skeletal events in women with bone metastases from breast cancer, but little is known about the prevalence and duration of bisphosphonate use.
Methods
Patients were identified from the Surveillance, Epidemiology, and End Results–Medicare database who were aged ≥65 years and were diagnosed with invasive breast cancer in 1995–2002. Healthcare Common Procedure Coding System codes were used to identify patients treated with pamidronate and zoledronic acid. Descriptive statistics were used to describe patterns of use. Multivariate analyses were performed to determine the predictors of bisphosphonate use.
Results
In total, 55,864 women with breast cancer were included, with 307,467 person-years of follow-up. Overall, 1.26% of women with all stages of breast cancer received i.v. bisphosphonates. In 2004, 2% of all breast cancer patients and 32% of patients with distant stage disease received bisphosphonates. Approximately two thirds of patients treated with bisphosphonates received zoledronic acid and one third received pamidronate in 2004. Multivariate analyses showed that patients who were ≥75 years old were less likely to receive bisphosphonates (75–79 years versus 65–69 years: odds ratio [OR], 0.81; 95% confidence interval [CI], 0.70–0.93; 80+ years versus 65–69 years: OR, 0.49; 95% CI, 0.42–0.57). The use of bisphosphonates dramatically increased over time. The majority of living patients were continued on i.v. bisphosphonates once started (83% at 1 year, 64% at 3 years, 50% at 5 years), but the median survival time after initiation of i.v. bisphosphonates was only 21 months.
Conclusions
i.v. bisphosphonates appear to be under- used in patients with metastatic breast cancer, particularly among those patients >75 years of age. The Oncologist 2008;13:494–502
Keywords: Breast cancer, Elderly, Metastatic
Introduction
It was estimated that approximately 40,000 women would die in 2007 as a result of metastatic breast cancer [1]. Bone is the most common site of metastatic lesions: more than half the women with metastatic breast cancer present with or develop bone metastases [2]. Bone metastases can be a source of significant pain for patients and place patients at risk for complications such as pathologic fractures, hypercalcemia, and cord compression [3, 4].
i.v. bisphosphonates, such as pamidronate or zoledronic acid, inhibit osteoclastic absorption of bone and are used to prevent complications of bone metastases. Randomized clinical trials have compared patients treated with pamidronate with those given placebo and have shown that pamidronate reduces the risk for skeletal events, delays the time to skeletal events, and reduces pain among women with metastatic breast cancer to the bone [5–8]. Zoledronic acid has been compared with pamidronate and is at least as effective as pamidronate in preventing skeletal events [9 –11]. The American Society of Clinical Oncology (ASCO) guidelines, which were originally published in 2000 and updated in 2003, recommend that women with evidence of metastatic bony destruction receive i.v. pamidronate or zoledronic acid every 3–4 weeks until there is a substantial decline in the patient’s performance status [12, 13].
To our knowledge, very limited data have been published on patterns of bisphosphonate use. It is unknown whether the majority of women with metastatic breast cancer are receiving bisphosphonates or how long such therapy is continued after initiation. In this study, we present data on patterns of bisphosphonate use in a population-based cohort of older women with breast cancer.
Methods
Data Source
We used the merged Surveillance, Epidemiology, and End Results (SEER)–Medicare database for this analysis. The SEER program, supported by the National Cancer Institute, is a population-based tumor registry that ascertains all newly diagnosed cancer cases that occur in selected geographic areas [14]. Over the years of this study, those included areas covered 14%–25% of the U.S. population. Data on patient demographics, tumor characteristics, stage at diagnosis, date of diagnosis, and date and cause of death are available through the SEER registry data. The Medicare program is administered by the Centers for Medicare and Medicaid services and covers hospital, physician, outpatient, and other medical services for 97% of the U.S. population aged ≥65 years [15]. SEER subjects were matched with Medicare’s master enrollment file, using the method described by Potosky et al. [15] to create the SEER–Medicare database.
Study Population
The study population included women aged ≥65 years who were diagnosed with breast cancer in 1995 through 2002. Patients were excluded if the breast cancer diagnosis was not the patient’s first cancer or if the histology was not confirmed. Patients without full coverage of both Medicare A and B for 1995–2004 were excluded, unless coverage was lost as a result of death. Women who belonged to a health maintenance organization were also excluded, because their claims data would be incomplete. Cases from Greater California, Kentucky, Louisiana, and New Jersey were excluded because those registries did not become part of the SEER program until 2000. All patients who were known to have received i.v. bisphosphonate treatment prior to their cancer diagnosis were also excluded from the study cohort.
The Healthcare Common Procedure Coding System drug administration codes J2430 (pamidronate) and J3487 (zoledronic acid) were used to identify patients who received i.v. bisphosphonate therapy during the period from January 1, 1995, to December 31, 2004. Patient demographic and tumor characteristics, such as age, ethnicity, marital status, census tract level social economic variables for education and poverty, tumor stage, and year of diagnosis, were obtained from the SEER–Medicare Patient Entitlement and Diagnosis Summary File. Medicare claims were available through December 2004.
Statistical Analysis
Among patients who met the eligibility criteria for this study, the prevalence of bisphosphonate use was calculated per person-year of follow-up for all patients and for those with metastatic disease. A univariate generalized estimating equation (GEE) method was used to calculate a p-value for the prevalence of bisphosphonate use for each covariate. We calculated the percentage of patients receiving i.v. bisphosphonates for each calendar year in 1995–2004 among all patients and among patients with distant stage disease.
A multivariate analysis was performed to determine the relationship between the covariates and bisphosphonate use. Annual bisphosphonate use was recorded starting from the diagnosis year and ending in the year of death or in 2004. Multiple logistic regression, implemented with a GEE method in order to account for multiple observations per individual, was performed to measure a dichotomous outcome of receiving bisphosphonate treatment or not, while adjusting for covariates. Because bisphosphonate use increased over time, an autoregressive correlation structure (first order) was specified in this model. The following covariates were included: age at diagnosis (categorical: 65–69 years, 70–74 years, 75–79 years, or ≥80 years), ethnicity (white, black, Hispanic, or other), marital status, census tract poverty level, census tract education level, historic stage (in situ, localized, regional, distant, or unstaged), bone metastases (no or yes), SEER region, and calendar year. For the census tract variables of poverty and education, quartiles were calculated in increasing order. The categories for percentage of persons ≥25 years of age with <12 years of education were 0%–7.76%, 7.77%–13.67%, 13.68%–21.29%, 21.3%–100%, and unknown. The categories for percentage of residents living below the poverty level were 0%–3.82%, 3.83%– 6.74%, 6.75%–11.89%, 11.9%–87.17%, and unknown. Census data from the 2000 files were supplemented with 1990 files if missing or unknown information was found.
Among patients who had claims for i.v. bisphosphonate use, we calculated the proportion of patients who continued bisphosphonates in subsequent years using the method of Kaplan and Meier. Dose was calculated such that claims for 4 mg of zoledronic acid and 90 mg of pamidronate were considered to be an equivalent single dose. The mean numbers of doses were calculated for patients by length of follow-up. The survival experience of patients from the first dose of bisphosphonates was calculated using the method of Kaplan and Meier. SAS software (SAS Institute, Inc., Cary, NC) was used for all statistical analyses. All statistical tests were two-sided.
The study was reviewed by the institutional review board (IRB) and was granted an exemption from IRB approval under Category 4 of the Code of Federal Regulations, because the data are without identifiers.
Results
In total, 55,864 women with breast cancer were included in this analysis, with a total of 307,467 person-years of follow-up. Overall, 1.26% of women with all stages of breast cancer received i.v. bisphosphonates. Univariate patterns of i.v. bisphosphonate use are shown in Table 1. i.v. bisphosphonates were more commonly used in younger patients, in patients with later stage disease, and in more recent years. In total, 2,773 women had distant disease at diagnosis, and overall, 16% of women with distant disease received bisphosphonates (Table 2).
Table 1.
Univariate patterns of i.v. bisphosphonate use
| n | Total person-years of follow-up | Percent bisphosphonate use | p-value | |
|---|---|---|---|---|
| Overall | 55,864 | 307,467 | 1.26 | |
|
| ||||
| Agea | <.001 | |||
| 65–69 | – | 39,277 | 1.25 | |
| 70–74 | – | 76,737 | 1.50 | |
| 75–79 | – | 80,665 | 1.35 | |
| 80+ | – | 110,788 | 1.04 | |
|
| ||||
| Race/ethnicity | .002 | |||
| White | 47,542 | 263,045 | 1.28 | |
| Black | 3575 | 18,010 | 1.23 | |
| Hispanic | 1,963 | 10,753 | 1.54 | |
| Other | 2,784 | 15,659 | 0.82 | |
|
| ||||
| Marital status | .968 | |||
| Married | 23,825 | 138,574 | 1.25 | |
| Unmarried | 1,746 | 159,678 | 1.27 | |
| Unknown | 30,293 | 9,215 | 1.25 | |
|
| ||||
| Poverty (from least to most) | .159 | |||
| 1st quartile | 13,880 | 78,139 | 1.38 | |
| 2nd quartile | 13,915 | 77,239 | 1.18 | |
| 3rd quartile | 13,904 | 76,160 | 1.20 | |
| 4th quartile | 13,885 | 74,089 | 1.27 | |
| Unknown | 280 | 1,840 | 1.90 | |
|
| ||||
| Education (from most to least) | .104 | |||
| 1st quartile | 13,898 | 76,568 | 1.33 | |
| 2nd quartile | 13,884 | 76,169 | 1.18 | |
| 3rd quartile | 13,903 | 76,422 | 1.36 | |
| 4th quartile | 13,899 | 76,468 | 1.17 | |
| Unknown | 280 | 1,840 | 1.90 | |
|
| ||||
| Stage at diagnosis | <.001 | |||
| In situ | 7771 | 45690 | 0.21 | |
| Localized | 32639 | 189123 | 0.57 | |
| Regional | 11761 | 60616 | 2.16 | |
| Distant | 2773 | 8193 | 16.09 | |
| Unknown | 920 | 3845 | 2.05 | |
|
| ||||
| Calendar yeara | <.001 | |||
| 1995 | – | 7,035 | 0.001 | |
| 1996 | – | 13,560 | 0.017 | |
| 1997 | – | 20,102 | 0.044 | |
| 1998 | – | 26,110 | 0.73 | |
| 1999 | – | 31,767 | 1.01 | |
| 2000 | – | 36,881 | 1.34 | |
| 2001 | – | 41,887 | 1.42 | |
| 2002 | – | 46,099 | 1.10 | |
| 2003 | – | 43,383 | 1.93 | |
| 2004 | – | 40,643 | 2.02 | |
Age and calendar year might change during the period of bisphosphonate use for each patient.
Table 2.
Univariate patterns of i.v. bisphosphonate use among patients with distant disease
| n | Total person-years of follow-up | Percent bisphosphonate use | p-value | |
|---|---|---|---|---|
| Overall | 2,773 | 8,193 | 16.1 | |
|
| ||||
| Agea | <.0001 | |||
| 65–69 | – | 1,469 | 16.4 | |
| 70–74 | – | 2,114 | 19.9 | |
| 75–79 | – | 1,953 | 18.2 | |
| 80+ | – | 2,657 | 11.3 | |
|
| ||||
| Race/ethnicity | <.0001 | |||
| White | 2,244 | 6,708 | 16.4 | |
| Black | 294 | 727 | 11.0 | |
| Hispanic | 132 | 442 | 23.1 | |
| Other | 103 | 316 | 11.7 | |
|
| ||||
| Marital status | <.0001 | |||
| Married | 887 | 2,939 | 19.2 | |
| Unmarried | 81 | 238 | 13.9 | |
| Unknown | 1,805 | 5,016 | 11.4 | |
|
| ||||
| Poverty (from least to most) | <.0001 | |||
| 1st quartile | 622 | 1,903 | 18.6 | |
| 2nd quartile | 637 | 1,993 | 15.2 | |
| 3rd quartile | 660 | 1,899 | 16.8 | |
| 4th quartile | 843 | 2,352 | 14.0 | |
| Unknown | 11 | 46 | 28.3 | |
|
| ||||
| Education (from most to least) | <.0001 | |||
| 1st quartile | 555 | 1,763 | 17.9 | |
| 2nd quartile | 640 | 1,971 | 14.8 | |
| 3rd quartile | 706 | 2,041 | 18.1 | |
| 4th quartile | 861 | 2,372 | 13.9 | |
| Unknown | 11 | 46 | 28.3 | |
|
| ||||
| Calendar yeara | <.0001 | |||
| 1995 | – | 343 | 0.3 | |
| 1996 | – | 610 | 2.1 | |
| 1997 | – | 774 | 4.7 | |
| 1998 | – | 881 | 9.7 | |
| 1999 | – | 970 | 14.7 | |
| 2000 | – | 1,042 | 18.8 | |
| 2001 | – | 1,085 | 21.2 | |
| 2002 | – | 1,089 | 16.9 | |
| 2003 | – | 800 | 30.0 | |
| 2004 | – | 599 | 31.7 | |
Age and calendar year might change during the period of bisphosphonate use for each patient.
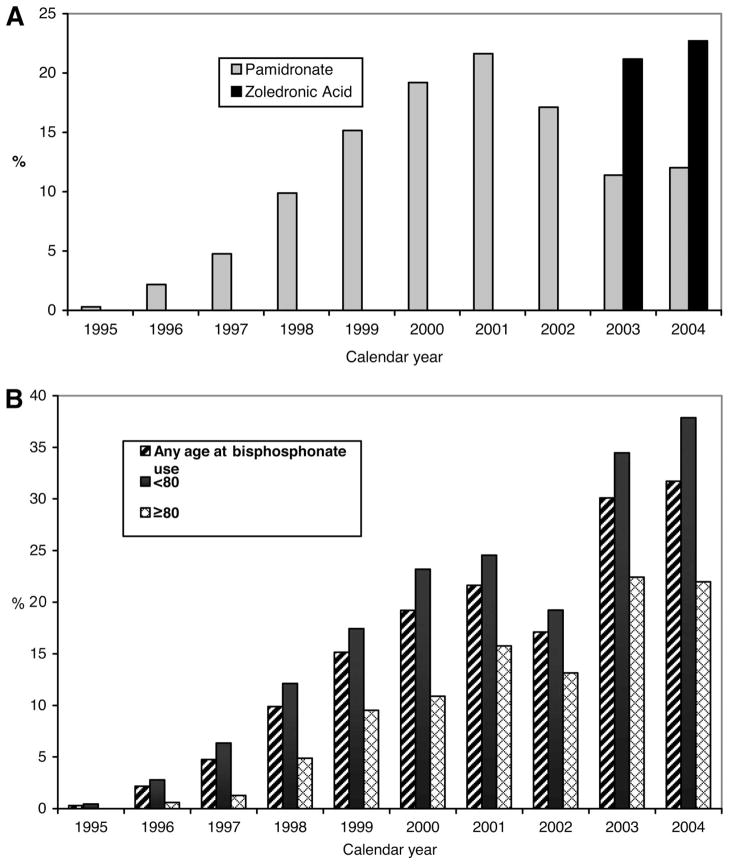
Pamidronate and zoledronic acid were initially approved by the U.S. Food and Drug Administration (FDA) in 1991 and 2001, respectively. Only a single patient received i.v. bisphosphonates in 1995. In contrast, in 2004, approximately 2% of all breast cancer patients received a bisphosphonate. We also evaluated use in the subset of patients with metastatic disease at diagnosis. Figure 1 shows the increasing use of pamidronate and zoledronic acid by calendar year among patients with metastatic disease. We note that the drop in bisphosphonate use in 2002 is likely artifactual because zoledronic acid was in use but did not have a separate billing code until January 1, 2003. In 2004, 32% of patients with distant disease at diagnosis received i.v. bisphosphonates. In 2003 and 2004, the years in which a billing code was available to identify zoledronic acid, zoledronic acid accounted for approximately two thirds of i.v. bisphosphonates administered and pamidronate accounted for one third. The use of bisphosphonates increased for both older and younger patients, but usage remained lower in women ≥80 years of age (Fig. 1B).
Figure 1.
Bisphosphonate use in breast cancer patients with distant disease by year. (A): Percentage of distant breast cancer patients receiving pamidronate or zoledronic acid in each calendar year. (B): Total bisphosphonate use for patients with distant disease in each calendar year, by patient age.
We then performed multivariate analyses to determine the predictors of bisphosphonate use. The results are shown in Table 3. As expected, the clinical characteristics of advanced stage disease and bone metastases were strong predictors of bisphosphonate use. Of note, patients ≥75 years old were significantly less likely to receive i.v. bisphosphonates, after adjustment for covariates including disease stage and presence of bone metastases and after accounting for length of follow-up. Patients living in census tract areas characterized by a lower educational level were also less likely to receive i.v. bisphosphonates. Consistent with the univariate findings, the use of bisphosphonates dramatically increased over time.
Table 3.
Multivariate analysis for predictors of i.v. bisphosphonate use
| Odds ratio | 95% confidence interval | |
|---|---|---|
| Age | ||
| 65–69 | 1.0 (ref) | |
| 70–74 | 0.88 | 0.78–1.01 |
| 75–79 | 0.81 | 0.70–0.93 |
| 80+ | 0.49 | 0.42–0.57 |
|
| ||
| Race/ethnicity | ||
| White | 1.00 (ref) | |
| Black | 0.78 | 0.62–0.98 |
| Hispanic | 0.81 | 0.61–1.06 |
| Other | 0.78 | 0.58–1.05 |
|
| ||
| Marital status | ||
| Married | 1.00 (ref) | |
| Unmarried | 1.00 | 0.90–1.11 |
| Unknown | 0.94 | 0.68–1.29 |
|
| ||
| Poverty (from least to most) | ||
| 1st quartile | 1.00 | |
| 2nd quartile | 0.91 | 0.78–1.05 |
| 3rd quartile | 0.95 | 0.80–1.12 |
| 4th quartile | 0.96 | 0.78–1.17 |
| Unknown | 1.74 | 0.98–3.10 |
|
| ||
| Education (from most to least) | ||
| 1st quartile | 1.00 (ref) | |
| 2nd quartile | 0.91 | 0.78–1.06 |
| 3rd quartile | 1.13 | 0.96–1.33 |
| 4th quartile | 0.86 | 0.71–1.05 |
|
| ||
| Stage at diagnosis | ||
| In situ | 1.00 (ref) | |
| Localized | 2.89 | 2.17–3.85 |
| Regional | 11.04 | 8.30–14.69 |
| Distant | 114.83 | 85.87–153.55 |
| Unknown | 12.65 | 7.77–20.59 |
|
| ||
| Calendar year | ||
| 1995 | 0.01 | 0.00–0.46 |
| 1996 | 0.14 | 0.08–0.26 |
| 1997 | 0.49 | 0.38–0.62 |
| 1998 | 1.0 (ref) | |
| 1999 | 1.52 | 1.32–1.75 |
| 2000 | 2.22 | 1.90–2.60 |
| 2001 | 2.55 | 2.17–3.00 |
| 2002 | 2.21 | 1.87–2.61 |
| 2003 | 4.46 | 3.79–5.24 |
| 2004 | 4.94 | 4.19–5.82 |
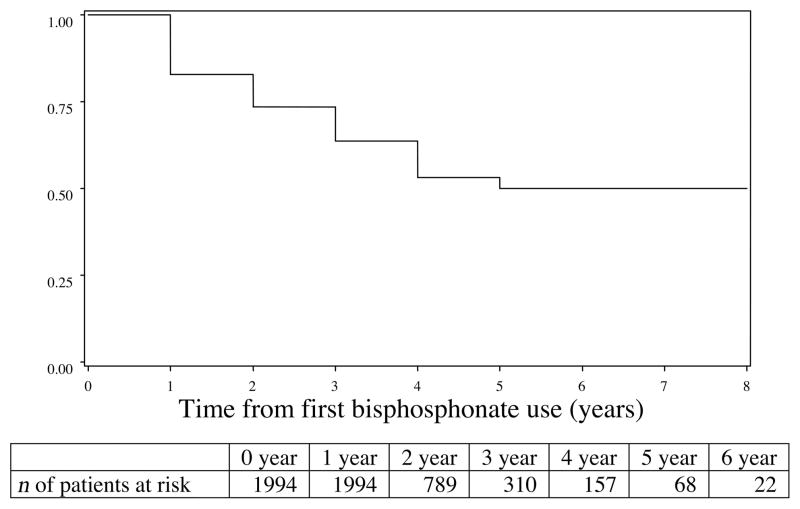
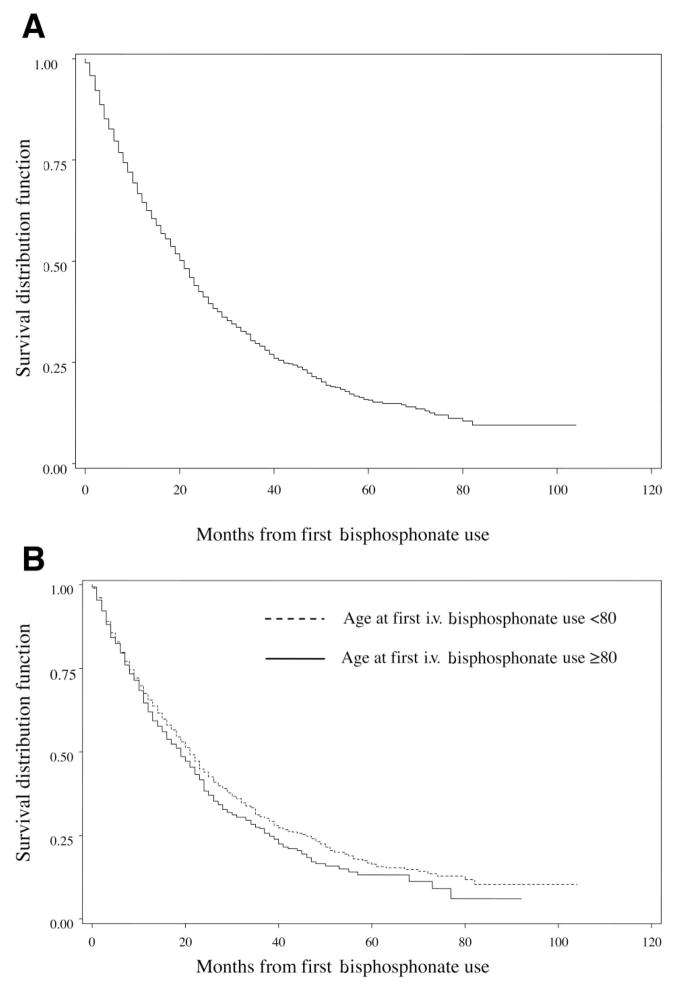
We next wished to evaluate how long patients were continued on i.v. bisphosphonates once such therapy was initiated. Figure 2 shows the proportion of patients who remained on bisphosphonates in the years after the initial dose. At 1 year, 83% of living patients were still receiving i.v. bisphosphonates. At 3 years, 64% of patients were still receiving bisphosphonates, and among patients followed for 5 years, 50% remained on i.v. bisphosphonates. At 3, 6, and 12 months of follow-up, the mean numbers of doses received were 2, 4, and 8, respectively. At 2 years, the mean number of bisphosphonate doses was 15, and at 5 years, the mean number was 27. However, most patients did not survive 5 years after the initiation of bisphosphonates (Fig. 3A). The median survival time was 21 months (95% CI 19–22 months), and 75% of patients were deceased at 42 months (95% confidence interval, 39–47 months). The survival duration after the initiation of bisphosphonates was similar for patients above and below the age of 80, although older women had slightly poorer survival rates (Fig. 3B).
Figure 2.
Proportion of patients remaining on i.v. bisphosphonates after first use.
Figure 3.
Survival probability for patients with i.v. bisphosphonates use. (A): All patients. (B): All patients, stratified by age <80 years versus age ≥80 years.
Discussion
In this manuscript, we report the first population-based analysis of the use of i.v. bisphosphonates among older women treated for breast cancer. Overall, the rate of bisphosphonate use was lower than we expected. Among patients with distant disease who were alive in 2004, only 32% of patients received an i.v. bisphosphonate. Given that more than half of the women with metastatic breast cancer are expected to have or develop bone metastases during the course of their illness, it appears that a substantial fraction of women with bone metastases are not receiving i.v. bisphosphonates. In each group of breast cancer patients, the use of i.v. bisphosphonates was increasing in more recent calendar years.
In the multivariate models, we found that bisphosphonate use significantly declined with age. Patients ≥80 years of age were 0.60 times less likely to be treated with bisphosphonates than women aged 65–69 years. These findings are consistent with previous studies that have documented less aggressive treatment of older women across many different modalities of therapy [16 –21]. However, given the high prevalence of osteoporosis and osteopenia among older women, the lower use of bisphosphonates is somewhat surprising. It is possible that concerns over renal toxicity among older women who have poorer renal function results in the lower use of bisphosphonates in the elderly.
In this study, we were also interested in exploring the length of therapy and cumulative dose of bisphosphonates among women who had been started on i.v. bisphosphonates. ASCO guidelines recommend that therapy be continued as long as the patient has a good performance status [12, 13]. However, the optimal length of therapy is under debate. The clinical trials that established the benefits of bisphosphonates treated patients for 12–24 months [6, 7, 22–24]. Recently, potential toxicities of bisphosphonates, such as osteonecrosis of the jaw and renal dysfunction, have increasingly been recognized and are thought to be dose-related [25–28]. Therefore, concerns have been raised about continuing i.v. bisphosphonates indefinitely, because an additional incremental benefit has not been proven but the toxicities appear to increase. In our study, we found that the majority of women were continued on bisphosphonates for the remainder of their lives. Our analysis of cumulative dose by length of follow-up indicates that patients tended to receive bisphosphonates less frequently than every 3– 4 weeks, particularly patients who continued on bisphosphonates for years (i.e., patients still on bisphosphonates at 24 months had received a mean of 17 doses).
The use of zoledronic acid versus pamidronate also increased over the years in this study. Although ASCO guidelines recommend either zoledronic acid or pamidronate, zoledronic acid has the advantage of a shorter infusion time (15 minutes versus 90 minutes). In addition, some data suggest that zoledronic acid may have superior efficacy in breast cancer patients when compared with pamidronate [10].
Our study has some limitations. First, it was conducted using claims data. From claims data, we could not identify patients with bone metastases who were clinically fit to receive i.v. bisphosphonates. We were able to reliably evaluate all patients with breast cancer or patients with distant disease at diagnosis based on SEER staging. Our study is also limited by the lack of a billing code for zoledronic acid in 2001 and 2002, after the drug was approved by the FDA and available for use. This resulted in an underestimate of i.v. bisphosphonate use in those 2 years. Finally, some of the bisphosphonate use could have been for treatment of hypercalcemia of malignancy, although hypercalcemia is uncommon without bone involvement.
In conclusion, we present data from a large population-based cohort of women with breast cancer. The use of i.v. bisphosphonates was lower than expected. Our data suggest that these highly effective supportive care medications are underused in older women with breast cancer. However, the majority of patients that are started on bisphosphonates are usually continued on bisphosphonates throughout their lifespan, consistent with guideline recommendations. Further research is needed to confirm our findings and to develop interventions to increase the use of bisphosphonates, so that skeletal complications in women with metastatic breast cancer can be minimized.
Learning Objectives.
After completing this course, the reader will be able to:
Make optimal use of i.v. bisphosphonates in women with breast cancer.
Uphold the current guidelines for the use of bisphosphonates among women with metastatic breast cancer.
Assess the relationship between bisphosphonate use and patient age.
Acknowledgments
We are in debted to the Applied Research Program, National Cancer Institute; to the Office of Research, Development, and Information, Centers for Medicare and Medicaid Services; to Information Management Services; and to the SEER Program for the creation of the SEER–Medicare database. The interpretation and reporting of the data are the sole responsibilities of the authors.
Footnotes
Disclosure: S.H.G. is supported by NIH 1K07 CA 109064–04. The funding sources had no role in the study design, conduct, data analysis, or manuscript preparation. No potential conflicts of interest were reported by the authors, planners, reviewers, or staff managers of this article.
Author Contributions
Conception/design: Sharon H. Giordano, Yong-Fang Kuo, Gabriel N. Hortobagyi, James S. Goodwin
Financial support: Sharon H. Giordano
Administrative support: Sharon H. Giordano
Provision of study materials or patients: Sharon H. Giordano
Collection/assembly of data: Sharon H. Giordano
Data analysis and interpretation: Sharon H. Giordano, Shenying Fang, Zhigang Duan, Yong-Fang Kuo, Gabriel N. Hortobagyi, James S. Goodwin
Manuscript writing: Sharon H. Giordano, Shenying Fang, Zhigang Duan
Final approval of manuscript: Sharon H. Giordano, Shenying Fang, Zhigang Duan, Yong-Fang Kuo, Gabriel N. Hortobagyi, James S. Goodwin
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Scheid V, Buzdar AU, Smith TL, et al. Clinical course of breast cancer patients with osseous metastasis treated with combination chemotherapy. Cancer. 1986;58:2589–2593. doi: 10.1002/1097-0142(19861215)58:12<2589::aid-cncr2820581206>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 3.Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55:61–66. doi: 10.1038/bjc.1987.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coleman RE, Rubens RD. Bone metastases and breast cancer. Cancer Treat Rev. 1985;12:251–270. doi: 10.1016/0305-7372(85)90008-8. [DOI] [PubMed] [Google Scholar]
- 5.Pavlakis N, Schmidt R, Stockler M. Bisphosphonates for breast cancer. Cochrane Database Syst Rev. 2005;(3):CD003474. doi: 10.1002/14651858.CD003474.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Hortobagyi GN, Theriault RL, Lipton A, et al. Long-term prevention of skeletal complications of metastatic breast cancer with pamidronate. Protocol 19 Aredia Breast Cancer Study Group. J Clin Oncol. 1998;16:2038–2044. doi: 10.1200/JCO.1998.16.6.2038. [DOI] [PubMed] [Google Scholar]
- 7.Hortobagyi GN, Theriault RL, Porter L, et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N Engl J Med. 1996;335:1785–1791. doi: 10.1056/NEJM199612123352401. [DOI] [PubMed] [Google Scholar]
- 8.Conte PF, Latreille J, Mauriac L, et al. Delay in progression of bone metastases in breast cancer patients treated with intravenous pamidronate: Results from a multinational randomized controlled trial. The Aredia Multinational Cooperative Group. J Clin Oncol. 1996;14:2552–2559. doi: 10.1200/JCO.1996.14.9.2552. [DOI] [PubMed] [Google Scholar]
- 9.Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: A randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98:1735–1744. doi: 10.1002/cncr.11701. [DOI] [PubMed] [Google Scholar]
- 10.Rosen LS, Gordon DH, Dugan W, Jr, et al. Zoledronic acid is superior to pamidronate for the treatment of bone metastases in breast carcinoma patients with at least one osteolytic lesion. Cancer. 2004;100:36–43. doi: 10.1002/cncr.11892. [DOI] [PubMed] [Google Scholar]
- 11.Berenson JR, Rosen LS, Howell A, et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer. 2001;91:1191–1200. doi: 10.1002/1097-0142(20010401)91:7<1191::aid-cncr1119>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 12.Hillner BE, Ingle JN, Berenson JR, et al. American Society of Clinical Oncology guideline on the role of bisphosphonates in breast cancer. American Society of Clinical Oncology Bisphosphonates Expert Panel. J Clin Oncol. 2000;18:1378–1391. doi: 10.1200/JCO.2000.18.6.1378. [DOI] [PubMed] [Google Scholar]
- 13.Hillner BE, Ingle JN, Chlebowski RT, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol. 2003;21:4042–4057. doi: 10.1200/JCO.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Hellman S. Stopping metastases at their source. N Engl J Med. 1997;337:996–997. doi: 10.1056/NEJM199710023371408. [DOI] [PubMed] [Google Scholar]
- 15.Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–748. [PubMed] [Google Scholar]
- 16.Greenfield S, Blanco DM, Elashoff RM, et al. Patterns of care related to age of breast cancer patients. JAMA. 1987;257:2766–2770. [PubMed] [Google Scholar]
- 17.Goodwin JS, Hunt WC, Samet JM. Determinants of cancer therapy in elderly patients. Cancer. 1993;72:594–601. doi: 10.1002/1097-0142(19930715)72:2<594::aid-cncr2820720243>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Samet J, Hunt WC, Key C, et al. Choice of cancer therapy varies with age of patient. JAMA. 1986;255:3385–3390. [PubMed] [Google Scholar]
- 19.Silliman RA, Guadagnoli E, Weitberg AB, et al. Age as a predictor of diagnostic and initial treatment intensity in newly diagnosed breast cancer patients. J Gerontol. 1989;44:M46–M50. doi: 10.1093/geronj/44.2.m46. [DOI] [PubMed] [Google Scholar]
- 20.Edge SB, Gold K, Berg CD, et al. Patient and provider characteristics that affect the use of axillary dissection in older women with stage I–II breast carcinoma. Cancer. 2002;94:2534–2541. doi: 10.1002/cncr.10540. [DOI] [PubMed] [Google Scholar]
- 21.Mandelblatt JS, Hadley J, Kerner JF, et al. Patterns of breast carcinoma treatment in older women: Patient preference and clinical and physical influences. Cancer. 2000;89:561–573. [PubMed] [Google Scholar]
- 22.Theriault RL, Lipton A, Hortobagyi GN, et al. Pamidronate reduces skeletal morbidity in women with advanced breast cancer and lytic bone lesions: A randomized, placebo-controlled trial. Protocol 18 Aredia Breast Cancer Study Group. J Clin Oncol. 1999;17:846–854. doi: 10.1200/JCO.1999.17.3.846. [DOI] [PubMed] [Google Scholar]
- 23.Hultborn R, Gundersen S, Ryden S, et al. Efficacy of pamidronate in breast cancer with bone metastases: A randomized, double-blind placebo-controlled multicenter study. Anticancer Res. 1999;19:3383–3392. [PubMed] [Google Scholar]
- 24.Kohno N, Aogi K, Minami H, et al. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: A randomized, placebo-controlled trial. J Clin Oncol. 2005;23:3314–3321. doi: 10.1200/JCO.2005.05.116. [DOI] [PubMed] [Google Scholar]
- 25.Ali SM, Esteva FJ, Hortobagyi G, et al. Safety and efficacy of bisphosphonates beyond 24 months in cancer patients. J Clin Oncol. 2001;19:3434–3437. doi: 10.1200/JCO.2001.19.14.3434. [DOI] [PubMed] [Google Scholar]
- 26.Maerevoet M, Martin C, Duck L. Osteonecrosis of the jaw and bisphosphonates. N Engl J Med. 2005;353:99–102. [PubMed] [Google Scholar]
- 27.Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: Incidence and risk factors. J Clin Oncol. 2005;23:8580–8587. doi: 10.1200/JCO.2005.02.8670. [DOI] [PubMed] [Google Scholar]
- 28.Wilkerson GS, Kuo YF, Freeman JL, et al. Intravenous bisphosphonate therapy and inflammatory conditions or surgery of the jaw: A population-based analysis. J Natl Cancer Inst. 2007;99:1016–1024. doi: 10.1093/jnci/djm025. [DOI] [PubMed] [Google Scholar]