Abstract
Purpose
To evaluate long-term outcomes after induction chemotherapy followed by “risk-based” local therapy for squamous cell carcinoma of the head and neck (SCCHN).
Methods
Forty-seven patients (stage IV, ≥N2b) were enrolled in a Phase II trial. Baseline and 24-months functional measures included modified barium swallow (MBS) studies, oropharyngeal swallow efficiency (OPSE), and the MD Anderson Dysphagia Inventory (MDADI). Functional status was assessed at 5 years.
Results
Five-year overall survival was 89% (95% CI: 81%-99%). A non-significant 13% average reduction in swallowing efficiency (OPSE) was observed at 24-months relative to baseline (p=0.191). MDADI scores approximated baseline at 24-months. Among 42 long-term survivors (median=5.9 years), 3 (7.1%) had chronic dysphagia. The rate of final gastrostomy-dependence was 4.8% (2/42).
Conclusion
Sequential chemoradiotherapy achieved favorable outcomes among patients with locally-advanced SCCHN, mainly of oropharyngeal origin. MBS and MDADI scores found modest swallowing deterioration at 2 years, and chronic aspiration was uncommon in long-term survivors.
INTRODUCTON
A majority of patients with squamous cell carcinomas of the head and neck (SCCHN) have stage III-IV disease at diagnosis.1 Among patients who are candidates for non-surgical therapy, concomitant chemoradiotherapy is a standard of care, with goals of locoregional tumor control and functional organ preservation. However, this strategy is often associated with substantial toxicity and adverse functional effects. Severe (grade 3-4) late laryngopharyngeal toxicity was reported in 43% of survivors who had adequate baseline functioning in a pooled analysis of 3 RTOG trials of concomitant chemoradiotherapy.2 Dysphagia is a consequence of persistent laryngopharyngeal toxicity, and is among the most commonly cited functional impairments after chemoradiotherapy3,4 with an estimated prevalence of 43% to 64%,5,6 and published rates of chronic gastrostomy dependence ranging from 4% to 26% dependent upon precise site and tumor stage.7-9 Thus, long-term swallowing outcomes are a key metric of functional success after combined therapy.
Although still investigational, sequential treatment with induction chemotherapy followed by radiotherapy for SCCHN has been shown to decrease the risk of distant metastases as a first site of tumor recurrence10 and may lead to favorable functional outcomes.11 In a phase II study previously reported, we treated patients with locally advanced nodal disease with induction chemotherapy consisting of weekly paclitaxel, carboplatin and cetuximab (PCC) followed by “risk-based” definitive treatment consisting of radiotherapy as a single modality, concomitant chemoradiotherapy, or surgical resection based on the site and stage of disease at diagnosis. Our hypothesis when we designed this study was that induction PCC followed by risk-based local therapy would achieve durable locoregional and distant disease control at 5-years with acceptable long-term functional outcomes. The early published results of this trial demonstrated a high overall response rate of 96% after 6 weekly cycles of PCC, and promising 3-year rates of progression-free and overall survival (87% and 91%, respectively).12 The objective of this current study is to evaluate long-term functional and survival outcomes of this trial.
METHODS
Eligibility and trial design
Forty-seven patients with previously untreated stage IV SCCHN (nodal-classification ≥N2b) were enrolled in a single-arm institutional phase II trial at The University of Texas MD Anderson Cancer Center (MDACC) between February, 2005 and December, 2005. Outcomes were ascertained through November, 2011. The informed consent and protocol were approved by the Institutional Review Board at The University of Texas MDACC.
Treatment protocol
Induction chemotherapy consisted of six weekly cycles of PCC (paclitaxel 135 mg/m2, carboplatin AUC 2, and cetuximab 400 mg/m2 in week 1 and then 250 mg/m2). The plan for local therapy was determined with respect to disease site and stage at diagnosis and included single modality radiation for primary T1-2 tumors, concurrent chemoradiation for T3-4 tumors, or surgery for primary tumors of the oral cavity. The protocol permitted adjustment of local treatment assignments in accordance with the treating physicians’ judgment, but the response to induction PCC did not dictate the local treatment assignment. Details of the induction PCC regimen, radiotherapy delivery, and concurrent therapy along with the CONSORT diagram (Figure 1) have been published previously.12 Patients were referred to Speech Pathology prior to definitive treatment for proactive swallowing therapy that included provision of swallowing exercises for patients to complete during radiotherapy. Patients were also referred for nutritional assessment by a Registered Dietitian prior to radiotherapy. Prophylactic feeding tubes were not placed unless nutritional compromise and/or dysphagia were identified in baseline assessments. A total of 33 patients (70%) received feeding tube placement. Of these, 26 patients required tube placement during radiotherapy.
Figure 1. CONSORT diagram.
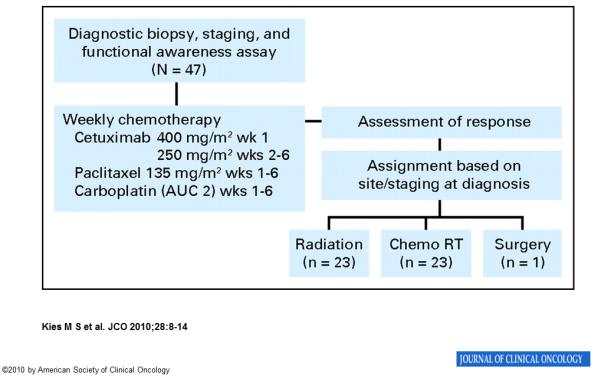
Abbreviations: AUC, area under the curve; chemoRT, chemoradiotherapy
Prospective functional assessment
Baseline and long-term (24-months) functional assessments were planned for all patients and included modified barium swallow (MBS) studies, the MD Anderson Dysphagia Inventory (MDADI), and the Performance Status Scale-Head and Neck (PSS-HN). The results of interim functional assessments at 6- and 12-months posttreatment intervals have been published previously.12
MBS Studies
Modified barium swallow (MBS) studies were conducted in standard format.13 Video-recordings of MBS studies were analyzed by blinded raters who met previously published reliability standards using two validated measures: Oropharyngeal Swallow Efficiency (OPSE) and the Penetration-Aspiration Scale (PAS). OPSE provides a continuous score (0 to ∞) as the ratio of the percent swallowed into the esophagus (100-[% residue + % aspiration]) divided by oropharyngeal transit time. Thus, a higher OPSE score indicates a safer and more efficient oropharyngeal swallow.14 OPSE scores were calculated independently for each bolus type (10-mL Varibar thin liquid, pudding, cracker), and averaged across consistencies to provide a global score of swallowing efficiency at baseline and 24 months assessments. Aspiration was rated according to the PAS on 10-mL volumes of Varibar thin liquid barium (PAS range: 0=normal, 8=silent aspiration; PAS ≥6 coded as aspiration).15
Questionnaires
Two questionnaires were administered at baseline and 24-months at the time of MBS studies: 1) the MD Anderson Dysphagia Inventory (MDADI), and 2) the Performance Status Scale-Head and Neck Cancer (PSS-HN). The MDADI is a validated 20-item measure of swallowing-related quality-of-life. MDADI global, composite, and subscale (Emotional, Physical, Functional) scores were calculated (range: 20 to 100); higher scores indicate superior perception of swallowing function. The composite MDADI score was chosen as the primary summary score of swallowing-related quality of life because it reflects overall performance on 19 MDADI items.16 The PSS-HN is a 3-item validated clinician-rated measure of disease-specific performance status based on semi-structured interview. Diet levels were rated according to the PSS HN Normalcy of Diet subscale, in which 0 indicates “non-oral nutrition” and 100 represents “full oral nutrition without restrictions”. Speech intelligibility was measured using the PSS-HN Understandability of Speech subscale, in which 0 indicates “never understandable” speech and 100 represents “always understandable” speech. Eating practices were rated according to the PSS-HN Public Eating subscale in which 0 indicates “always eats alone” and 100 means “no restriction of food, place, or companion”.17 The MDADI was completed by written questionnaire at the time of MBS studies, and the PSS-HN per interview by the speech pathologist conducting the MBS study. Patients who did not return for MBS studies at 24-months were mailed both questionnaires (MDADI and PSS-HN). Mailed PSSHN scores (n=6) did not significantly differ from clinician-rated PSS-HN scores at 24-months (p>0.05).
Final functional outcomes
Final functional outcomes were ascertained from the medical record of long-term disease-free survivors. Final diet level, gastrostomy dependence, tracheostomy dependence, and laryngectomy status were recorded a minimum of 5 years after enrollment. Medical records were also reviewed to identify cases of pneumonia per radiographic infiltrates and stricture per endoscopic examination, one year or more after treatment. A composite endpoint of chronic dysphagia was defined by chronic (≥ 1 year) aspiration or stricture per MBS, and/or permanent gastrostomy dependence. Osteoradionecrosis was graded per dental oncology records and radiographic imaging as: grade 1, minimal bone exposure with conservative management only; grade 2, minor debridement received; grade 3, hyperbaric oxygen needed; grade 4, major surgery required.18
Statistical methods
Descriptive statistics were calculated to describe the sample characteristics, toxicity, and functional outcomes. Survival distributions were estimated using the Kaplan-Meier method. Statistical differences between paired data were analyzed using the nonparametric sign-rank test. Statistical significance was considered α-level 0.05. Statistical analyses were performed using the STATA data analysis software, version 10.0 (StataCorp LP, College Station, TX).
RESULTS
Sample characteristics
Forty-seven patients with previously untreated stage IV SCCHN (nodal-classification ≥N2b) were enrolled. The median age was 53 years; 33 patients were male. Most had oropharyngeal tumors (41/47, 87.2%), and 63.8% (30/47) had T1 or T2 primary tumors. Local therapy after induction PCC included single modality radiation (23/47, 48.9%), concurrent chemoradiation (23/47, 48.9%), or surgery with adjuvant radiotherapy (1/47, 2.1%). Table 1 describes patient and treatment characteristics.
Table 1.
Patient Characteristics (n=47)
| No. of patients (%) | |
|---|---|
| Sex | |
| Male | 33 (70.2) |
| Female | 14 (29.8) |
| Primary site | |
| Oral cavity | 1 (2.1) |
| Nasopharynx | 1 (2.1) |
| Oropharynx | 41 (87.2) |
| Hypopharynx | 2 (4.3) |
| Larynx (supraglottic) | 2 (4.3) |
| T-classification | |
| 1 | 16 (34.0) |
| 2 | 14 (29.8) |
| 3 | 12 (25.5) |
| 4 | 5 (10.6) |
| N-classification | |
| 2b | 30 (65.2) |
| 2c | 13 (28.3) |
| 3 | 3 (6.5) |
| X | 1 (2.1) |
| Local treatment after induction | |
| Radiotherapy alone | 23 (48.9) |
| Concurrent chemoradiotherapy | 23 (48.9) |
| Surgery and radiotherapy | 1 (2.1) |
| Smoking History at Diagnosis | |
| Never | 18 (38.3) |
| Former | 16 (34.0) |
| Current | 13 (27.7) |
| HPV status | |
| HPV+* | 12 (25.5) |
| HPV− | 14 (29.8) |
| Not tested | 21 (44.7) |
HPV-16 positive tumor specimen per in situ hybridization
Survival outcomes
The 5-year overall survival rate was 89% (95% CI: 81%-99%), with median follow-up of 5.9 years. No patient developed new locoregional recurrence or distant metastasis since the initial report of 3-year outcomes.12 One patient died of disease 3.9 years after enrollment and 1 patient developed a second primary spindle cell sarcoma of the scalp 5.5 years after enrollment. Figure 2 shows overall survival.
Figure 2.
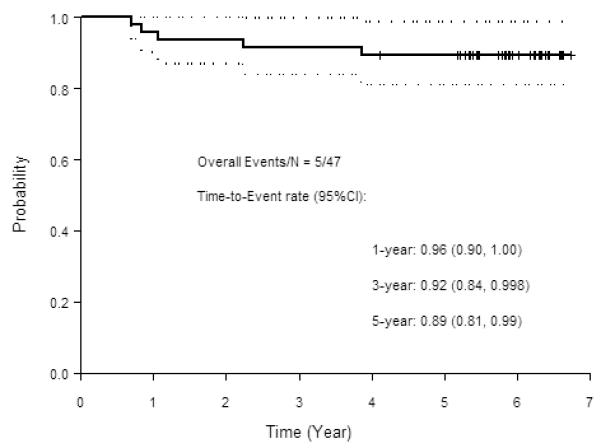
Overall survival (OS)
Longitudinal swallowing data (baseline to 24-months)
Swallowing data prospectively collected at baseline and 2 years are summarized in Table 2. Baseline data from MBS studies were available in all 47 patients, and in 26 of 42 (62%) long-term survivors at 24-months. MBS studies found a non-significant 13% average reduction in swallowing efficiency (OPSE) across all bolus consistencies at 24-months relative to baseline (baseline: 92.7±20.1; 24-months: 80.7±28.4; p=0.191). Only pudding OPSE scores were significantly lower at 24-months (baseline: 93.4±29.2; 24-months: 75.8±35.7; p=0.035), owing to greater percentages of pharyngeal residue. Similarly, mean composite MDADI scores approximated baseline values at 24-months (baseline: 83.8±14.3; 24-months=83.0±12.9; p=0.101), but 24-month physical subscale MDADI scores were significantly lower than baseline (baseline: 84.4±19.2; 24-months: 78.5±16.3; p=0.001). Figure 3 illustrates change in OPSE per MBS and MDADI scores from baseline to 2 years.
Table 2.
Results of Prospective Swallowing Assessments at Baseline and 24-months Follow-up (n = 47)
| Baseline | 24-mos. | % change | p | |
|---|---|---|---|---|
| n (eligible for functional assessment) | 47 | 42 | ||
| n (MBS) | 47 | 26 (61.9%) | ||
| n (Questionnaire)* | 46 | 36 (85.7%) | ||
| Median (range) mos. post-RT | -- | 24 (22-37) | ||
| OPSE, mean (SD) | ||||
| 10 mL liquid | 99.2 (23.9) | 87.8 (23.7) | −11.5% | 0.713 |
| Pudding | 93.4 (29.2) | 75.8 (35.7) | −18.8% | 0.035 |
| Cracker | 87.9 (34.7) | 81.1 (40.3) | −7.7% | 0.638 |
| All consistencies | 92.7 (20.1) | 80.7 (28.4) | −12.9% | 0.191 |
| Aspiration, No. of patients(%) | ||||
| 10 mL liquid | 1 (2.0%) | 2 (7.6%) | −- | |
| Pen-Asp Scale, median (Range) | ||||
| 10 mL liquid | 1 (1-8) | 1 (1-7) | -- | 0.040 |
| MDADI, mean (SD) | ||||
| Global score | 86.1 (22.3) | 86.7 (22.4) | 0.7% | 0.301 |
| Composite score | 83.8 (14.3) | 83.0 (12.9) | 1.0% | 0.101 |
| Emotional subscale | 84.9 (12.9) | 86.5 (12.8) | 1.9% | 0.959 |
| Functional subscale | 81.7 (12.6) | 85.4 (12.8) | 4.5% | 0.408 |
| Physical subscale | 84.4 (19.2) | 78.5 (16.3) | −7.0% | 0.001 |
| Feeding Tube, No. of patients (%) | ||||
| No | 43 (91.5%) | 35 (93.3%) | -- | |
| Yes | 4 (8.5%) | 1 (2.8%) | -- | 0.564 |
| PSS-HN Normalcy of Diet, No. of patients (%) | ||||
| Full diet, no restriction (PSSHN=100) | 35 (74.5%) | 10 (27.8%) | -- | |
| Full diet, liquid assist (PSSHN=90) | 2 (4.3%) | 20 (55.5%) | -- | |
| Solids, w/ restrictions (PSSHN=50-80) | 5 (10.6%) | 5 (13.8%) | -- | |
| Non-chewable/pureed foods (PSSHN=30-40) | 3 (6.4%) | 1 (2.8%) | -- | |
| Liquids only (PSSHN=10-20) | 0 | 0 | -- | |
| Non-oral feeding (PSSHN=0) | 2 (4.3%) | 0 | -- | |
| Median (range) PSSHN score | 100 (0-100) | 90 (30-100) | -- | 0.101 |
| PSS-HN Eating in Public | ||||
| Median (range) PSSHN score | 100 (0-100) | 100 (25-100) | -- | 0.253 |
| PSS-HN Understandability of Speech | ||||
| Median (range) PSSHN score | 100 (50- 100) |
100 (75-100) | -- | 0.586 |
Questionnaires (MDADI and PSS-HN) mailed to patients who missed MBS; OPSE, aspiration, Penetration-Aspiration Scale per analysis of MBS studies.
Abbreviations: MBS, modified barium swallow, OPSE, oropharyngeal swallowing efficiency, MDADI, MD Anderson Dysphagia Inventory, PSS-HN, Performance Status Scale-Head and Neck
Figure 3. Results of prospective 24-months swallowing measures.
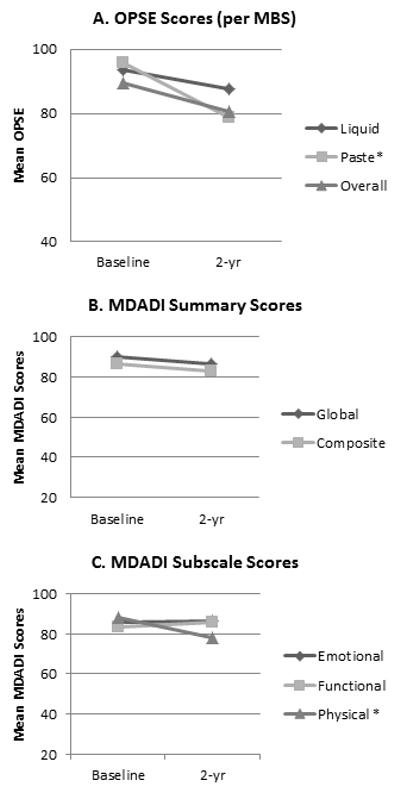
*Significantly lower OPSE scores on paste bolus during MBS (p=0.035) and on physical subscale per MDADI questionnaire (p=0.001) at 2 years relative to baseline.
Abbreviations: OPSE, Oropharyngeal Swallowing Efficiency; MBS, modified barium swallow study; MDADI, MD Anderson Dysphagia Inventory
Final functional outcomes and late toxicity in long-term survivors
Final functional outcomes are summarized in Table 3. Functional outcomes were evaluable in 42 long-term survivors (89.4%) at a median follow-up time of 5.9 years. Overall, 3 (7.1%) long-term survivors (2 hypopharynx/supraglottic, 1 tonsil) had chronic dysphagia defined by either chronic aspiration per MBS and/or permanent gastrostomy dependence, 2 of whom had baseline dysphagia per MBS studies. Among chronic dysphagia cases, 2 patients (2/42, 4.8%) experienced recurrent episodes of pneumonia in long-term follow-up (>12 months post treatment); 1 of whom had significant baseline tumor-related dysphagia and the other developed progressive pharyngeal dysphagia within the first year of treatment. The rate of final gastrostomy dependence was 4.8% (2/42) and 92.9% (39/42) tolerated a soft or regular oral diet.
Table 3.
Final Functional Outcomes in Long-term Survivors (n=42)
| All patients No. of patients (%) |
Oropharynx patients No. of patients (%) |
|
|---|---|---|
| Diet * | ||
| NPO | 1 (2.4) | 1 (2.6) |
| TF + PO | 1 (2.4) | 0 (0) |
| Liquid only | 1 (2.4) | 1 (2.6) |
| Soft | 5 (11.9) | 5 (12.8) |
| Regular/Full | 34 (81.0) | 32 (82.1) |
| Feeding-tube dependent * | 2 (4.8) | 1 (2.6) |
| Laryngectomy * | 1 (2.4) | 1 (2.6) |
| Tracheostomy dependent * | 0 (0) | 0 (0) |
| Pneumonia ** | 2 (4.8) | 1 (2.6) |
| Stricture ** | 0 (0) | 0 (0) |
| Chronic dysphagia *** | 3 (7.1) | 1 (2.6) |
| Total | 42 | 39 |
Rated at last follow-up
12 months or more post-treatment
Composite endpoint defined by chronic aspiration or stricture per MBS, and/or permanent gastrostomy
One patient with a base of tongue primary tumor required tracheostomy for stridor 19 months after chemoradiation; this patient was subsequently found to have laryngeal carcinoma in situ and underwent total laryngectomy 2 months later. No patient required permanent tracheostomy. No patient required total laryngectomy for swallowing dysfunction, and none had chronic stricture. Two patients (4.2%) developed early post-treatment esophageal stricture, and both were managed successfully with dilation within the first year of treatment. No cases of late-onset dysphagia or stricture were observed at last follow-up.
Notably, acute grade 2 peripheral neuropathy affected 15% of patients with a single patient sustaining grade 3. However, only 3 long-term survivors had persistent peripheral neuropathy (grade 1 = 2 patients; and grade 2 = 1 patient). Performance status (Zubrod) at last follow-up was 0 in 25 patients, 1 in 13 patients, and 2 in 4 patients.
Four patients (9.5%) developed mandibular osteoradionecrosis. Two resolved after multiple sequestrectomies (grade 2), 1 resolved with hyperbaric oxygen treatment (grade 3), and one required mandibulectomy with free flap reconstruction (grade 4). No patients developed secondary radiation-related tumors.
Final functional outcomes in long-term oropharyngeal cancer survivors
The majority of long-term survivors (39/42) had oropharyngeal primary tumors. Final functional outcomes for the cancer survivors are presented in Table 3. Only 1 (2.6%) long-term oropharyngeal cancer survivor (T3N2b SCCA tonsil with parapharyngeal space, pharyngeal wall and tongue base extension) had chronic dysphagia. This patient had evidence of tumor-related dysphagia and aspiration on baseline MBS that progressed on post-treatment studies.
DISCUSSION
The long-term results of this phase II trial are favorable with respect to disease control, but also demonstrate encouraging long-term functional outcomes. We have observed a 5-year overall survival rate of 89% after induction PCC followed by risk-based local therapy for patients with locally advanced (N-classification ≥2b) SCCHN. Cetuximab12 was added to a 6-week regimen of induction paclitaxel and carboplatin,19 and treatment planning allowed for individualized local therapy based on disease-site and stage at diagnosis. Early results of this trial12 demonstrated 3-year overall survival of 91% and moderate acute toxicities, most commonly grade 2 or 3 rash/folliculitis after induction PCC (83% of patients) and grade 2 or 3 mucositis attributed to definitive RT or CRT (92% of patients). Herein, we describe durable locoregional control at 5 years with acceptable long-term toxicity.
Very encouraging long-term functional outcomes were observed in 42 five-year survivors after induction PCC and local therapy. Prospective functional assessments found modest, non-significant changes in global swallowing metrics (i.e., overall OPSE and composite MDADI) at 2 years, and chronic aspiration and pneumonia were rare events (<5% of patients). Together, the functional outcomes we observed compare favorably to rates of aspiration (24% to 64%) and gastrostomy dependence (4% to 26%) reported after chemoradiotherapy.7-9
Statistically significant differences identified on subscales of OPSE (paste bolus) and MDADI (physical subscale) evaluations are indicative of decreased swallowing efficiency 2 years after treatment relative to baseline performance. That is, pharyngeal residue resulting from physiologic impairments led to significantly lower OPSE scores for paste swallows despite non-significant changes for liquid swallows and airway protection. Likewise, among the MDADI subscales, only the physical domain scores (example items: “swallowing takes great effort”, “it takes me longer to eat because of my swallowing problem”) were significantly lower than baseline at 2 years. Near-baseline performance on other MDADI domains suggests that patients might develop coping strategies that lessen the social and functional impact of dysphagia over time, despite persistent physical differences in swallowing abilities. Jointly, long-term performance on OPSE and MDADI suggest acceptable changes in swallowing efficiency for most patients with adjustments and adaptations to “new normal” levels of functioning.
Functional outcomes are influenced by a number of patient, clinical, and treatment factors. Baseline dysfunction, laryngeal or hypopharyngeal tumors, and T4 staging have been shown to portend unfavorable functional outcomes after organ preservation.20-22 A majority of patients in this trial (64%) had T1-T2 disease, few (9%) had laryngeal or hypopharyngeal tumors, and only 2 (4%) had MBS-evidence of baseline aspiration, explaining in part the satisfactory long-term functional outcomes we observed. Local therapy decisions likely also contributed to better than expected long-term functioning after the induction PCC regimen. There is evidence that induction chemotherapy does not adversely impact swallowing physiology, and notably may improve important aspects of functioning including diet levels and swallowing-related quality of life prior to local therapy.11 Conversely, both clinical and population-based studies have found highest rates of dysphagia after concurrent chemoradiotherapy.6,23 The risk-based approach used to select definitive local therapy at diagnosis successfully avoided concurrent chemotherapy in almost half of patients enrolled. In addition, our institution commonly employs a split-beam IMRT approach for oropharynx cancers that has been shown to achieve a lower laryngeal and esophageal inlet dose in comparison to full IMRT fields and may be associated with low rates of aspiration in this trial.24-25 Finally, prophylactic swallowing therapy was provided as a standard of care to patients enrolled in this trial, and included provision of targeted swallowing exercises and avoidance of NPO intervals during treatment.3 It is likely that complex interactions between baseline function, tumor burden, supportive care, and the intensity of local therapy influenced our functional outcomes, and these associations require further assessment.
Favorable early results of this trial were maintained at 5 years, with a 5-year OS rate of 89% after induction PCC and risk-based local therapy. However, induction chemotherapy as a component of sequential treatment for selected patients with locally advanced squamous head and neck cancer continues under study. Two prospective trials,26-27 with patients randomized to receive docetaxel, cisplatin and infusional fluorouracil (TPF) or not followed by concomitant chemoradiotherapy, were presented at the 2012 American Society of Oncology general meeting this year. There was no reported significant difference in tumor control or overall survival between the experimental and control groups. This, in part, may have been related to better than expected outcomes in the control chemoradiotherapy arms. Tumor testing for HPV-positivity was not required in these studies and the influence of this factor on results is not known. The indication for use of induction chemotherapy remains a study question.
In conclusion, we observed modest changes in swallowing efficiency 2 years after treatment, and a low prevalence of chronic aspiration and gastrostomy-dependence after sequential chemoradiotherapy for patients with locally advanced SCCHN, mainly of oropharyngeal origin. A comparative phase 2 trial is ongoing to further investigate the efficacy of PCC versus docetaxel, cisplatin, infusional fluorouracil and cetuximab28 followed by risk-based local therapy in patients with squamous cancers of the head and neck of any primary site, but again with advanced cervical nodal disease. HPV status is a stratification factor and affects planning for definitive radiotherapy. HPV-positive patients with T1-3 staging receive radiotherapy as a single modality. We continue to study sequential chemoradiotherapy for subgroups of patients at moderate to high risk of developing distant metastases with attention to overall disease control and maintenance of function.
Acknowledgments
Supported by peer-reviewed funding from Specialized Program of Research Excellence in Head and Neck Cancer Grant No. P50 CA97007 from the National Cancer Institute and the “Clinician Investigator Program in Translational Research” Grant No. K12 CA88084 (F.C.H.).
Additional support was provided by The University of Texas MD Anderson Cancer Center Support Grant No. CA 16672, Bristol-Myers Squibb Oncology Investigator Initiated Trials program and Grant No. CS 2004-00011435 WC from Imclone Systems.
Additional support was provided by the UT Health Innovation for Cancer Prevention Research Fellowship, The University of Texas School of Public Health – Cancer Prevention and Research Institute of Texas (CPRIT) grant #RP101503 (K.A.H).
The authors thank Ms. Bich Tran for help in preparation of the manuscript.
REFERENCES
- 1.Cooper JS, Porter K, Mallin K, et al. National Cancer Database report on cancer of the head and neck: 10-year update. Head Neck. 2009;31:748–58. doi: 10.1002/hed.21022. [DOI] [PubMed] [Google Scholar]
- 2.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26:3582–9. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenthal DI, Lewin JS, Eisbruch A. Prevention and treatment of dysphagia and aspiration after chemoradiation for head and neck cancer. J Clin Oncol. 2006;24:2636–2643. doi: 10.1200/JCO.2006.06.0079. [DOI] [PubMed] [Google Scholar]
- 4.Eisbruch A, Lyden T, Bradford CR, et al. Objective assessment of swallowing dysfunction and aspiration after radiation concurrent with chemotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2002;53:23–8. doi: 10.1016/s0360-3016(02)02712-8. [DOI] [PubMed] [Google Scholar]
- 5.Caudell JJ, Schaner PE, Meredith RF, et al. Factors associated with long-term dysphagia after definitive radiotherapy for locally advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2009;73:410–5. doi: 10.1016/j.ijrobp.2008.04.048. [DOI] [PubMed] [Google Scholar]
- 6.Francis DO, Weymuller EA, Jr., Parvathaneni U, Merati AL, Yueh B. Dysphagia, stricture, and pneumonia in head and neck cancer patients: does treatment modality matter? Ann Otol Rhinol Laryngol. 2010;119:391–7. doi: 10.1177/000348941011900605. [DOI] [PubMed] [Google Scholar]
- 7.Lefebvre JL, Rolland F, Tesselaar M, et al. Phase 3 randomized trial on larynx preservation comparing sequential vs alternating chemotherapy and radiotherapy. J Natl Cancer Inst. 2009;101:142–52. doi: 10.1093/jnci/djn460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garden AS, Harris J, Trotti A, et al. Long-term results of concomitant boost radiation plus concurrent cisplatin for advanced head and neck carcinomas: a phase II trial of the radiation therapy oncology group (RTOG 99-14) Int J Radiat Oncol Biol Phys. 2008;71:1351–5. doi: 10.1016/j.ijrobp.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Givens DJ, Karnell LH, Gupta AK, et al. Adverse events associated with concurrent chemoradiation therapy in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009;135:1209–17. doi: 10.1001/archoto.2009.174. [DOI] [PubMed] [Google Scholar]
- 10.Brockstein B, Haraf DJ, Rademaker AW, et al. Patterns of failure, prognostic factors and survival in locoregionally advanced head and neck cancer treated with concomitant chemoradiotherapy: a 9-year, 337 patient, multi-institutional experience. Ann Oncol. 2004;15:1179–86. doi: 10.1093/annonc/mdh308. [DOI] [PubMed] [Google Scholar]
- 11.Barringer DA, Hutcheson KA, Sturgis EM, Kies MS, Lewin JS. Effect of induction chemotherapy on speech and swallowing function in patients with oral tongue cancer. Head Neck. 2009;31:611–7. doi: 10.1002/hed.20989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kies MS, Holsinger FC, Lee JJ, et al. Induction chemotherapy and cetuximab for locally advanced squamous cell carcinoma of the head and neck: results from a phase II prospective trial. J Clin Oncol. 2010;28:8–14. doi: 10.1200/JCO.2009.23.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Logemann J. A Manual for Videofluoroscopic Swallow Studies. 2nd Pro-Ed; Austin, TX: 1993. [Google Scholar]
- 14.Rademaker AW, Pauloski BR, Logemann JA, Shanahan TK. Oropharyngeal swallow efficiency as a representative measure of swallowing function. J Speech Hear Res. 1994;37:314–25. doi: 10.1044/jshr.3702.314. [DOI] [PubMed] [Google Scholar]
- 15.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–8. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 16.Chen AY, Frankowski R, Bishop-Leone J, et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg. 2001;127:870–6. [PubMed] [Google Scholar]
- 17.List MA, Ritter-Sterr C, Lansky SB. A performance status scale for head and neck cancer patients. Cancer. 1990;66:564–9. doi: 10.1002/1097-0142(19900801)66:3<564::aid-cncr2820660326>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 18.Tsai CJ, Hofstede TM, Sturgis EM, et al. Osteoradionecrosis and Radiation Dose to the Mandible in Patients With Oropharyngeal Cancer. Int J Radiat Oncol Biol Phys. 2012 Jul 12; doi: 10.1016/j.ijrobp.2012.05.032. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Vokes EE, Stenson K, Rosen FR, et al. Weekly carboplatin and paclitaxel followed by concomitant paclitaxel, fluorouracil, and hydroxyurea chemoradiotherapy: curative and organ-preserving therapy for advanced head and neck cancer. J Clin Oncol. 2003;21:320–6. doi: 10.1200/JCO.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Logemann JA, Rademaker AW, Pauloski BR, et al. Site of disease and treatment protocol as correlates of swallowing function in patients with head and neck cancer treated with chemoradiation. Head Neck. 2006;28:64–73. doi: 10.1002/hed.20299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutcheson KA, Barringer DA, Rosenthal DI, May AH, Roberts DB, Lewin JS. Swallowing outcomes after radiotherapy for laryngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2008;134:178–83. doi: 10.1001/archoto.2007.33. [DOI] [PubMed] [Google Scholar]
- 22.Solares CA, Wood B, Rodriguez CP, et al. Does vocal cord fixation preclude nonsurgical management of laryngeal cancer? Laryngoscope. 2009;119:1130–4. doi: 10.1002/lary.20225. [DOI] [PubMed] [Google Scholar]
- 23.Hutcheson KA, Lewin JS. Functional Outcomes after Chemoradiotherapy of Laryngeal and Pharyngeal Cancers. Curr Oncol Rep. 2012 doi: 10.1007/s11912-012-0216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz DL, Hutcheson K, Barringer D, et al. Candidate dosimetric predictors of long-term swallowing dysfunction after oropharyngeal intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:1356–65. doi: 10.1016/j.ijrobp.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: early dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2007;68:1289–98. doi: 10.1016/j.ijrobp.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 26.Haddad RI, Rabinowits G, Tisher RB. The PARADIGM trial: A phase III study comparing sequential therapy (ST) to concurrent chemoradiotherapy (CRT) in locally advanced head and neck cancer (LANHC). Presented at the American Society of Clinical Oncology Annual Meeting; Chicago, IL. 2012 May 30. [Google Scholar]
- 27.Cohen EE, Karrison T, Kocherginsky M. DeCide: A phase III randomized trial of docetaxel (d), cisplatin (P), 5-fluorouracil (F) (TPF) induction chemotherapy (IC) in patients with N2/N3 locally advanced squamous cell carcinoma of the head and neck (SSCHN). Presented at the American Society of Clinical Oncology Annual Meeting; Chicago, IL. 2012 May 30. [Google Scholar]
- 28.Haddad RI, Tishler RB, Norris C, et al. Phase I study of C-TPF in patients with locally advanced squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27:4448–53. doi: 10.1200/JCO.2009.22.1333. [DOI] [PubMed] [Google Scholar]


