Abstract
Glyceollins are soy-derived phytoalexins that have been proposed to be candidate cancer preventive compounds. The effect of the glyceollins on prostate cancer is unknown. The present study examined the molecular effects of soy phytoalexin, glyceollins, on human prostate cancer cell LNCaP to further elucidate its potential effects on prostate cancer prevention. We found that the glyceollins inhibited LNCaP cell growth similar to that of the soy isoflavone genistein. The growth inhibitory effects of the glyceollins appeared to be due to an inhibition of G1/S progression and correlated with an up-regulation of cyclin-dependent kinase inhibitor 1 A and B mRNA and protein levels. By contrast, genistein only up-regulates cyclin-dependent kinase inhibitor 1A. In addition, glyceollin treatments led to down-regulated mRNA levels for androgen responsive genes. In contrast to genistein, this effect of glyceollins on androgen responsive genes appeared to be mediated through modulation of an estrogen- but not androgen-mediated pathway. Hence, the glyceollins exerted multiple effects on LNCaP cells that may be considered cancer preventive and the mechanisms of action appeared to be different from other soy-derived phytochemicals.
Keywords: androgen, estrogen, cancer prevention, cell cycle, gene expression
Introduction
Prostate cancer is the most common non-cutaneous cancer among American men and is ranked third as a cause of cancer deaths [1]. Since there is currently no effective cure for this disease, there is much interest in developing preventive strategies to reduce prostate cancer's occurrence and impact [2]. Population and experimental studies have implicated dietary components in both the cause as well as prevention of prostate cancer [3,4]. In particular, consumption of a diet that is rich in fruits, vegetables, and legumes is associated with a decreased risk for prostate and other forms of cancer [3,4]. Hence, there is much interest in pursuing the development of food-derived products or compounds as chemopreventive agents due to their expected safety and the fact that they are perceived as supportive of medical therapies [4]. The molecular targets of phytochemicals, as well as the mechanisms that contribute to their beneficial effects on cancer, remain elusive. Further elucidation of the molecular targets and mechanisms would be important in fulfilling their cancer preventive properties.
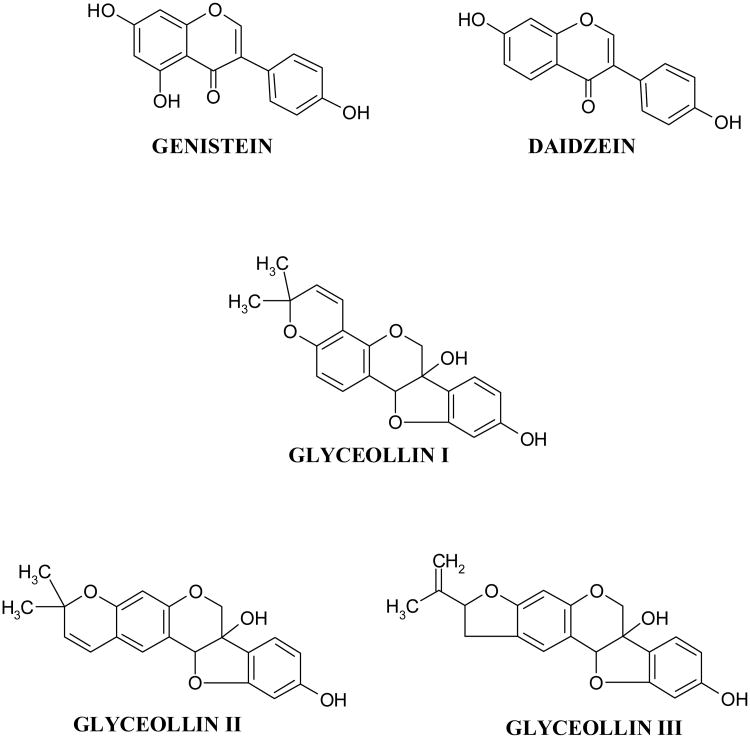
Of potential interest among the diet-derived compounds are the isoflavones, including genistein and daidzein (Figure 1) that are rich in soy products [5]. The isoflavones are also known as phytoalexins [6]. Phytoalexins constitute a chemically heterogeneous group of low molecular weight antimicrobial compounds that are synthesized de novo and accumulate in plants in response to stress [7,8]. Soy contains several phytoalexins including the constitutive isoflavones daidzein and genistein that are considered as candidate diet-derived prostate cancer preventive compounds [9]. Initial interest in these compounds arose from studies that correlate consumption of soy products in Asian countries with a decreased incidence of hormone dependent cancers such as those of the mammary and prostate glands [10, 11]. Hence, a possible use for these compounds in mammary and prostate cancer chemoprevention has been suggested [3, 5, 10]. Of the various soy-derived compounds, genistein has received the most interest due to its potent biological activity [12, 13]. Consumption of genistein has been shown to be protective against prostate cancer in animal models [12, 13]. We, as well as others, have reported an effect of genistein on various cellular pathways in cell culture models, including proliferation, apoptosis, cell cycle, and steroid hormone-mediated pathways [12-17]. Genistein and daidzein can exert universal inhibitory effects on androgen responsive genes including prostate specific antigen (PSA) in the androgen responsive human prostate cancer cell LNCaP [16, 17]. The mechanisms by which genistein and daidzein exert their effects appeared to be through both androgen as well as estrogen receptor (ER) beta-mediated events [16, 17].
Figure 1.
Structures of the soy isoflavone phytoalexins genistein, daidzein, glyceollin I, glyceollin II, and glyceollin III.
In addition to genistein and daidzein, the glyceollins represent another group of phytoalexins whose biosynthesis is increased in response to stress signals [7, 8]. The glyceollin isomers I–III (Figure 1) have core structures similar to that of coumestrol and are derived from the precursor daidzein. The glyceollins (I-III) can be derived from exposure of soybean to the fungus Aspergillus sojae, a nontoxin-producing Aspergillus strain commonly used in the fermentation of soybeans to produce soy sauce and miso [18]. Compared with genistein and daidzein, purified glyceollins show greater inhibition of estradiol's effects on proliferation and ER signaling in breast cancer cells [19]. Glyceollins also have enhanced antagonism toward ER-α relative to ER-β, and lack the estrogen agonist activity of genistein and daidzein seen in low-estrogen conditions [19]. These findings suggest that soy protein enriched with glyceollins may have distinct estrogen-modulating properties compared with standard soy protein. However, the effects of the glyceollins towards prostate cancer remain unclear.
In the present study, we tested the hypothesis that glyceollins, due to their structural similarity to genistein, may have similar activity towards human androgen responsive prostate cancer cells LNCaP. We found glyceollins exert growth inhibitory effects on LNCaP cells. The mechanisms by which the glyceollins exert its effects appeared to be through inhibition of cell cycle by up-regulation of multiple cyclin inhibitors. In addition, we also observed inhibitory effects of glyceollins on the androgen-responsive genes, including PSA mRNA and protein levels. These effects of glyceollins appeared to be through a mechanism(s) involving inhibition of only the estrogen-mediated pathway that is different from genistein.
Materials and Methods
Chemicals
Dihydrotestosterone (DHT), dimethylsulfoxide (DMSO), and genistein, 17β-estradiol were from Sigma Chemical Co. (St. Louis, MO). Cell culture media and reagents were purchased from Invitrogen (Carlsbad, CA)
Soybean treatment and harvesting
Aspergillus sojae (SRRC 1125) cultures were grown at 25°C in the dark on potato dextrose agar. After 5 days, inoculum was prepared by harvesting conidia (3.4 × 107/ml) in 15 ml sterile, distilled H2O. Seeds from commercial soybean variety Asgrow 5902 were surface-sterilized for 3 min in 70% ethanol followed by a quick deionized-H2O rinse and two 2 min rinses in deionized-H20. Seeds were presoaked in sterile deionized-H2O for 4-5 hr, and then chopped for 2 min in a Cuisinart food processor. Aspergillus. sojae spore suspension (300 ml) was applied to the cut surface of seeds on each tray. All trays were stored at 25° C in the dark for three days, rinsed with water to remove spores, and oven dried at 40° C for 24 hrs. Seeds were ground using a Waring blender before extraction.
Isolation of glyceollins (I-III)
The glyceollins I, II, and III were extracted from the 300g ground seeds with 1L methanol. The glyceollins were isolated using preparative scale HPLC using two Waters 25 mm 10 mm particle size mBondapak C18 radial compression column segments combined using an extension tube. HPLC was performed on a Waters 600E System Controller combined with a Waters UV-VIS 996 detector. Elution was carried out at a flow rate of 8.0 ml/min with the following solvent system: A = acetonitrile, B = water; 5% A for 10 min, then 5% A to 90% A in 60 min followed by holding at 90% A for 20 min. The injection volume was 20 mL. The fraction containing the glyceollins was concentrated under vacuum and freeze-dried. The glyceollins were confirmed by UV-VIS spectrophotometry, mass spectrometry, and NMR as described previously [19]. The solvents acetonitrile (HPLC grade) and methanol were purchased from Aldrich Chemical Company. Water was obtained using a Millipore system and used during sample preparation procedures and HPLC analyses. A mixture of glyceollins I (68%), II (21%), and III (11%) were isolated (see Fig 1) and used in treatments. An average MW of 338 was use to calculate the concentration of glyceollins used in all cell culture experiments.
Cells and cell culture
LNCaP and PC-3 human prostate cancer cells were obtained from the American Type Culture Collection (Manassas, VA) and maintained in Media A [RPMI 1640 medium with phenol red (Invitrogen, Carlsbad, CA), 2 mM L-glutamine (Sigma), 100 U/mL penicillin and 100 μg/mL streptomycin (BioSource International, Camarillo, CA) with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA)]. Cells were incubated in the presence of 5% CO2 in air at 37 °C.
Cell growth assay
LNCaP or PC-3 cells (5×104 cells/well) were plated in 24-well plates (Costar); treatments were begun 24h later. Cells were treated with 0, 1, 5, 10 or 25 μM glyceollins or genistein (DMSO as vehicle) for 0-72 h, and the medium containing test compound was replaced every 24 h. Cell growth was analyzed using the sulforhodamine B (SRB) assay [16]. For experiments using the DHT or 17β-estradiol, cells were switched to Media B [RPMI 1640 medium without phenol red, 2 mM L-glutamine (Sigma), 100 U/mL penicillin and 100 μg/mL streptomycin with 10% charcoal dextran-treated FBS (CDS, Hyclone, Logan, UT)] 24 h after plating to minimize the effect of serum hormones. The cells were then incubated in Media B for an additional 24 hours before the treatments were begun.
Cell cycle analysis using flow cytometry
LNCaP or PC3 cells (3 ×106 cell) were seeded into T-175 flask in Medium A. Twenty-four hours later the medium was changed to that containing vehicle or test compounds. Concentration dependent effects of glyceollins (0- 25 μM was studied in LNCaP cell. In PC-3 cell, comparisons were made between cells treated with or without 25μM glyceollins. For genistein, comparison were made between cell treated with or without 25 μM genistein in both LNCaP and PC-3 cells. Cells were treated for 72 hours and harvested, transferred into centrifuge tubes (50 mL polypropylene) pellet (1000×g), wash 1× in PBS (no Ca or Mg) and pelleted again. Cell pellets were then re-suspended in 1.5 mL PBS. To re-suspended cells, 15 mL of 70% ethanol was added and the capped tubes vortexed gently. The ethanol fixed cells were pelleted and washed one time in PBS. Washed cells were fixed in ethanol and stained for DNA content using propidium iodide (PI) [20]. The cellular DNA was then analyzed by flow cytometry. DNA content of the cells was determined flow cytometry using a FACScalibur cytometer (Becton Dickinson, San Jose, CA). Flow cytometric data files were collected and analyzed using the CELLQuest program (Becton Dickinson). A total of 10,000 cell events were collected for DNA analyses. Cell cycle distribution percentages of stained nuclei were calculated using Modfit LT software (Version 3.0, Verity Software House, Inc., Topsham, ME). Calibration standards (LinearFlow Green and DNA QC Particle Kit) for verification of instrument performance were purchased from Molecular Probes (Eugene, OR) and Becton Dickinson, respectively.
Determination of the effects of glyceollins on gene expression in LNCaP cells using RT-PCR
To examine the effects of glyceollins on cyclin inhibitor CDKN1A and B mRNA expression, LNCaP cells were plated in 6-well plates (0.25 × 106 cells/well) in Media A. After twenty-four hours the medium was removed and replaced with fresh medium containing vehicle, 1, 5, or 25 μM glyceollins or genistein. For experiments examining the effects of glyceollins on steroid hormone, LNCaP cells were plated in 6-well plates (0.25 × 106 cells/well) in Media A and switched to Media B containing 10% CDS 24 h after plating to minimize the effect of serum hormones. Twenty-four hours later, the medium was replaced with fresh medium containing 1 nM DHT or 17β-estradiol with or without 0-25 μM glyceollin. For all experiments fresh medium containing the test compounds was changed daily and cells were harvested for total RNA isolation using the Trizol method (Invitrogen) after 48 h [16, 17]. Taqman real-time PCR was used to quantify expression of the mRNA [16, 17]. Taqman real-time PCR Primer and probes for glyceraldehydes-3-phosphate dehydrogenase (G3PDH), PSA, cyclin-dependent kinase inhibitor (CDKN) 1A and CDKN1B, NKX3.1(NK3 homeobox 1), Bcl-2, Bax and insulin like growth factor-1 receptor (IGF-1R) were purchased form Applied Biosystems (Foster City, CA).
Apoptosis assay
Activation of caspase was used as an additional method to flow cytometry to detect apoptosis. LNCaP cells (1×106 cells/well) were plated in 6-well plates and 24 hrs later the glyceollins (25 μM final concentration) were added. After 72 hrs of treatment with or without the test compounds, cells were washed with PBS once and lysed in cell lysis buffer (Biosource, Camarilo, CA). Protein was determined using the BCA method (Pierce, Rockford, IL) according to manufacturer's protocol. Fifty μg of lysate was used for determination of caspase activity using the Caspase-Glo 3/7 Assay (Promega, Medison, WI) following manufacturer's protocol.
Western Blots
LNCaP cells were plated in 100mm × 20mm cell culture dish in Media A and switched to Media B containing 10% CDS 24 hours after plating to minimize the effect of serum hormones. Twenty-four hours later, the medium was replaced with fresh medium containing vehicle (DMSO) or 25 μM glyceollins. Fresh medium containing the test compound was changed daily and cells were harvested for Western Blot analysis after 72 hours of treatments. The lysed extracts were collected, than centrifuged at 10000 × g for 10 minutes. The supernatants were used to determine the protein concentration. Following this, the supernatant, sample buffer, and reducing agent were added and the samples were heated at 105°C and loaded onto a 4-12% gradient SDS-PAGE gel (Invitrogen, Carlsbad, CA). Gels were then transferred to nitrocellulose membranes and the membranes were probed with mouse anti-p21Waf1/Cip1and rabbit anti-p27Kip1 at a 1:1000 dilution as primary antibodies (Cell Signaling, Danvers, MA) followed by incubation with IR-tagged secondary antibodies (LiCor Biosciences, Lincoln, NE). The blots were analyzed using the Odyssey Infrared Imaging System (LiCor Biosciences, Lincoln, NE). The conditions for PSA western analysis were performed as described previously [16].
Statistics
All treatments were repeated at least 3 times and representative experiments were presented. Experimental data were analyzed using the Prism 4 statistical software package (GraphPad software.). Unpaired t tests were used for two group comparisons. For multiple group comparisons, ANOVA followed by post hoc analysis using Bonferroni's test were employed. Treatments effects with a p value of < 0.05 were considered significant.
Results
Glyceollins inhibited LNCaP cell growth and affected cell cycle progression
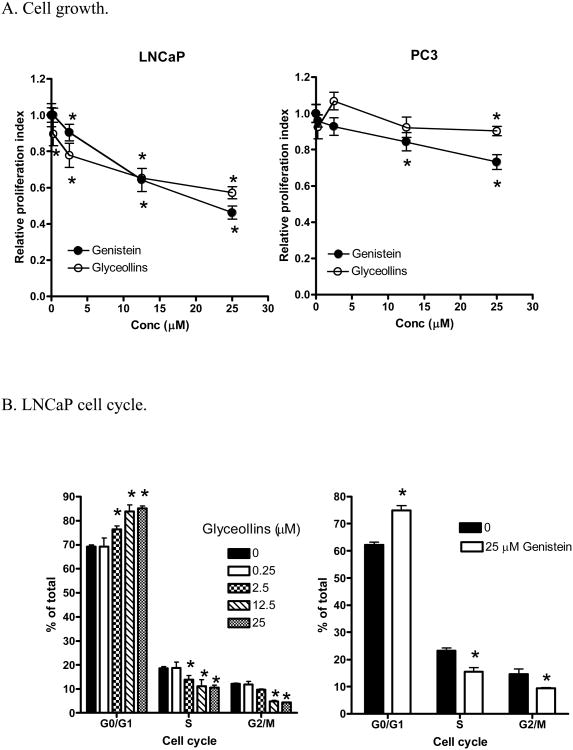
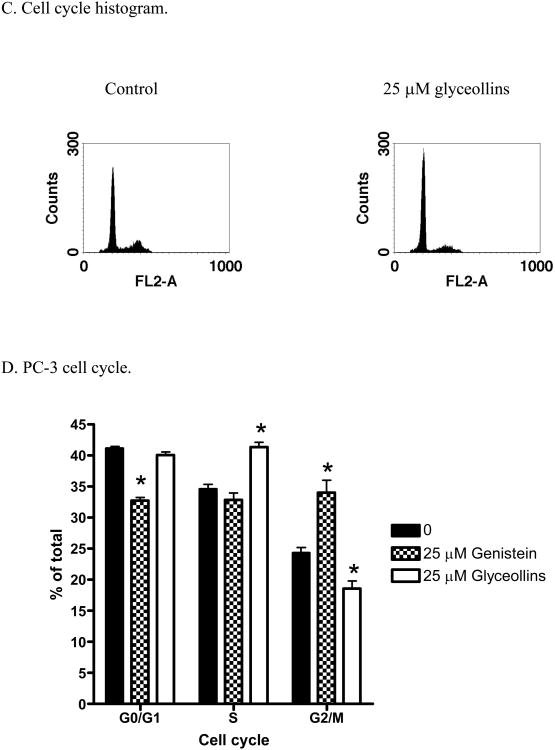
To examine the effects of glyceollins on prostate cancer prevention, we first tested the effects of glyceollin on LNCaP cell growth. As shown in Figure 2A (left panel), after 72 hours treatment, glyceollins inhibited LNCaP cell growth in a concentration-dependent manner. The inhibitory effects of the glyceollins can be observed at 2.5 μM. The growth inhibitory effects of the glyceollins on LNCaP cells were similar to that observed for genistein (Figure 2 A, left panel, ref.16). However, in the androgen non-responsive prostate cancer cell PC-3 (Figure 2 A, right panel), the effects of glyceollins were attenuated. Similar attenuated effects were also observed for genistein in PC-3 cells (Figure 2A, right panel). To further elucidate the mechanism(s) by which the glyceollins treatment resulted in growth inhibition, we also examined the effects of the glyceollins on cell cycle progression. As shown in Figure 2B left panel and 2C, treatment of LNCaP cells with the glyceollins for 72 hours led to concentration-dependent effects on G1/S arrest. Similarly, treatment of LNCaP cells with genistein (25 μM) for 72 hours also lead to G1/G0 arrest (Figure 2B right panel). By contrast, genistein (25 μM) and not glyceollins (25 μM) treatments for 72 hours lead to G2/M blockage in PC-3 cells (Figure D). Glyceollins treatment appeared to lead to S phase blockages in PC3 Cells (Figure 2D). The cell cycle analysis did not reveal any significant effects of the glyceollins on apoptotic events as indicated by lack of sub-2N PI staining of DNA (Figure 2C). Additionally, we also did not observe induction of the caspase 3/7 activation in glyceollins treated LNCaP cells.
Figure 2.
Effects of glyceollins and genistein on prostate cancer cell growth and cell cycle.
A. Effect of glyceollins and genistein on prostate cancer cell growth. LNCaP or PC-3 cells (0.25×106 cells/well) were plated on 6-well plates. Cell treatments with varied concentrations (0-25 μM) of glyceollins or genistein were started 24 hours later for an additional 72 hours, and cell number determined as described in Materials and Methods.
* represent significantly different from control at p<0.05 (n=6). Left panel: LNCaP; Right Panel: PC-3. B. Effect of glyceollins and genistein on cell cycle in LNCaP cells. LNCaP cells (3 ×106 cell) were plated in T-175 flask and treated with 0, 0.25, 2.5, 12.5, or 25 μM glyceollins or with and without genistein (25 μM) for 72 hr, cell cycle analysis performed as described in Material and Methods. Results are expressed as % of total cells (n=3). Left panel: effects of glyceollins. Right panel: effects of genistein. C.
Representative histogram of effects of glyceollins (25 μM) on LNCaP cells. Histogram illustration of results for control and LNCaP cells treated with 25 μM glyceollins from panel B. D. Effects of glyceollins and genistein on cell cycle in PC-3. PC-3 cells (3 ×106 cell) were plated in T-175 flask and treated with or without 25 μM of glyceollins or genistein for 72 hr, cell cycle analysis performed as described in Material and Methods. Results are expressed as % of total cells (n=3).
Glyceollins modulate cyclin-dependent kinase inhibitors mRNA levels in LNCaP cells
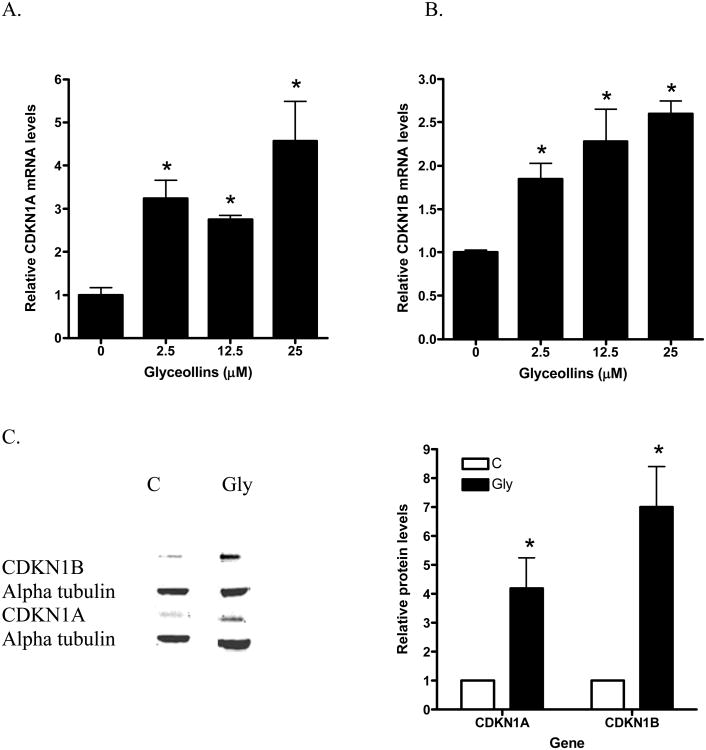
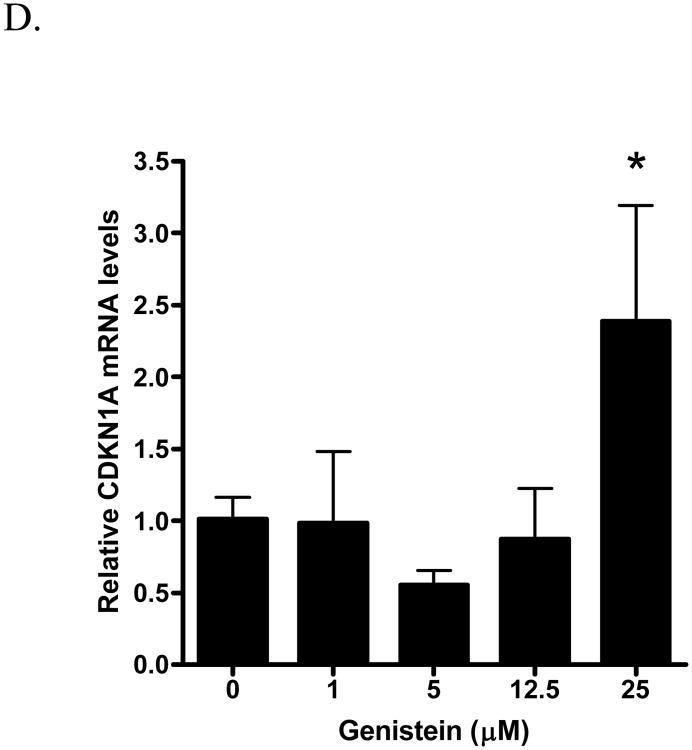
The cyclin-dependent kinase inhibitors CDKN1A and B mRNA expression are modulated during cell cycle progression and are involved in G1/S arrest [21, 22]. To gain additional perspective at the molecular levels of the glyceollins growth inhibitory effects, we also determined the effects of the gylceollins on CDKN1A and B mRNA levels in LNCaP cells. As shown in Figures 3 A and B, after 48 hours treatment, glyceollins appeared to induce both CDKN1A and B mRNA levels. There were significant changes at 2.5 μM for both CDKN1A and B mRNA levels. Up regulation of these cyclin inhibitors were confirmed at the protein level (Figure 3C). By contrast we only observed an induction of CDKN1A mRNA by genistein at 25 μM (Figure 3D), there were no changes in CDKN1B mRNA levels in LNCaP cells treated with genistein at all concentrations (0-25 μM) tested. Consistent with lack of effect of the glyceollins on apoptosis, we also did not detect alteration in either Bax or Bcl-2, two well documented regulators of apoptosis pathways [23], mRNA expression.
Figure 3.
Effect of glyceollins and genistein on cyclin inhibitors CDKN1A and B mRNA levels in LNCaP cells.
A. Effect of glyceollins on CDKN1A mRNA levels. LNCaP cells cultured in 10% FBS were treated with 0, 2.5, 12.5, or 25 μM glyceollins for 48 h, total RNA isolated and mRNA levels of CDKN1A determined as described in Materials and Methods. Results are expressed as mean +/- SD (n=3). B. Effects of glyceollins on CDKN1B mRNA levels. LNCaP cells cultured in 10% FBS were treated with 0, 2.5, 12.5, or 25 μM glyceollins for 48 h, total RNA isolated and mRNA levels of CDKN1A determined as described in Materials and Methods. Results are expressed as mean +/- SD (n=3). C. Effects of glyceollins on CDKN1A and B protein levels. LNCaP cell were treated with and without 25 μM Glyceollins for 72 hours, cell were harvested and CDKN1A and B protein determined by western analysis as described in Materials and Methods. Intensity of the bands were normalized to control and expressed as % of control (mean +/- SD, n=3). * represents significantly different from vehicle (DMSO) control at p<0.05. D. Effects of genistein on CDKN1A mRNA levels. LNCaP cells cultured in 10% FBS were treated with 0, 1, 5, 12.5 or 25 μM genistein for 48 h, total RNA isolated and mRNA levels of CDKN1A determined as described in Materials and Methods. Results are expressed as mean +/- SD (n=3).
Effects of glyceollins on androgen- and estrogen-induced growth
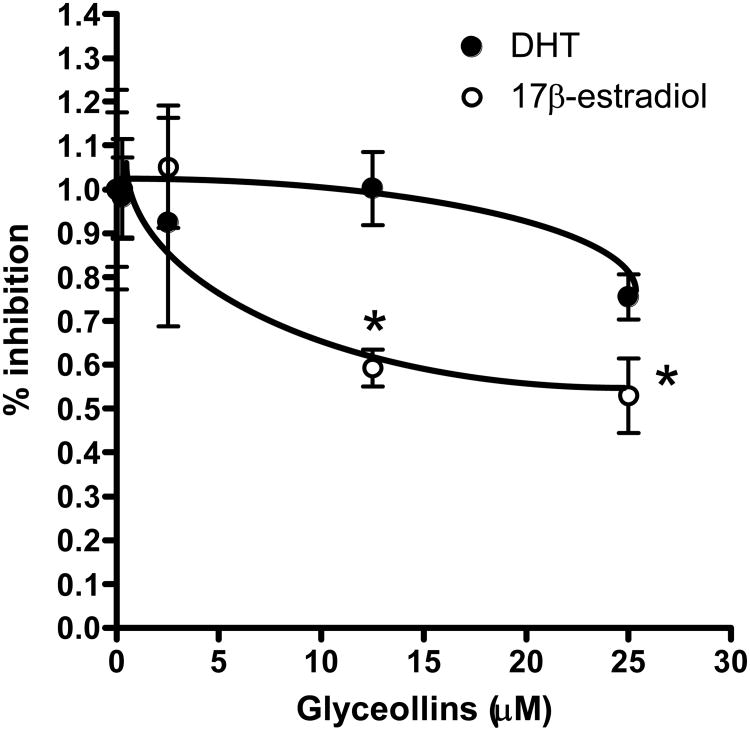
Prostate cancer LNCaP cell growth can be subject to modulation by androgen as well as estrogen [24]. To further identify proximal events modulated by the glyceollins that result in cell cycle arrest and growth inhibition, we examined the effects of the glyceollins on DHT (1 nM) and 17β-estradiol (10 nM) induced LNCaP cell growth. The concentration of steroid hormones was chosen based on their physiological achievable levels as well as in-vitro efficacy [24-26]. As shown in Figure 4, after 72 hr treatment of LNCaP cells with the glyceollins led to an inhibition of 17β-estradiol-induced growth, but not DHT-induced growth of LNCaP cells.
Figure 4.
Effect of glyceollins on DHT- and 17β-estradiol-mediated growth in LNCaP cells.
LNCaP cells (0.25×106 cells/well) were plated in 6-well plate. Twenty-four hours later media was change to Media B that containing 10% CDS, followed 24 hours later with addition of varied concentrations (0-25 μM) of glyceollins in presence or absence of DHT (1 nM) or 17 β -estradiol (10 nM) begin. Cells were treated for 72 hours and cell number determined as described in Materials and methods. * represents significantly different from DHT or 17β-estradiol control at p<0.05 (n=6)
Effects of glyceollins on androgen-and estrogen-induced genes mRNA levels in LNCaP cells
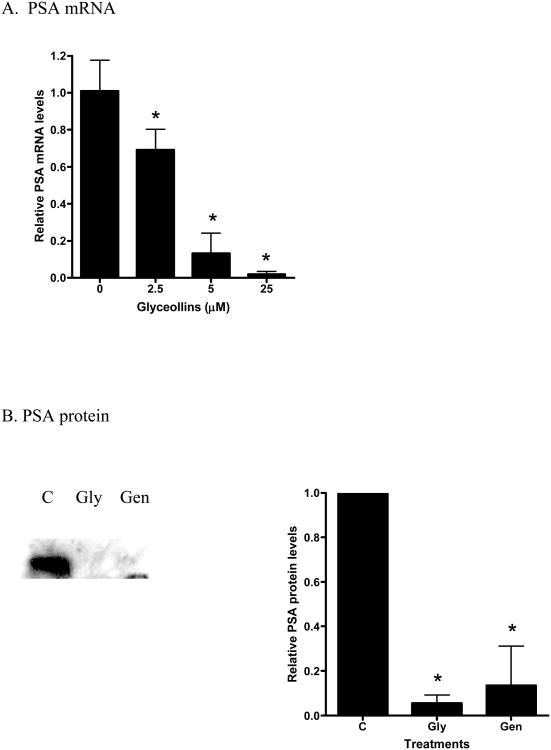
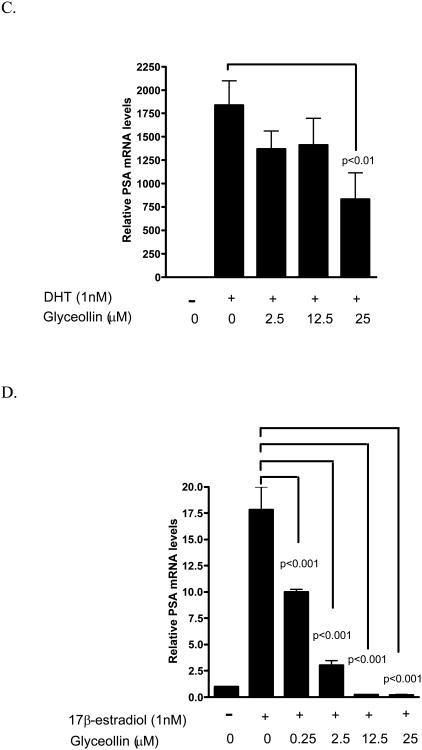
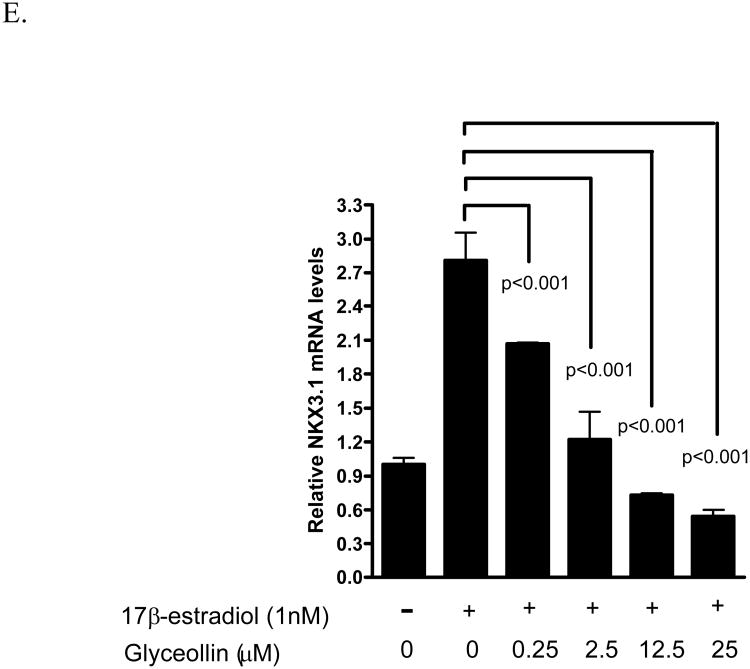
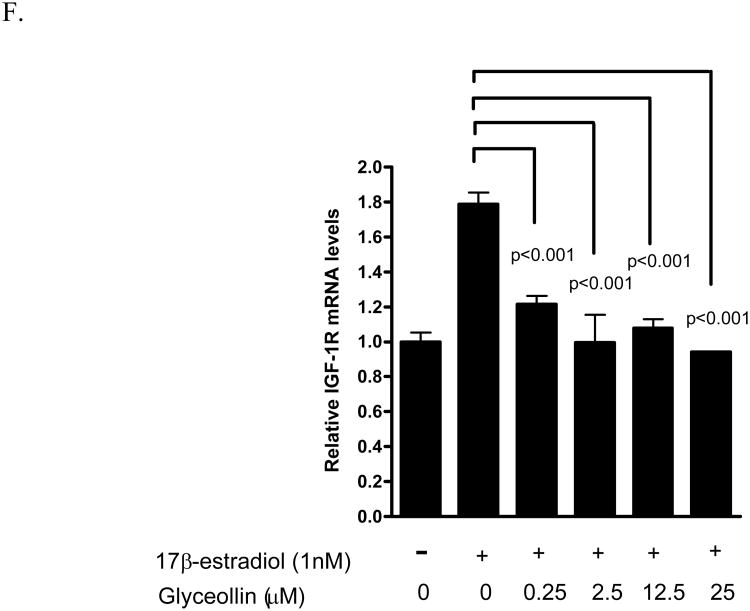
We have previously shown that the androgen responsive genes PSA not only respond to androgen, but also to 17β-estradiol through ER- β-mediated events [24]. Hence, we examined the effects of glyceollins on this gene as a surrogate end point to further elucidate the effects of the glyceollins on the androgen- and estrogen- responsive pathways. As shown in Figure 5 A and B, glyceollins treatment of LNCaP cells cultured in 10% FBS for 48 hr led to an attenuation of PSA mRNA and protein levels supporting the hypothesis of possible hormonal regulation. This effect of glyceollins is similar to that of genistein on PSA mRNA we reported earlier [16] and protein (Figure 5B). We also ask whether this effect of glyceollins is through an androgen- or estrogen-dependent pathway. As shown in Figure 5C, the glyceollins appeared not to be effective in inhibiting the DHT-induced increase in PSA mRNA levels. It required at least 25 μM of glyceollins to produce a significant inhibition of DHT-induced PSA mRNA levels. In contrast, the glyceollins effectively inhibited the 17β-estradiol–induced increase in PSA mRNA levels (Figure 5D). The effect can be seen starting at a concentration of 0.25 μM. Furthermore we also observed inhibition of 17β-estradiol-induced increase in two other androgen/estrogen responsive genes, NKX3.1 (Figure 5 E) and IGF-1R (Figure 5F) mRNA levels. The concentration effects of glyceollins on these two genes were similar to the effect on PSA mRNA and occur at 0.25 μM.
Figure 5.
Effect of glyceollins on DHT- and 17 β -estradiol-mediated genes expressions.
A. Effect of glyceollins on PSA mRNA levels in LNCaP cell cultured in 10% FBS. LNCaP cells cultured in 10% FBS were treated with 0, 2.5, 12.5, or 25 μM glyceollins for 48 h, total RNA isolated and mRNA levels of CDKN1A determined as described in Materials and Methods. Results are expressed as mean +/- SD (n=3). B. Effect of glyceollins and genistein on PSA protein levels in LNCaP cell cultured in 10% FBS. LNCaP cells cultured in 10% FBS were treated with or with 25 μM glyceollins or genistein for 72 hours, cell harvested and PSA protein determined using western analysis as described in Materials and Methods. Intensity of the bands were normalized to control and expressed as % of control (mean +/- SD, n=3). * represents significantly different from vehicle (DMSO) control at p<0.05. C. Effect of glyceollins on DHT-induced increase in PSA mRNA levels. LNCaP cells were plated in 6-well plates; 24 h after plating the medium was switched to Media B, which contains 10% CDS, for an additional 24 h. Cells were then treated with or without DHT (1 nM) in the presence or absence of glyceollins (0, 2.5, 12.5, 25 μM) for 48 h, total RNA isolated, and mRNA levels of PSA were determined as described in Materials and Methods. Results are expressed as mean+/- SD (n=3). Lines and the respective p value indicate comparison of treatments that are significantly different from each other D. Effect of glyceollins on 17β-estradiol induced-increase in PSA mRNA levels. LNCaP cells were plated in 6-well plates; 24 h after plating the medium was switched to Media B, which contains 10% CDS, for an additional 24 h. Cells were then treated with or without 17β-estradiol (1 nM) in the presence or absence of glyceollins (0, 0.25, 2.5, 12.5, 25 μM) for 48 h, total RNA isolated, and mRNA levels of PSA were determined as described in Materials and Methods. Results are expressed as mean +/- SD (n=3). Lines and the respective p value indicate comparison of treatments that are significantly different from each other E. Effect of glyceollins on 17β-estradiol induced-increase in NKX3.1 mRNA levels. LNCaP cells were plated in 6-well plates; 24 h after plating the medium was switched to Media B, which contains 10% CDS, for an additional 24 h. Cells were then treated with or without 17β-estradiol (1 nM) in the presence or absence of glyceollins (0, 0.25, 2.5, 12.5, 25 μM) for 48 h, total RNA isolated, and mRNA levels of selected NKX3.1 were determined as described in Materials and Methods. Results are expressed as mean +/- SD (n=3). Lines and the respective p value indicate comparison of treatments that are significantly different from each other F. Effect of glyceollins on 17β-estradiol induced-increase in IGF-1R mRNA levels. LNCaP cells were plated in 6-well plates; 24 h after plating the medium was switched to Media B, which contains 10% CDS, for an additional 24 h. Cells were then treated with or without 17β-estradiol (1 nM) in the presence or absence of glyceollins (0, 0.25, 2.5, 12.5, 25 μM) for 48 h, total RNA isolated, and mRNA levels of IGF-1R were determined as described in Materials and Methods. Results are expressed as mean +/- SD (n=3). Lines and the respective p value indicate comparison of treatments that are significantly different from each other.
Discussion
Currently there is much research examining the use of soy as a rich source of cancer preventive phytochemicals [5, 10, 12,13]. In addition to the isoflavones genistein and daidzein, the glyceollins may represent candidate cancer preventive agents [19, 27]. However, available information on the effects of the glyceollins on prostate cancer remains scarce. In the present study, we demonstrated that the glyceollins exert growth inhibitory effects on the human androgen responsive prostate cancer cells LNCaP (Figure 2A). The effects of the glyceollins on cell growth appeared to be through modulation of the cell cycle because treatment of LNCaP cells with the glyceollins led to G1/S arrest (Figure 2B and C). These effects of the glyceollins on LNCaP cells are similar to the effects of genistein (Figure 2A,B, C) that we and others have previously described [13, 16, 28, 29]. The molecular effects of the glyceollins that resulted in cell cycle inhibition were associated with induction of both CDKN1A and CDKN1B, cyclin-dependent kinase inhibitors that are involved in G1/S arrest [21, 22], mRNA and protein levels (Figure 3A-C). Both cyclin-dependent kinase inhibitors are transcriptionally regulated to induce cell cycle arrest [21, 22]. The up-regulation of mRNA and protein for these cyclin inhibitors by glyceollins are consistent with the overall inhibitory effects of the glyceollins on cell cycle and growth. This effect of glyceollins appeared to be different from that of genistein, as treatments of LNCaP cells with genistein only led to induction of CDKN1A (Figure 3D), but not CDKN1B mRNA levels. Thus, mechanistically the two phytoalexins appeared to act through different pathways that lead to cell cycle arrest and growth inhibition. Moreover, the effects of the glyceollins appeared not to be involved in apoptosis, as we did not observe an increase in PI staining of DNA less than 2N (Figure 2C), lack of caspase 3/7 activation, as well as lack of changes in mRNA of apoptosis related proteins Bax and Bcl-2. By contrast, genistein at high concentration (27 μM) are known to up-regulate Bax and induce apoptosis [28].
We have previously shown both androgen and estrogen can contribute to the growth of LNCaP cells [24]. The inhibitory effects of the glyceollins on LNCaP cell growth and cell cycle appeared to be due, in part, to the effects of the glyceollins on a 17β-estradiol-mediated event and not an androgen-mediated event (Figure 4). This is supported by our observation that the glyceollins inhibited 17β-estradiol, but not DHT-induced LNCaP cell growth in 10% CDS (Figure 5). Moreover, in the androgen non-responsive cell PC-3, the growth inhibitory effects of glyceollins as well as genistein were attenuated. This is consistent with our previous observation that 17β-estradiol-mediated event requires androgen receptors [24]. This effect of the glyceollins is also different from that of other soy isoflavones, such as genistein, which appear to be able to block both androgen as well as estrogen-induced growth in LNCaP cells [16]. Interestingly, unlike their activity in breast cancer cells such as estrogen receptor positive MCF-7 cells [19, 30], where genistein is estrogenic and glyceollin is anti-estrogenic, both genistein [16] and the glyceollins appear to act as anti-estrogens in LNCaP cells. The effects of glyceollins and genistein on PC-3 cell cycle also appeared different. Genistein act on G2/M but glyceollins appeared to act on S blockage. The significant of these observations warrant further study.
Consistent with the glyceollins inhibition of 17β-estradiol-induced growth (Figure 4), glyceollins inhibited the induction of PSA mRNA levels by 17β-estradiol (Figure 5C). Also consistent with the lack of effect of glyceollins on androgen-stimulated growth (Figure 4), the glyceollins are at least 100-fold less effective in inhibiting DHT-induced increase in PSA mRNA levels than that of 17β-estradiol -induced increase in PSA in LNCaP cells (Figure 5B). Further supporting an effect of glyceollins on estrogen-mediated pathway, we observed inhibition of 17β-estradiol-induced increase in NKX3.1 and IGF-1R mRNA levels (Figure 5C and D). The effect of the glyceollins on gene expression and growth is similar to that of genistein. Despite genistein's known estrogenic activity [30], the biological effects of genistein on LNCaP cells appeared to be as an anti-estrogen and inhibited 17β-estradiol induction of androgen responsive genes and growth. Although these two phytoalexins appeared to share similar properties at the estrogen-mediated events [16, 19, 30], the glyceollins appeared to be different from genistein or daidzein. The glyceollins showed very little effect on androgen (DHT)-stimulated events in LNCaP cells. In contrast, genistein and daidzein appeared to affect both androgen as well as estrogen-mediated events in LNCaP cells [16].
We have previously shown that 17β-estradiol induced PSA protein and mRNA through an ER-β-dependent event [24]. Exposure to estrogen is thought to be involved in prostate carcinogenesis [31]. It is possible that the glyceollins may exert prostate cancer protective effects through its anti-estrogenic activity that involves ER-β. The observed anti-estrogenic effects of glyceollins are consistent with other observations that the glyceollins appear to serve as an antagonist for ER-α as well as ER-β [19]. The effects of the glyceollins on IGF-1R may also be important since IGF-1R mediates the effects of IGF-1 [32] that are involved in prostate cancer carcinogenesis [32, 33]. Thus, the glyceollins as well as other soy phytochemicals such as the constitutive soy isoflavones, appear to modulate signaling events that are involved in promoting prostate cancer. This data suggests that the phytoalexins from soy as a whole may affect hormone-responsive pathways, thereby contributing to soy's prostate cancer protective effects.
In summary, the present study provides molecular and cellular data on glyceollin's effects in the androgen-responsive prostate cancer cells LNCaP. The effects of the glyceollins appear to be mediated through interaction with an estrogen-responsive pathway. In addition, soy glyceollins appear to act differently when compared to genistein. Hence, the glyceollins inhibitory effects on prostate cancer cells warrant further consideration of their use in soy-based foods and supplements for prostate cancer prevention
Acknowledgments
Funding supports: This work was supported by U.S appropriated funds to USDA project number 1235-51530-052-00 (TTYW, NWS), 59-6435-7-188 (TEC, SMB) and the National Cancer Institute (YSK).
Abbreviations
- CDS
charcoal dextran-treated FBS
- CDKN
cyclin-dependent kinase inhibitor
- DHT
Dihydrotestosterone
- DMSO
dimethylsulfoxide
- ER
estrogen receptor
- FBS
fetal bovine serum
- G3PDH
glyceraldehydes-3-phosphate dehydrogenase
- IGF-1R
insulin like growth factor-1 receptor
- NK3 homeobox 1
NKX3.1
- PI
propidium iodide
- PSA
prostate specific antigen
- SRB
sulforhodamine B
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Shirai T, Asamoto M, Takahashi S, Imaida K. Diet and prostate cancer. Toxicology. 2002:181–182. 89–94. doi: 10.1016/s0300-483x(02)00260-3. [DOI] [PubMed] [Google Scholar]
- 3.Greenwald P, Clifford CK, Milner JA. Diet and cancer prevention. Eur J Cancer. 2001;37:948–965. doi: 10.1016/s0959-8049(01)00070-3. [DOI] [PubMed] [Google Scholar]
- 4.Greenwald P. Clinical trials of breast and prostate cancer prevention. J Nutr. 2001;131:176S–178S. doi: 10.1093/jn/131.1.176S. [DOI] [PubMed] [Google Scholar]
- 5.Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutr Cancer. 2006;55(1):1–12. doi: 10.1207/s15327914nc5501_1. [DOI] [PubMed] [Google Scholar]
- 6.Dakora FD, Phillips DA. Diverse functions of isoflavonoids in legumes transcend anti-microbial definitions of phytoalexins. Physiol Molecular Plant Physiol. 1996;49:1–20. [Google Scholar]
- 7.Darvill AG, Albersheim P. Phytoalexins and their elicitors: a defense against microbial infection in plants. Annu Rev Plant Physiol. 1984;35:243–275. [Google Scholar]
- 8.Paxton JD. Biosynthesis and accumulation of legume phytoalexins. In: Sharma RP, Salunkhe DK, editors. Mycotoxins and phytoalexins. Boca Raton (FL): C RCPress; 1991. pp. 485–499. [Google Scholar]
- 9.Bemis DL, Katz AE, Buttyan R. Clinical trials of natural products as chemopreventive agents for prostate cancer. Expert Opin Investig Drugs. 2006;15(10):1191–1200. doi: 10.1517/13543784.15.10.1191. Review. [DOI] [PubMed] [Google Scholar]
- 10.Messina MJ. Emerging evidence on the role of soy in reducing prostate cancer risk. Nutr Rev. 2003;61:117–131. doi: 10.1301/nr.2003.apr.117-131. [DOI] [PubMed] [Google Scholar]
- 11.Ziegler RG, Hoover RN, Pike MC, et al. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993;85:1819–1827. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]
- 12.Lamartiniere CA, Cotroneo MS, Fritz WA, Wang J, Mentor-Marcel R, Elgavish A. Genistein chemoprevention: timing and mechanisms of action in murine mammary and prostate. J Nutr. 2002;132:552S–558S. doi: 10.1093/jn/132.3.552S. [DOI] [PubMed] [Google Scholar]
- 13.Sarkar FH, Li Y. Mechanisms of cancer chemoprevention by soy isoflavone genistein. Cancer Metastasis Rev. 2002;21:265–280. doi: 10.1023/a:1021210910821. [DOI] [PubMed] [Google Scholar]
- 14.El Touny LH, Banerjee PP. Akt GSK-3 pathway as a target in genistein-induced inhibition of TRAMP prostate cancer progression toward a poorly differentiated phenotype. Carcinogenesis. 2007;28(8):1710–1707. doi: 10.1093/carcin/bgm103. [DOI] [PubMed] [Google Scholar]
- 15.Guo Y, Wang S, Hoot DR, Clinton SK. Suppression of VEGF-mediated autocrine and paracrine interactions between prostate cancer cells and vascular endothelial cells by soy isoflavones. J Nutr Biochem. 2007;18(6):408–417. doi: 10.1016/j.jnutbio.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi Y, Hursting SD, Perkins SN, Wang TC, Wang TT. Genistein affects androgen-responsive genes through both androgen- and estrogen-induced signaling pathways. Mol Carcinog. 2006;45(1):18–25. doi: 10.1002/mc.20153. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi Y, Lavigne JA, Hursting SD, Chandramouli GV, Perkins SN, Barrett JC, Wang TT. Using DNA microarray analyses to elucidate the effects of genistein in androgen-responsive prostate cancer cells: identification of novel targets. Mol Carcinog. 2004;41(2):108–119. doi: 10.1002/mc.20045. [DOI] [PubMed] [Google Scholar]
- 18.Chang TS, Ding HY, Tai SS, Wu CY. Metabolism of the soy isoflavones daidzein and genistein by fungi used in the preparation of various fermented soybean foods. Biosci Biotechnol Biochem. 2007;71(5):1330–1333. doi: 10.1271/bbb.60573. [DOI] [PubMed] [Google Scholar]
- 19.Burow ME, Boué SM, Collins-Burow BM, Melnik LI, Duong BN, Carter-Wientjes CH, Li S, Wiese TE, Cleveland TE, McLachlan JA. Phytochemical glyceollins, isolated from soy, mediate antihormonal effects through estrogen receptor α and β. J Clin Endocrinol Metab. 2001;86:1750–1758. doi: 10.1210/jcem.86.4.7430. [DOI] [PubMed] [Google Scholar]
- 20.Schoene NW, Kelly MA, Polansky MM, Anderson RA. Water-soluble polymeric polyphenols from cinnamon inhibit proliferation and alter cell cycle distribution patterns of hematologic tumor cell lines. Cancer Lett. 2005;230(1):134–140. doi: 10.1016/j.canlet.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 21.Hong C, Kim HA, Firestone GL, Bjeldanes LF. 3,3′-Diindolylmethane (DIM) induces a G(1) cell cycle arrest in human breast cancer cells that is accompanied by Sp1-mediated activation of p21(WAF1/CIP1) expression. Carcinogenesis. 2002;23(8):1297–1305. doi: 10.1093/carcin/23.8.1297. [DOI] [PubMed] [Google Scholar]
- 22.Fabiani R, Rosignoli P, De Bartolomeo A, Fuccelli R, Morozzi G. Inhibition of cell cycle progression by hydroxytyrosol is associated with upregulation of cyclin-dependent protein kinase inhibitors p21(WAF1/Cip1) and p27(Kip1) and with induction of differentiation in HL60 cells. J Nutr. 2008;138(1):42–48. doi: 10.1093/jn/138.1.42. [DOI] [PubMed] [Google Scholar]
- 23.van Delft MF, Huang DC. How the Bcl-2 family of proteins interact to regulate apoptosis. Cell Res. 2006;16(2):203–213. doi: 10.1038/sj.cr.7310028. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi Y, Perkins SN, Hursting SD, Wang TT. 17beta-Estradiol differentially regulates androgen-responsive genes through estrogen receptor-beta- and extracellular-signal regulated kinase-dependent pathways in LNCaP human prostate cancer cells. Mol Carcinog. 2007;46(2):117–129. doi: 10.1002/mc.20254. [DOI] [PubMed] [Google Scholar]
- 25.Langley RE, Godsland IF, Kynaston H, Clarke NW, Rosen SD, Morgan RC, Pollock P, Kockelbergh R, Lalani elN, Dearnaley D, Parmar M, Abel PD. Early hormonal data from a multicentre phase II trial using transdermal oestrogen patches as first-line hormonal therapy in patients with locally advanced or metastatic prostate cancer. BJU Int. 2008;102(4):442–445. doi: 10.1111/j.1464-410X.2008.07583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castagnetta LA, Miceli MD, Sorci CM, Pfeffer U, Farruggio R, Oliveri G, Calabrò M, Carruba G. Growth of LNCaP human prostate cancer cells is stimulated by estradiol via its own receptor. Endocrinology. 1995 May;136(5):2309–19. doi: 10.1210/endo.136.5.7536668. [DOI] [PubMed] [Google Scholar]
- 275.Salvo VA, Boué SM, Fonseca JP, Elliott S, Corbitt C, Collins-Burow BM, Curiel TJ, Shih BY, Carter-Wientjes C, Wood CE, Erhardt P, Beckman B, McLachlan JA, Cleveland TE, Burow ME. Antiestrogenic glyceollins suppress human breast and ovarian carcinoma tumorigenesis. Clinical Cancer Research. 2006;12(23):7159–7164. doi: 10.1158/1078-0432.CCR-06-1426. [DOI] [PubMed] [Google Scholar]
- 28.Onozawa M, Fukuda K, Ohtani M, Akaza H, Sugimura T, Wakabayashi K. Effects of soybean isoflavones on cell growth and apoptosis of the human prostatic cancer cell line LNCaP. Jpn J Clin Oncol. 1998;28:360–363. doi: 10.1093/jjco/28.6.360. [DOI] [PubMed] [Google Scholar]
- 29.Davis JN, Muqim N, Bhuiyan M, Kucuk O, Pienta KJ, Sarkar FH. Inhibition of prostate specific antigen expression by genistein in prostate cancer cells. Int J Oncol. 2000;16:1091–1097. doi: 10.3892/ijo.16.6.1091. [DOI] [PubMed] [Google Scholar]
- 30.Wang TT, Sathyamoorthy N, Phang JM. Molecular effects of genistein on estrogen receptor mediated pathways. Carcinogenesis. 1996;17:271–275. doi: 10.1093/carcin/17.2.271. [DOI] [PubMed] [Google Scholar]
- 31.Risbridger GP, Ellem SJ, McPherson SJ. Estrogen action on the prostate gland: a critical mix of endocrine and paracrine signaling. J Mol Endocrinol. 2007;39(3):183–188. doi: 10.1677/JME-07-0053. [DOI] [PubMed] [Google Scholar]
- 31.Gennigens C, Menetrier-Caux C, Droz JP. Insulin-Like Growth Factor (IGF) family and prostate cancer. Crit Rev Oncol Hematol. 2006;58(2):124–145. doi: 10.1016/j.critrevonc.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Meinbach DS, Lokeshwar BL. Insulin-like growth factors and their binding proteins in prostate cancer: cause or consequence? Urol Oncol. 2006;24(4):294–306. doi: 10.1016/j.urolonc.2005.12.004. [DOI] [PubMed] [Google Scholar]